Effect of Heat Treatment Temperature on the Microstructure and Properties of Titanium-Clad Steel Plate Prepared by Vacuum Hot Rolling
Abstract
1. Introduction
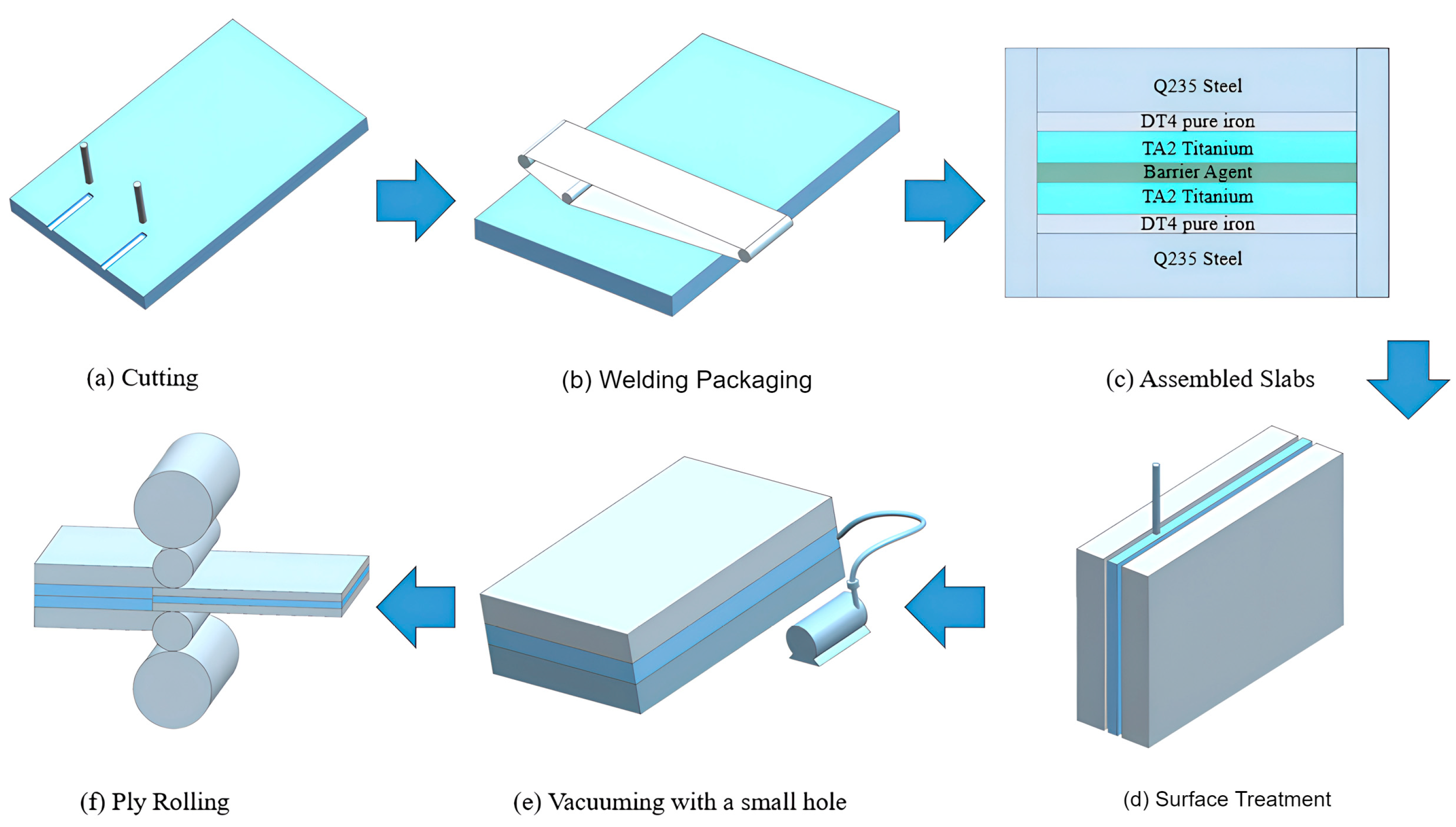
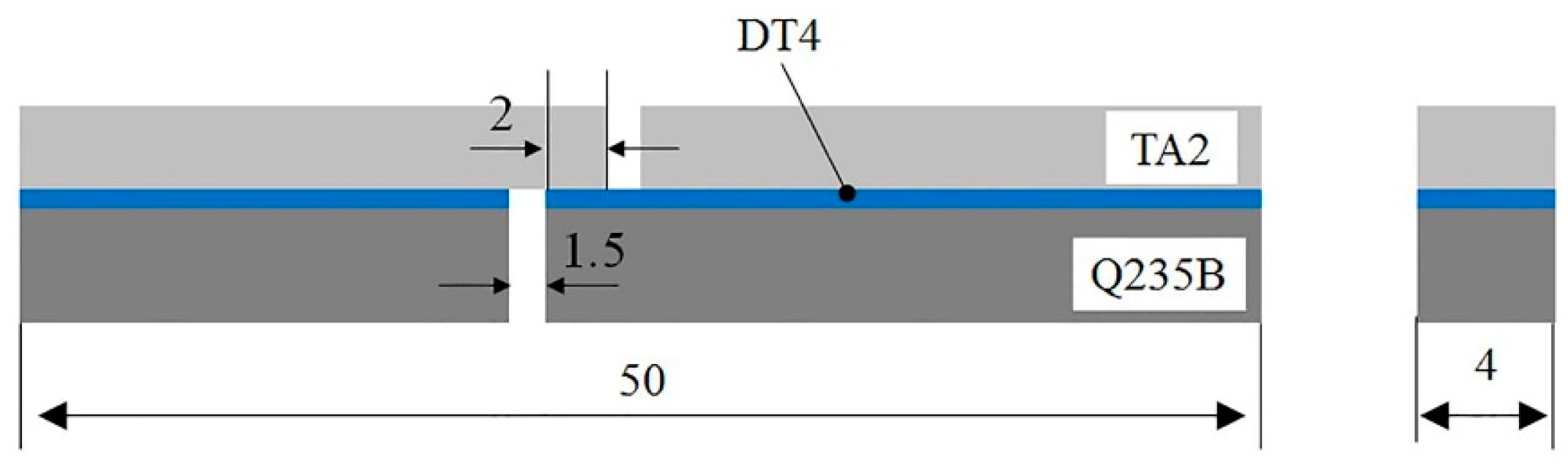
2. Experimental Materials and Methods
3. Results and Discussion
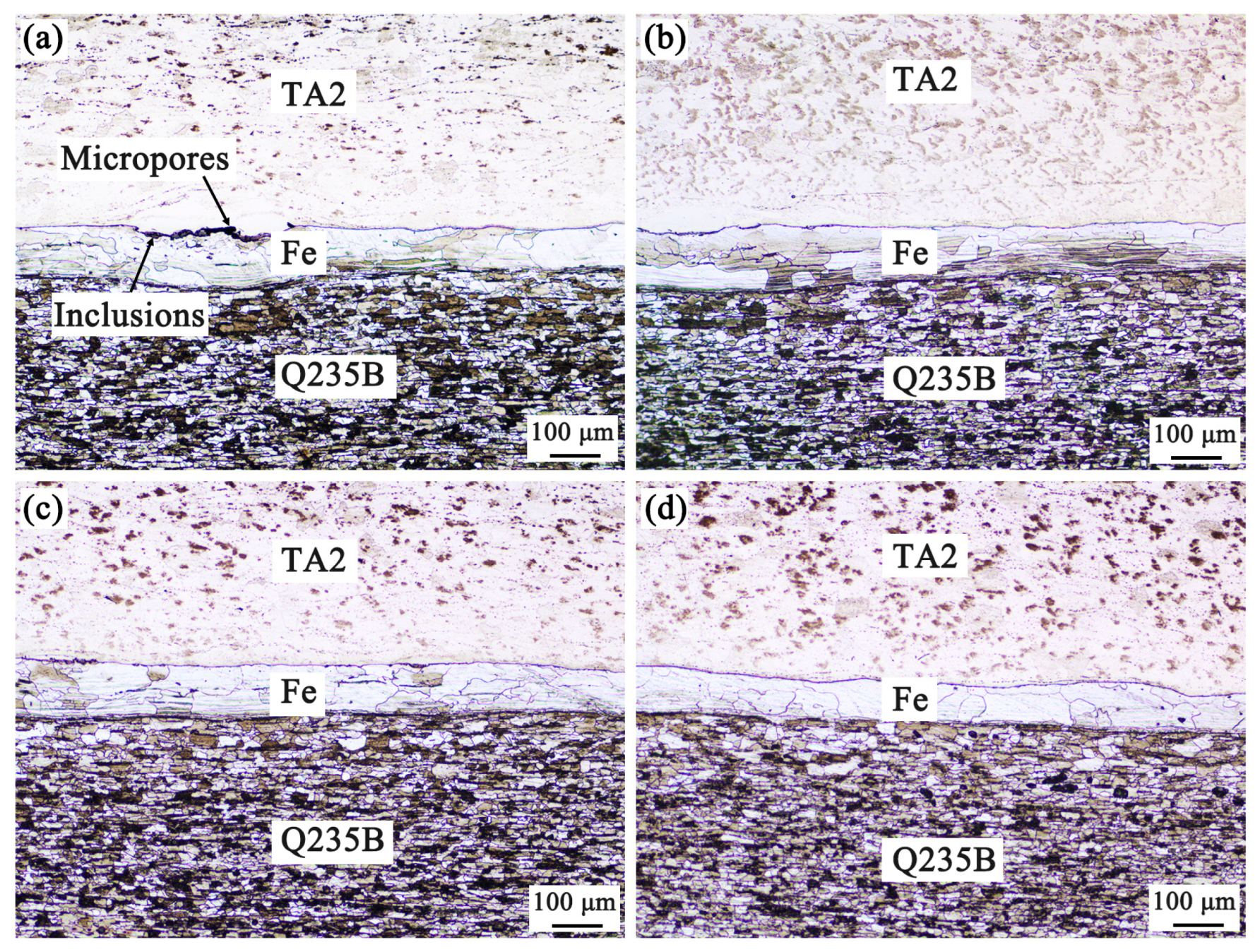
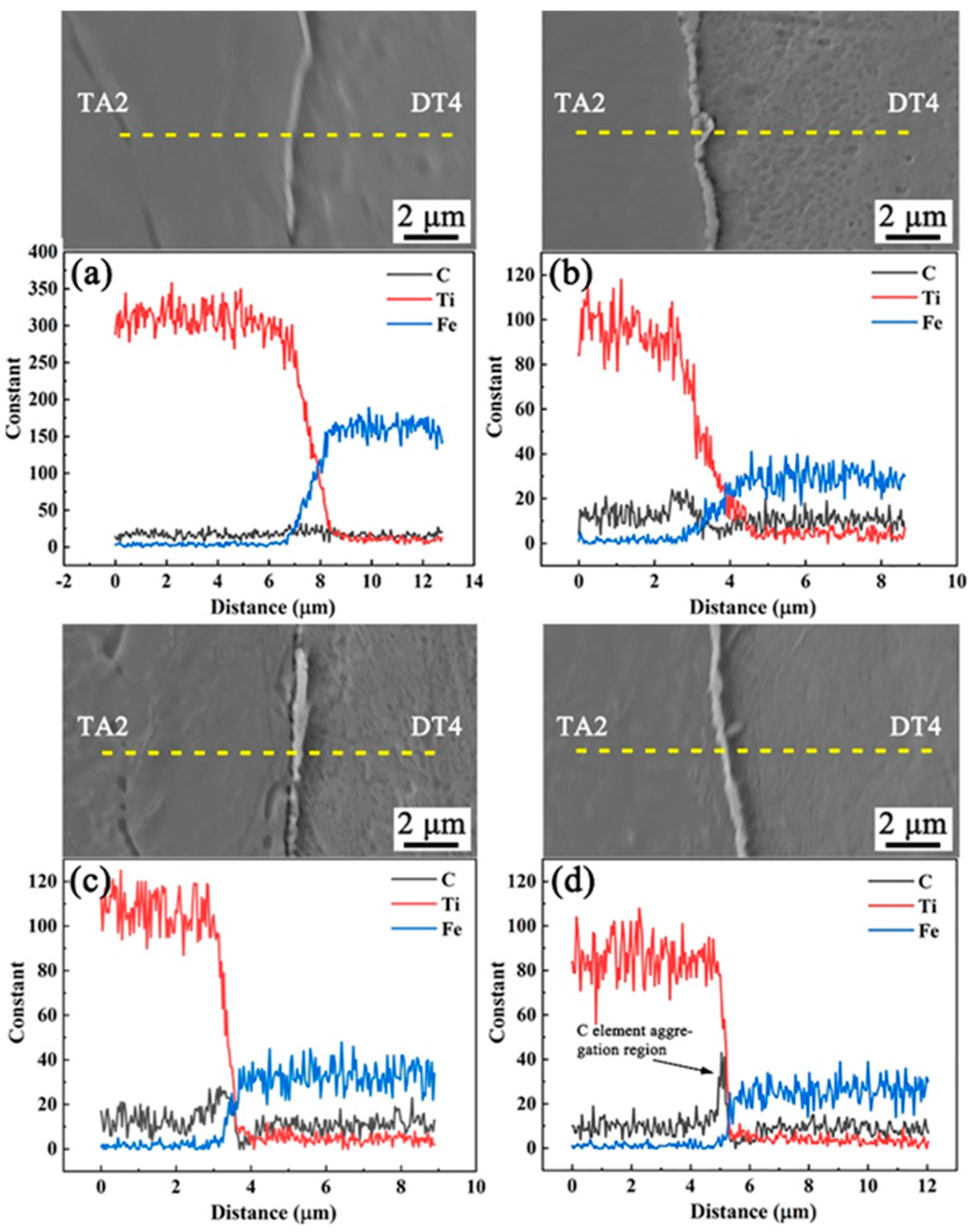
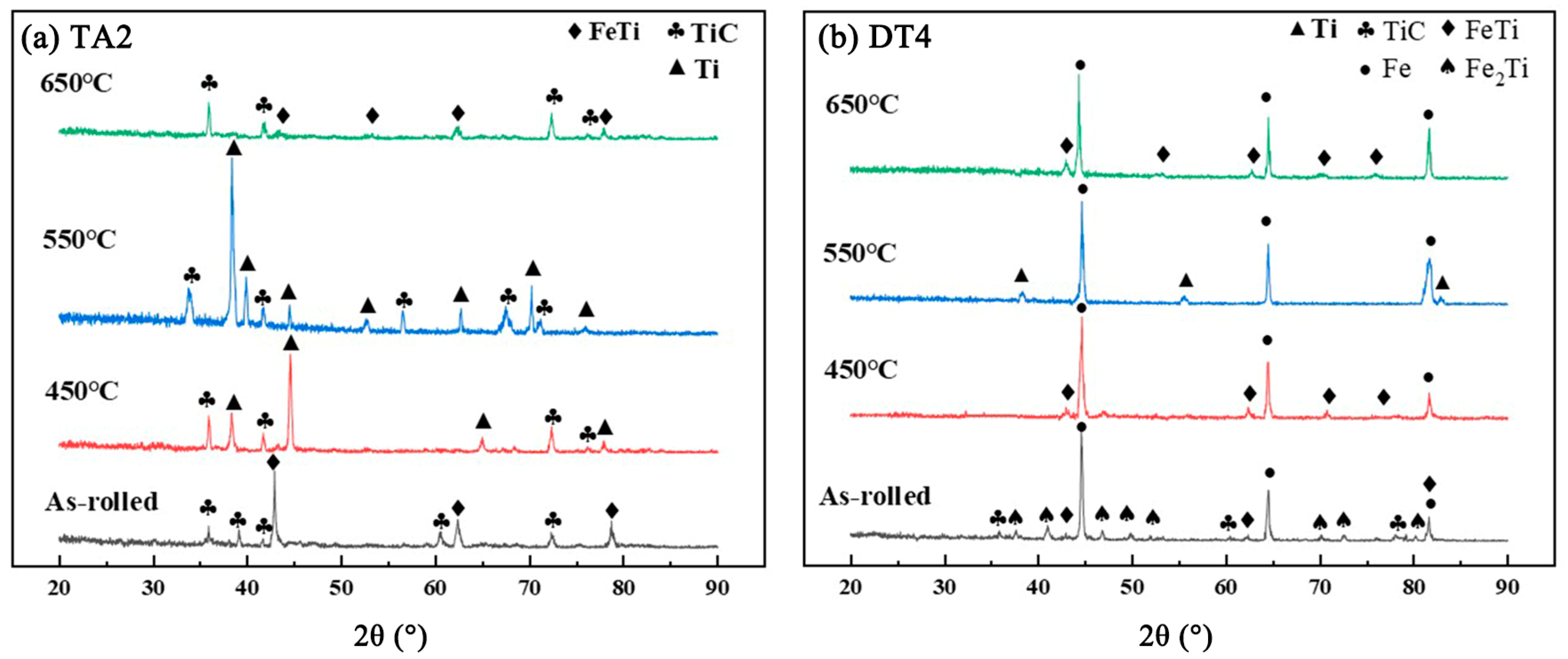
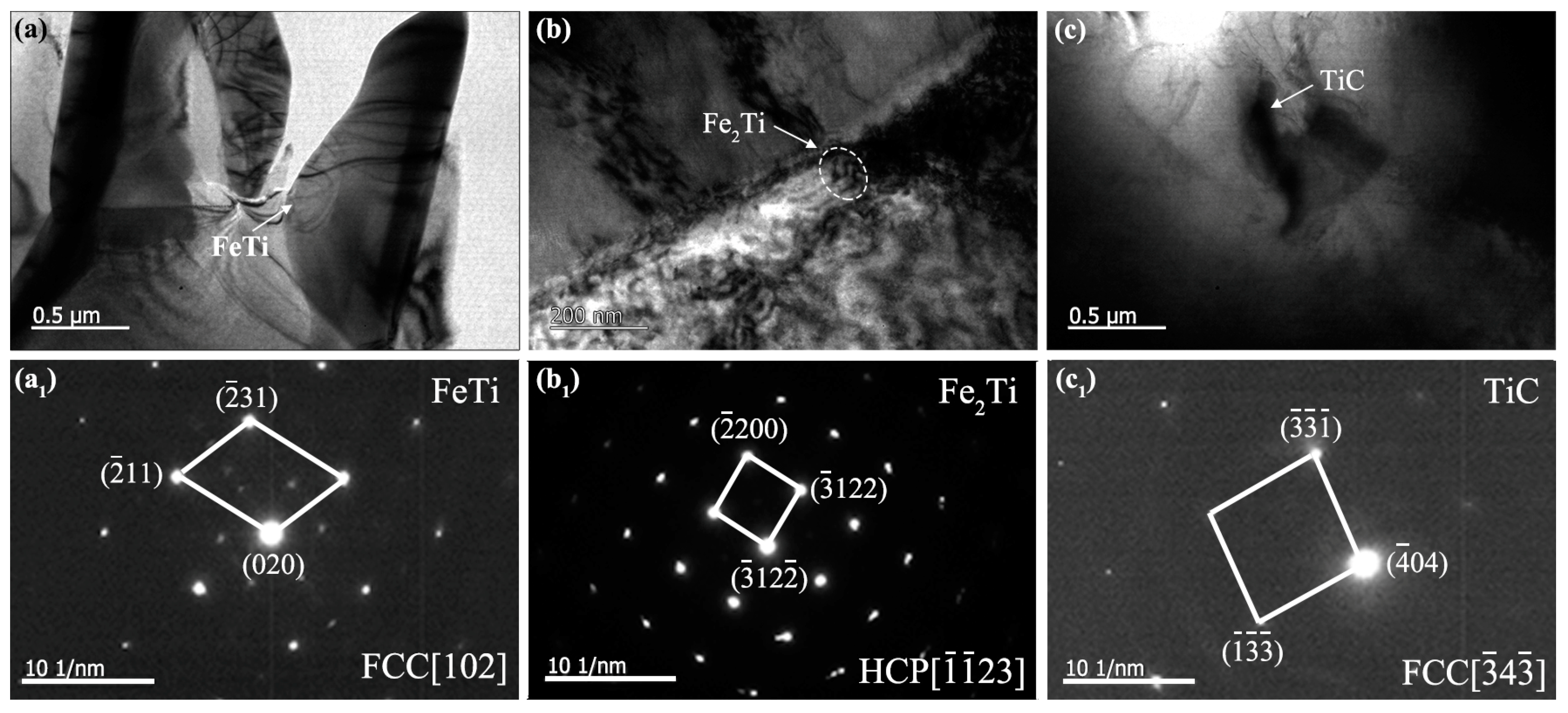
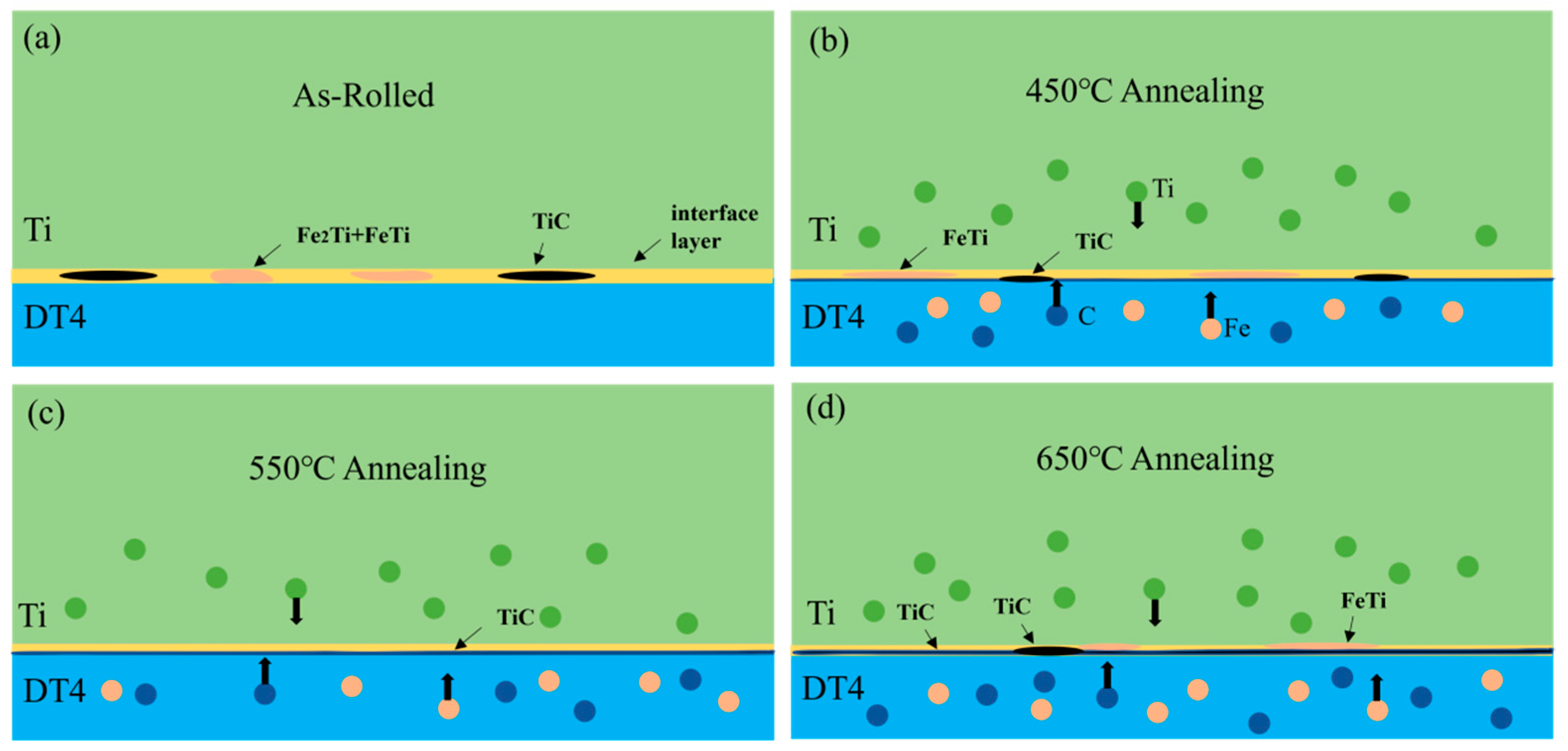
3.1. Microstructure Analysis of Titanium-Clad Steel Plates
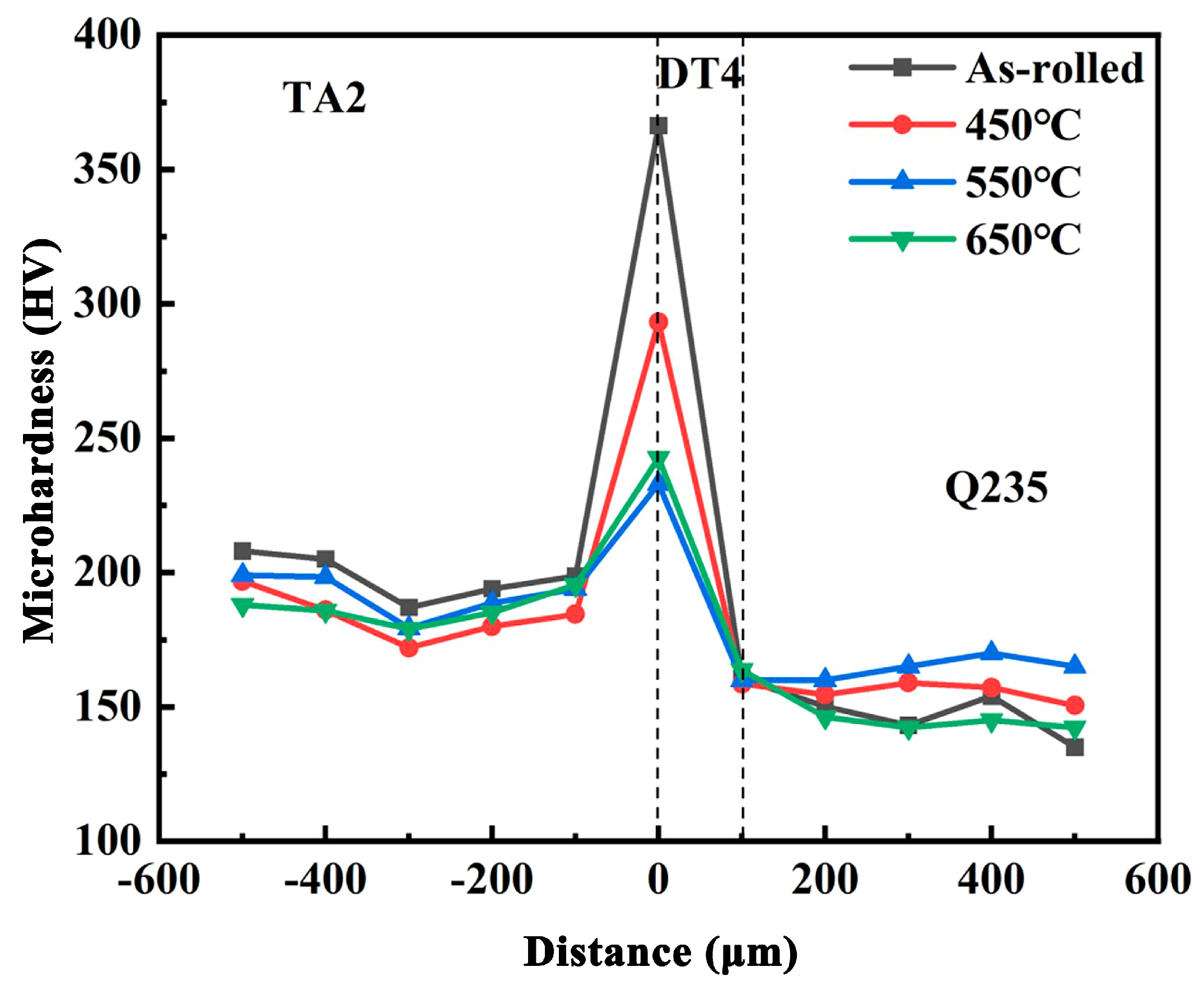
3.2. Microhardness Analysis of Titanium-Clad Steel Plates
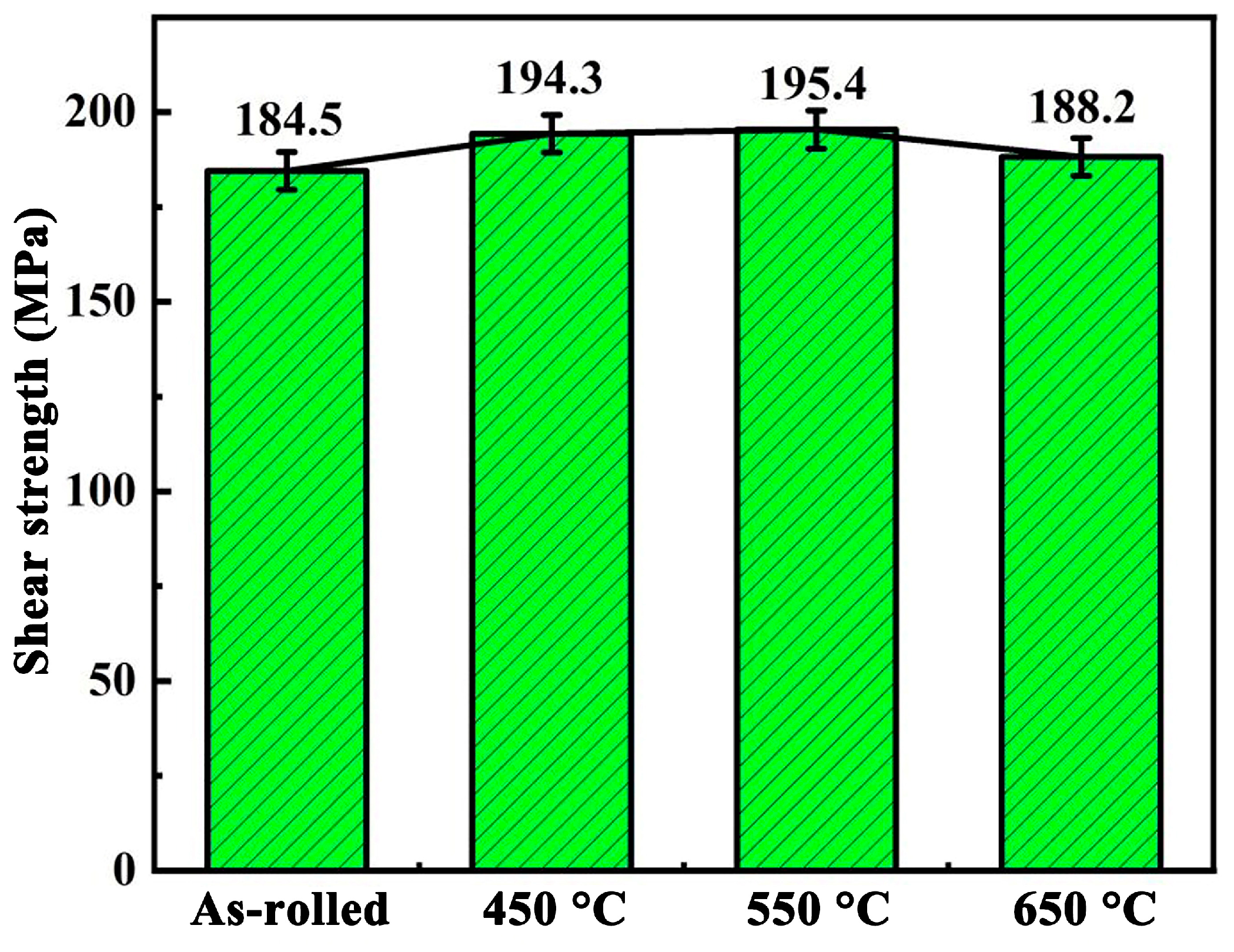
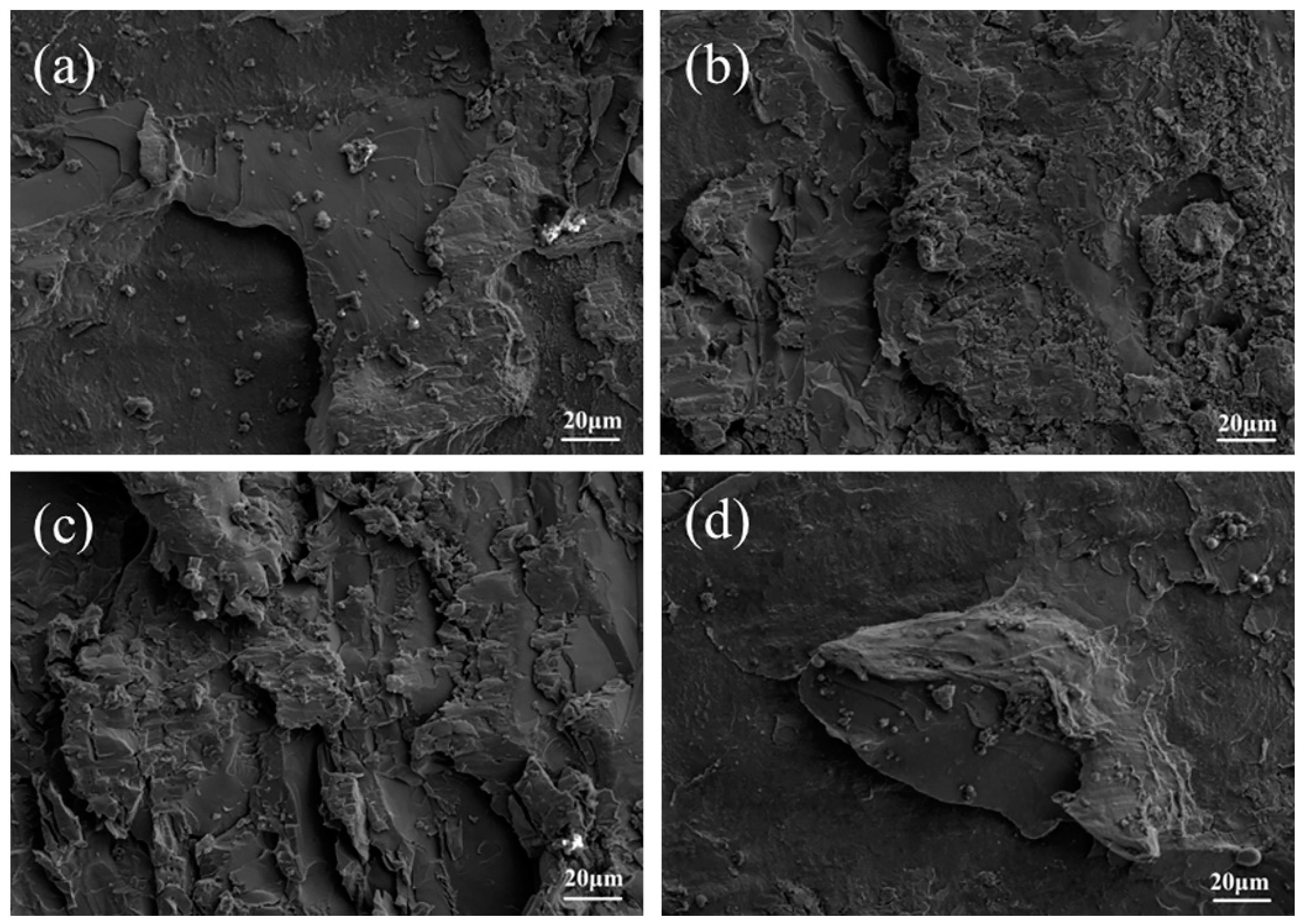
3.3. Analysis of the Shear Strength of Titanium-Clad Steel Plates
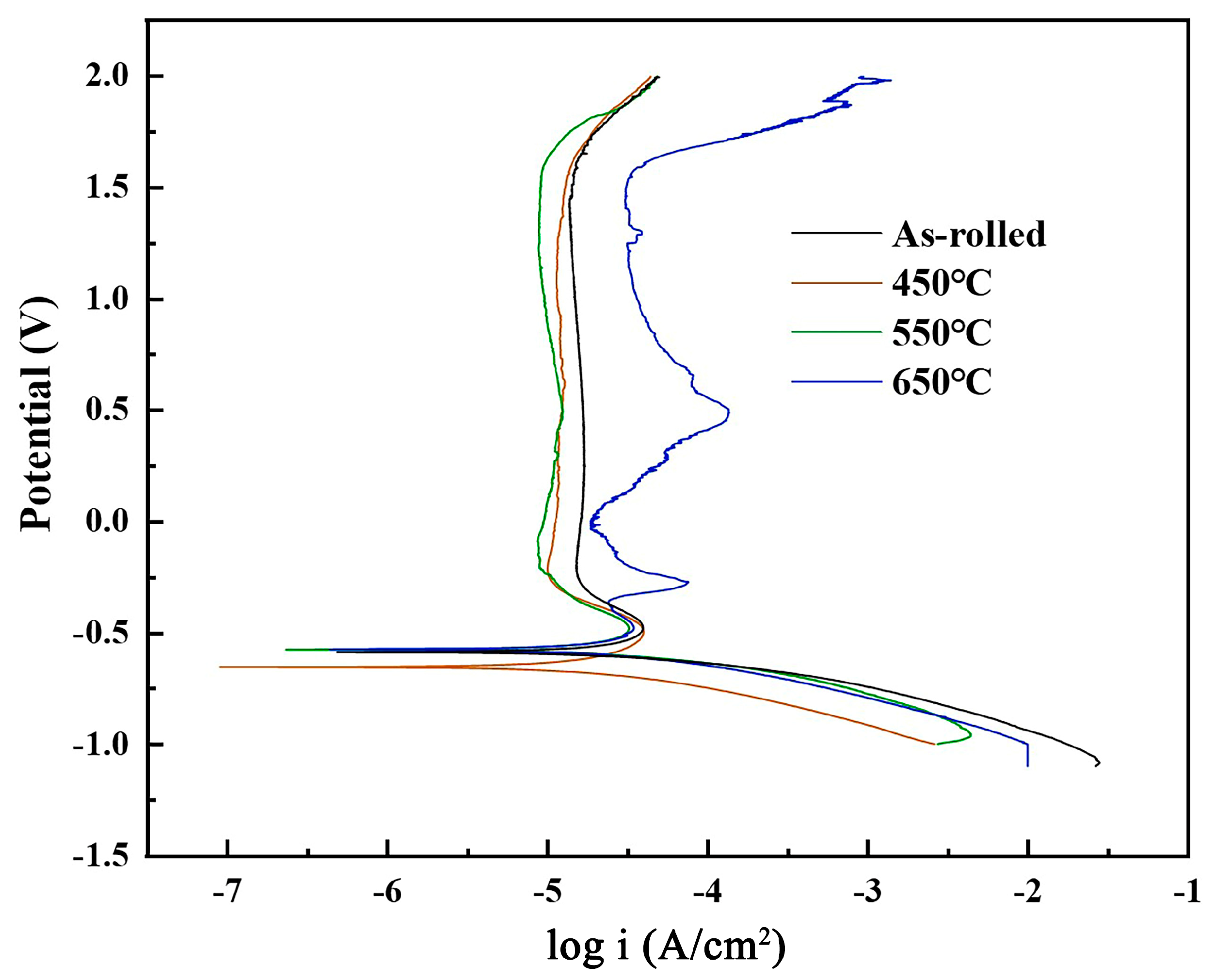
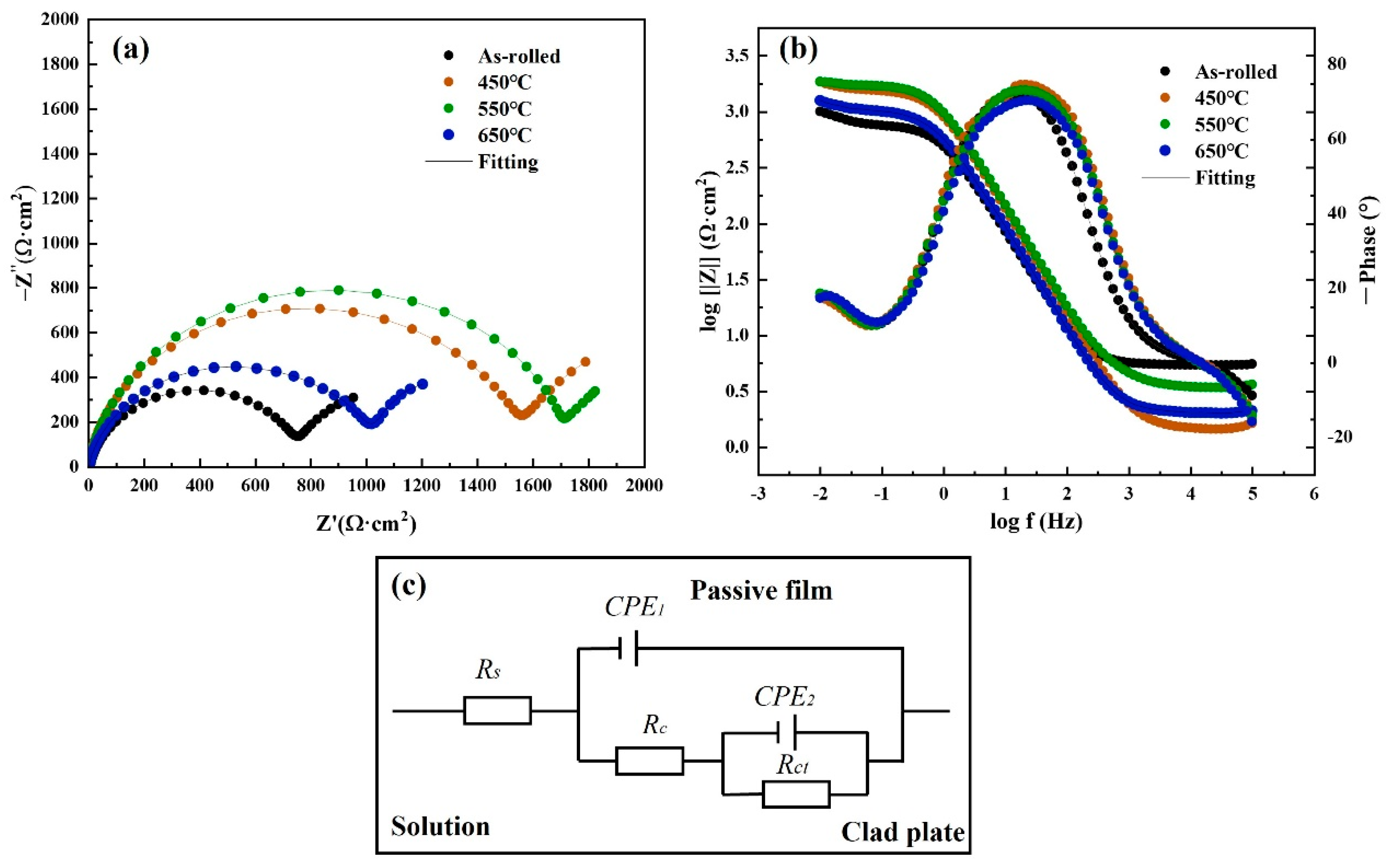
3.4. Corrosion Resistance Analysis on TA2 Titanium Plates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, H.; Luo, X.B.; Chai, F.; Shen, J.C.; Sun, X.J.; Lu, F. Manufacturing Technology and Application Trends of Titanium Clad Steel Plates. J. Iron Steel Res. Int. 2015, 22, 977–982. [Google Scholar] [CrossRef]
- Kundu, S.; Sam, S.; Chatterjee, S. Interface microstructure and strength properties of Ti–6Al–4V and microduplex stainless steel diffusion bonded joints. Mater. Des. 2011, 32, 2997–3003. [Google Scholar] [CrossRef]
- Jiang, H.T.; Yan, X.Q.; Liu, J.X.; Zeng, S.W.; Duan, X.G. Diffusion Behavior and Mathematical Model of Ti-Steel Explosive Clad Plate during Heat Treatment. Rare Met. Mater. Eng. 2015, 44, 972–976. [Google Scholar]
- Zu, G.Y.; Sun, X.; Zhang, J.H. Interfacial Bonding Mechanism and Mechanical Performance of Ti/Steel Bimetallic Clad Sheet Produced by Explosive Welding and Annealing. Rare Met. Mater. Eng. 2017, 46, 906–911. [Google Scholar]
- Jiang, C.; Long, W.M.; Feng, J.; Zhang, L.; Zhang, S. Interfacial microstructure and mechanical properties of copper/stainless steel fabricated by explosive welding. Weld. Join. 2021, 09, 22–27. [Google Scholar]
- Sun, J.F.; Liang, X.J.; Jiao, S.H. Study on the interface of direct hot rolling titanium-clad steel plates. Baosteel Tech. Res. 2017, 11, 32–39. [Google Scholar]
- Bai, Y.L.; Liu, X.F.; Wang, W.J.; Yang, Y.H. Current status and research trends in processing and application of titanium/steel composite plate. Chin. J. Eng. 2021, 43, 85–96. [Google Scholar]
- Yu, C.; Fu, L.; Xiao, H.; Lv, Q.; Gao, B.X. Effect of carbon content on the microstructure and bonding properties of hot-rolling pure titanium clad carbon steel plates. Mater. Sci. End. A 2021, 820, 141572. [Google Scholar] [CrossRef]
- Chai, X.Y.; Shi, Z.R.; Chai, F.; Su, H.; Yang, Z.G.; Yang, C.F. Effect of Heating Temperature on Microstructure and Mechanical Properties of Titanium Clad Steel by Hot Roll Bonding. Rare Met. Mater. Eng. 2019, 48, 2701–2710. [Google Scholar]
- Wang, G.L.; Luo, Z.A.; Xie, G.M.; Wang, L.P.; Zhao, K. Effect of heating temperture on the bonding property of the Titanium/Stainless Steel Plate by Hot-Rolling Bonding. Rare Met. Mater. Eng. 2013, 42, 387–391. [Google Scholar]
- Luo, Z.A.; Xie, G.M.; Wang, G.L.; Wang, G.D. Effect of interfacial Microstructure on Mechanical Properties of Vacuum Rolling Clad Pure Titanium/High Strength Low Alloy Steel. Chin. J. Mater. Res. 2013, 27, 569–575. [Google Scholar]
- Gloc, M.; Wachowski, M.; Plocinski, T.; Kurzydlowski, K.J. Microstructural and microanalysis investigations of bond titanium grade1/low alloy steel st52-3N obtained by explosive welding. J. Alloys Compd. 2016, 671, 446–451. [Google Scholar] [CrossRef]
- Yang, D.H.; Luo, Z.A.; Xie, G.M.; Jiang, T.; Zhao, S.; Misra, R.D.K. Interfacial microstructure and properties of a vacuum roll-cladding titanium-steel clad plate with a nickel interlayer. Mater. Sci. End. A 2019, 753, 49–58. [Google Scholar] [CrossRef]
- Luo, Z.A.; Wang, G.L.; Xie, G.M.; Wang, L.P.; Zhao, K. Interfacial microstructure and properties of a vacuum hot roll-bonded Titanium-Stainless Steel Clad Plate with a Niobium Interlayer. Acta Metall. Sin. Engl. Lett. 2013, 26, 754–760. [Google Scholar] [CrossRef]
- Jiang, C.; Long, W.M.; Zhong, S.J.; Fan, X.G.; Liao, Z.Q.; Wei, Y.Q. Interface microstructure and properties of TA1/Q235R explosive welding clad plate. Electr. Weld. Mach. 2023, 53, 65–70. [Google Scholar]
- Chu, Q.L.; Zhang, M.; Li, J.H.; Yan, C. Experimental and numerical investigation of microstructure and mechanical behavior of titanium/steel interfaces prepared by explosive welding. Mater. Sci. Eng. A 2017, 689, 323–331. [Google Scholar] [CrossRef]
- Yan, J.C.; Zhao, D.S.; Wang, C.W.; Wang, L.Y.; Wang, Y.; Yang, S.Q. Vacuum hot roll bonding of titanium alloy and stainless steel using nickel interlayer. Mater. Sci. Technol. 2013, 25, 914–918. [Google Scholar] [CrossRef]
- Li, X.B.; Li, X.H.; Li, M.; Zhang, S.; Jiang, Q.W. Effect of annealing on element diffusion of TA1/Q235 clad sheet. Trans. Mater. Heat Treat. 2017, 38, 35–40. [Google Scholar]
- Chai, X.Y.; Pan, T.; Chai, F.; Luo, X.B.; Su, H.; Yang, Z.G.; Yang, C.F. Interlayer engineering for titanium clad steel by hot roll bonding. J. Iron Steel Res. Int. 2018, 25, 739–745. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, H.; Yu, H.; Qi, Z.C.; Xu, C. Mechanical properties and interfacial structure of hot-roll bonding TA2/Q235B plate using DT4 interlayer—ScienceDirect. Mater. Sci. End. A 2017, 695, 120–125. [Google Scholar] [CrossRef]
- Li, B.X.; He, W.J.; Chen, Z.J.; Mo, T.Q.; Peng, L.; Li, J.; Liu, Q. Influence of annealing on the microstructure, interfacial compounds and mechanical properties of hot rolling bonded Ti/steel clad plate with bimetallic interlayered steel and vanadium. Mater. Sci. End. A 2019, 764, 138221–138227. [Google Scholar] [CrossRef]
- Jiang, H.T.; Yan, X.Q.; Liu, J.X.; Duan, X.G. Effect of heat treatment on microstructure and mechanical property of Ti-Steel explosive-rolling clad plate. Trans. Nonferrous Met. Soc. China 2014, 24, 697–704. [Google Scholar] [CrossRef]
- Wang, J.Z.; Yan, X.B.; Wang, W.Q.; Yan, J.Y.; Rong, Y.; Yan, P. Titanium cladding steel plates with interlayer by explosion and rolling bonding. Rare Met. Mater. Eng. 2010, 39, 309–313. [Google Scholar]
- Zhao, G.H.; Tang, Q.Y.; Guo, Q.C.; Li, J.; Ma, L.F.; Ma, L.W. Effect of annealing temperature on interfacial microstructure and tensile fracture behavior of titanium-steel clad plate. Mater. Charact. 2024, 215, 114128. [Google Scholar] [CrossRef]
- Bai, Y.L.; Liu, X.F. Interfacial reaction behavior of titanium/steel composite plate formed by cold-hot rolling. Mater. Charact. 2023, 202, 113030. [Google Scholar] [CrossRef]
- Meng, K.; Guo, K.; Yu, Q.; Miao, D.; Yao, C.; Wang, Q.F.; Wang, T.S. Effect of annealing temperature on the microstructure and corrosion behavior of Ti-6Al-3Nb-2Zr-1Mo alloy in hydrochloric acid solution. Corros. Sci. 2021, 183, 109320. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yuan, X.G.; Huang, H.J.; Zuo, X.J.; Cheng, Y.L. Influence of chloride ion concentration and temperature on the corrosion of Cu–Al composite plates in salt fog. J. Alloys Compd. 2020, 821, 153249. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, M.F.; Pu, J.; Shan, Q.; Sun, Y.B.; Wang, S.Q.; Hermann, S.K.U.G. Effect of Cu Coating on Microstructure and Properties of Al/Steel Welding–Brazing Joints Obtained by Cold Metal Transfer (CMT). Coatings 2022, 12, 1123. [Google Scholar] [CrossRef]
- Pu, J.; Xie, P.; Long, W.M.; Wu, M.F.; Sheng, Y.W.; Sheng, J. Effect of current on corrosion resistance of duplex stainless steel layer obtained by plasma arc cladding. Crystals 2022, 12, 341. [Google Scholar] [CrossRef]
- Meenakshi, K.S.; Kumar, S.A. Corrosion resistant behaviour of titanium–Molybdenum alloy in sulphuric acid environment. Mater. Today Proc. 2022, 65, 3282–3287. [Google Scholar] [CrossRef]
- Mao, T.; Li, L.; Huang, H.B.; Yu, Z.M.; Zhong, Y.; Chen, S.L. Corrosion behaviors of TA2 and TA36 titanium alloys in a high-sulfur environment. Int. J. Electrochem. Sci. 2024, 19, 100462. [Google Scholar]
- Liu, H.D.; Pu, J.; Wu, M.F.; Zhang, C.; Rao, J.W.; Long, W.M.; Shen, Y.X. Research on the Microstructure and Properties of Al Alloy/Steel CMT Welding-Brazing Joints with Al–Si Flux-Cored. Coatings 2023, 13, 1590. [Google Scholar] [CrossRef]
- Wei, Y.; Pan, Z.M.; Fu, Y.; Yu, W.; He, S.L.; Yuan, Q.Y.; Luo, H.; Li, X.G. Effect of annealing temperatures on microstructural evolution and corrosion behavior of Ti-Mo titanium alloy in hydrochloric acid. Corros Sci. 2022, 197, 110079. [Google Scholar] [CrossRef]

| Elements | C | Si | Mn | Fe | N | H | Ti | O | Al | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TA2 | 0.030 | - | - | 0.300 | 0.009 | 0.002 | Bal. | 0.25 | - | - | - |
| Q235B | 0.2 | 0.2 | 0.2 | Bal. | - | - | - | - | 0.03 | 0.03 | |
| DT4 | 0.148 | 0.15 | 0.6 | Bal. | - | - | - | - | 0.023 | 0.003 | 0.017 |
| Materials | Density (g/cm3) | Thermal Conductivity W/(m·K) | Coefficient of Linear Expansion (10−6·K−1) | Melting Points (°C) | Tensile Strength (MPa) | Elongation after Fracture A (%) |
|---|---|---|---|---|---|---|
| TA2 | 4.51 | 21.9 | 8.6 | 1668 | 441 | 20 |
| Q235B | 7.85 | 49.8 | 12 | 1468 | 487 | 30 |
| Temperature (°C) | Ecorr (mV) | Icorr (A·cm2) |
|---|---|---|
| As-rolled | −0.654 | 4.546 × 10−5 |
| 450 | −0.575 | 4.020 × 10−5 |
| 550 | −0.573 | 3.614 × 10−5 |
| 650 | −0.587 | 4.264 × 10−5 |
| Temperature (°C) | RS (Ω·cm2) | CPE1 × 10−4 (Ω−1cm−2S−n) | n1 | Rc (Ω·cm2) | CPE2 × 10−2 (Ω−1cm−2S−n) | n2 | Rct (Ω·cm2) | χ2 × 10−4 |
|---|---|---|---|---|---|---|---|---|
| As-rolled | 5.385 | 2.66 | 0.914 | 767.7 | 2.79 | 0.927 | 758.7 | 3.18 |
| 450 | 1.492 | 1.48 | 0.943 | 1535 | 1.74 | 0.807 | 2112 | 6.32 |
| 550 | 3.529 | 1.50 | 0.918 | 1770 | 4.30 | 0.986 | 2356 | 1.95 |
| 650 | 2.017 | 0.23 | 0.923 | 1047 | 1.89 | 0.781 | 1762 | 7.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, J.; Chen, T.; Sun, Y.; Long, W.; Sun, H.; Chen, Y. Effect of Heat Treatment Temperature on the Microstructure and Properties of Titanium-Clad Steel Plate Prepared by Vacuum Hot Rolling. Coatings 2024, 14, 1096. https://doi.org/10.3390/coatings14091096
Pu J, Chen T, Sun Y, Long W, Sun H, Chen Y. Effect of Heat Treatment Temperature on the Microstructure and Properties of Titanium-Clad Steel Plate Prepared by Vacuum Hot Rolling. Coatings. 2024; 14(9):1096. https://doi.org/10.3390/coatings14091096
Chicago/Turabian StylePu, Juan, Tingmu Chen, Yubo Sun, Weimin Long, Huawei Sun, and Yunxia Chen. 2024. "Effect of Heat Treatment Temperature on the Microstructure and Properties of Titanium-Clad Steel Plate Prepared by Vacuum Hot Rolling" Coatings 14, no. 9: 1096. https://doi.org/10.3390/coatings14091096
APA StylePu, J., Chen, T., Sun, Y., Long, W., Sun, H., & Chen, Y. (2024). Effect of Heat Treatment Temperature on the Microstructure and Properties of Titanium-Clad Steel Plate Prepared by Vacuum Hot Rolling. Coatings, 14(9), 1096. https://doi.org/10.3390/coatings14091096


.jpg)



