Impact of Calcium Chloride Addition on the Microstructural and Physicochemical Properties of Pea Protein Isolate-Based Films Plasticized with Glycerol and Sorbitol
Abstract
1. Introduction
- electrostatic shielding (ion binding neutralizes the repulsive charges, facilitating protein oligomerization),
- crosslinking of negatively charged carboxyl groups (in the C-terminus of each polypeptide chain and also the one in the aspartate and glutamate side chain) via protein-Ca2+-protein bridges,
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Conditioning of Films
2.2.2. pH and Viscosity of FFSs
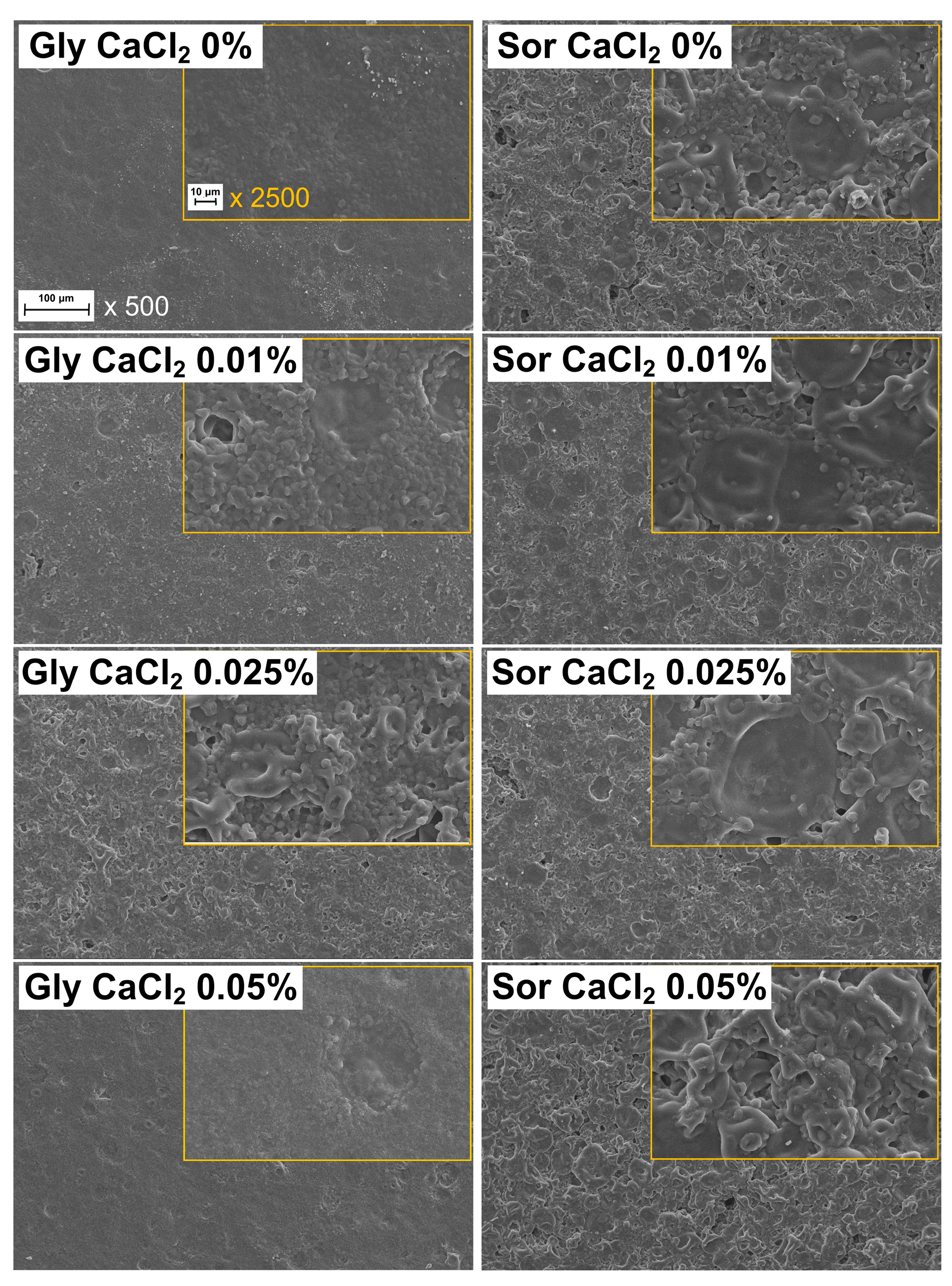
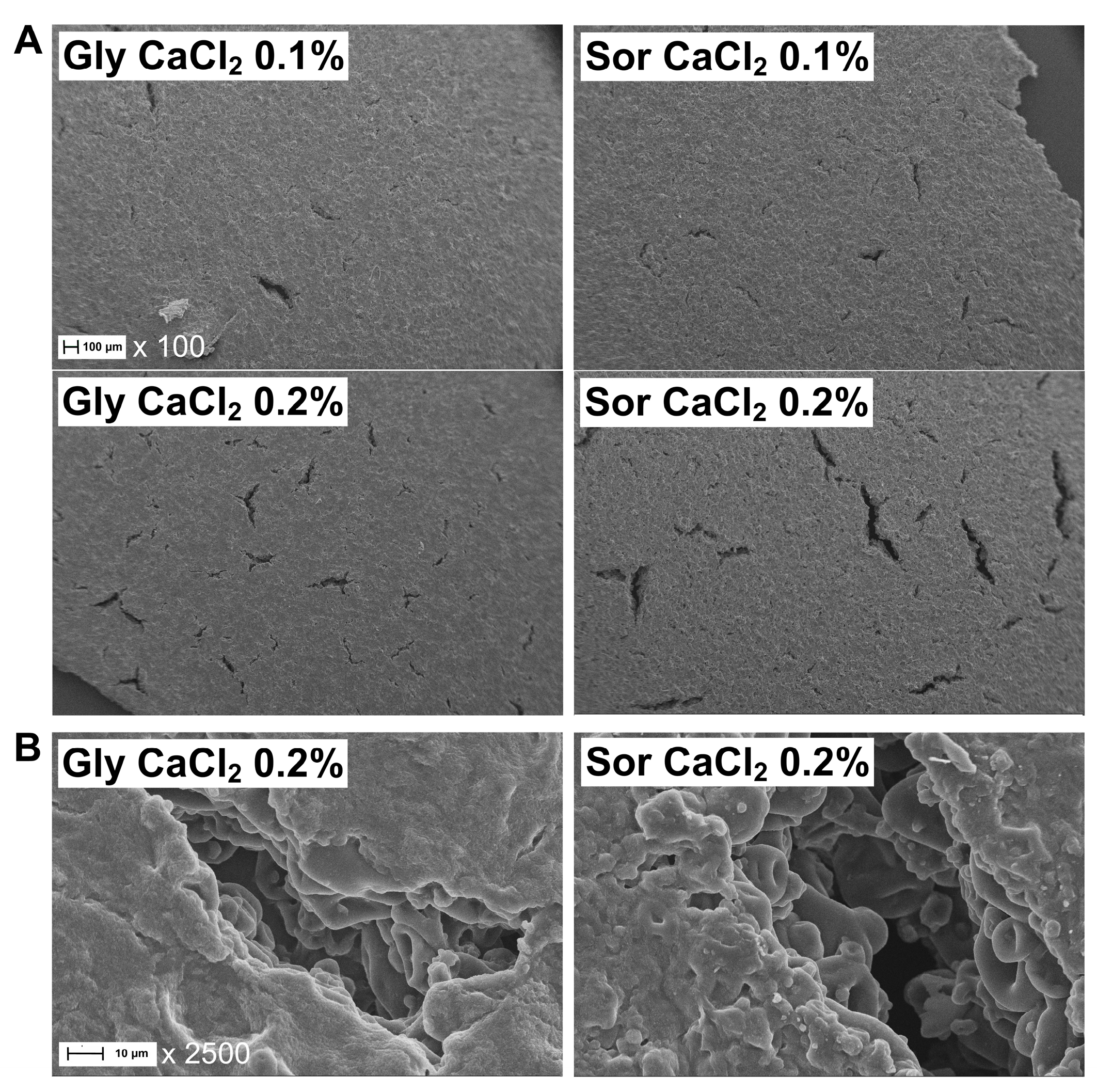
2.2.3. Microstructure
2.2.4. pH of the Films
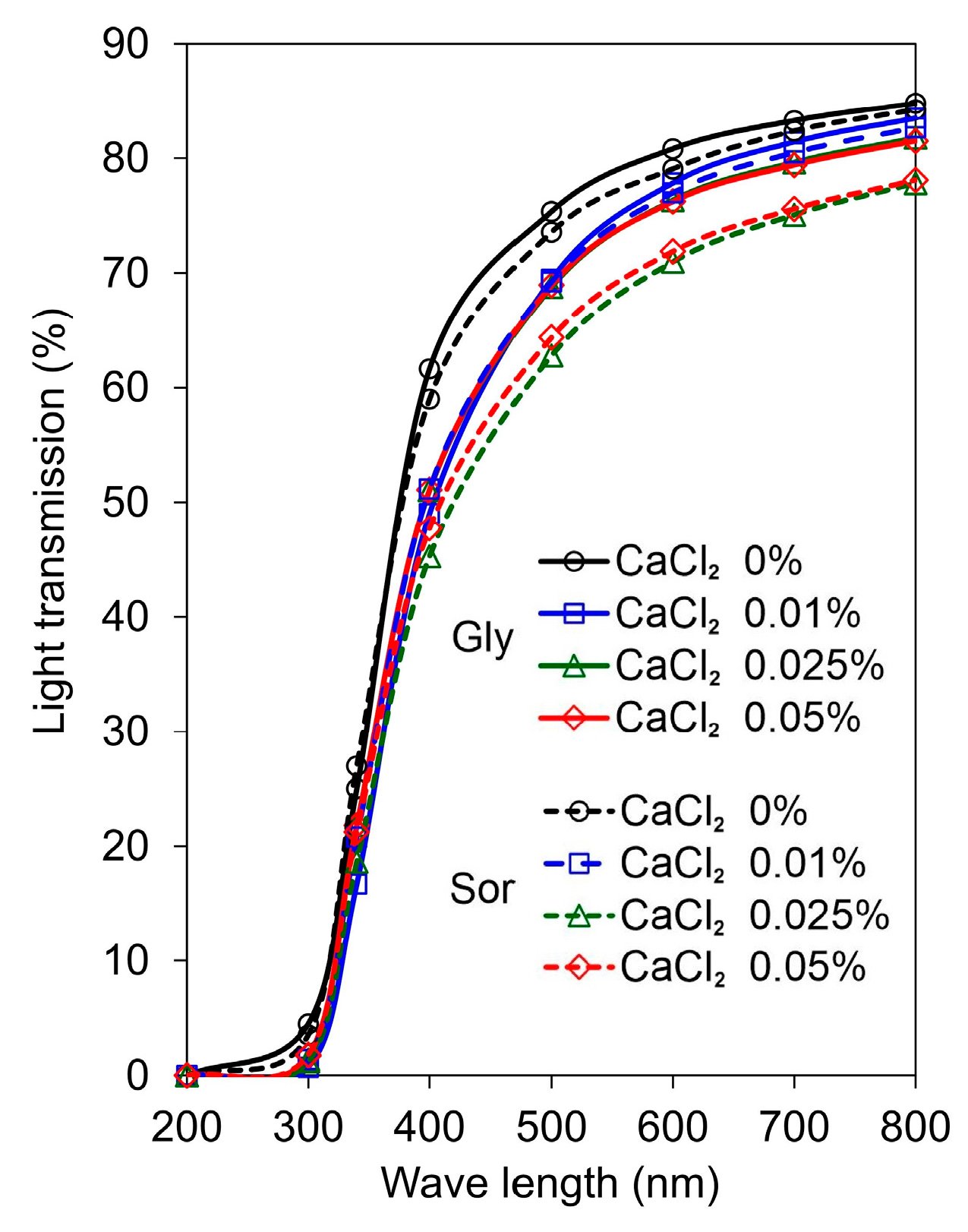
2.2.5. Optical Properties
2.2.6. Water Affinities
2.2.7. Mechanical Properties
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. pH and Viscosity of the FFSs
3.2. Microstructure
3.3. Optical Properties
3.4. Water Affinities
3.5. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. OJ L 155 2019, 12.6.2019, pp. 1–19. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019L0904 (accessed on 25 July 2024).
- Nair, S.S.; Trafiałek, J.; Kolanowski, W. Edible Packaging: A Technological Update for the Sustainable Future of the Food Industry. Appl. Sci. 2023, 13, 8234. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The Biomass Distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lan, Y. A Comparative Study on Carbon Footprints between Plant- and Animal-Based Foods in China. J. Clean. Prod. 2016, 112, 2581–2592. [Google Scholar] [CrossRef]
- Linares-Castañeda, A.; Sánchez-Chino, X.M.; Yolanda de las Mercedes Gómez y Gómez; Jiménez-Martínez, C.; Martínez Herrera, J.; Cid-Gallegos, M.S.; Corzo-Ríos, L.J. Cereal and Legume Protein Edible Films: A Sustainable Alternative to Conventional Food Packaging. Int. J. Food Prop. 2023, 26, 3197–3213. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, Physicochemical Properties of Pea Protein and Its Application in Functional Foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA Relevance. OJ L 304 2011, 22.11.2011, pp. 18–63. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj (accessed on 25 July 2024).
- Kowalczyk, D.; Baraniak, B. Effects of Plasticizers, pH and Heating of Film-Forming Solution on the Properties of Pea Protein Isolate Films. J. Food Eng. 2011, 105, 295–305. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Gustaw, W.; Świeca, M.; Baraniak, B. A Study on the Mechanical Properties of Pea Protein Isolate Films. J. Food Process Preserv. 2014, 38, 1726–1736. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Baraniak, B. Effect of Selected Polysaccharides on Physicochemical Propetrties of Edible Films Produced on the Basis of Pea Proteins. Food Sci. Technol. Qual. 2012, 5, 99–112. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Gustaw, W.; Zieba, E.; Lisiecki, S.; Stadnik, J.; Baraniak, B. Microstructure and Functional Properties of Sorbitol-Plasticized Pea Protein Isolate Emulsion Films: Effect of Lipid Type and Concentration. Food Hydrocoll. 2016, 60, 353–363. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in Polysaccharide and Protein Films and Coatings for Food Contact—A Review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Ma, T.; Xiong, Y.L.; Jiang, J. Calcium-Aided Fabrication of Pea Protein Hydrogels with Filler Emulsion Particles Coated by pH 12-Shifting and Ultrasound Treated Protein. Food Hydrocoll. 2022, 125, 107396. [Google Scholar] [CrossRef]
- Batoulis, H.; Schmidt, T.H.; Weber, P.; Schloetel, J.G.; Kandt, C.; Lang, T. Concentration Dependent Ion-Protein Interaction Patterns Underlying Protein Oligomerization Behaviours. Sci. Rep. 2016, 6, 24131. [Google Scholar] [CrossRef] [PubMed]
- Mezgheni, E.; D’aprano, G.; Lacroix, M. Formation of Sterilized Edible Films Based on Caseinates: Effects of Calcium and Plasticizers. J. Agric. Food Chem. 1998, 46, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Avena-Bustillos, R.J.; Krochta, J.M. Water Vapor Permeability of Caseinate-Based Edible Films as Affected by pH, Calcium Crosslinking and Lipid Content. J. Food Sci. 1993, 58, 904–907. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Influence of Calcium on Tensile, Optical and Water Vapour Permeability Properties of Sodium Caseinate Edible Films. J. Food Eng. 2010, 96, 356–364. [Google Scholar] [CrossRef]
- Park, S.K.; Rhee, C.O.; Bae, D.H.; Hettiarachchy, N.S. Mechanical Properties and Water-Vapor Permeability of Soy-Protein Films Affected by Calcium Salts and Glucono-δ-Lactone. J. Agric. Food Chem. 2001, 49, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tung, M.A.; Britt, I.J.; Yada, S.; Dalgleish, D.G. Tensile and Barrier Properties of Edible Films Made from Whey Proteins. J. Food Sci. 2002, 67, 188–193. [Google Scholar] [CrossRef]
- Chai, Z.; Shang, J.; Jiang, Y.; Ren, F.; Leng, X. Effects of the Free and Pre-Encapsulated Calcium Ions on the Physical Properties of Whey Protein Edible Film. Int. J. Food Sci. Technol. 2010, 45, 1532–1538. [Google Scholar] [CrossRef]
- Ren, W.; Xia, W.; Gunes, D.Z.; Ahrné, L. Heat-induced Gels From Pea Protein Soluble Colloidal Aggregates: Effect of Calcium Addition or pH Adjustment on Gelation behavior and Rheological Properties. Food Hydrocoll. 2024, 147 Pt A, 109417. [Google Scholar] [CrossRef]
- Babault, N.; Païzis, C.; Deley, G.; Guérin-Deremaux, L.; Saniez, M.H.; Lefranc-Millot, C.; Allaert, F.A. Pea Proteins Oral Supplementation Promotes Muscle Thickness Gains During Resistance Training: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial vs. Whey Protein. J. Int. Soc. Sports Nutr. 2015, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- PN-ISO 2528:2000; Sheet Materials—Determination of Water Vapour Transmission Rate—Gravimetric (Dish) Method. Polish Committee for Standardization: Warsaw, Poland, 2000.
- Shu, L.; Obagbemi, I.J.; Liyanaarachchi, S.; Navaratna, D.; Parthasarathy, R.; Aim, R.B.; Jegatheesan, V. Why Does pH Increase with CaCl2 as Draw Solution during forward Osmosis Filtration. Process. Saf. Environ. Prot. 2016, 104, 465–471. [Google Scholar] [CrossRef]
- Tarapata, J.; Smoczyński, M.; Maciejczyk, M.; Zulewska, J. Effect of Calcium Chloride Addition on Properties of Acid-Rennet Gels. Int. Dairy. J. 2020, 106, 104707. [Google Scholar] [CrossRef]
- Peng, Y.; Kyriakopoulou, K.; Keppler, J.K.; Venema, P.; van der Goot, A.J. Effect of Calcium Enrichment on the Composition, Conformation, and Functional Properties of Soy Protein. Food Hydrocoll. 2022, 123, 107191. [Google Scholar] [CrossRef]
- Shin, J.-S.; Kim, B.-H.; Baik, M.-Y. Applicable Plant Proteins and Dietary Fibers for Simulate Plant-Based Yogurts. Foods 2021, 10, 2305. [Google Scholar] [CrossRef]
- Xiao, Y.; Kang, S.; Liu, Y.; Guo, X.; Li, M.; Xu, H. Effect and Mechanism of Calcium Ions on the Gelation Properties of Cellulose Nanocrystals-Whey Protein Isolate Composite Gels. Food Hydrocoll. 2021, 111, 106401. [Google Scholar] [CrossRef]
- Edelenbos, M.; Christensen, L.P.; Grevsen, K. HPLC Determination of Chlorophyll and Carotenoid Pigments in Processed Green Pea Cultivars (Pisum sativum L.). J. Agric. Food Chem. 2001, 49, 4768–4774. [Google Scholar] [CrossRef]
- Kapoor, R.; Karabulut, G.; Mundada, V.; Feng, H. Unraveling the Potential of Non-Thermal Ultrasonic Contact Drying for Enhanced Functional and Structural Attributes of Pea Protein Isolates: A Comparative Study with Spray and Freeze-Drying Methods. Food Chem. 2024, 439, 138137. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Color Difference ΔE: A Survey. Machine Graphics and Vision 2011, 20(4), 383–411. [Google Scholar]
- Jonauskaite, D.; Mohr, C.; Antonietti, J.-P.; Spiers, P.M.; Althaus, B.; Anil, S.; Dael, N. Most and Least Preferred Colours Differ According to Object Context: New Insights from an Unrestricted Colour Range. PLoS ONE 2016, 11, e0152194. [Google Scholar] [CrossRef]
- Demchenko, A.P. Spectroscopic Properties of Protein Chromophores. In Ultraviolet Spectroscopy of Proteins; Springer: Berlin/Heidelberg, Germany, 1986; pp. 5–26. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Bartkowiak, A.; Łupina, K. A Comprehensive Study on Gelatin- and Whey Protein Isolate-Based Edible Films as Carriers of Fireweed (Epilobium angustifolium L.). Extract. Food Bioproc. Tech. 2022, 15, 2547–2561. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kordowska-Wiater, M.; Nowak, J.; Baraniak, B. Characterization of Films Based on Chitosan Lactate and Its Blends with Oxidized Starch and Gelatin. Int. J. Biol. Macromol. 2015, 77, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Jacucci, G.; Schertel, L.; Zhang, Y.; Yang, H.; Vignolini, S. Light Management with Natural Materials: From Whiteness to Transparency. Adv. Mater. 2021, 33, 2001215. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Baraniak, B. Effect of Candelilla Wax on Functional Properties of Biopolymer Emulsion Films—A Comparative Study. Food Hydrocoll. 2014, 41, 195–209. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamada, M.; Machida, Y.; Tsuda, Y. A New Method. to Evaluate the Softening Effect of Cosmetic Ingredients on the Skin. J. Soc. Cosmet. Chem. 1984, 35, 171–181. [Google Scholar]
- Yan, J.; Yin, L.; Qu, Y.; Yan, W.; Zhang, M.; Su, J.; Jia, X. Effect of Calcium Ions Concentration on the Properties and Microstructures of Doubly Induced Sorghum Arabinoxylan/Soy Protein Isolate Mixed Gels. Food Hydrocoll. 2022, 133, 107997. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, L.; Yang, J.; Chen, X.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. Effect of Calcium Chloride on Heat-Induced Mesona Chinensis Polysaccharide-Whey Protein Isolation Gels: Gel Properties and Interactions. LWT 2022, 155, 112907. [Google Scholar] [CrossRef]
- Arabestani, A.; Kadivar, M.; Shahedi, M.; Goli, S.A.H.; Porta, R. Properties of a New Protein Film from Bitter Vetch (Vicia ervilia) and Effect of CaCl2 on Its Hydrophobicity. Int. J. Biol. Macromol. 2013, 57, 118–123. [Google Scholar] [CrossRef]
- Galietta, G.; Di Gioia, L.; Guilbert, S.; Cuq, B. Mechanical and Thermomechanical Properties of Films Based on Whey Proteins as Affected by Plasticizer and Crosslinking Agents. J. Dairy. Sci. 1998, 81, 3123–3130. [Google Scholar] [CrossRef]
- Valdivia-López, M.A.; Tecante, A.; Granados-Navarrete, S.; Martínez-García, C. Preparation of Modified Films with Protein from Grouper Fish. Int. J. Food Sci. 2016, 2016, 3926847. [Google Scholar] [CrossRef]
- Duan, B.; Yang, M.; Chao, Q.; Wang, L.; Zhang, L.; Gou, M.; Li, Y.; Liu, C.; Lu, K. Preparation and Properties of Egg White Dual Cross-Linked Hydrogel with Potential Application for Bone Tissue Engineering. Polymers 2022, 14, 5116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, C.; Yuan, D.; Liu, Q.; Zhao, J. Effects of the Incorporation of Calcium Chloride on the Physical and Oxidative Stability of Filled Hydrogel Particles. Foods 2022, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]

| Plasticizer | CaCl2 (%) | pH | η (mPa·s) |
|---|---|---|---|
| Gly | 0 | 6.90 ± 0.01 j | 7.14 ± 0.07 g |
| 0.01 | 6.88 ± 0.01 i | 6.32 ± 0.18 f | |
| 0.025 | 6.81 ± 0.01 g | 5.55 ± 0.18 e | |
| 0.05 | 6.73 ± 0.01 e | 5.06 ± 0.09 d | |
| 0.1 | 6.59 ± 0.01 c | 4.40 ± 0.07 c | |
| 0.2 | 6.34 ± 0.01 a | 3.39 ± 0.05 a | |
| Sor | 0 | 6.94 ± 0.01 k | 7.71 ± 0.19 h |
| 0.01 | 6.89 ± 0.01 ij | 6.30 ± 0.21 f | |
| 0.025 | 6.86 ± 0.01 h | 6.19 ± 0.21 f | |
| 0.05 | 6.77 ± 0.01 f | 5.76 ± 0.01 e | |
| 0.1 | 6.64 ± 0.01 d | 4.39 ± 0.15 c | |
| 0.2 | 6.42 ± 0.01 b | 3.77 ± 0.09 b |
| Plasticizer | CaCl2 (%) | L* | a* | b* | h (°) | C* | ΔE* |
|---|---|---|---|---|---|---|---|
| Gly | 0 | 41.39 ± 0.01 cd | −0.21 ± 0.03 c | 2.13 ± 0.07 cd | 95.77 ± 0.39 a | 2.14 ±0.07 bc | - |
| 0.01 | 37.60 ± 3.37 b | −0.69 ± 0.13 b | 2.50 ± 0.16 d | 105.45 ± 2.49 bc | 2.60 ± 0.18 d | 3.97 ± 3.15 a | |
| 0.025 | 30.87 ± 1.24 a | −1.00 ± 0.09 a | 1.79 ± 0.51 bc | 120.09 ± 5.28 d | 2.06 ± 0.49 bc | 10.56 ± 1.23 b | |
| 0.05 | 29.80 ± 0.40 a | −1.04 ± 0.02a | 1.44 ± 0.20 ab | 126.17 ± 4.42 d | 1.78 ± 0.15 ab | 11.64 ± 0.40 b | |
| Sor | 0 | 41.72 ± 0.04 d | -0.28 ± 0.02 c | 2.30 ± 0.06 d | 96.85 ± 0.32 ab | 2.32 ± 0.06 cd | - |
| 0.01 | 38.46 ± 3.23 bc | −0.77 ± 0.24 b | 2.27 ±0.48 d | 109.61 ± 9.33 c | 2.42 ± 0.39 cd | 3.36 ± 3.19 a | |
| 0.025 | 30.16 ± 0.62 a | −1.04 ± 0.08 a | 1.80 ± 0.11 bc | 126.69 ± 7.96 d | 2.08 ± 0.05 bc | 11.60 ± 0.61 b | |
| 0.05 | 30.03 ± 0.44 a | −0.97 ± 0.08 a | 1.25 ± 0.06 a | 127.85 ± 2.80 d | 1.58 ± 0.06 a | 11.76 ± 0.45 b |
| Plasticizer | CaCl2 (%) | pH | Op (A600/mm) | MC (%) | WVP (*) | So (%) |
|---|---|---|---|---|---|---|
| Gly | 0 | 6.50 ± 0.02 d | 0.90 ± 0.02 a | 29.46 ± 1.14 d | 18.60 ± 0.88 c | 100.00 ± 0.00 a |
| 0.01 | 6.49 ± 0.02 d | 1.09 ± 0.05 bc | 26.88 ± 1.42 c | 14.91 ± 0.77 b | 100.00 ± 0.00 a | |
| 0.025 | 6.41 ± 0.01c | 1.19 ± 0.06 d | 27.63 ± 1.32 c | 14.43 ± 0.98 b | 100.00 ± 0.00 a | |
| 0.05 | 6.35 ± 0.01b | 1.19 ± 0.05 d | 27.34 ± 1.50 c | 15.51 ± 1.93 b | 100.00 ± 0.00 a | |
| Sor | 0 | 6.54 ± 0.01 e | 1.02 ± 0.08 b | 11.50 ± 0.44 b | 1.40 ± 0.04 a | 100.00 ± 0.00 a |
| 0.01 | 6.48 ± 0.02 d | 1.13 ± 0.06 cd | 6.71 ± 0.12 a | 0.81 ± 0.20 a | 100.00 ± 0.00 a | |
| 0.025 | 6.37 ± 0.01b | 1.40 ± 0.08 e | 6.62 ± 0.42 a | 1.19 ± 0.16 a | 100.00 ± 0.00 a | |
| 0.05 | 6.23 ± 0.03a | 1.43 ± 0.01 e | 6.69 ± 0.57 a | 1.43 ± 0.07 a | 100.00 ± 0.00 a |
| Plasticizer | CaCl2 (%) | σmax (MPa) | EM (MPa) | εb (%) |
|---|---|---|---|---|
| Gly | 0 | 1.70 ± 0.17 b | 51.03 ± 3.35 b | 73.79 ± 4.58 d |
| 0.01 | 1.43 ± 0.18 a | 44.14 ± 8.00 ab | 60.80 ± 4.89 c | |
| 0.025 | 1.42 ± 0.16 a | 42.52 ± 7.22 ab | 57.52 ± 3.90 c | |
| 0.05 | 1.41 ± 0.20 a | 39.49 ± 4.00 a | 59.49 ± 4.00 c | |
| Sor | 0 | 6.31 ± 0.24 e | 219.15 ± 12.36 e | 37.01 ± 3.70 a |
| 0.01 | 5.40 ± 0.27 d | 193.52 ± 14.36 d | 32.67 ± 2.72 a | |
| 0.025 | 3.99 ± 0.26 c | 129.23 ± 7.46 c | 55.23 ± 3.06 c | |
| 0.05 | 4.14 ± 0.25 c | 133.39 ± 10.62 c | 46.14 ± 8.88 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, D.; Kazimierczak, W. Impact of Calcium Chloride Addition on the Microstructural and Physicochemical Properties of Pea Protein Isolate-Based Films Plasticized with Glycerol and Sorbitol. Coatings 2024, 14, 1116. https://doi.org/10.3390/coatings14091116
Kowalczyk D, Kazimierczak W. Impact of Calcium Chloride Addition on the Microstructural and Physicochemical Properties of Pea Protein Isolate-Based Films Plasticized with Glycerol and Sorbitol. Coatings. 2024; 14(9):1116. https://doi.org/10.3390/coatings14091116
Chicago/Turabian StyleKowalczyk, Dariusz, and Waldemar Kazimierczak. 2024. "Impact of Calcium Chloride Addition on the Microstructural and Physicochemical Properties of Pea Protein Isolate-Based Films Plasticized with Glycerol and Sorbitol" Coatings 14, no. 9: 1116. https://doi.org/10.3390/coatings14091116
APA StyleKowalczyk, D., & Kazimierczak, W. (2024). Impact of Calcium Chloride Addition on the Microstructural and Physicochemical Properties of Pea Protein Isolate-Based Films Plasticized with Glycerol and Sorbitol. Coatings, 14(9), 1116. https://doi.org/10.3390/coatings14091116








