β-Cyclodextrin-Modified Cotton Fabric for Medical and Hospital Applications with Photodynamic Antibacterial Activity Using Methylene Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cross-Linking of β-CD in Textiles
2.2.2. Methylene Blue Dyeing Method
2.2.3. Statistical Treatment
2.2.4. Characterization of the Materials
Scanning Electron Microscopy
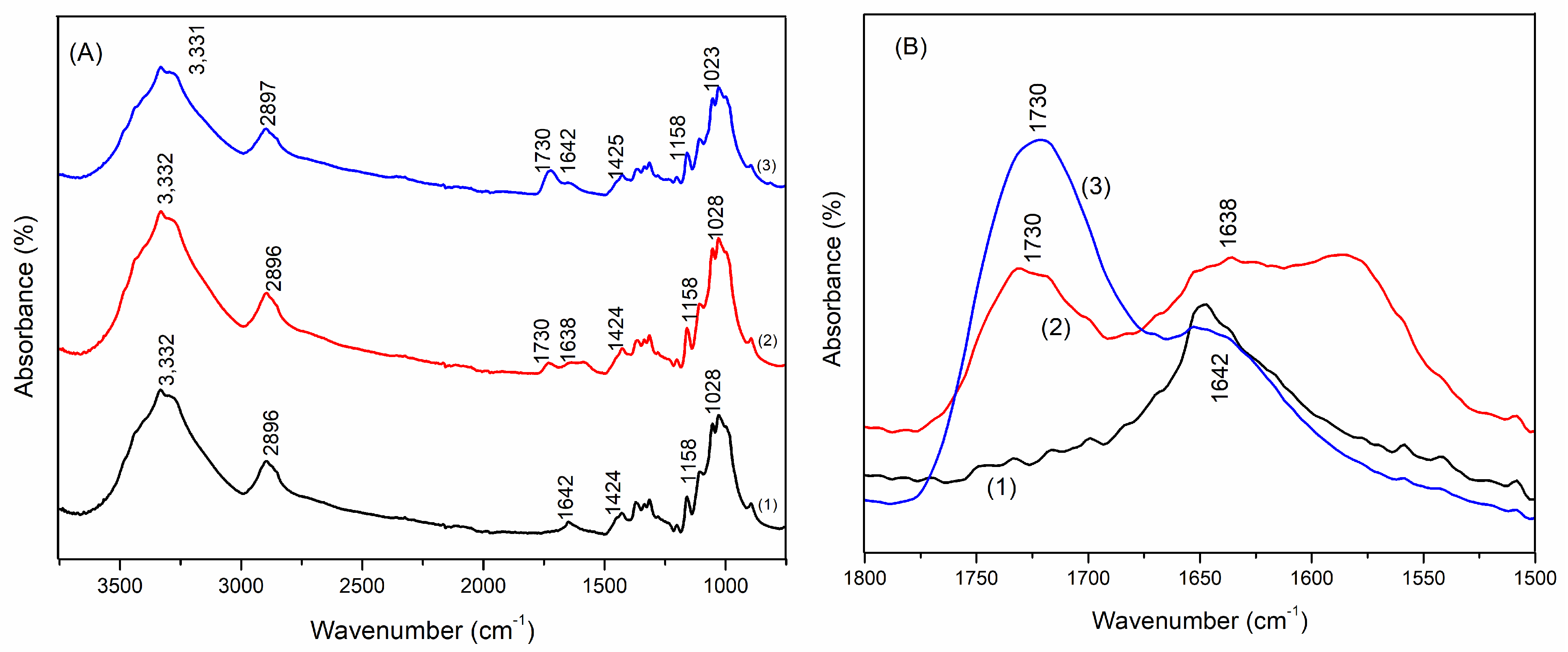
Fourier Transform Attenuated Reflection Infrared Spectroscopy
Photodegradation Test
ABDA Degradation Kinetics
Microbiological Analysis
3. Results and Discussion
3.1. Analysis of the Fabric Treatment
3.2. Statistical Analysis of the Dyeing Process
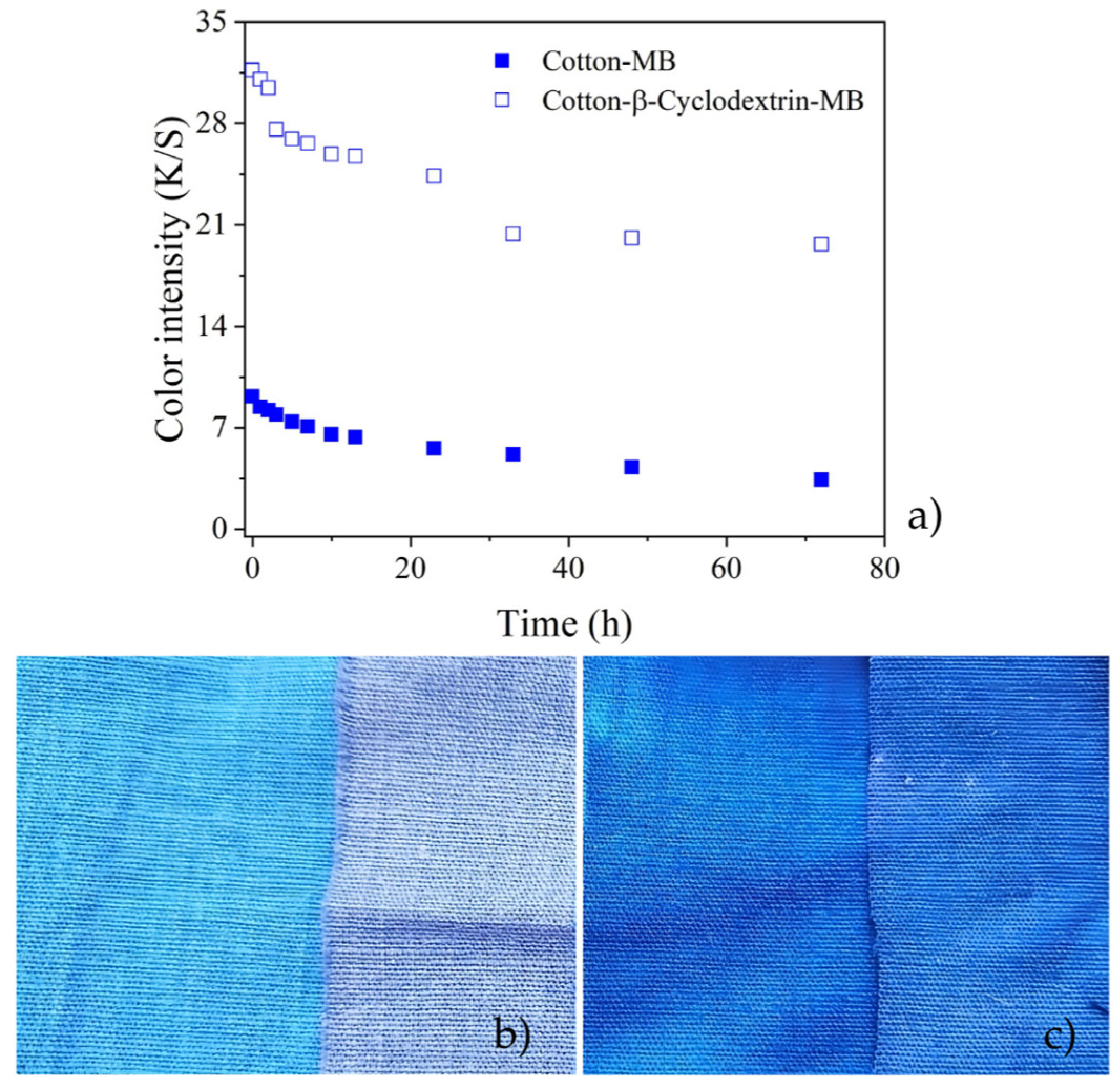
3.3. Photobleaching Tests
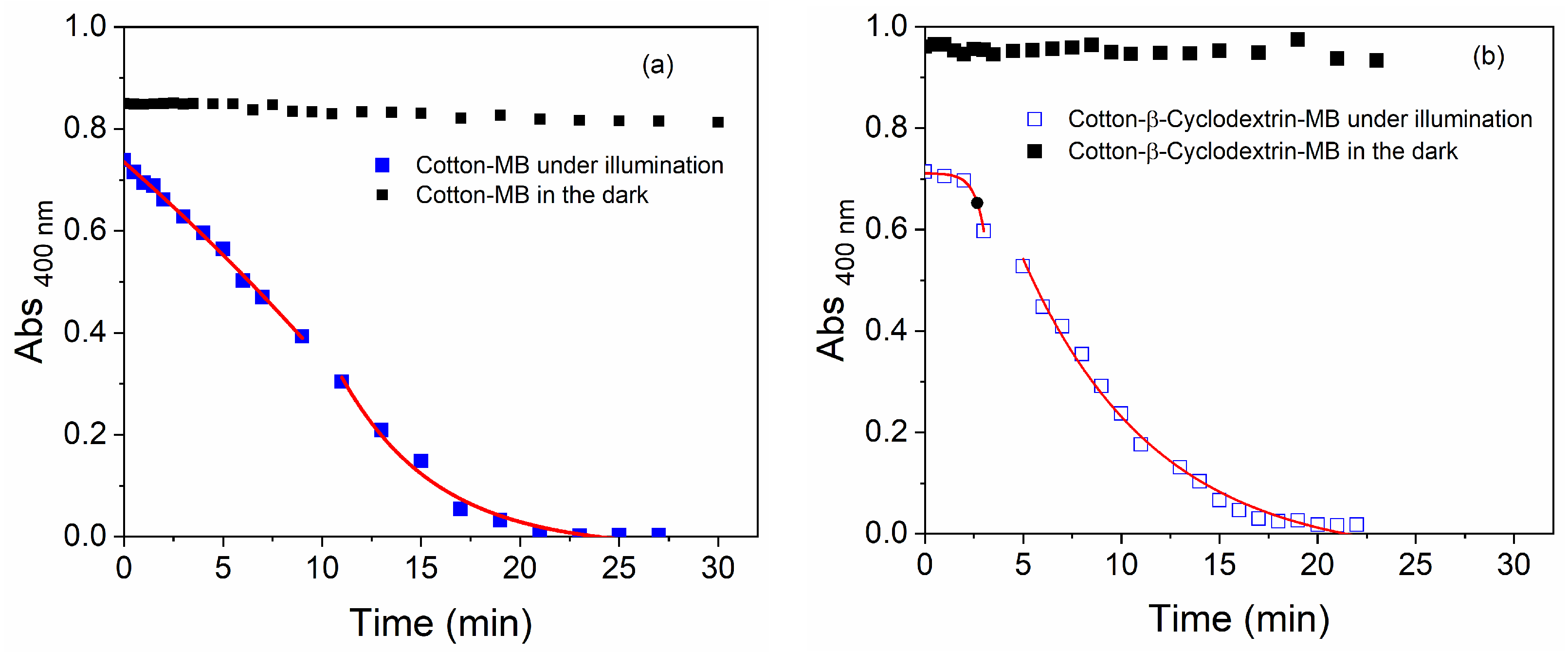
3.4. Kinetics of ABDA Degradation via Singlet Oxygen
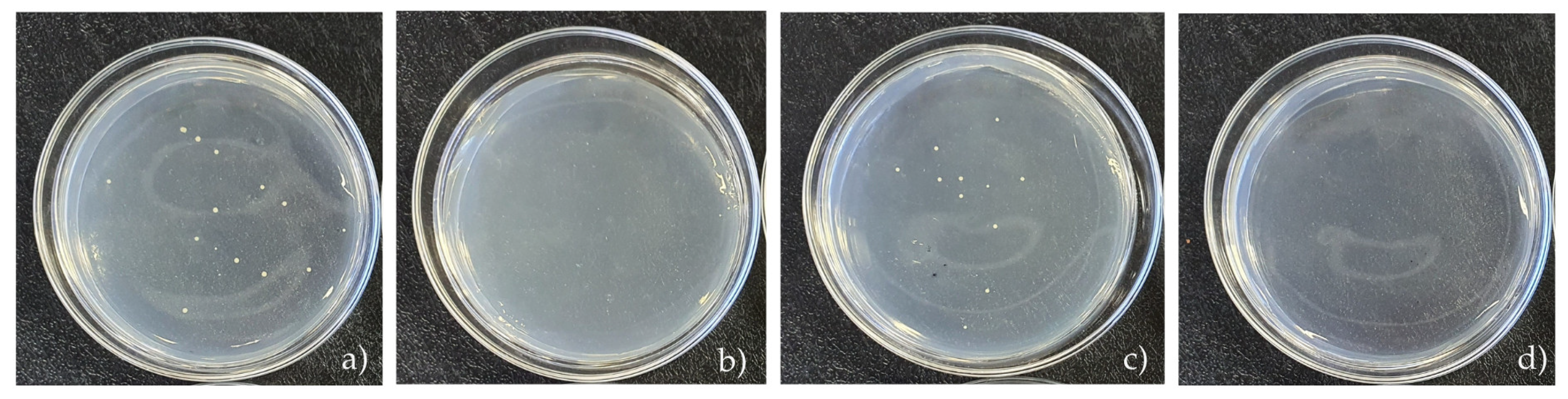
3.5. Microbiological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Fan, J.; Xia, Y.; Kou, X.; Ke, Q.; Zhao, Y. Preparation of Aromatic β-Cyclodextrin Nano/Microcapsules and Corresponding Aromatic Textiles: A Review. Carbohydr. Polym. 2023, 308, 120661. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Pirzada, B.M.; Price, G.; Shibiru, A.L.; Qurashi, A. Applications of Nanotechnology in Smart Textile Industry: A Critical Review. J. Adv. Res. 2022, 38, 55–75. [Google Scholar] [CrossRef]
- Mendes, S.; Catarino, A.; Zille, A.; Fernandes, N.; Bezerra, F.M. Vehiculation of Methyl Salicylate from Microcapsules Supported on Textile Matrix. Materials 2021, 14, 1087. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wei, Y.-Y.; Li, J.; Sun, Y.-Y.; Liu, S.R.; Ma, K.M.; Leung, P.H.-M.; Tao, X.M. Clinical Study of Antibacterial Medical Textiles Containing Polyhydroxyalkanoate Oligomers for Reduction of Hospital-Acquired Infections. J. Hosp. Infect. 2024, 149, 144–154. [Google Scholar] [CrossRef]
- Valle, J.A.B.; Curto Valle, R.D.C.S.; Da Costa, C.; Maestá, F.B.; Lis Arias, M.J. Reservoir Effect of Textile Substrates on the Delivery of Essential Oils Microencapsulated by Complex Coacervation. Polymers 2024, 16, 670. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Chevalier, Y.; Majdoub, M. Isosorbide-Based Microcapsules for Cosmeto-Textiles. Ind. Crops Prod. 2014, 52, 150–157. [Google Scholar] [CrossRef]
- Emam, H.E.; Abdelhameed, R.M. In-Situ Modification of Natural Fabrics by Cu-BTC MOF for Effective Release of Insect Repellent (N,N-Diethyl-3-Methylbenzamide). J. Porous Mater. 2017, 24, 1175–1185. [Google Scholar] [CrossRef]
- Jiao, M.; Zhang, Y.; Dong, Z.; Zhang, H.; Jiang, Y. Microencapsulation of Multi-Component Traditional Chinese Herbs Extracts and Its Application to Traditional Chinese Medicines Loaded Textiles. Colloids Surf. B Biointerfaces 2024, 240, 113970. [Google Scholar] [CrossRef]
- Da Silva, A.C.P.; Arruda, L.M.; Moreira, I.P.; Scacchetti, F.A.P.; De Oliveira, H.P.M.; Samulewski, R.B.; Fangueiro, R.; Tessaro, A.L. Functionalization of Fibrous Substrates with Mesoporous Silica Nanoparticles as a Strategy to Obtain Photodynamic Antibacterial Textiles. Dye. Pigment. 2024, 230, 112342. [Google Scholar] [CrossRef]
- Rahman, M.; Kabir, M.; Chen, S.; Wu, S. Developments, Applications, and Challenges of Metal–Organic Frameworks@textile Composites: A State-of-Art Review. Eur. Polym. J. 2023, 199, 112480. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Lis, M.J.; Firmino, H.B.; Dias Da Silva, J.G.; Curto Valle, R.D.C.S.; Borges Valle, J.A.; Scacchetti, F.A.P.; Tessaro, A.L. The Role of β-Cyclodextrin in the Textile Industry—Review. Molecules 2020, 25, 3624. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Z.; Wang, X.; Wu, J.-Z.; Li, S.-D. Development of Thermosensitive Microgel-Loaded Cotton Fabric for Controlled Drug Release. Appl. Surf. Sci. 2017, 403, 509–518. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Nardello-Rataj, V.; Leclercq, L. Encapsulation of Biocides by Cyclodextrins: Toward Synergistic Effects against Pathogens. Beilstein J. Org. Chem. 2014, 10, 2603–2622. [Google Scholar] [CrossRef]
- Fernández, M.A.; Silva, O.F.; Vico, R.V.; De Rossi, R.H. Complex Systems That Incorporate Cyclodextrins to Get Materials for Some Specific Applications. Carbohydr. Res. 2019, 480, 12–34. [Google Scholar] [CrossRef]
- Sehgal, V.; Pandey, S.P.; Singh, P.K. Prospects of Charged Cyclodextrins in Biomedical Applications. Carbohydr. Polym. 2024, 323, 121348. [Google Scholar] [CrossRef] [PubMed]
- Alishahi, M.; Aboelkheir, M.; Chowdhury, R.; Altier, C.; Shen, H.; Uyar, T. Functionalization of Cotton Nonwoven with Cyclodextrin/Lawsone Inclusion Complex Nanofibrous Coating for Antibacterial Wound Dressing. Int. J. Pharm. 2024, 652, 123815. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Alfatease, A.; Hani, U.; Haider, N.; Akbar, M.J.; Talath, S.; Angolkar, M.; Paramshetti, S.; Osmani, R.A.M.; Gundawar, R. Drug Eluting Protein and Polysaccharides-Based Biofunctionalized Fabric Textiles- Pioneering a New Frontier in Tissue Engineering: An Extensive Review. Int. J. Biol. Macromol. 2024, 268, 131605. [Google Scholar] [CrossRef]
- Rasheed, A. Cyclodextrins as Drug Carrier Molecule: A Review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Nostro, P.L.; Fratoni, L.; Ridi, F.; Baglioni, P. Surface Treatments on Tencel Fabric: Grafting with Β-cyclodextrin. J. Appl. Polym. Sci. 2003, 88, 706–715. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Carvalho Cotre, D.S.D.; Plath, A.; Firmino, H.B.; Lima, M.A.D.; Lis, M.; Samulewski, R.B.; Moisés, M.P. β -Cyclodextrin: Disperse Yellow 211 Complexes Improve Coloristic Intensity of Polyamide Dyed Knits. Text. Res. J. 2022, 92, 2194–2204. [Google Scholar] [CrossRef]
- Ferreira, B.T.M.; Espinoza-Quiñones, F.R.; Borba, C.E.; Módenes, A.N.; Santos, W.L.F.; Bezerra, F.M. Use of the β-Cyclodextrin Additive as a Good Alternative for the Substitution of Environmentally Harmful Additives in Industrial Dyeing Processes. Fibers Polym. 2020, 21, 1266–1274. [Google Scholar] [CrossRef]
- Crupi, V.; Ficarra, R.; Guardo, M.; Majolino, D.; Stancanelli, R.; Venuti, V. UV–Vis and FTIR–ATR Spectroscopic Techniques to Study the Inclusion Complexes of Genistein with β-Cyclodextrins. J. Pharm. Biomed. Anal. 2007, 44, 110–117. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S.; Alfaifi, A.Y.A. Cotton Fabric Finished with β-Cyclodextrin: Inclusion Ability toward Antimicrobial Agent. Carbohydr. Polym. 2014, 102, 550–556. [Google Scholar] [CrossRef]
- Marques, H.M.C. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour. Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as Encapsulation Agents for Plant Bioactive Compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Maestá Bezerra, F.; García Carmona, Ó.; García Carmona, C.; Souza Plath, A.M.; Lis, M. Biofunctional Wool Using β-Cyclodextrins as Vehiculizer of Citronella Oil. Process Biochem. 2019, 77, 151–158. [Google Scholar] [CrossRef]
- Radu, C.-D.; Parteni, O.; Ochiuz, L. Applications of Cyclodextrins in Medical Textiles—Review. J. Control. Release 2016, 224, 146–157. [Google Scholar] [CrossRef]
- Huang, T.; Hu, X.; Huang, S.; Liu, D.; Chen, L.; Wu, Q.; Zhu, W.; Zhang, X.; Liu, M.; Wei, Y. One-Step Synthesis of Cyclodextrin-Based Fluorescent Hyperbranched Polymer as Type I Photosensitizer for Effective Photodynamic Therapy. Dye. Pigment. 2024, 223, 111928. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Y.; Shi, R.; Zhou, M.; Yu, Y.; Wang, P.; Wang, Q. Protein-Based Coating Strategy for Preparing Durable Sunlight-Driven Rechargeable Antibacterial, Super Hydrophilic, and UV-Resistant Textiles. Int. J. Biol. Macromol. 2024, 258, 128761. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, H.; Ran, P.; Liu, Y.; Wang, J.; Xu, X.; Zhou, Z. Regulating Molecular Brush Structure on Cotton Textiles for Efficient Antibacterial Properties. Int. J. Biol. Macromol. 2024, 267, 131486. [Google Scholar] [CrossRef]
- Mali, S.B.; Dahivelkar, S.D.; Mahale, S.A. Nanotechnology in Photodynamic Therapy. Oral. Oncol. Rep. 2024, 10, 100307. [Google Scholar] [CrossRef]
- Lu, B.; Lu, X.; Mu, M.; Meng, S.; Feng, Y.; Zhang, Y. Novel Near-Infrared BODIPY-Cyclodextrin Complexes for Photodynamic Therapy. Heliyon 2024, 10, e26907. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Antimicrobial Photodynamic Inactivation: A Bright New Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Zeyada, H.M.; El-Mallah, H.M.; Atwee, T.; El-Damhogi, D.G. Spectroscopic Studies of UV Irradiated Erythrosine B Thin Films Prepared by Spin Coating Technique. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Michielsen, S. Photodynamic Activity of Nanostructured Fabrics Grafted with Xanthene and Thiazine Dyes against Opportunistic Fungi. J. Photochem. Photobiol. B Biol. 2015, 150, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; Borges, A.; Freitas, C.F.; Hioka, N.; Mikcha, J.M.G.; Simões, M. Antimicrobial Photodynamic Inactivation Mediated by Rose Bengal and Erythrosine Is Effective in the Control of Food-Related Bacteria in Planktonic and Biofilm States. Molecules 2018, 23, 2288. [Google Scholar] [CrossRef]
- Slyusareva, E.; Sizykh, A.; Tyagi, A.; Penzkofer, A. Spectral and Photophysical Properties of Fluorone Dyes in Bio-Related Films and Methanol. J. Photochem. Photobiol. A Chem. 2009, 208, 131–140. [Google Scholar] [CrossRef]
- Leyva-Castillo, J.-M.; McGurk, A.; Geha, M.D.R. Allergic Skin Inflammation and S. Aureus Skin Colonization Are Mutually Reinforcing. Clin. Immunol. 2020, 218, 108511. [Google Scholar] [CrossRef]
- Lima, W.G.; De Brito, J.C.M.; Cardoso, V.N.; Fernandes, S.O.A. In-Depth Characterization of Antibacterial Activity of Melittin against Staphylococcus Aureus and Use in a Model of Non-Surgical MRSA-Infected Skin Wounds. Eur. J. Pharm. Sci. 2021, 156, 105592. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswami, V.; Natarajan, B.; Sethuraman, V.; Natesan, S.; RajSelvaraj, B. Nano Based Photodynamic Therapy to Target Tumor Microenvironment. Nano Trends 2023, 1, 100003. [Google Scholar] [CrossRef]
- Dey, A.; Singhvi, G.; Puri, A.; Kesharwani, P.; Dubey, S.K. An Insight into Photodynamic Therapy towards Treating Major Dermatological Conditions. J. Drug Deliv. Sci. Technol. 2022, 76, 103751. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Zúñiga, T.; Palavecino, C.E. Photodynamic Therapy for Treatment of Staphylococcus Aureus Infections. Photodiagn. Photodyn. Ther. 2021, 34, 102285. [Google Scholar] [CrossRef] [PubMed]
- Sadraeian, M.; Zhang, L.; Aavani, F.; Biazar, E.; Jin, D. Photodynamic Viral Inactivation Assisted by Photosensitizers. Mater. Today Phys. 2022, 28, 100882. [Google Scholar] [CrossRef]
- Jiang, J.; Lv, X.; Cheng, H.; Yang, D.; Xu, W.; Hu, Y.; Song, Y.; Zeng, G. Type I Photodynamic Antimicrobial Therapy: Principles, Progress, and Future Perspectives. Acta Biomater. 2024, 177, 1–19. [Google Scholar] [CrossRef]
- Boltes Cecatto, R.; Siqueira De Magalhães, L.; Fernanda Setúbal Destro Rodrigues, M.; Pavani, C.; Lino-dos-Santos-Franco, A.; Teixeira Gomes, M.; Fátima Teixeira Silva, D. Methylene Blue Mediated Antimicrobial Photodynamic Therapy in Clinical Human Studies: The State of the Art. Photodiagn. Photodyn. Ther. 2020, 31, 101828. [Google Scholar] [CrossRef]
- Alasqah, M.N. Efficacy of Methylene Blue-Mediated Antimicrobial Photodynamic Therapy on Clinical and Radiographic Outcomes among Patients with Periodontal Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Photodiagn. Photodyn. Ther. 2024, 46, 104000. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; De Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; De Fátima Turchiello, R.; Baptista, M.S. Methylene Blue in Photodynamic Therapy: From Basic Mechanisms to Clinical Applications. Photodiagn. Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Garapati, C.; Boddu, S.H.; Jacob, S.; Ranch, K.M.; Patel, C.; Babu, R.J.; Tiwari, A.K.; Yasin, H. Photodynamic Therapy: A Special Emphasis on Nanocarrier-Mediated Delivery of Photosensitizers in Antimicrobial Therapy. Arab. J. Chem. 2023, 16, 104583. [Google Scholar] [CrossRef]
- Bergmann, E.V.; Capeloto, O.A.; Catanio, A.T.S.; Flizikowski, G.A.S.; Kimura, N.M.; Freitas, C.F.; Herculano, L.S.; Astrath, N.G.C.; Malacarne, L.C. Photoactivation of Erythrosine in Simulated Body Fluids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119867. [Google Scholar] [CrossRef] [PubMed]
- Entradas, T.; Waldron, S.; Volk, M. The Detection Sensitivity of Commonly Used Singlet Oxygen Probes in Aqueous Environments. J. Photochem. Photobiol. B Biol. 2020, 204, 111787. [Google Scholar] [CrossRef] [PubMed]
- Rukmani, A.; Sundrarajan, M. Inclusion of Antibacterial Agent Thymol on β-Cyclodextrin-Grafted Organic Cotton. J. Ind. Text. 2012, 42, 132–144. [Google Scholar] [CrossRef]
- Alzate-Sánchez, D.M.; Smith, B.J.; Alsbaiee, A.; Hinestroza, J.P.; Dichtel, W.R. Cotton Fabric Functionalized with a β-Cyclodextrin Polymer Captures Organic Pollutants from Contaminated Air and Water. Chem. Mater. 2016, 28, 8340–8346. [Google Scholar] [CrossRef]
- Lis, M.J.; García Carmona, Ó.; García Carmona, C.; Maestá Bezerra, F. Inclusion Complexes of Citronella Oil with β-Cyclodextrin for Controlled Release in Biofunctional Textiles. Polymers 2018, 10, 1324. [Google Scholar] [CrossRef]
- Medronho, B.; JM Valente, A.; Costa, P.; Romano, A. Inclusion Complexes of Rosmarinic Acid and Cyclodextrins: Stoichiometry, Association Constants, and Antioxidant Potential. Colloid. Polym. Sci. 2014, 292, 885–894. [Google Scholar] [CrossRef]
- Voncina, B.; Le Marechal, A.M. Grafting of Cotton with Β-cyclodextrin via Poly(Carboxylic Acid). J. Appl. Polym. Sci. 2005, 96, 1323–1328. [Google Scholar] [CrossRef]
- Wainwright, M. Tricyclic Cationic Chromophores as Models for New Photoantimicrobials. J. Braz. Chem. Soc. 2015, 26, 2390–2404. [Google Scholar] [CrossRef]
- Miazaki, J.B.; Dos Santos, A.R.; De Freitas, C.F.; Stafussa, A.P.; Mikcha, J.M.G.; Bergamasco, R.D.C.; Tonon, L.A.C.; Madrona, G.S.; Caetano, W.; Da Silva, L.H.; et al. Edible Coatings and Application of Photodynamics in Ricotta Cheese Preservation. LWT 2022, 165, 113697. [Google Scholar] [CrossRef]
- Hakimov, S.; Kylychbekov, S.; Harness, B.; Neupane, S.; Hurley, J.; Brooks, A.; Banga, S.; Er, A.O. Evaluation of Silver Nanoparticles Attached to Methylene Blue as an Antimicrobial Agent and Its Cytotoxicity. Photodiagn. Photodyn. Ther. 2022, 39, 102904. [Google Scholar] [CrossRef]
- Feng, Y.; Tonon, C.C.; Hasan, T. Dramatic Destruction of Methicillin-Resistant Staphylococcus Aureus Infections with a Simple Combination of Amoxicillin and Light-Activated Methylene Blue. J. Photochem. Photobiol. B Biol. 2022, 235, 112563. [Google Scholar] [CrossRef] [PubMed]


| Sample | pH | Time (min) | Temperature (°C) | NaCl Concentration (g/L) | RDC (g/L) | K/S |
|---|---|---|---|---|---|---|
| 1 | 4 | 30 | 50 | 0 | 0.001171 | 26.1172 |
| 2 | 10 | 30 | 50 | 0 | 0.000283 | 30.61208 |
| 3 | 4 | 60 | 50 | 0 | 0.001032 | 25.79435 |
| 4 | 10 | 60 | 50 | 0 | 0.00022 | 34.56379 |
| 5 | 4 | 30 | 90 | 0 | 0.001236 | 21.71599 |
| 6 | 10 | 30 | 90 | 0 | 0.000175 | 34.16909 |
| 7 | 4 | 60 | 90 | 0 | 0.000437 | 23.52474 |
| 8 | 10 | 60 | 90 | 0 | 0.000387 | 3458468 |
| 9 | 4 | 30 | 50 | 60 | 0.000839 | 29.78177 |
| 10 | 10 | 30 | 50 | 60 | 0.000839 | 28.35535 |
| 11 | 4 | 60 | 50 | 60 | 0.000741 | 31.69385 |
| 12 | 10 | 60 | 50 | 60 | 0.000592 | 26.93975 |
| 13 | 4 | 30 | 90 | 60 | 0.001179 | 26.45997 |
| 14 | 10 | 30 | 90 | 60 | 0.001088 | 24.78423 |
| 15 | 4 | 60 | 90 | 60 | 0.001188 | 25.09617 |
| 16 | 10 | 60 | 90 | 60 | 0.001194 | 26.29626 |
| 17 | 7 | 45 | 70 | 30 | 0.001213 | 22.49068 |
| 18 | 7 | 45 | 70 | 30 | 0.001166 | 23.94341 |
| 19 | 7 | 45 | 70 | 30 | 0.001249 | 25.09617 |
| 20 | 7 | 60 | 70 | 30 | 0.001084 | 26.39438 |
| 21 | 7 | 30 | 70 | 30 | 0.001255 | 22.51928 |
| 22 | 7 | 45 | 70 | 30 | 0.001173 | 27.99113 |
| 23 | 7 | 45 | 50 | 30 | 0.00101 | 27.02324 |
| 24 | 7 | 45 | 70 | 0 | 0.000359 | 35.98995 |
| 25 | 7 | 45 | 70 | 30 | 0.001185 | 23.62882 |
| 26 | 10 | 45 | 70 | 30 | 0.001082 | 25.42733 |
| 27 | 7 | 45 | 70 | 60 | 0.000924 | 28.70613 |
| 28 | 7 | 45 | 90 | 30 | 0.001442 | 23.65863 |
| 29 | 7 | 45 | 70 | 30 | 0.001215 | 25.04917 |
| 30 | 4 | 45 | 70 | 30 | 0.00127 | 26.08476 |
| 31 | 7 | 45 | 70 | 30 | 0.00115 | 24.98661 |
| Sample | Initial Concentration (mg/L) | Final Concentration (mg/L) | Adsorption (%) |
|---|---|---|---|
| Untreated | 25.60 | 3.50 ± 0.20 | 86.14 ± 0.94% |
| Treated with β-cyclodextrin | 25.60 | 0.60 ± 0.17 | 97.73 ± 0.57% |
| Material | Kinetic Rate (First Phase) | Kinetic Rate (Second Phase) |
|---|---|---|
| Untreated | 0.025 min−1 | 0.207 min−1 |
| Treated with β-cyclodextrin | 0.148 min−1 | 2.000 min−1 |
| ratio | 592.00% | 966.20% |
| Samples | N. of Bacteria (CFU mL−1) in 1 h | Reduction (%) |
|---|---|---|
| MB (no light) | 1.5 × 103 | 99.42 |
| MB | 0 | 100 |
| MB + β-CD (no light) | 1.1 × 103 | 99.57 |
| MB + β-CD | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firmino, H.B.; Volante, E.K.T.S.; da Silva, A.C.P.; Scacchetti, F.A.P.; Lis, M.J.; Martí, M.; Saxena, S.; Tessaro, A.L.; Bezerra, F.M. β-Cyclodextrin-Modified Cotton Fabric for Medical and Hospital Applications with Photodynamic Antibacterial Activity Using Methylene Blue. Coatings 2024, 14, 1100. https://doi.org/10.3390/coatings14091100
Firmino HB, Volante EKTS, da Silva ACP, Scacchetti FAP, Lis MJ, Martí M, Saxena S, Tessaro AL, Bezerra FM. β-Cyclodextrin-Modified Cotton Fabric for Medical and Hospital Applications with Photodynamic Antibacterial Activity Using Methylene Blue. Coatings. 2024; 14(9):1100. https://doi.org/10.3390/coatings14091100
Chicago/Turabian StyleFirmino, Helen Beraldo, Emilly Karoline Tonini Silva Volante, Ana Claudia Pedrozo da Silva, Fabio Alexandre Pereira Scacchetti, Manuel José Lis, Meritxell Martí, Siddanth Saxena, André Luiz Tessaro, and Fabrício Maestá Bezerra. 2024. "β-Cyclodextrin-Modified Cotton Fabric for Medical and Hospital Applications with Photodynamic Antibacterial Activity Using Methylene Blue" Coatings 14, no. 9: 1100. https://doi.org/10.3390/coatings14091100
APA StyleFirmino, H. B., Volante, E. K. T. S., da Silva, A. C. P., Scacchetti, F. A. P., Lis, M. J., Martí, M., Saxena, S., Tessaro, A. L., & Bezerra, F. M. (2024). β-Cyclodextrin-Modified Cotton Fabric for Medical and Hospital Applications with Photodynamic Antibacterial Activity Using Methylene Blue. Coatings, 14(9), 1100. https://doi.org/10.3390/coatings14091100











