The Influence of Surface Nanocrystallization of TA2 Titanium Alloy on Its Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Characterization
2.1. SN Experiment
2.2. Characterization
3. Result and Discussion
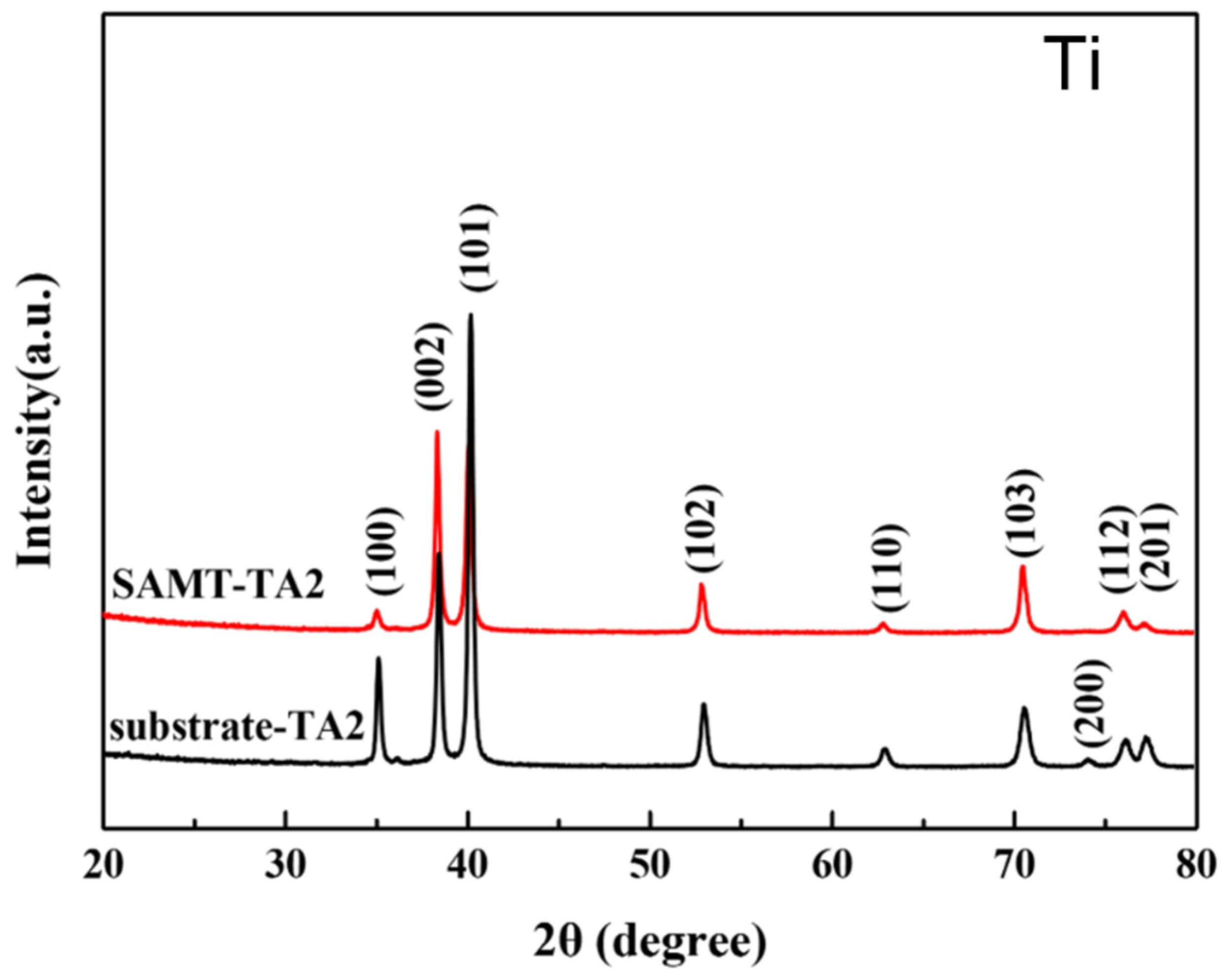
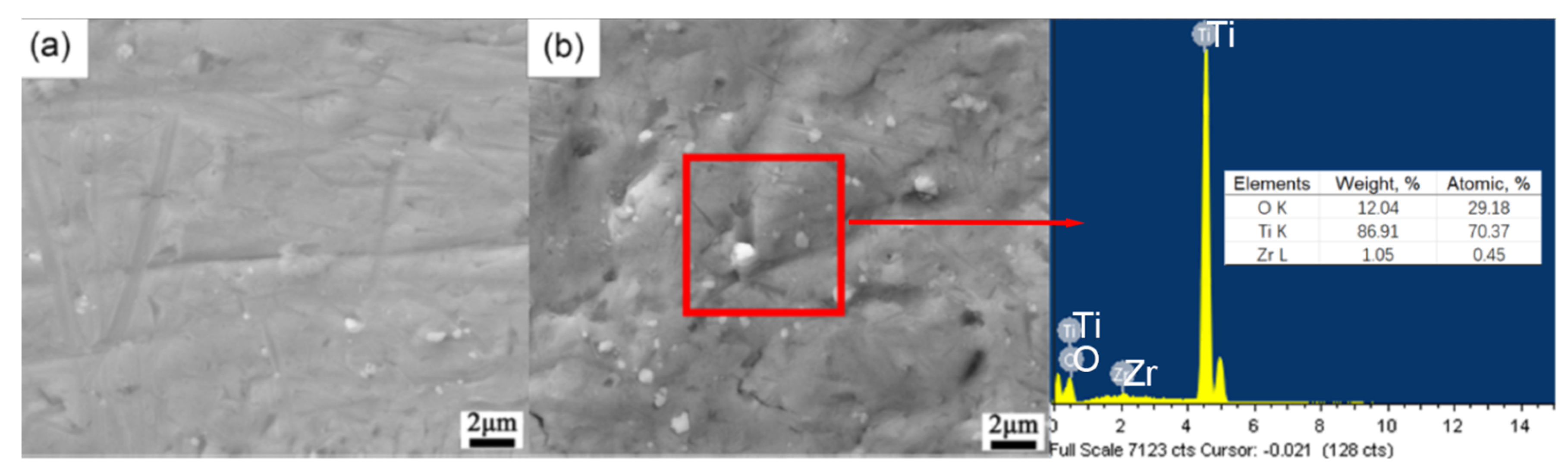
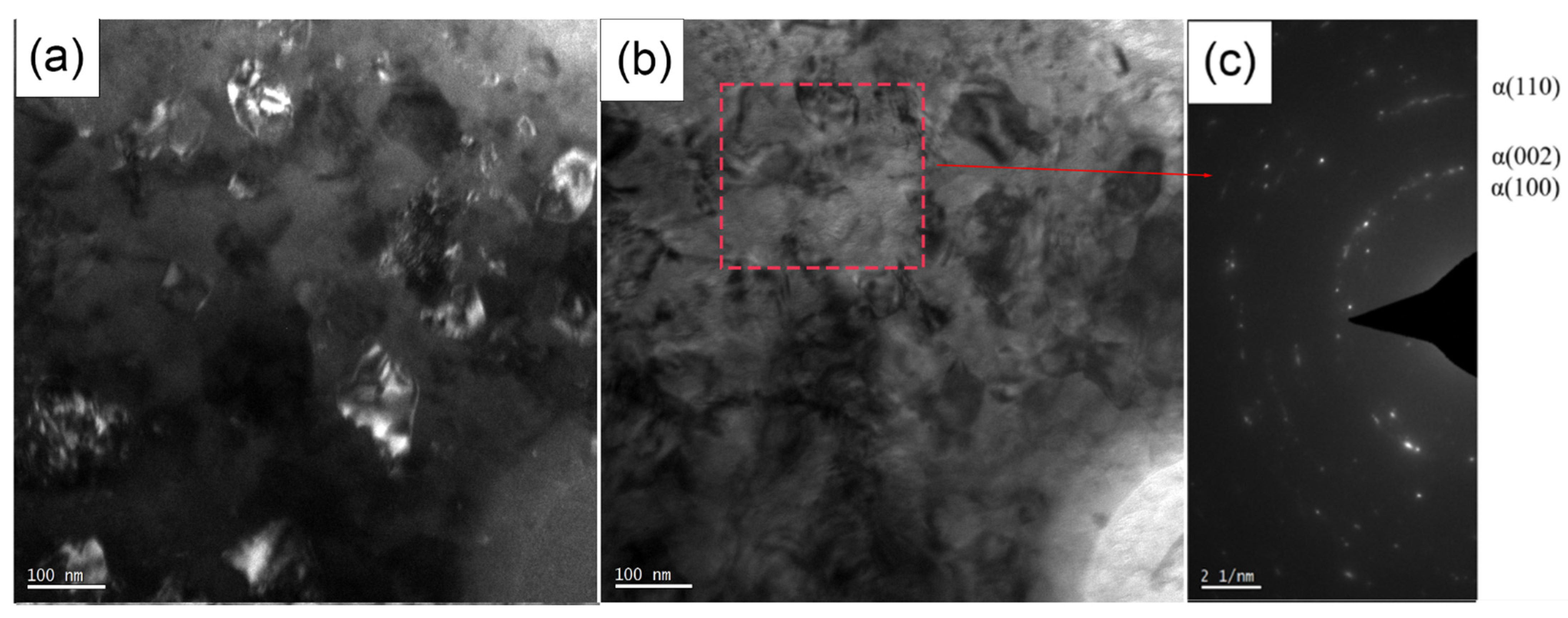
3.1. Characterization of Microstructure of TA2 by SMAT
3.2. Electrochemical Behavior of TA2/SMAT-TA2
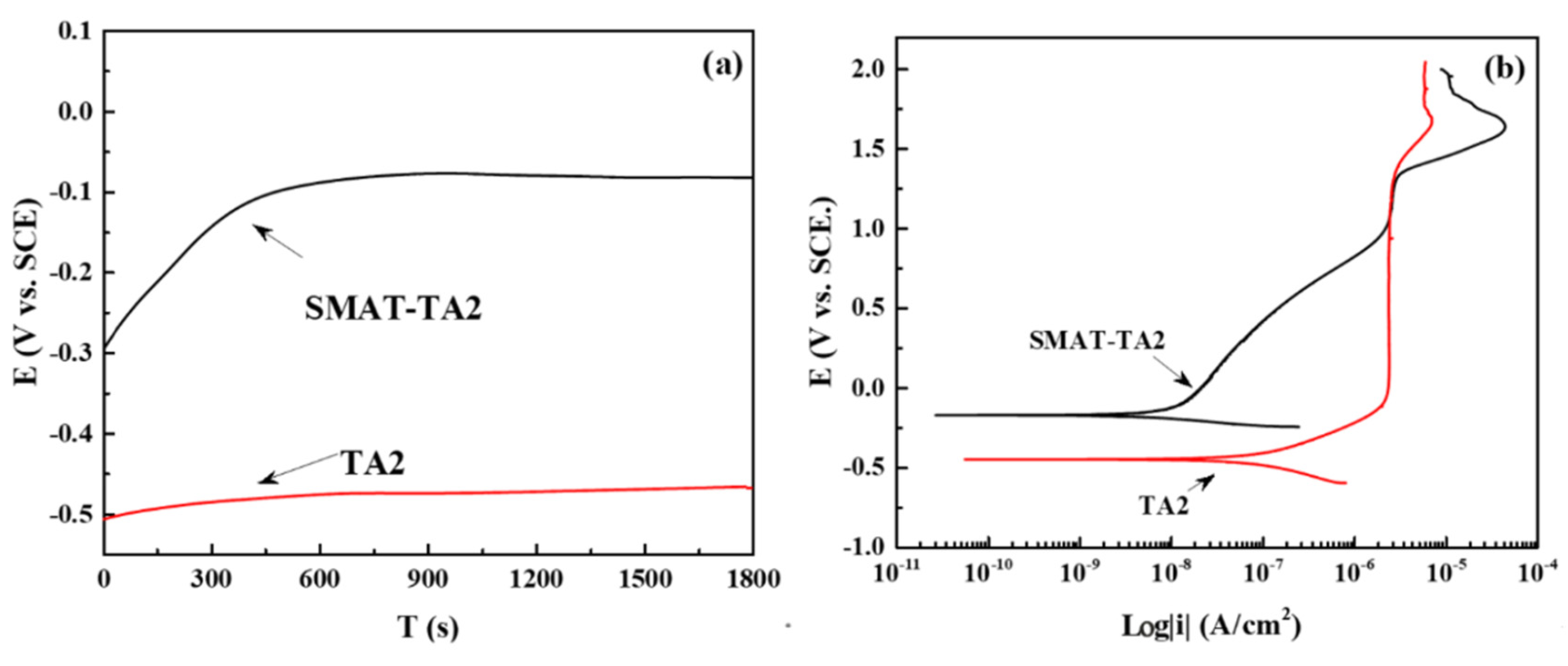
3.2.1. OCP and Polarization Curve
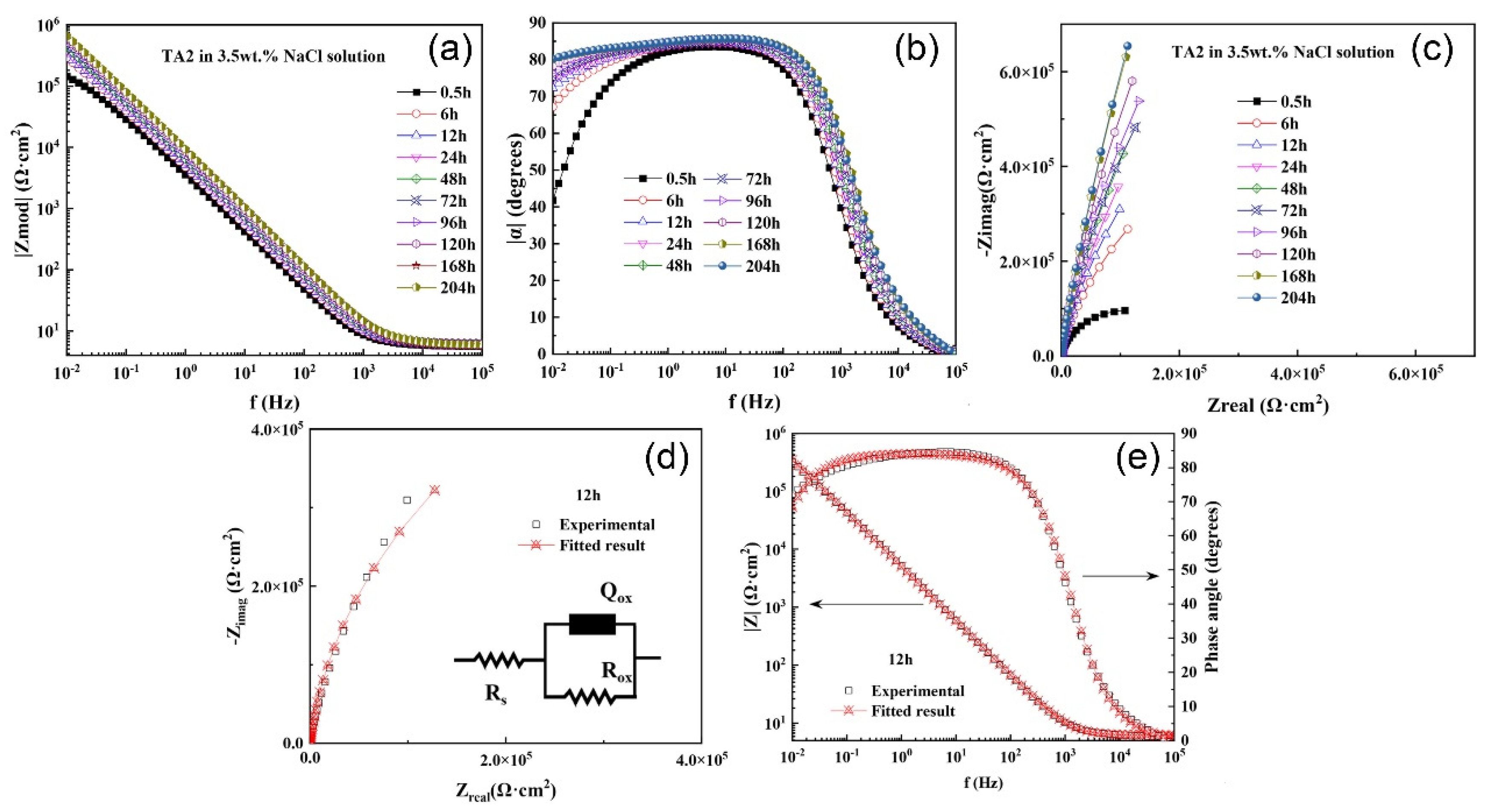
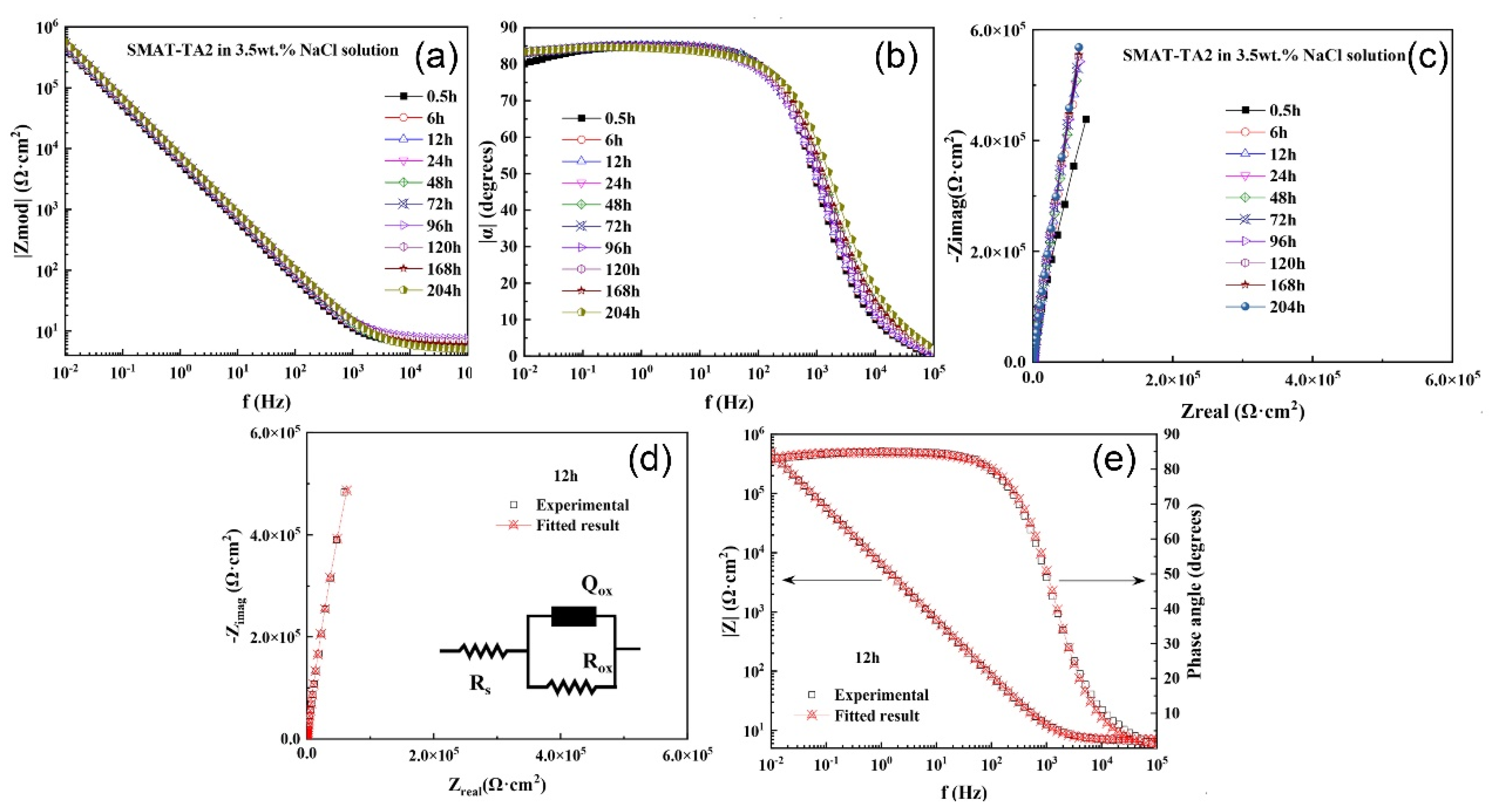
3.2.2. EIS
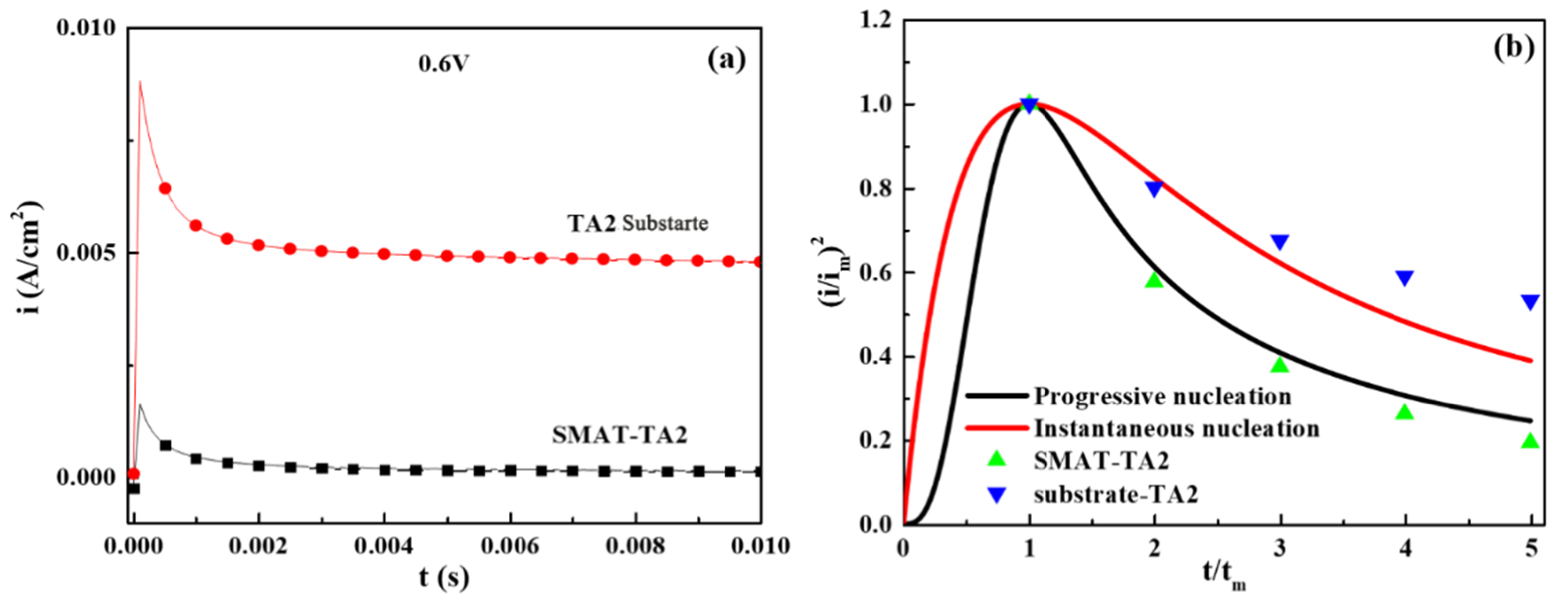
3.3. Nucleation Behavior of TA2/SMAT-TA2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, X.; Ma, J.; Tian, A.; Wang, Y.; Li, Y.; Dong, B.; Tong, X.; Ma, X. Surface modification techniques of titanium and titanium alloys for biomedical orthopaedics applications: A review. Colloids Surf. B Biointerfaces 2023, 227, 113339. [Google Scholar] [CrossRef]
- Li, L.; Sun, L.; Li, M. Diffusion bonding of dissimilar titanium alloys via surface nanocrystallization treatment. J. Mater. Res. Technol. 2022, 17, 1274–1288. [Google Scholar] [CrossRef]
- Al-Samarai, R.A.; Al-Douri, Y. Friction and Wear in Metals; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Gurrappa, I. Characterization of titanium alloy Ti-6Al-4V for chemical, marine and industrial applications. Mater. Charact. 2003, 51, 131–139. [Google Scholar] [CrossRef]
- Gurrappa, I.; Reddy, D.V. Characterisation of titanium alloy, IMI-834 for corrosion resistance under different environmental conditions. J. Alloys Compd. 2005, 390, 270–274. [Google Scholar] [CrossRef]
- Codaro, E.N.; Nakazato, R.Z.; Horovistiz, A.L.; Ribeiro, L.M.; Ribeiro, R.B.; Hein, L.D. An image analysis study of pit formation on Ti-6Al-4V. Mater. Sci. Eng. A 2003, 341, 202–210. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chen, K.M.; Xu, X.J.; Zhang, X.F.; Liu, G.L. Wear Failure and Surface wear Treatment Technology of Titanium Alloy Ti-6Al-4V. Light Met. 2005, 5, 54–58. [Google Scholar]
- Wang, J.; Chen, J.; Chen, B.; Yan, F.; Xue, Q. Wear behaviors and wear mechanisms of several alloys under simulated deep-sea environment covering seawater hydrostatic pressure. Tribol. Int. 2012, 56, 38–46. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Wang, L. Surface modification of titanium and titanium alloys: Technologies, developments, and future interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Moeinfar, K.; Khodabakhshi, F.; Kashani-Bozorg, S.; Mohammadi, M.; Gerlich, A. A review on metallurgical aspects of laser additive manufacturing (LAM): Stainless steels, nickel superalloys, and titanium alloys. J. Mater. Res. Technol. 2022, 16, 1029–1068. [Google Scholar] [CrossRef]
- Morshed-Behbahani, K.; Zakerin, N. A review on the role of surface nanocrystallization in corrosion of stainless steel. J. Mater. Res. Technol. 2022, 19, 1120–1147. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, K.; Lan, J.; Xu, S.; Chen, H.; Fu, A.; Lin, J.; Cao, H.; Zheng, G. Surface nanocrystallization and biomedical performance of Ti-Ta laminated composite processed by surface mechanical grinding treatment. Appl. Surf. Sci. 2023, 623, 157051. [Google Scholar] [CrossRef]
- Song, X.; Lu, M.; Wang, H.; Wang, X.C.; Sun, F.H. Fracture mechanics of microcrystalline/nanocrystalline composited multilayer chemical vapor deposition self-standing diamond films. Ceram. Int. 2022, 48, 21868–21878. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Lan, X. Microstructure and Properties of Surface High-energy Shot Peening Nanocrystallization on TC4 Titanium Alloy. Hot Work. Technol. 2006, 35, 5–7. [Google Scholar]
- Yang, C. Effects of Surface Mechanical Attrition Treatment on Surface Nanocrystallization Process of HCP Structural Metal Magnesium. Hot Work. Technol. 2018, 47, 156–158. [Google Scholar]
- Zhao, Y.; Wang, T. Surface Micronano Crystal-lization of 2024 Alloy Induced by Machinery Multi-Grinding. Spec. Cast. Nonferrous Alloys 2018, 38, 813–815. [Google Scholar]
- Pacquentin, W.; Caron, N.; Oltra, R. Effect of microstructure and chemical composition on localized corrosion resistance of a AISI 304L stainless steel after nanopulsed-laser surface melting. Appl. Surf. Sci. 2015, 356, 561–573. [Google Scholar] [CrossRef]
- Sun, Q.; Cao, F.; Wang, S. Nanoscale corrosion investigation of surface nanocrystallized 7150 Al alloy in 3.5 wt% NaCl solution by using FIB-TEM techniques. Corros. Sci. 2022, 195, 110021. [Google Scholar] [CrossRef]
- Zhao, W.; Li, C.; Lin, T.; Gao, J.; Si, X.; Qi, J.; Dai, X.; Cao, J. Low-temperature diffusion bonding of Ti6Al4V alloy via nanocrystallization and hydrogenation surface treatment. J. Mater. Res. Technol. 2023, 24, 7599–7613. [Google Scholar] [CrossRef]
- Pramod, K.; Mahobia, G.; Chattopadhyay, K. Surface nanocrystallization of β-titanium alloy by ultrasonic shot peening. Mater. Today Proc. 2020, 28, 486–490. [Google Scholar]
- Chen, G.; Chu, T.; Cui, Y.; Wu, Y.; Liu, X.; Lin, Q. Effect of surface nanocrystallization on high-cycle fatigue behavior of Ti–2Al–2.5 Zr alloy tube. Int. J. Fatigue 2022, 158, 106735. [Google Scholar] [CrossRef]
- Olugbade Olumide, T.; Lu, J. Literature review on the mechanical properties of materials after surface mechanical attrition treatment (SMAT). Nano Mater. Sci. 2020, 2, 3–31. [Google Scholar] [CrossRef]
- Lu, K.; Lu, J. Nanostructured surface layer on metallic materials induced by surface mechanical attrition treatment. Mater. Sci. Eng. A 2004, 375, 38–45. [Google Scholar] [CrossRef]
- Lu, K. Surface nanocrystallization (SNC) of metallic materials-presentation of the concept behind a new approach. J. Mater. Sci. Technol. 1999, 15, 193–197. [Google Scholar]
- Maleki, E.; Maleki, N.; Fattahi, A.; Unal, O.; Guagliano, M.; Bagherifard, S. Mechanical characterization and interfacial enzymatic activity of AISI 316L stainless steel after surface nanocrystallization. Surf. Coat. Technol. 2021, 405, 126729. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Huang, H.; Gan, J.; Yang, Y.; He, J.; Shi, M. Effect of high-energy shot peening on surface nanocrystallization and integrity of AISI 4140 Steel: A Numerical and Experimental Investigation. J. Mater. Eng. Perform. 2021, 30, 5579–5592. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Wen, A. Effect of High Energy shot peening surface nano on fatigue properties of industrial pure titanium. In Proceedings of the 6th International Conference on Transportation, Chongqing, China, 24 September 2006; Volume 2. [Google Scholar]
- Yang, L.; Liu, X. Analysis of Surface roughness of pure Titanium after nano-sized high-energy shot peening. Heavy Ind. Lift. Technol. 2007, 1, 4–6. [Google Scholar]
- Ortiz, A.L.; Shaw, L. X-ray diffraction analysis of a severely plastically deformed aluminum alloy. Acta Mater. 2004, 52, 2185–2197. [Google Scholar] [CrossRef]
- Hills, G.J.; Peter, L.M.; Scharifker, B.R.; Pereira, M.D. The nucleation and growth of two-dimensional anodic films under galvanostatic conditions. J. Electroanal. Chem. Interfacial Electrochem. 1981, 124, 247–262. [Google Scholar] [CrossRef]

| Element | Fe | C | N | H | Others | Ti |
|---|---|---|---|---|---|---|
| Content (wt.%) | 0.03 | 0.01 | <0.01 | 0.001 | <0.49 | 99.45 |
| Specimen | Ecorr (mV vs. SCE) | icorr (nA·cm−2) |
|---|---|---|
| TA2 | −454.0 | 134.5 |
| SMAT-TA2 | −177.0 | 9.2 |
| Immersion Time (h) | Rs (Ω·cm2) | Qox (10−5 cm2snΩ) | nox | Rox (105 Ω·cm2) |
|---|---|---|---|---|
| 0.5 | 5.811 | 5.241 | 0.9264 | 2.053 |
| 6 | 6.148 | 3.903 | 0.9356 | 8.285 |
| 12 | 6.156 | 3.594 | 0.9355 | 12.650 |
| 24 | 6.137 | 3.220 | 0.9362 | 17.320 |
| 48 | 6.360 | 2.806 | 0.9417 | 23.620 |
| 72 | 6.199 | 2.494 | 0.9444 | 25.030 |
| 96 | 6.120 | 2.281 | 0.9463 | 29.620 |
| 120 | 6.408 | 2.166 | 0.9461 | 39.060 |
| 168 | 5.825 | 2.016 | 0.9478 | 51.990 |
| 204 | 6.314 | 1.943 | 0.9471 | 56.040 |
| Immersion Time (h) | Rs (Ω·cm2) | Qox (10−5 cm2snΩ) | nox | Rox (105 Ω·cm2) |
|---|---|---|---|---|
| 0.5 | 6.524 | 3.059 | 0.9456 | 41.98 |
| 6 | 6.856 | 2.859 | 0.9423 | 117.2 |
| 12 | 6.962 | 2.749 | 0.9417 | 132.5 |
| 24 | 6.676 | 2.661 | 0.9403 | 176.1 |
| 48 | 6.079 | 2.623 | 0.9357 | 282.1 |
| 72 | 6.914 | 2.525 | 0.9358 | 417.1 |
| 96 | 7.971 | 2.461 | 0.9337 | 617.1 |
| 120 | 7.000 | 2.400 | 0.9350 | 525.5 |
| 168 | 7.001 | 2.390 | 0.9343 | 550.7 |
| 204 | 7.506 | 2.160 | 0.9378 | 580.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, Y.; Zhang, J.; Guo, Y.; Wen, L. The Influence of Surface Nanocrystallization of TA2 Titanium Alloy on Its Corrosion Resistance. Coatings 2024, 14, 1114. https://doi.org/10.3390/coatings14091114
Li Z, Wang Y, Zhang J, Guo Y, Wen L. The Influence of Surface Nanocrystallization of TA2 Titanium Alloy on Its Corrosion Resistance. Coatings. 2024; 14(9):1114. https://doi.org/10.3390/coatings14091114
Chicago/Turabian StyleLi, Zhengyi, Yanzhao Wang, Jiangong Zhang, Yiwei Guo, and Lei Wen. 2024. "The Influence of Surface Nanocrystallization of TA2 Titanium Alloy on Its Corrosion Resistance" Coatings 14, no. 9: 1114. https://doi.org/10.3390/coatings14091114





