Regulations of Thermal Expansion Coefficients of Yb1−xAlxTaO4 for Environmental Barrier Coatings Applications
Abstract
:1. Introduction
2. Experiments
2.1. Sample Synthesis
2.2. Structural Analysis
2.3. Properties Measurements
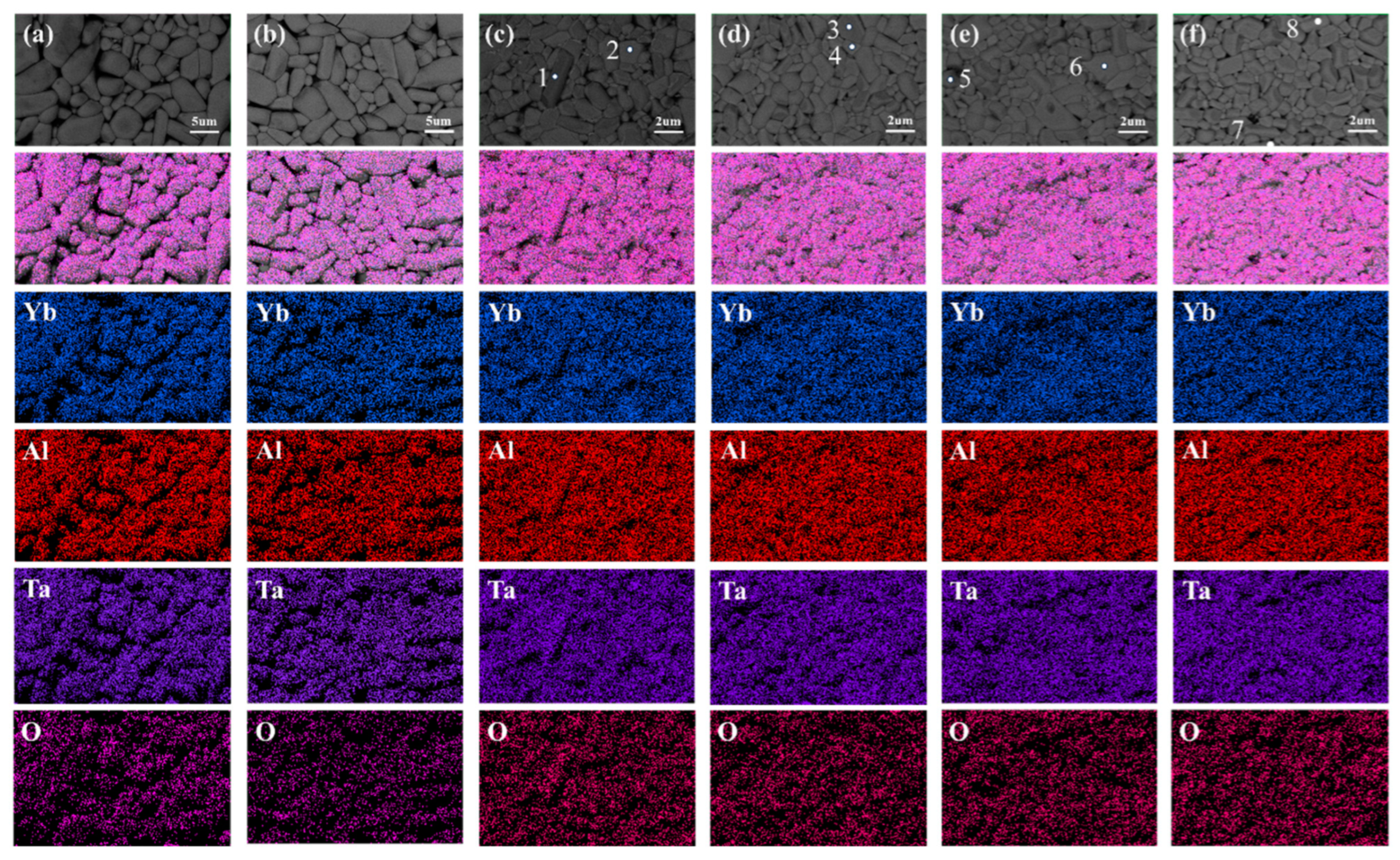
3. Results and Discussion
3.1. Crystal and Microstructure
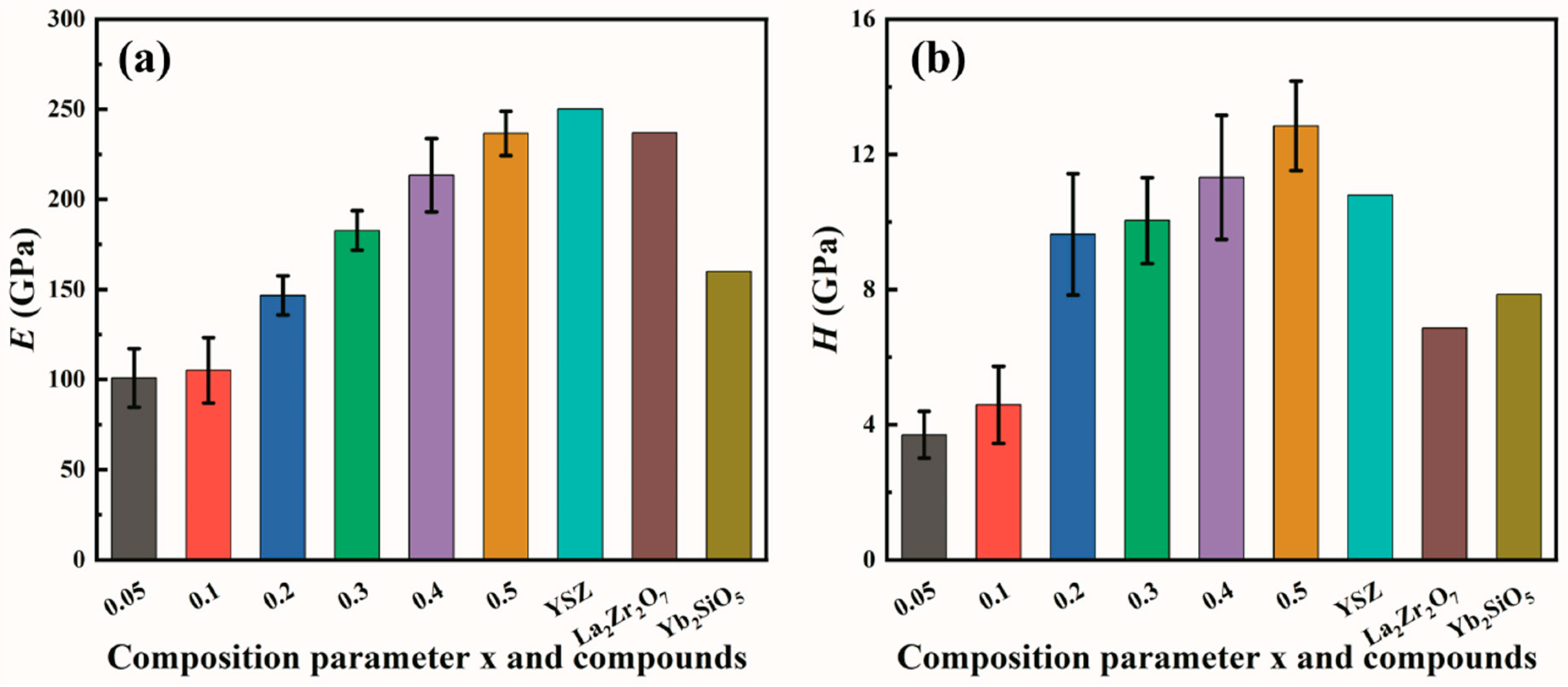
3.2. Mechanical Properties
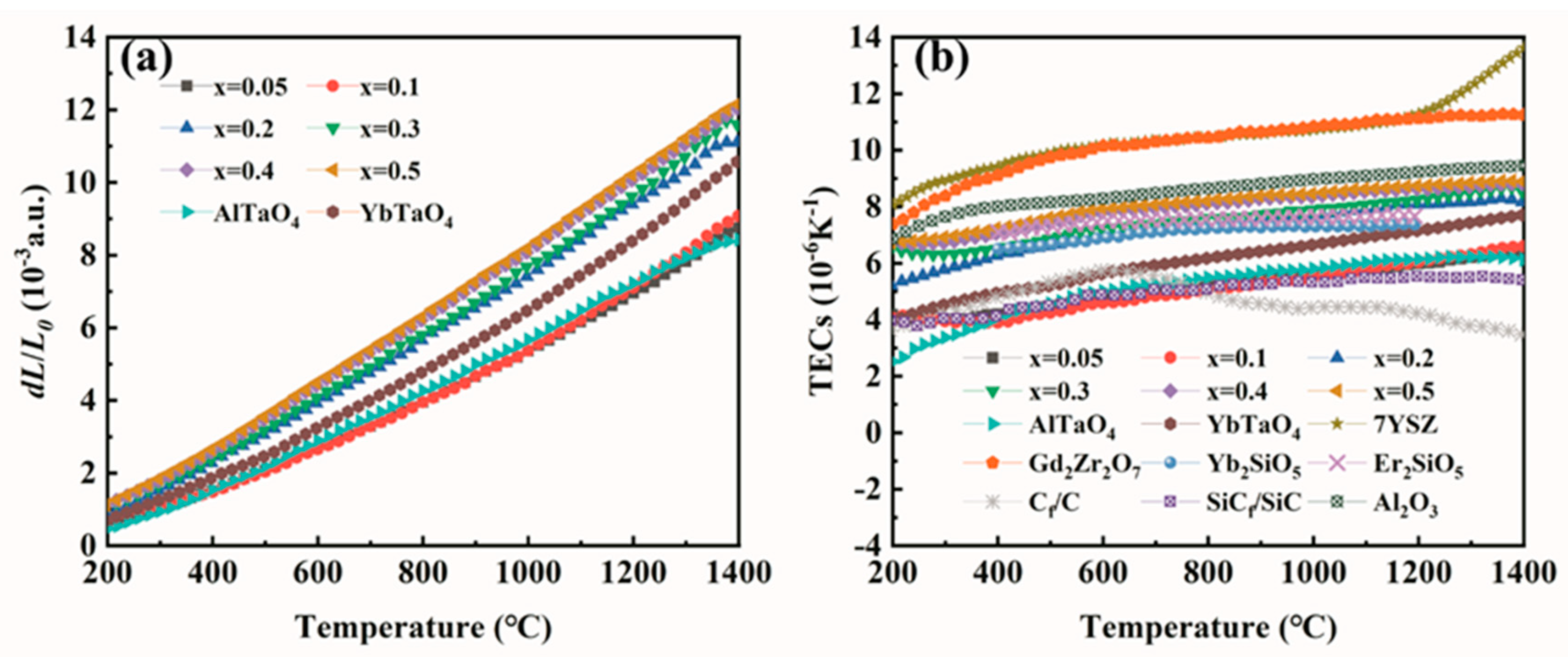
3.3. Thermal Expansion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaßen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on Advanced Thermal Barrier Coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Zhao, P. Mechanical Properties, Thermophysical Properties and Electronic Structure of Yb3+ or Ce4+-Doped La2Zr2O7-Based TBCs. J. Rare Earths 2023, 41, 588–598. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, X.; He, D. Research Progress of CMAS Corrosion and Protection Method for Thermal Barrier Coatings in Aero-engines. China Surf. Eng. 2023, 36, 1–13. [Google Scholar]
- Wang, Y.; Ouyang, J.; Wei, T.; Cao, G.; Liu, Z.; Ding, Z.; Wang, Y.; Wang, Y. Mechanical Properties, Thermal Conductivity and Defect Formation Energies of Samarium Immobilization in Gd2Zr2O7: First-Principles Study and Irradiation Experiment. J. Rare Earths 2023, 41, 422–433. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, L.; Zheng, W. Environmental Barrier Coating for Aeroengines: Materials and Properties. Adv. Ceram. 2019, 40, 331–344. [Google Scholar]
- Chen, W.; Duan, H.; Shi, J.-L.; Qian, Y.; Wan, J.; Zhang, X.; Sheng, H.; Guan, B.; Wen, R.; Yin, Y.; et al. Bridging Interparticle Li+ Conduction in a Soft Ceramic Oxide Electrolyte. J. Am. Chem. Soc. 2021, 143, 5717–5726. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Xiao, G.; Wei, D.; Du, Y. Thermal Performance Affected by the Mesoscopic Characteristics of the Ceramic Matrix Composite for Hypersonic Vehicle. J. Phys. Conf. Ser. 2021, 1786, 012004. [Google Scholar]
- Wang, X.; Gao, X.; Zhang, Z.; Cheng, L.; Ma, H.; Yang, W. Advances in Modifications and High-Temperature Applications of Silicon Carbide Ceramic Matrix Composites in Aerospace: A Focused Review. J. Eur. Ceram. Soc. 2021, 41, 4671–4688. [Google Scholar] [CrossRef]
- Yan, Z.; Peng, H.; Ji, X.; Zhang, D. Preparation of Yb2Si2O7 Precursor and Its Agglomerated Powder for Low Pressure Plasma Spraying. Therm. Spray Technol. 2022, 14, 38–45+22. [Google Scholar]
- Kimmel, J.; Miriyala, N.; Price, J.; More, K.; Tortorelli, P.; Eaton, H.; Linsey, G.; Sun, E. Evaluation of CFCC Liners with EBC after Field Testing in a Gas Turbine. J. Eur. Ceram. Soc. 2002, 22, 2769–2775. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Meng, H.; Chu, Y. Atomic-Level Insights into the Initial Oxidation Mechanism of High-Entropy Diborides by First-Principles Calculations. J. Mater. 2024, 10, 423–430. [Google Scholar] [CrossRef]
- Wen, Z.; Tang, Z.; Liu, Y.; Zhuang, L.; Yu, H.; Chu, Y. Ultrastrong and High Thermal Insulating Porous High-Entropy Ceramics up to 2000 °C. Adv. Mater. 2024, 36, 2311870. [Google Scholar] [CrossRef]
- Du, J.; Liu, R.; Wan, F.; Li, D.; Li, J.; Wang, Y. Failure Mechanism of Ytterbium Silicate/Silicon Bi-Layer Environmental Barrier Coatings on SiCf/SiC Composites upon Long-Time Water Vapor and Oxygen Corrosion Test. Surf. Coat. Technol. 2022, 447, 128871. [Google Scholar] [CrossRef]
- Yang, F.; Fan, Y.; Zhao, J.; Liu, Y.; Chu, K.; Li, Y.; Li, W.; Liu, B. Mechanical and Thermal Properties for Corrosion Products of Lutetium Silicates against CMAS. J. Am. Ceram. Soc. 2024, 107, 5285–5297. [Google Scholar] [CrossRef]
- Fan, Y.; Bai, Y.; Li, Q.; Lu, Z.; Chen, D.; Liu, Y.; Li, W.; Liu, B. Compositional Design and Phase Formation Capability of High-Entropy Rare-Earth Disilicates from Machine Learning and Decision Fusion. npj Comput. Mater. 2024, 10, 95. [Google Scholar] [CrossRef]
- Richards, B.T.; Sehr, S.; De Franqueville, F.; Begley, M.R.; Wadley, H.N.G. Fracture Mechanisms of Ytterbium Monosilicate Environmental Barrier Coatings during Cyclic Thermal Exposure. Acta Mater. 2016, 103, 448–460. [Google Scholar] [CrossRef]
- Yuan, K.; Hou, W.; Ji, X.; Lu, X. Study on Microstructure Evolution in Ytterbium Silicate Environmental Barrier Coatings under Thermal Shock Tests. Therm. Spray Technol. 2023, 15, 13–18. [Google Scholar]
- Tian, J.; Chen, L.; Chen, X.; Luo, K.; Li, B.; Zhang, D.; Wang, M.; Xu, B.; Ren, Z.; Yan, S.; et al. Regulation of Crystal and Microstructures of RETaO4 (RE = Nd, Sm, Gd, Ho, Er) Powders Synthesized via Co-Precipitation. J. Rare Earths 2024, in press. [Google Scholar] [CrossRef]
- Deijkers, J.A.; Wadley, H.N.G. A Duplex Bond Coat Approach to Environmental Barrier Coating Systems. Acta Mater. 2021, 217, 117167. [Google Scholar] [CrossRef]
- Chen, L.; Li, B.; Guo, J.; Zhu, Y.; Feng, J. High-Entropy Perovskite RETa3O9 Ceramics for High-Temperature Environmental/Thermal Barrier Coatings. J. Adv. Ceram. 2022, 11, 556–569. [Google Scholar] [CrossRef]
- Turcer, L.R.; Sengupta, A.; Padture, N.P. Low Thermal Conductivity in High-Entropy Rare-Earth Pyrosilicate Solid-Solutions for Thermal Environmental Barrier Coatings. Scr. Mater. 2021, 191, 40–45. [Google Scholar] [CrossRef]
- Federer, J.I. Alumina Base Coatings for Protection of SiC Ceramics. J. Mater. Eng. 1990, 12, 141–149. [Google Scholar] [CrossRef]
- Lee, K.N.; Miller, R.A. Development and Environmental Durability of Mullite and Mullite/YSZ Dual Layer Coatings for SiC and Si3N4 Ceramics. Surf. Coat. Technol. 1996, 86–87, 142–148. [Google Scholar] [CrossRef]
- Jiang, C.; Feng, M.; Yu, C.; Bao, Z.; Zhu, S.; Wang, F. Thermal Cycling Behavior of Nanostructured and Conventional Yttria-Stabilized Zirconia Thermal Barrier Coatings via Air Plasma Spray. Rare Met. 2023, 42, 3859–3869. [Google Scholar] [CrossRef]
- Cojocaru, C.V.; Kruger, S.E.; Moreau, C.; Lima, R.S. Elastic Modulus Evolution and Behavior of Si/Mullite/BSAS-Based Environmental Barrier Coatings Exposed to High Temperature in Water Vapor Environment. J. Therm. Spray Technol. 2011, 20, 92–99. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, P.; Li, Z.; Wang, Y.; Tang, X.; Li, Y. Water Vapor Corrosion Behavior and Failure Mechanism of Air Sprayed Bi-Layer Yb2Si2O7/SiC and Tri-Layer Yb2Si2O7/(SiCw-Mullite)/SiC Environmental Barrier Coating. Adv. Powder Mater. 2023, 2, 100064. [Google Scholar] [CrossRef]
- Dong, Y.; Ren, K.; Wang, Q.; Shao, G.; Wang, Y. Interaction of Multicomponent Disilicate (Yb0.2Y0.2Lu0.2Sc0.2Gd0.2)2Si2O7 with Molten Calcia-Magnesia-Aluminosilicate. J. Adv. Ceram. 2022, 11, 66–74. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Li, B.; Luo, K.; Feng, J. Simultaneous Manipulations of Thermal Expansion and Conductivity in Symbiotic ScTaO4/SmTaO4 Composites via Multiscale Effects. J. Adv. Ceram. 2023, 12, 1625–1640. [Google Scholar] [CrossRef]
- Meng, H.; Yu, R.; Tang, Z.; Wen, Z.; Yu, H.; Chu, Y. Formation Ability Descriptors for High-Entropy Diborides Established through High-Throughput Experiments and Machine Learning. Acta Mater. 2023, 256, 119132. [Google Scholar] [CrossRef]
- Gatzen, C.; Mack, D.E.; Guillon, O.; Vaßen, R. YAlO3—A Novel Environmental Barrier Coating for Al2O3/Al2O3–Ceramic Matrix Composites. Coatings 2019, 9, 609. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, C.; Zheng, W.; Song, X.; Jiang, C.; Niu, Y.; Zeng, Y. Influence of Average Radii of RE3+ Ions on Phase Structures and Thermal Expansion Coefficients of High-Entropy Pyrosilicates. J. Adv. Ceram. 2023, 12, 1090–1104. [Google Scholar] [CrossRef]
- Song, C.; Ye, F.; Cheng, L.; Liu, Y.; Zhang, Q. Long-Term Ceramic Matrix Composite for Aeroengine. J. Adv. Ceram. 2022, 11, 1343–1374. [Google Scholar] [CrossRef]
- Chen, L.; Li, B.; Feng, J. Rare-Earth Tantalates for Next-Generation Thermal Barrier Coatings. Prog. Mater Sci. 2024, 144, 101265. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Guo, J.; Chong, X.; Feng, J. Mechanical and Thermal Properties of RETaO4 (RE = Yb, Lu, Sc) Ceramics with Monoclinic-Prime Phase. J. Mater. Sci. Technol. 2020, 52, 20–28. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Zhang, A.; Guo, S.; Mao, J.; Deng, C.; Wang, P.; Deng, C.; Feng, J.; Liu, M.; et al. Al-Modification for PS-PVD 7YSZ TBCs to Improve Particle Erosion and Thermal Cycle Performances. J. Adv. Ceram. 2022, 11, 1093–1103. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, X.; Fan, X.; Mao, J.; Deng, C.-M.; Liu, M.; Zhou, K.; Zhang, X. Thermal Insulation Performance of 7YSZ TBCs Adjusted via Al Modification. Rare Met. 2023, 42, 994–1004. [Google Scholar] [CrossRef]
- Shi, X.; Liu, W.; Li, M.; Sun, Q.; Xu, S.; Du, D.; Zou, J.; Chen, Z. A Solvothermal Synthetic Environmental Design for High-Performance SnSe-Based Thermoelectric Materials. Adv. Energy Mater. 2022, 12, 2200670. [Google Scholar] [CrossRef]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld Refinement Guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Feng, J. Defect-Dominated Phonon Scattering Processes and Thermal Transports of Ferroelastic (Sm1-XYbX)TaO4 Solid Solutions. Mater. Today Phys. 2023, 35, 101118. [Google Scholar] [CrossRef]
- Dolan, M.D.; Harlan, B.; White, J.S.; Hall, M.; Misture, S.T.; Bancheri, S.C.; Bewlay, B. Structures and anisotropic thermal expansion of the α, β, γ, and δ polymorphs of Y2Si2O7. Power Diffr. 2008, 23, 20–25. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Zheng, X.; Feng, J. Characteristics of Ferroelastic Domains and Thermal Transport Limits in HfO2 Alloying YTaO4 Ceramics. Acta Mater. 2023, 251, 118870. [Google Scholar] [CrossRef]
- Kong, F.; Tao, S.; Qian, B.; Gao, L. Facile Synthesis of MTaO4 (M = Al, Cr and Fe) Metal Oxides and Their Application as Anodes for Lithium-Ion Batteries. Ceram. Int. 2018, 44, 8827–8831. [Google Scholar] [CrossRef]
- Nie, G.; Bao, Y.; Wan, D.; Tian, Y. Evaluating High Temperature Elastic Modulus of Ceramic Coatings by Relative Method. J. Adv. Ceram. 2017, 6, 288–303. [Google Scholar] [CrossRef]
- Ren, X.; Pan, W. Mechanical Properties of High-Temperature-Degraded Yttria-Stabilized Zirconia. Acta Mater. 2014, 69, 397–406. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Zhu, C.; Xiang, H.; Chen, H.; Sun, L.; Gao, Y.; Zhou, Y. Advances on Strategies for Searching for next Generation Thermal Barrier Coating Materials. J. Mater. Sci. Technol. 2019, 35, 833–851. [Google Scholar] [CrossRef]
- Ren, X.; Tian, Z.; Zhang, J.; Wang, J. Equiatomic Quaternary (Y1/4Ho1/4Er1/4Yb1/4)2SiO5 Silicate: A Perspective Multifunctional Thermal and Environmental Barrier Coating Material. Scr. Mater. 2019, 168, 47–50. [Google Scholar] [CrossRef]
- Ruffa, A.R. Temperature Dependence of the Elastic Shear Moduli of the Cubic Metals. Phys. Rev. B 1977, 16, 2504–2514. [Google Scholar] [CrossRef]
- Quane, D. Crystal Lattice Energy and the Madelung Constant. J. Chem. Educ. 1970, 47, 396. [Google Scholar] [CrossRef]
- Yang, K.; Chen, L.; Wu, F.; Zheng, Q.; Li, J.; Song, P.; Wang, Y.; Liu, R.; Feng, J. Thermophysical Properties of Yb(TaxNb1−x)O4 Ceramics with Different Crystal Structures. Ceram. Int. 2020, 46, 28451–28458. [Google Scholar] [CrossRef]
- Han, Y.; Yu, R.; Liu, H.; Chu, Y. Synthesis of the Superfine High-Entropy Zirconate Nanopowders by Polymerized Complex Method. J. Adv. Ceram. 2022, 11, 136–144. [Google Scholar] [CrossRef]
- Anderson, O.L. A Simplified Method for Calculating the Debye Temperature from Elastic Constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Hazen, R.M.; Prewitt, C.T. Effects of Temperature and Pressure on Interatomic Distances in Oxygen-Based Minerals. Am. Mineral. 1977, 62, 309–315. [Google Scholar]
- Hazen, R.M. Effects of Temperature and Pressure on the Crystal Structure of Ferromagnesian Olivine. Am. Mineral. 1977, 62, 286–295. [Google Scholar]
- Ridley, M.; Gaskins, J.; Hopkins, P.; Opila, E. Tailoring Thermal Properties of Multi-Component Rare Earth Monosilicates. Acta Mater. 2020, 195, 698–707. [Google Scholar] [CrossRef]
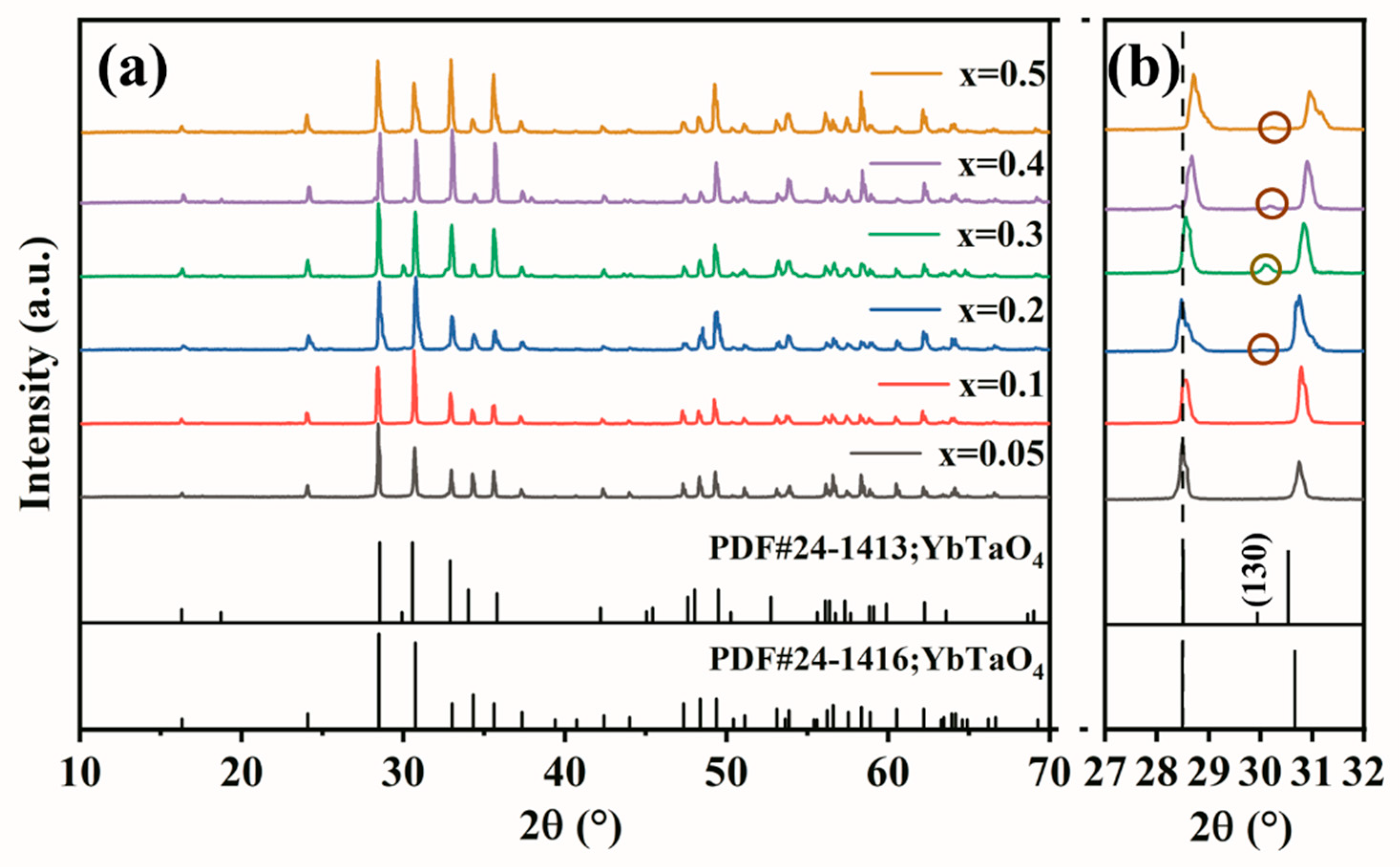
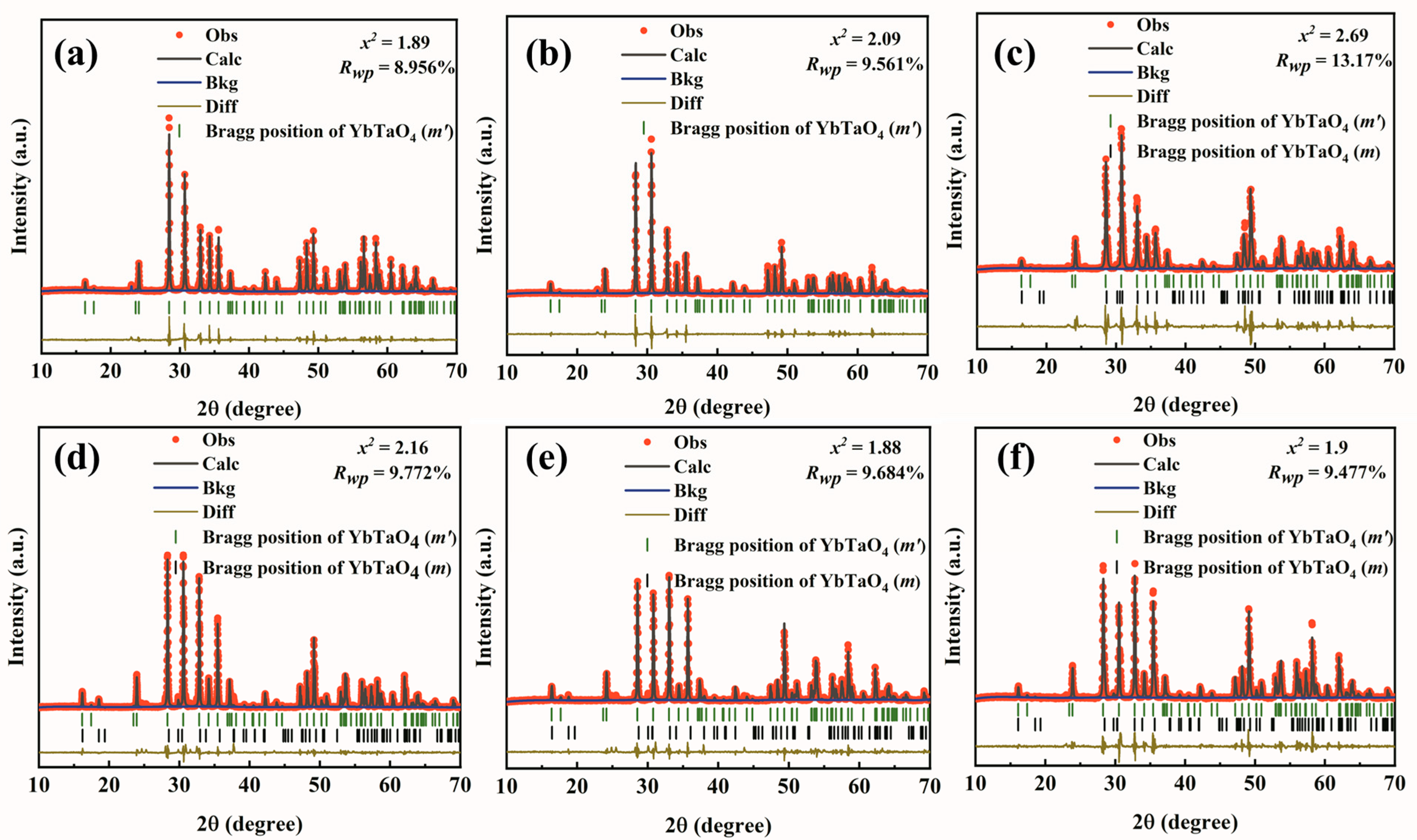
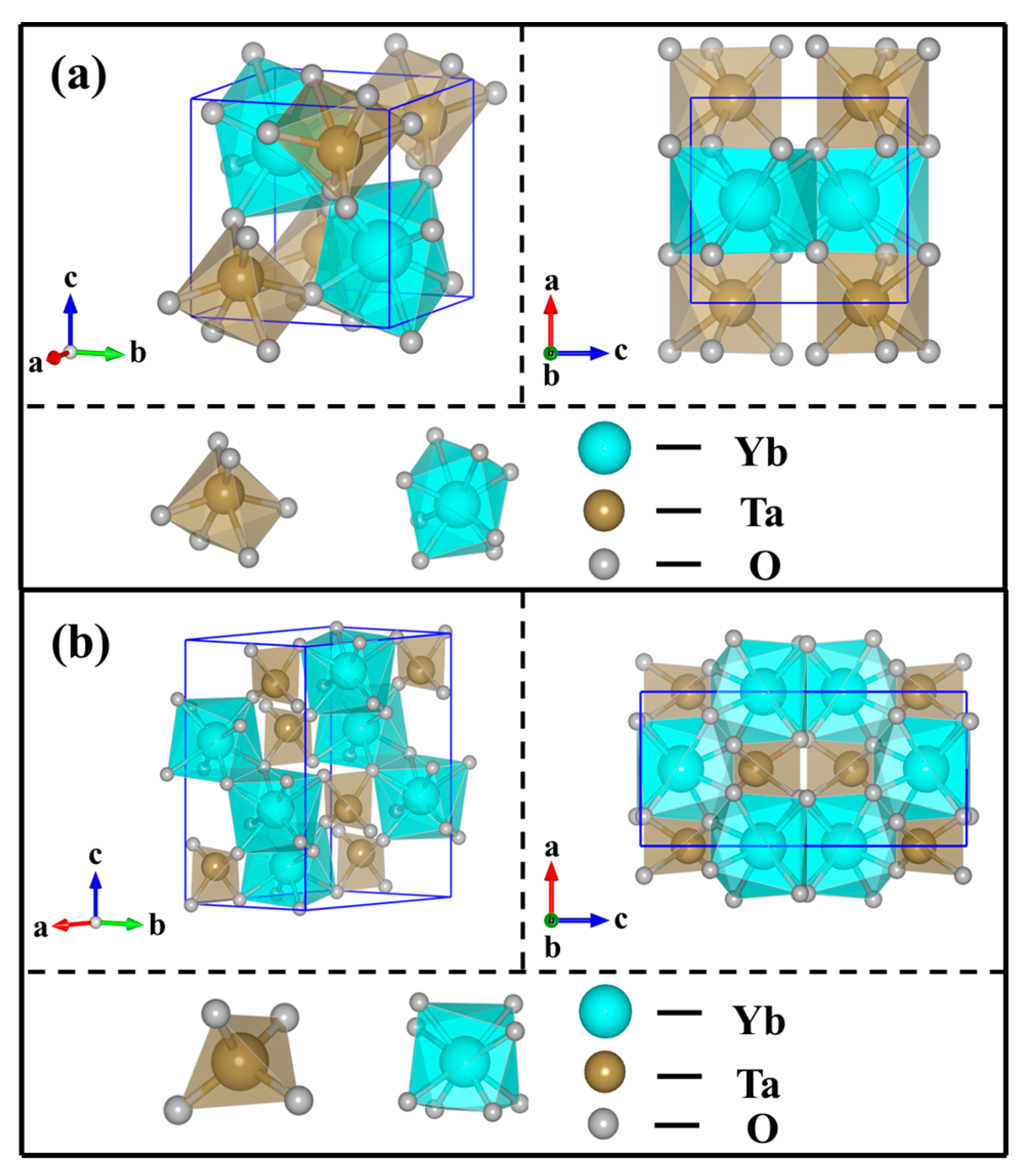
| Samples | Phase | Content | a | b | c | β | V | ρ | ρR |
|---|---|---|---|---|---|---|---|---|---|
| 0.05 | m′ | 100 | 5.07 | 5.26 | 5.44 | 96.17 | 144.192 | 9.496 | 97 |
| 0.1 | m′ | 100 | 5.07 | 5.43 | 5.26 | 96.18 | 144.061 | 9.357 | 97 |
| 0.2 | m′ | 91.5 | 5.07 | 5.43 | 5.25 | 96.16 | 144.005 | 8.972 | 98 |
| m | 8.5 | 6.89 | 5.27 | 10.86 | 133.13 | 287.507 | |||
| 0.3 | m′ | 85.5 | 5.07 | 5.43 | 5.25 | 96.16 | 143.999 | 8.650 | 97 |
| m | 14.5 | 6.81 | 5.25 | 10.89 | 132.95 | 285.041 | |||
| 0.4 | m′ | 80.9 | 5.07 | 5.25 | 5.43 | 96.18 | 143.643 | 8.309 | 97 |
| m | 19.1 | 6.86 | 5.23 | 10.84 | 133.03 | 284.464 | |||
| 0.5 | m′ | 76.0 | 5.07 | 5.25 | 5.43 | 96.16 | 143.518 | 8.017 | 96 |
| m | 24.0 | 6.83 | 5.19 | 10.85 | 132.92 | 281.963 |
| Samples | Position | Yb (at%) | Al (at%) | Ta (at%) | O (at%) |
|---|---|---|---|---|---|
| Yb0.8Al0.2TaO4 | 1 | 3.8 | 19.73 | 15.22 | 61.25 |
| 2 | 10.95 | 20.84 | 19.53 | 48.68 | |
| Yb0.7Al0.3TaO4 | 3 | 5.62 | 20.66 | 17.98 | 55.47 |
| 4 | 9.93 | 15.75 | 16.56 | 57.77 | |
| Yb0.6Al0.4TaO4 | 5 | 3.86 | 20.71 | 17.39 | 58.04 |
| 6 | 11.44 | 17.86 | 18.66 | 52.04 | |
| Yb0.5Al0.5TaO4 | 7 | 3.61 | 17.30 | 15.67 | 63.41 |
| 8 | 10.39 | 15.82 | 17.44 | 56.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Chen, L.; Zhang, L.; Chen, X.; Xu, C.; Li, T.; Feng, J. Regulations of Thermal Expansion Coefficients of Yb1−xAlxTaO4 for Environmental Barrier Coatings Applications. Coatings 2024, 14, 1097. https://doi.org/10.3390/coatings14091097
Liao J, Chen L, Zhang L, Chen X, Xu C, Li T, Feng J. Regulations of Thermal Expansion Coefficients of Yb1−xAlxTaO4 for Environmental Barrier Coatings Applications. Coatings. 2024; 14(9):1097. https://doi.org/10.3390/coatings14091097
Chicago/Turabian StyleLiao, Jiaxin, Lin Chen, Luyang Zhang, Xunlei Chen, Cheng Xu, Tianyu Li, and Jing Feng. 2024. "Regulations of Thermal Expansion Coefficients of Yb1−xAlxTaO4 for Environmental Barrier Coatings Applications" Coatings 14, no. 9: 1097. https://doi.org/10.3390/coatings14091097




