Rapid Synthesis and Sintering of La2O2S and Its Physical, Optical, and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results
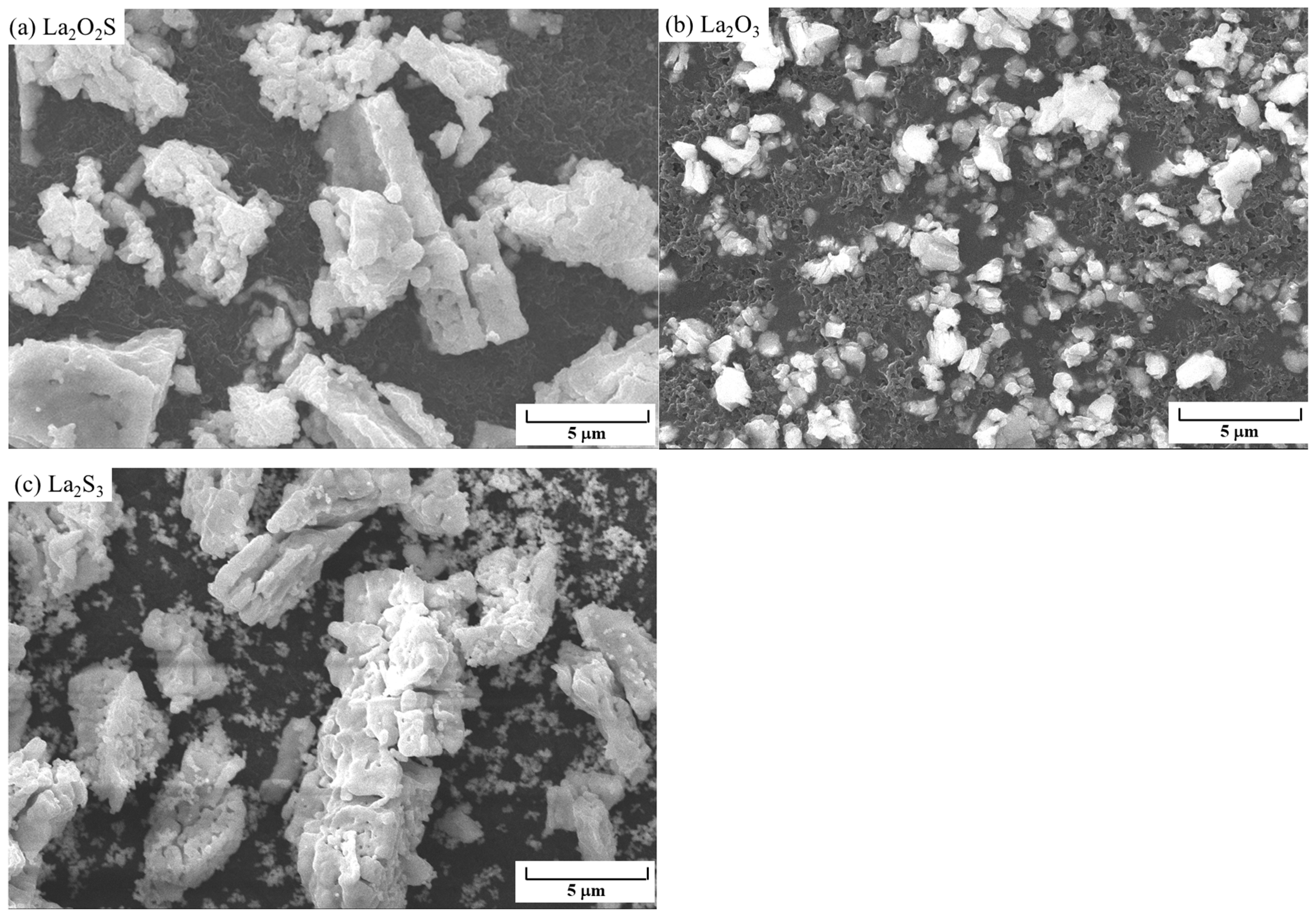
3.1. XRD and SEM of Synthetic La2O2S Powders
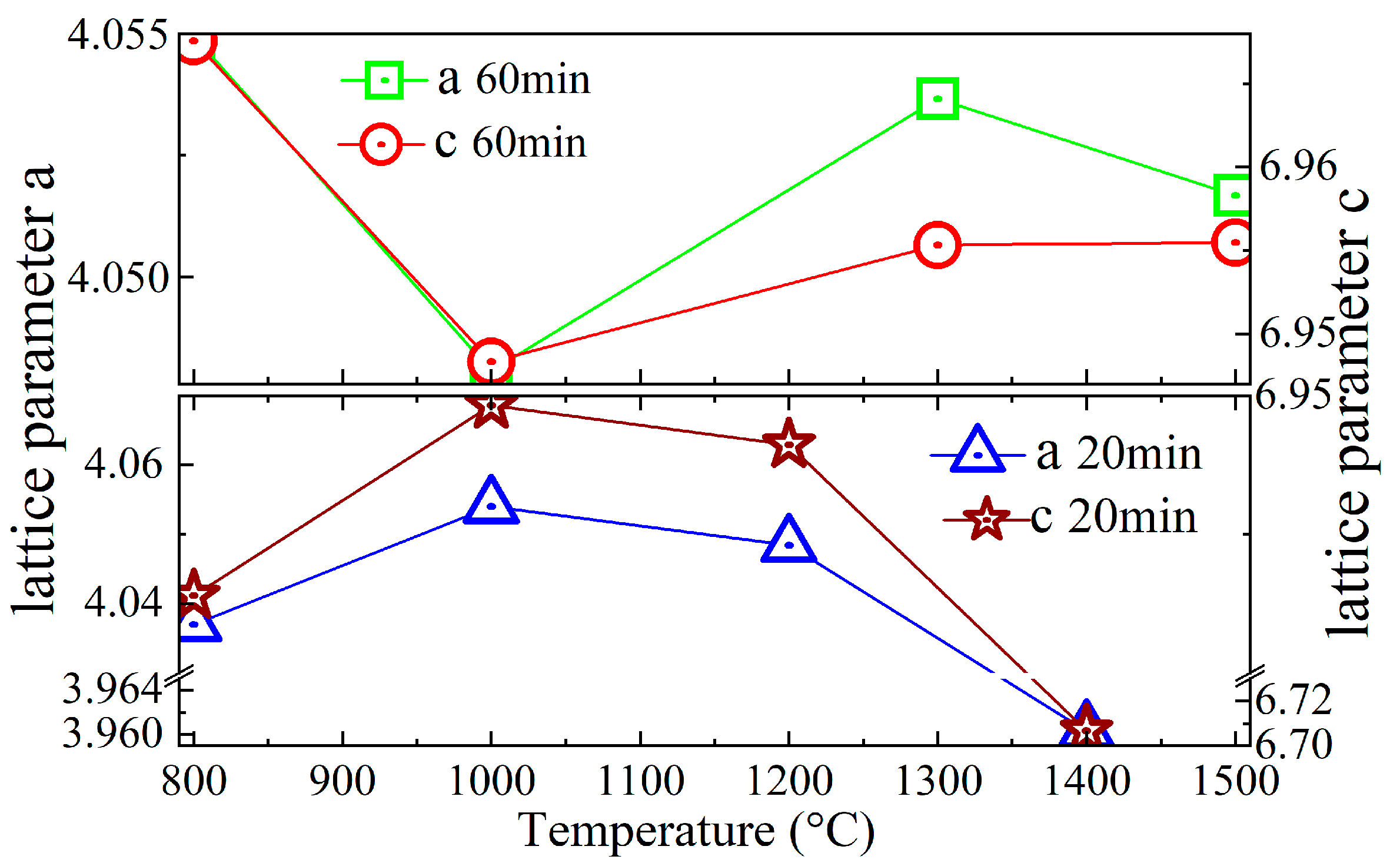
3.2. XRD and SEM of Synthetic La2O2S Bulks
3.3. Specific Heat of Synthetic La2O2S Bulks
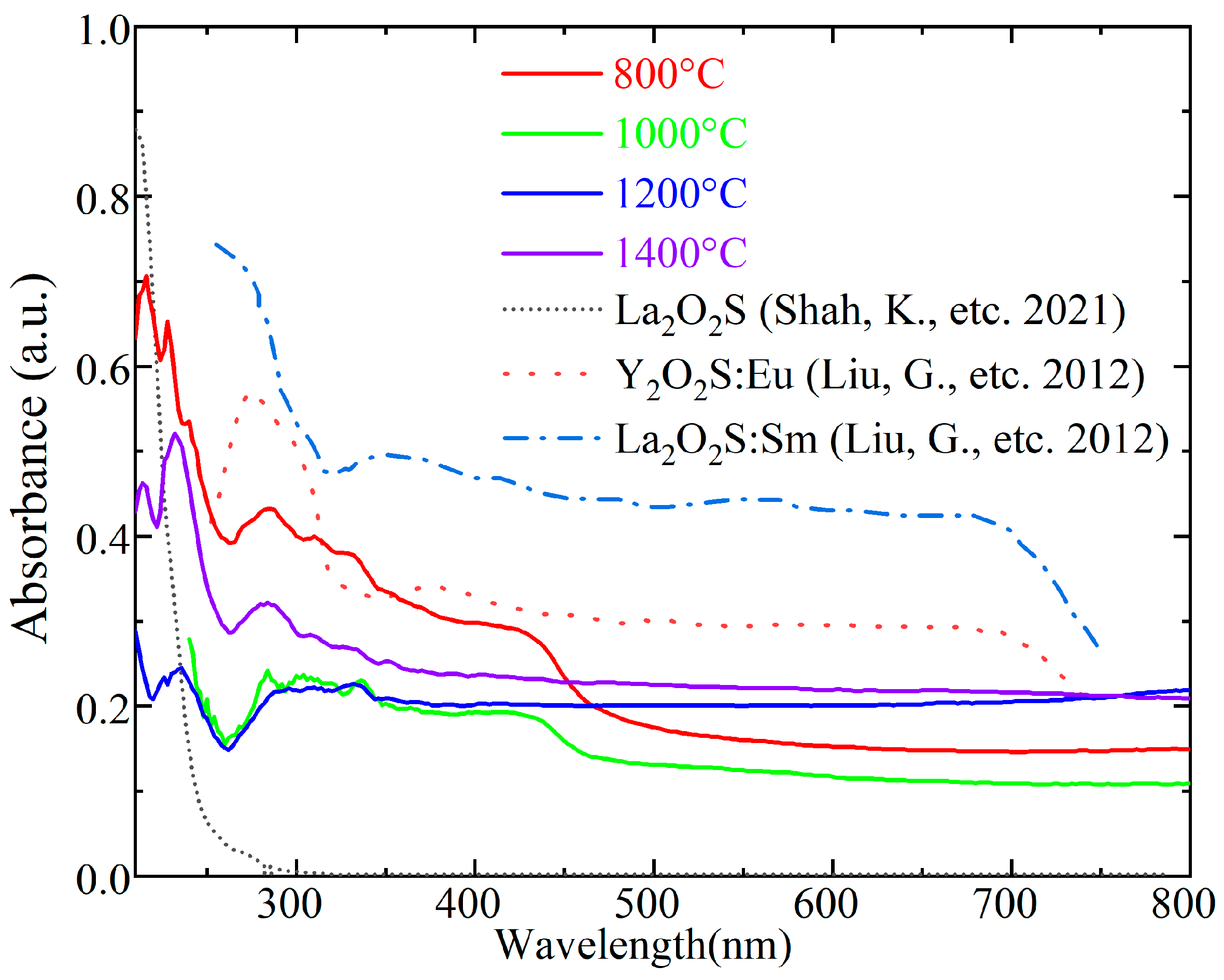
3.4. UV-Vis Spectra and Raman Spectroscopy of Synthetic La2O2S Bulks
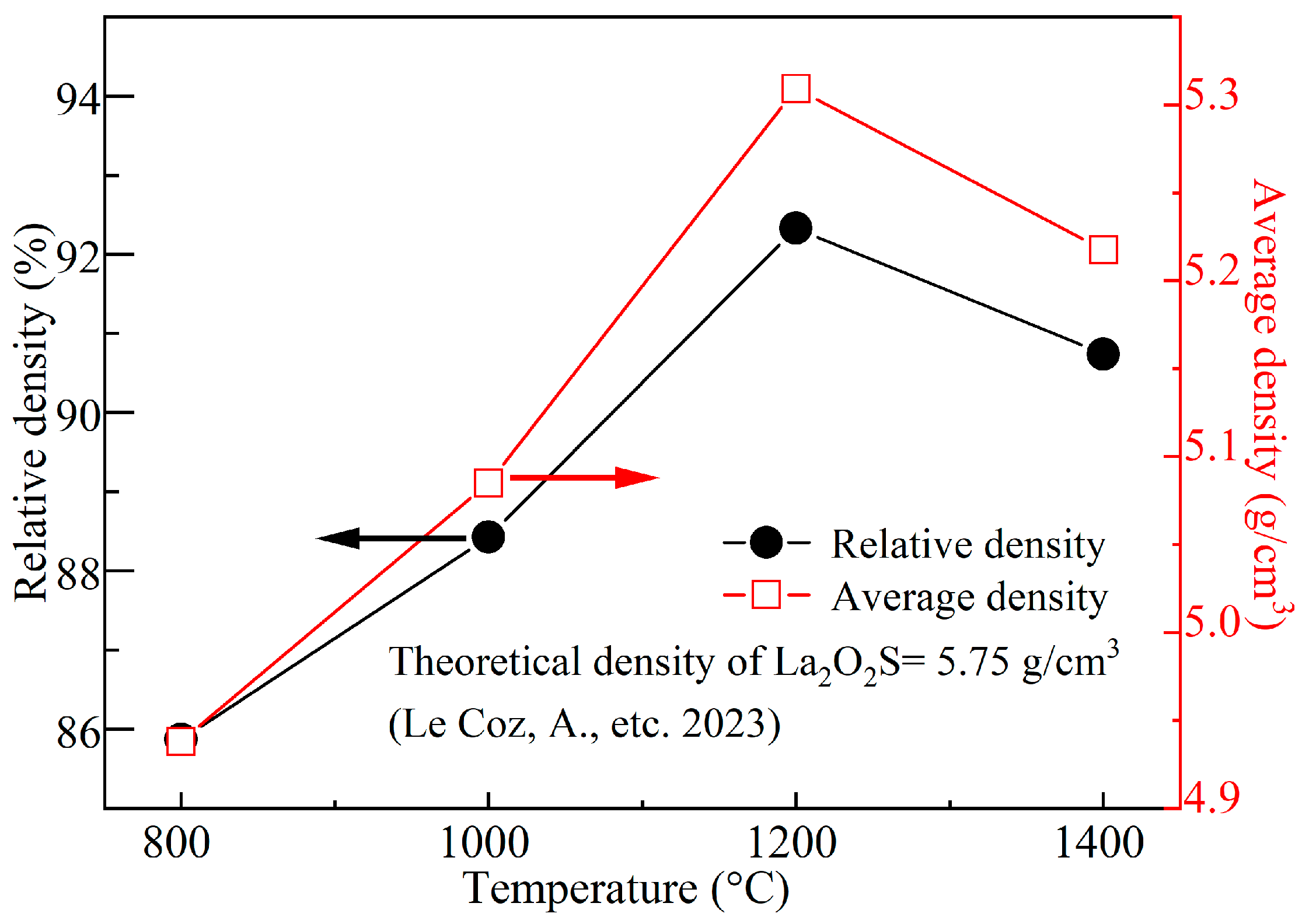
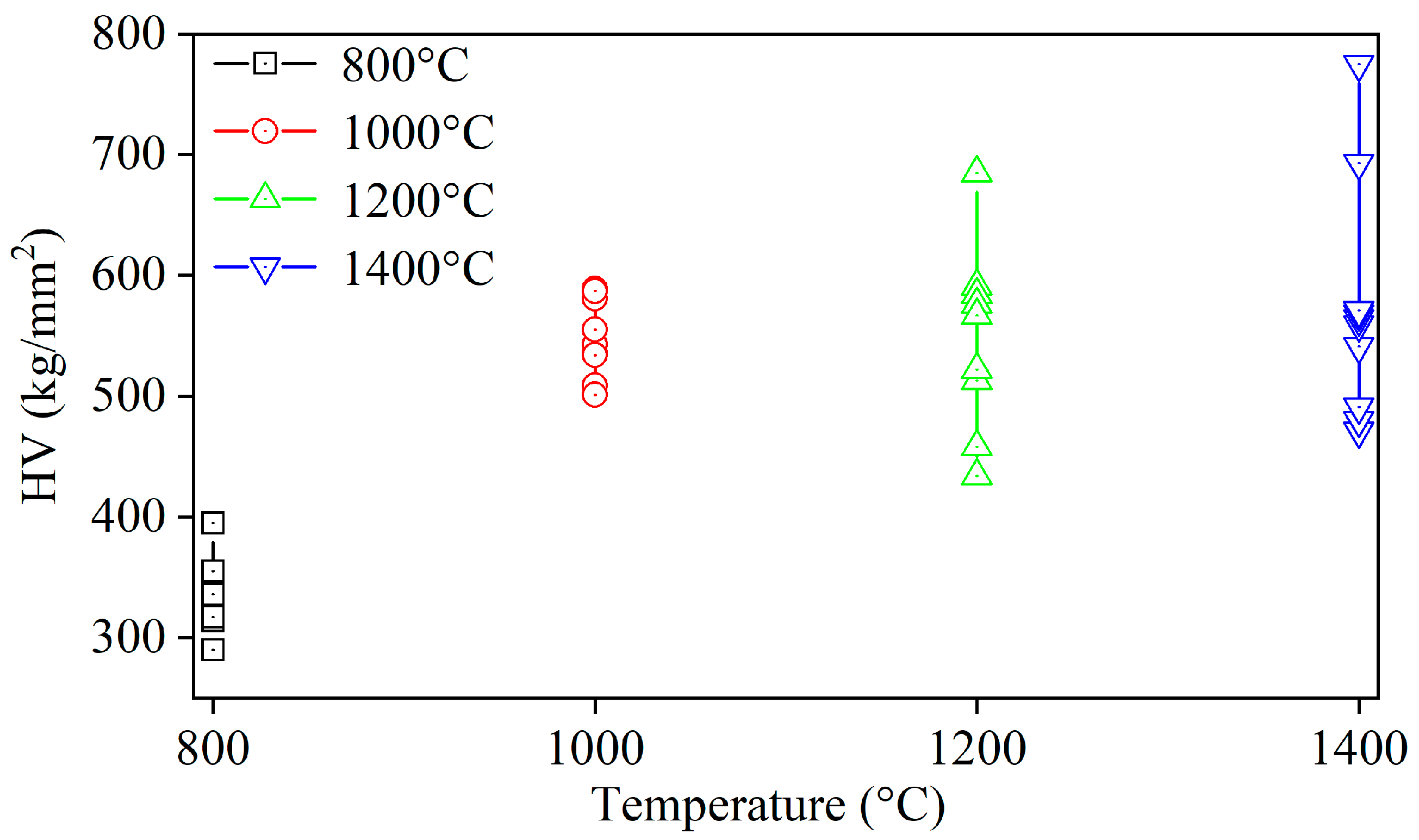
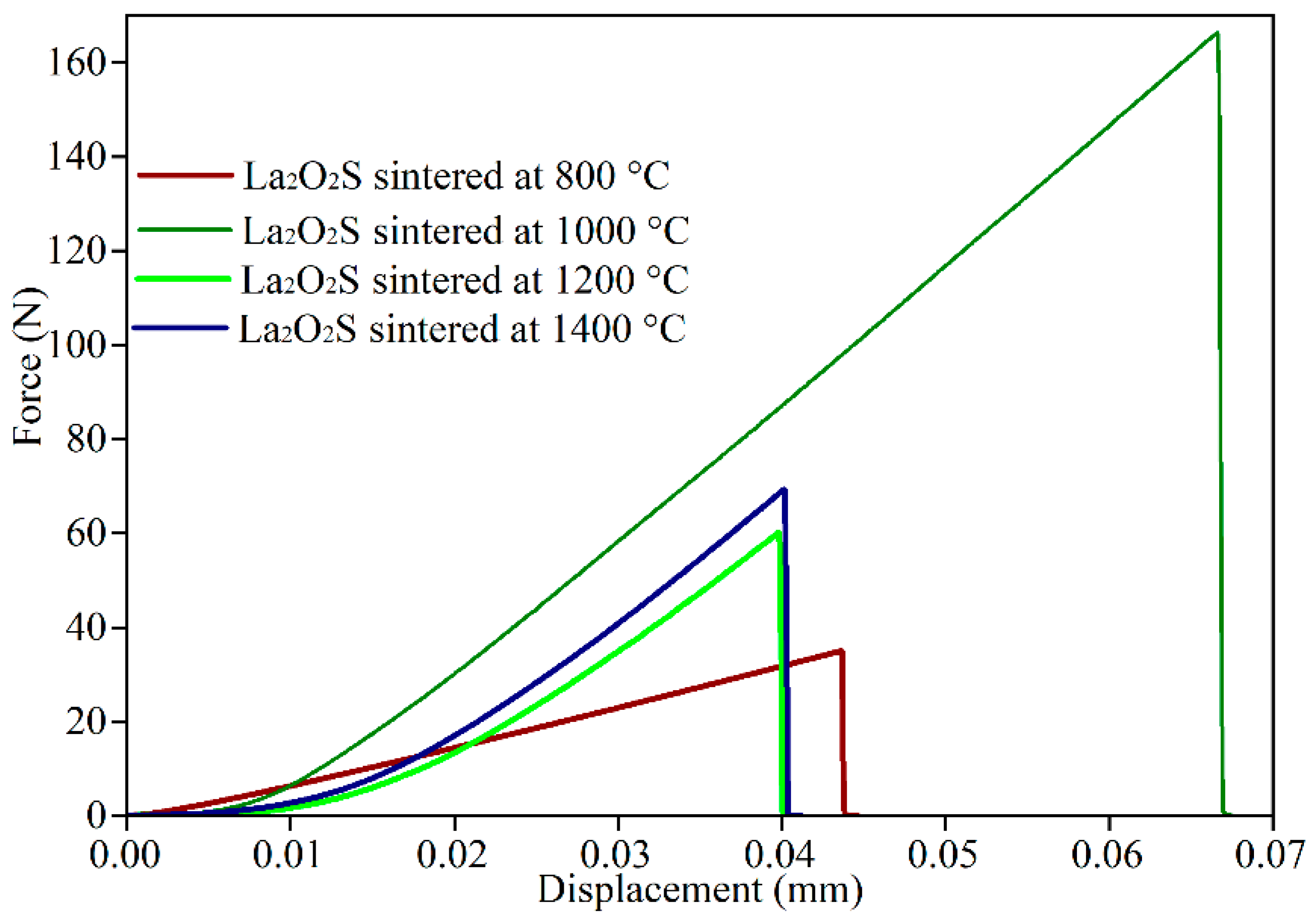
3.5. Density, Hardness, and Bending Strength of Synthetic La2O2S Bulks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Mi, C.; Yu, F.; Su, X.; Guo, C.; Li, G.; Zhang, J.; Liu, L.; Liu, Y.; Li, X. Optical thermometry based on the upconversion fluorescence from Yb3+/Er3+ codoped La2O2S phosphor. Ceram. Int. 2014, 40, 9875–9880. [Google Scholar] [CrossRef]
- Andreev, P.O.; Sal’nikova, E.I.; Kovenskii, I.M. Preparation of Ln2O2S (Ln = Gd, Dy, Y, Er, Lu) in flowing hydrogen and hydrogen sulfide. Inorg. Mater. 2014, 50, 1018–1023. [Google Scholar] [CrossRef]
- Jaffar, B.M.; Swart, H.C.; Seed Ahmed, H.A.A.; Yousif, A.; Kroon, R.E. Optical properties and stability of Bi doped La2O2S. Opt. Mater. 2019, 95, 109260. [Google Scholar] [CrossRef]
- Biondo, V.; Sarvezuk, P.W.C.; Ivashita, F.F.; Silva, K.L.; Paesano, A., Jr.; Isnard, O. Geometric magnetic frustration in RE2O2S oxysulfides (RE = Sm, Eu and Gd). Mater. Res. Bull. 2014, 54, 41–47. [Google Scholar] [CrossRef]
- Li, L.; Hirai, S.; Nakamura, E.; Yuan, H. Influences of Eu2O3 characters and sulfurization conditions on the preparation of EuS and its large magnetocaloric effect. J. Alloys Compd. 2016, 687, 413–420. [Google Scholar] [CrossRef]
- Li, L.; Hirai, S.; Yuan, H. Influences of Yb2O3 characters and sulfurization conditions on preparation of Yb2S3. J. Alloy. Compd. 2015, 618, 742–749. [Google Scholar] [CrossRef]
- Andreev, P.; Sal’nikova, E.; Andreev, O.; Kovenskii, I. Kinetic schemes of chemical transformations and particle morphology upon interaction between Ln2(SO4)3 (Ln = La, Pr, Nd, Sm) and hydrogen. Russ. J. Phys. Chem. A 2016, 90, 25–30. [Google Scholar] [CrossRef]
- Andreev, P.; Sal’nikova, E.; Kislitsyn, A. Kinetics of the transformation of Ln2O2SO4 into Ln2O2S (Ln= La, Pr, Nd, and Sm) in a hydrogen flow. Russ. J. Phys. Chem. A 2013, 87, 1482–1487. [Google Scholar] [CrossRef]
- Larquet, C. Nanoparticles of Lanthanide and Transition Metal Oxysulfides: From Colloidal Synthesis to Structure, Surface, Optical and Magnetic Properties. Ph.D. Thesis, Sorbonne Université, Paris, France, 2018. [Google Scholar]
- Sal’nikova, E.; Andreev, P.; Antonov, S. Kinetic diagrams of Ln2O2SO4 phase transformations in a H2 flow (Ln = La, Pr, Nd, Sm). Russ. J. Phys. Chem. A 2013, 87, 1280–1283. [Google Scholar] [CrossRef]
- Leskelä, M.; Leskelä, T. Thermal stability of rare earth oxysulfide solid solutions in air. Thermochim. Acta 1981, 48, 43–50. [Google Scholar] [CrossRef]
- Kay, D.A.R.; Subramanian, S.V.; Kumar, V.; Meng, V.; Dwivedi, R.K. The use of RE-O-S phase stability diagrams in gaseous desulfurisation and iron and steel production. Inorg. Chim. Acta 1984, 94, 132–134. [Google Scholar] [CrossRef]
- Le Coz, A.; Durand, G.R.; Cornet, L.; Herbert, N.; Gouttefangeas, F.; Joanny, L.; Célarié, F.; Cheviré, F.; Merdrignac-Conanec, O. First La2O2S infrared transparent ceramics. J. Eur. Ceram. Soc. 2023, 43, 2133–2142. [Google Scholar] [CrossRef]
- Ohta, M.; Hirai, S.; Kato, H.; Sokolov, V.V.; Bakovets, V.V. Thermal decomposition of NH4SCN for preparation of Ln2S3 (Ln = La and Gd) by sulfurization. Mater. Trans. 2009, 50, 1885–1889. [Google Scholar] [CrossRef]
- Shah, K.; Ćirić, A.; Murthy, K.; Chakrabarty, B. Investigation of a new way of synthesis for Nano crystallites of La2O2S & 1% Ln3+ (Ln = Pr, Eu, Tb, Dy, Er) doped La2O2S and study their structural and optical properties. J. Alloy. Compd. 2021, 851, 156725. [Google Scholar]
- Kim, S.W.; Hasegawa, T.; Abe, T.; Nakagawa, H.; Hasegawa, S.; Seki, K.; Toda, K.; Uematsu, K.; Ishigaki, T.; Sato, M. Abnormal improvement in emission of lanthanum oxysulfide phosphor La2O2S: Tb3+ synthesized by a novel method, thermal decomposition in eutectic molten salt. Ceram. Int. 2016, 42, 10389–10392. [Google Scholar] [CrossRef]
- Hakmeh, N.; Chlique, C.; Merdrignac-Conanec, O.; Fan, B.; Cheviré, F.; Zhang, X.; Fan, X.; Qiao, X. Combustion synthesis and up-conversion luminescence of La2O2S: Er3+, Yb3+ nanophosphors. J. Solid State Chem. 2015, 226, 255–261. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Zhang, S.; Zhang, S.; Jin, H. Energy transfer of Eu2+→ Eu3+ improves the photoluminescence properties of orange-red phosphor La2O2S: Eu. J. Alloy. Compd. 2023, 969, 172394. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Zhang, Q.; Song, B.; Wong, C. Luminescence properties of La2O2S: Tb3+ phosphors and phosphor-embedded polymethylmethacrylate films. Mater. Des. 2017, 125, 100–108. [Google Scholar] [CrossRef]
- Erasmus, L.; Swart, H.; Terblans, J. La2O2S: Eu3+ stability as temperature sensor. Appl. Surf. Sci. 2019, 487, 41–51. [Google Scholar] [CrossRef]
- Sasaki, S.; Caldes, M.T.; Guillot-Deudon, C.; Braems, I.; Steciuk, G.; Palatinus, L.; Gautron, E.; Frapper, G.; Janod, E.; Corraze, B. Design of metastable oxychalcogenide phases by topochemical (de) intercalation of sulfur in La2O2S2. Nat. Commun. 2021, 12, 3605. [Google Scholar] [CrossRef]
- Morosin, B. La2O2S structure refinement and crystal field. Struct. Sci. 1973, 29, 2647–2648. [Google Scholar] [CrossRef]
- Larquet, C.; Carenco, S. Metal oxysulfides: From bulk compounds to nanomaterials. Front. Chem. 2020, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Polfus, J.M.; Norby, T.; Bredesen, R. Protons in oxysulfides, oxysulfates, and sulfides: A first-principles study of La2O2S, La2O2SO4, SrZrS3, and BaZrS3. J. Phys. Chem. C 2015, 119, 23875–23882. [Google Scholar] [CrossRef]
- Aktas, B.; Tekeli, S.; Salman, S. Synthesis and properties of La2O3-doped 8 mol% yttria-stabilized cubic zirconia. J. Mater. Eng. Perform. 2014, 23, 294–301. [Google Scholar] [CrossRef]
- Aktas, B.; Tekeli, S.; Salman, S. Crystallization and grain growth behavior of La2O3-doped yttria-stabilized zirconia. Adv. Mater. Lett. 2014, 5, 260–264. [Google Scholar] [CrossRef]
- Zhang, X.; Wenhua, G.; Qian, W.; Qingfeng, Z. Structural, phonon, mechanical, optical, and thermodynamic properties of stable β-La2S3 from first-principles calculations. J. Rare Earths 2017, 35, 271–279. [Google Scholar] [CrossRef]
- Westrum, E.F., Jr.; Burriel, R.; Gruber, J.B.; Palmer, P.E.; Beaudry, B.J.; Plautz, W. Thermophysical properties of the lanthanide sesquisulfides. I. Schottky functions and magnetic and electronic properties of γ-La2S3, γ-Ce2S3, γ-Nd2S3, and γ-Gd2S3. J. Chem. Phys. 1989, 91, 4838–4848. [Google Scholar] [CrossRef]
- Amano, T.; Beaudry, B.; Gschneidner, K., Jr. High-temperature thermodynamic properties of alpha and gamma lanthanum sesquisulfides and related compounds. J. Appl. Phys. 1986, 59, 3437–3440. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Q.; Wang, H.; Li, Y. A reddish La2O2S-based long-afterglow phosphor with effective absorption in the visible light region. Mater. Sci. Eng. B 2012, 177, 316–320. [Google Scholar] [CrossRef]
- Mikami, M.; Nakamura, S. Electronic structure of rare-earth sesquioxides and oxysulfides. J. Alloy. Compd. 2006, 408, 687–692. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Kumar, R.; Almas, T.; Sandal, P.; Al-Assiri, M.; Mahnashi, M.H.; AlFarhan, B.; Baskoutas, S. Fern shaped La2O3 nanostructures as potential scaffold for efficient hydroquinone chemical sensing application. Ceram. Int. 2020, 46, 5141–5148. [Google Scholar] [CrossRef]
- Wang, N.; Liu, J.; Gu, W.; Song, Y.; Wang, F. Toward synergy of carbon and La2O3 in their hybrid as an efficient catalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 77786–77795. [Google Scholar] [CrossRef]
- Anan’eva, G.; Gorokhova, E.; Demidenko, V.; Parfinskii, V.; Khristich, O. Optical properties of Gd2O2S-based ceramic. J. Opt. Technol. 2005, 72, 58–61. [Google Scholar] [CrossRef]
- Kumta, P.N.; Risbud, S.H. Low-temperature chemical routes to formation and IR properties of lanthanum sesquisulfide (La2S3) ceramics. J. Mater. Res. 1993, 8, 1394–1410. [Google Scholar] [CrossRef]
- Ji, H.; Fang, M.; Huang, Z.; Chen, K.; Xu, Y.; Liu, Y.G.; Huang, J. Effect of La2O3 additives on the strength and microstructure of mullite ceramics obtained from coal gangue and γ-Al2O3. Ceram. Int. 2013, 39, 6841–6846. [Google Scholar] [CrossRef]
- Ren, X.; Peng, Z.; Wang, C.; Miao, H. Influence of nano-sized La2O3 addition on the sintering behavior and mechanical properties of WC–La2O3 composites. Ceram. Int. 2015, 41, 14811–14818. [Google Scholar] [CrossRef]
- Hu, X.; Chen, W.; Lu, D.; Lu, L.; Jia, J.; Li, H.; Zou, J.; Hu, Q. Microstructure and Mechanical Properties of Modified M2 High-Speed Steel by Adding La2O3 in the Electroslag Casting Process. Steel Res. Int. 2024, 95, 2300035. [Google Scholar] [CrossRef]





| Material | Method | a/Å | c/Å | Ref. |
|---|---|---|---|---|
| La2O2S compact | 800 °C 20 min | 4.036934 | 6.9139 | |
| La2O2S compact | 1000 °C 20 min | 4.053987 | 6.94828 | |
| La2O2S compact | 1200 °C 20 min | 4.048406 | 6.941172 | |
| La2O2S compact | 1400 °C 20 min | 3.960311 | 6.70675 | |
| La2O2S compact | 800 °C 60 min | 4.054946 | 6.967556 | |
| La2O2S compact | 1000 °C 60 min | 4.048065 | 6.948317 | |
| La2O2S compact | 1300 °C 60 min | 4.053658 | 6.955327 | |
| La2O2S compact | 1500 °C 60 min | 4.05167 | 6.955472 | |
| La2O2S | first-principles study | 4.06/4.03 | 6.95/6.91 | [24] |
| La2O2S | furnace combustion | 4.0313 | 6.9097 | [15] |
| La2O2S | one-step flux method | 4.0350 | 6.914 | [19] |
| La2O2S | single crystal | 4.049 | 6.939 | [22] |
| La2O2S | annealing | 4.04 | 6.99 | [20] |
| La2O2S | sulfurized powder | 4.052 | 6.946 | [13] |
| La1.99O2S:0.01Eu | solid-state reaction | 4.049 | 6.944 | [18] |
| La2O2S:1%Er | combustion synthesis | 4.0506 | 6.9456 | [17] |
| (La0.95Tb0.05)2O2S | two-step flux method | 4.0509 | 6.9432 | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, L.; Li, J.; Han, K. Rapid Synthesis and Sintering of La2O2S and Its Physical, Optical, and Mechanical Properties. Coatings 2024, 14, 1120. https://doi.org/10.3390/coatings14091120
Chen Y, Li L, Li J, Han K. Rapid Synthesis and Sintering of La2O2S and Its Physical, Optical, and Mechanical Properties. Coatings. 2024; 14(9):1120. https://doi.org/10.3390/coatings14091120
Chicago/Turabian StyleChen, Yuqi, Liang Li, Jin Li, and Kun Han. 2024. "Rapid Synthesis and Sintering of La2O2S and Its Physical, Optical, and Mechanical Properties" Coatings 14, no. 9: 1120. https://doi.org/10.3390/coatings14091120






