Fabrication and Properties of Superhydrophobic Colored Stainless Steel Surface for Decoration and Anti-Corrosion
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Fabrication of Nano-Silica Particle
2.3. Sample Characterization
2.4. Contact Angle Measurements
2.5. Electrochemical Measurements
3. Results and Discussion
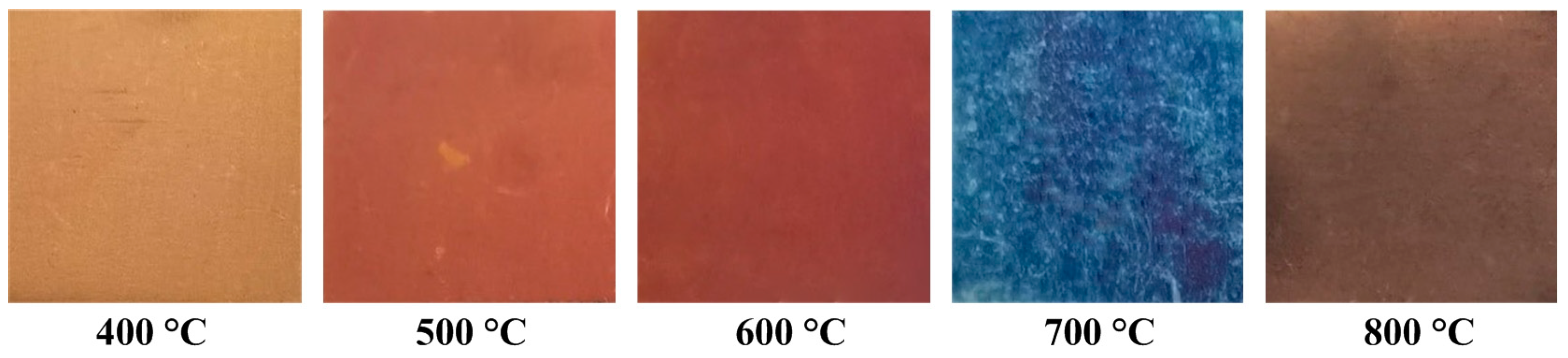
3.1. Optical Micrographs of Oxidized Stainless Steel Surfaces
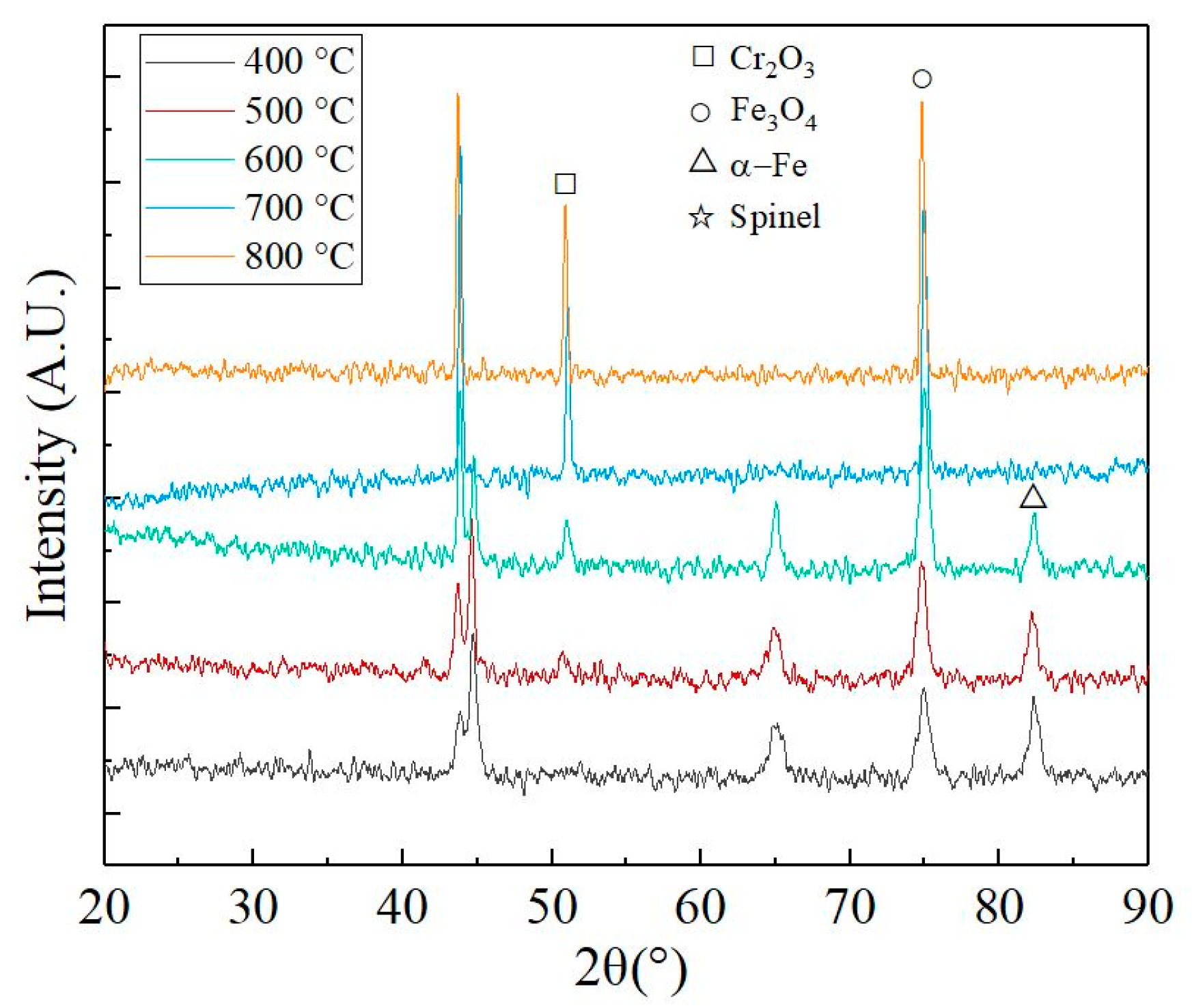
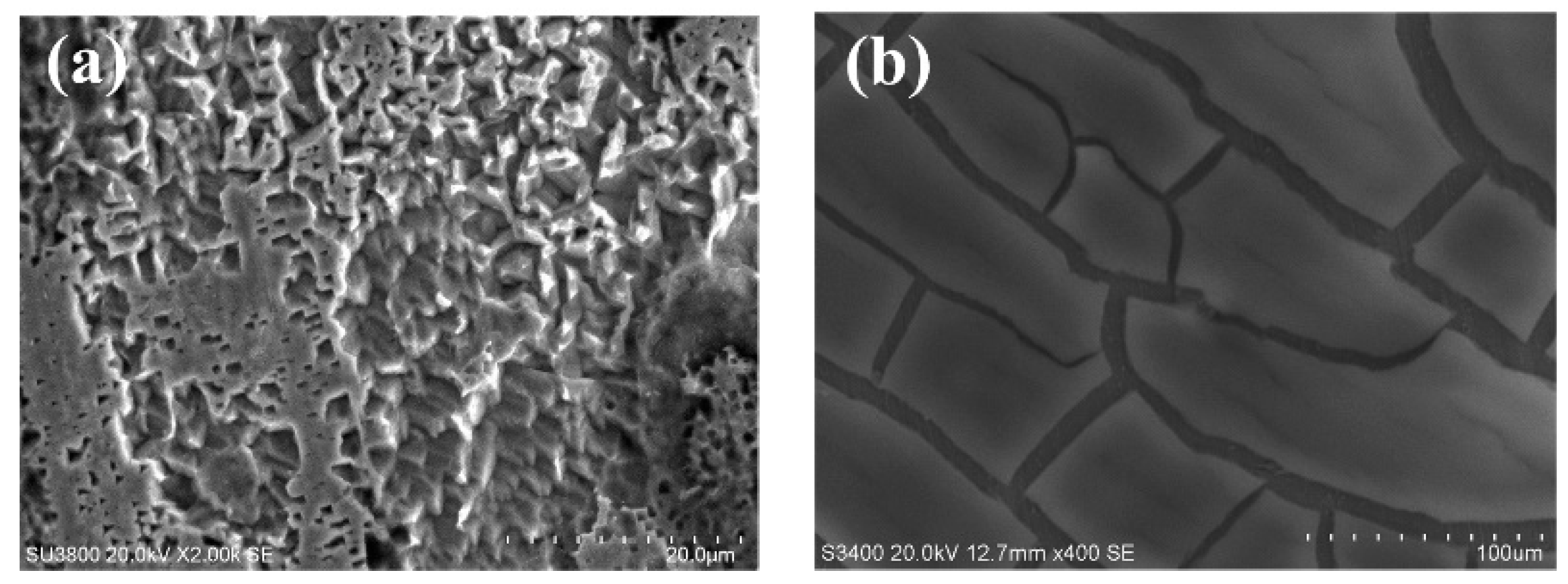
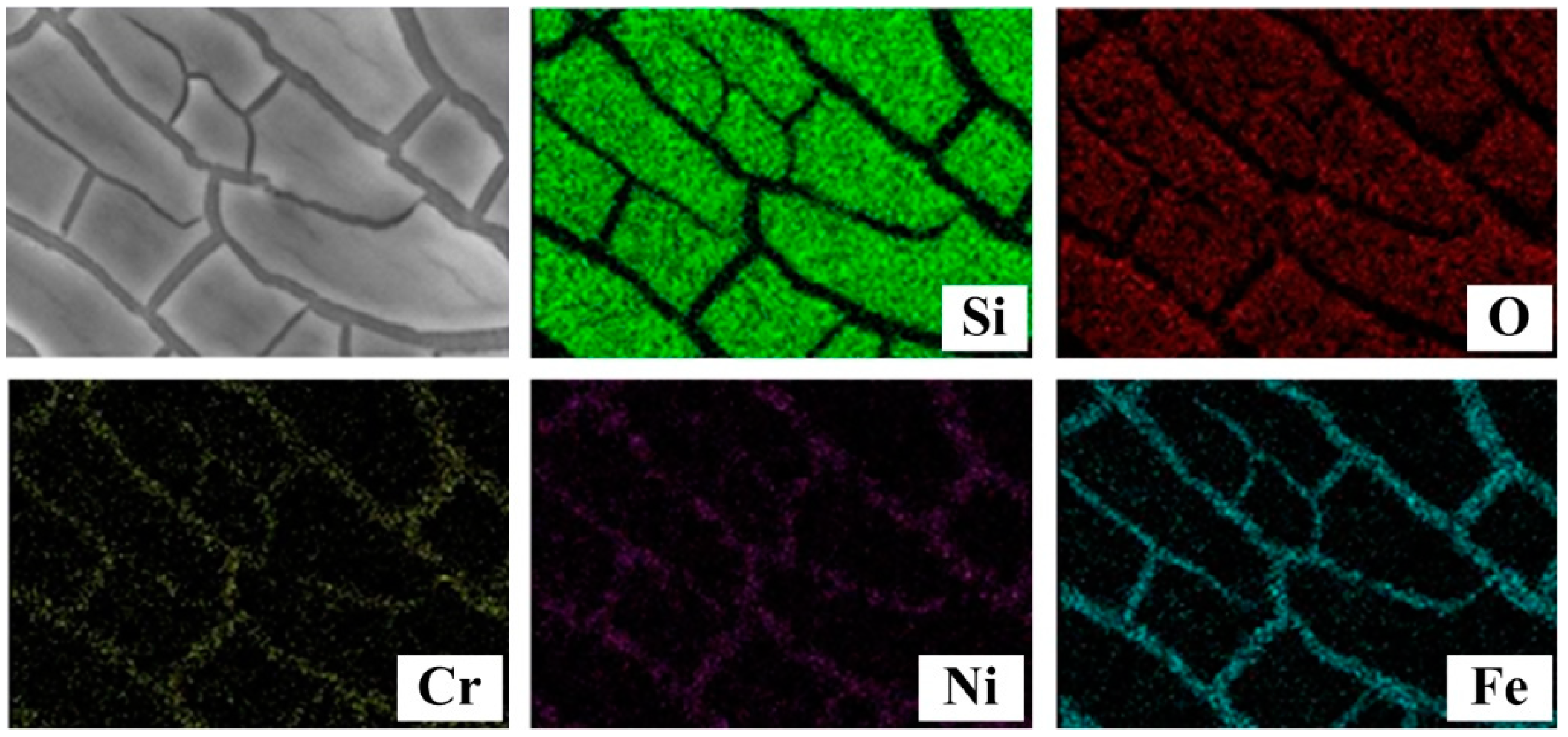
3.2. Surface Characterization
3.3. Contact Angle Measurements
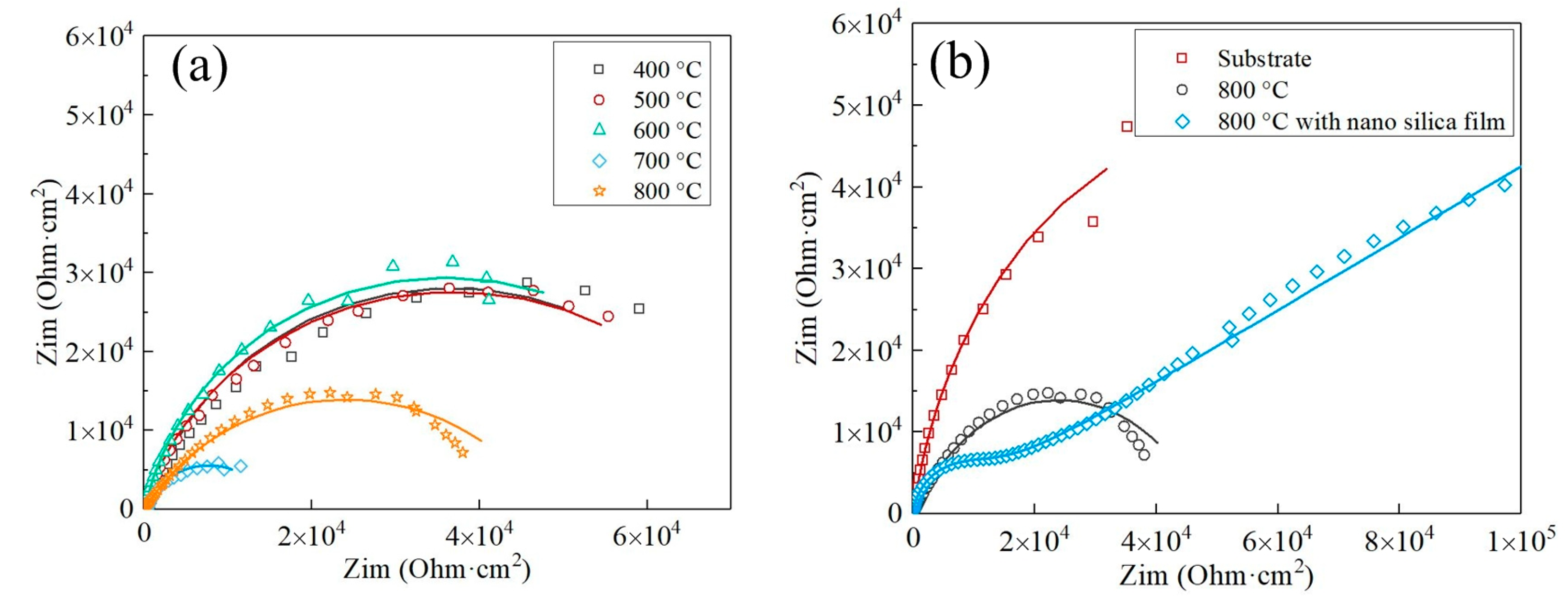
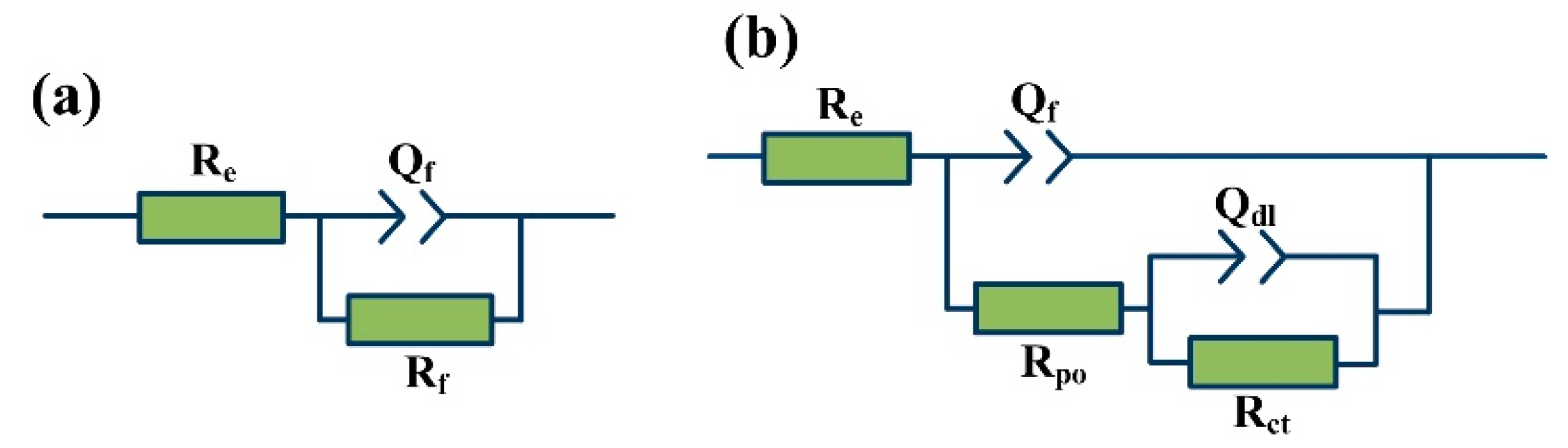
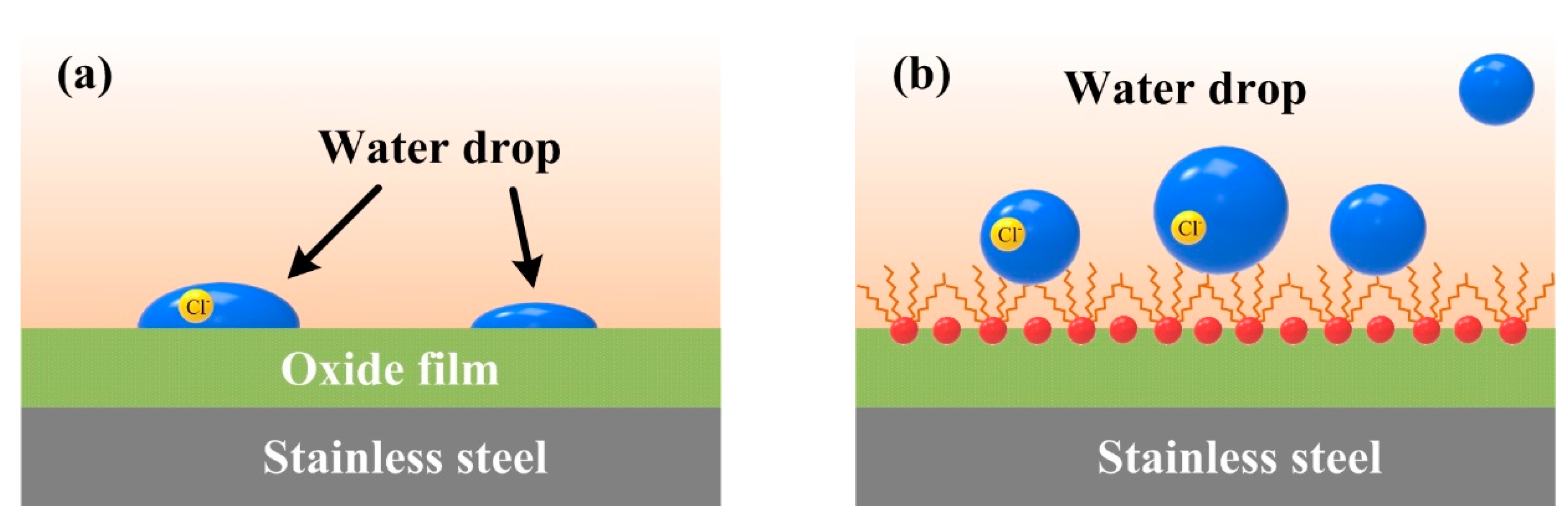
3.4. Dynamic Polarization and Electrochemical Impedance Spectroscopy Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, J.; Yu, C.; Nie, C.; Wang, H.; Cao, Z.; Li, Y.; Wang, X. A new environmentally friendly approach to prepare superhydrophobic colored stainless steel surface for decoration, anti-corrosion and self-cleaning. J. Mater. Sci. 2020, 56, 854–869. [Google Scholar] [CrossRef]
- Alliott, G.T.; Higginson, R.L.; Wilcox, G.D. Producing a thin coloured film on Stainless Steels—A review. part 2: Non-electrochemical and Laser Processes. Trans. IMF 2023, 101, 72–78. [Google Scholar] [CrossRef]
- Gaidys, M.; Selskis, A.; Gečys, P.; Gedvilas, M. Stainless steel colouring using burst and BIBURST mode ultrafast laser irradiation. Opt. Laser Technol. 2024, 174, 110561. [Google Scholar] [CrossRef]
- Conrrado, R.; Bocchi, N.; Rocha-Filho, R.C.; Biaggio, S.R. Corrosion resistance of colored films grown on stainless steel by the alternating potential pulse method. Electrochim. Acta 2003, 48, 2417–2424. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, J.; Qiao, Y.; Cao, C.; Zhang, J.; Zhou, G. The corrosion and passivation of SS304 stainless steel under square wave electric field. Mater. Chem. Phys. 2003, 79, 43–48. [Google Scholar] [CrossRef]
- Sharma, V.C. A comparison of thermal performance of austenitic stainless steel solar absorber plates coloured by chemical and thermal oxidation techniques. Energy 1981, 6, 133–138. [Google Scholar] [CrossRef]
- Huang, Q.; Ang, Y.; Ye, S.; Xiang, X. Color of SUS304 stainless steel in high temperature oxidation. Met. Funct. Mater. 2017, 24, 39–43. [Google Scholar]
- de Oliveira Vasconcelos, K.; Bocchi, N.; Rocha-Filho, R.C.; Biaggio, S.R. An environmentally friendly and practical method for obtaining color on stainless steel by interference. J. Electrochem. Soc. 2005, 152, 11. [Google Scholar]
- Luo, F.; Ong, W.; Guan, Y.; Li, F.; Sun, S.; Lim, G.C.; Hong, M. Study of micro/nanostructures formed by a nanosecond laser in gaseous environments for stainless steel surface coloring. Appl. Surf. Sci. 2015, 328, 405–409. [Google Scholar] [CrossRef]
- Guay, J.-M.; Calà Lesina, A.; Côté, G.; Charron, M.; Poitras, D.; Ramunno, L.; Berini, P.; Weck, A. Laser-induced plasmonic colours on metals. Nat. Commun. 2017, 8, 16095. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Facile fabrication of superhydrophobic surfaces from austenitic stainless steel (Aisi 304) by Chemical Etching. Appl. Surf. Sci. 2018, 439, 598–604. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, Y.; Li, X.; Wu, X. Facile fabrication of durable superhydrophobic silica/epoxy resin coatings with compatible transparency and stability. Surf. Coat. Technol. 2018, 347, 191–198. [Google Scholar] [CrossRef]
- Ye, Y.; Kang, Z.; Wang, F.; Long, Y.; Guo, T.; Chen, D.; Kong, J.; Xu, L. Achieving Hierarchical Structure with Superhydrophobicity and Enhanced Anti-Corrosion via Electrochemical Etching and Chemical Vapor Deposition. Appl. Surf. Sci. 2022, 610, 155362. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yang, J.; Yue, Y.; Zhang, H. Fabrication of Superhydrophobic Surface on Stainless Steel by Two-Step Chemical Etching. Chem. Phys. Lett. 2022, 797, 139567. [Google Scholar] [CrossRef]
- Li, L.; Breedveld, V.; Hess, D.W. Creation of superhydrophobic stainless steel surfaces by acid treatments and hydrophobic film deposition. ACS Appl. Mater. Interfaces 2012, 4, 4549–4556. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Emelyanenko, A.M.; Boinovich, L.B. Laser Obtained Superhydrophobic State for Stainless Steel Corrosion Protection, a Review. Coatings 2023, 13, 194. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, Y.; Wang, L.; Luo, X.; Wang, B.; Song, Q.; Liu, Z. Fabrication of Superhydrophobic Stainless Steel via Hybrid Femtosecond Laser-Chemical Method with Wear-Resistance and Anti-Corrosion Properties. Opt. Laser Technol. 2023, 164, 109474. [Google Scholar] [CrossRef]
- Wysard, M.; Vasconcelos, R.; de Souza, E.A.; Costa, M.E.H.M.; Camargo, S.S. Very Simple Method to Produce Superhydrophobic Stainless Steel Surfaces at Room Temperature. Mater. Chem. Phys. 2023, 307, 128203. [Google Scholar] [CrossRef]
- Park, B.; Hwang, W. A facile fabrication method for corrosion-resistant micro/nanostructures on stainless steel surfaces with tunable wettability. Scripta. Mater. 2016, 113, 118–121. [Google Scholar] [CrossRef]
- Kim, J.; Sim, S.O.; Park, H.W. Fabrication of durable hydrophobic micropatterns on stainless steel using a hybrid irradiation process. Surf. Coat. Technol. 2016, 302, 535–542. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, F.; Yin, Z.; Xue, M.; Chen, Y.; He, P.; Wu, J.; Ou, J.; Wang, F.; Luo, Y.; et al. Controllable fabrication of superhydrophobic alloys surface on 304 stainless steel substrate for anti-icing performance. Ceram. Int. 2023, 49, 25135–25143. [Google Scholar] [CrossRef]
- Qu, J.; Yu, C.; Cui, R.; Qin, J.; Wang, H.; Cao, Z. Preparation of super-hydrophobic and corrosion resistant colored films on chemically etched 304 stainless steel substrate. Surf. Coat. Technol. 2018, 354, 236–245. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, D.; Song, J. Electrochemical fabrication of superhydrophobic 304 stainless steel by neutral electrolyte. J. Mater. Sci. 2022, 57, 20069–20081. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Ming, P.; Zhao, D.; Song, J. Wire electrochemical etching of superhydrophobic 304 stainless steel surfaces based on high local current density with neutral electrolyte. Appl. Surf. Sci. 2022, 571, 151269. [Google Scholar] [CrossRef]
- Renner, F.U.; Ankah, G.N.; Bashir, A.; Ma, D.; Biedermann, P.U.; Shrestha, B.R.; Nellessen, M.; Khorashadizadeh, A.; Losada-Pérez, P.; Duarte, M.J.; et al. Star-Shaped Crystallographic Cracking of Localized Nanoporous Defects. Adv. Mater. 2015, 27, 4877–4882. [Google Scholar] [CrossRef]
- Gardiner, D.J.; Littleton, C.J.; Thomas, K.M.; Strafford, K.N. Distribution and characterization of high temperature air corrosion products on iron-chromium alloys by Raman Microscopy. Oxid. Met. 1987, 27, 57–72. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Q.; Li, Y. Characterization of microstructure, mechanical properties and corrosion resistance of dissimilar welded joint between 2205 duplex stainless steel and 16MNR. Mater. Design 2011, 32, 831–837. [Google Scholar] [CrossRef]
- Niu, W.; Lillard, R.S.; Li, Z.; Ernst, F. Properties of the passive film formed on interstitially hardened AISI 316L stainless steel. Electrochim. Acta 2015, 176, 410–419. [Google Scholar] [CrossRef]
- Yu, F.; Chen, S.; Chen, Y.; Li, H.; Yang, L.; Chen, Y.; Yin, Y. Experimental and theoretical analysis of polymerization reaction process on the polydopamine membranes and its corrosion protection properties for 304 Stainless Steel. J. Mol. Struct. 2010, 982, 152–161. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.F.; Li, X.G.; Xiao, K. The electrochemical behaviour of 2205 duplex stainless steel in alkaline solutions with different ph in the presence of chloride. Electrochim. Acta 2012, 64, 211–220. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Yao, K.-F.; Chen, Y.-B.; Wang, M.-H.; Ge, X.-Y. Influence of heat treatment on the microstructure and corrosion resistance of 13 wt PCT CR-type martensitic stainless steel. Metall. Mater. Trans. A 2015, 46, 6090–6102. [Google Scholar] [CrossRef]
- Westin, E.M.; Olsson, C.-O.A.; Hertzman, S. Weld Oxide Formation on lean duplex stainless steel. Corros. Sci. 2008, 50, 2620–2634. [Google Scholar] [CrossRef]
- Kokalj, A.; Lozinšek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Stavber, S.; Crespo, D.; Renner, F.U.; et al. Simplistic Correlations between Molecular Electronic Properties and Inhibition Efficiencies: Do They Really Exist? Corros. Sci. 2021, 179, 108856. [Google Scholar] [CrossRef]




| Element | S | P | C | Mo | Cu | Si | Mn | Ni | Cr | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.007 | 0.027 | 0.057 | 0.15 | 0.23 | 0.59 | 1.05 | 8.09 | 18.14 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.; Wang, X.; Wang, W.; Meng, D.; Zhan, X.; Yin, X.; Liu, Y. Fabrication and Properties of Superhydrophobic Colored Stainless Steel Surface for Decoration and Anti-Corrosion. Coatings 2024, 14, 1117. https://doi.org/10.3390/coatings14091117
Fan C, Wang X, Wang W, Meng D, Zhan X, Yin X, Liu Y. Fabrication and Properties of Superhydrophobic Colored Stainless Steel Surface for Decoration and Anti-Corrosion. Coatings. 2024; 14(9):1117. https://doi.org/10.3390/coatings14091117
Chicago/Turabian StyleFan, Changfeng, Xue Wang, Wei Wang, Dechao Meng, Xianghua Zhan, Xiaoli Yin, and Yancong Liu. 2024. "Fabrication and Properties of Superhydrophobic Colored Stainless Steel Surface for Decoration and Anti-Corrosion" Coatings 14, no. 9: 1117. https://doi.org/10.3390/coatings14091117
APA StyleFan, C., Wang, X., Wang, W., Meng, D., Zhan, X., Yin, X., & Liu, Y. (2024). Fabrication and Properties of Superhydrophobic Colored Stainless Steel Surface for Decoration and Anti-Corrosion. Coatings, 14(9), 1117. https://doi.org/10.3390/coatings14091117






