Preparation and Properties of Waterborne Acrylic-Modified Epoxy Phosphate Resin and Its Coating
Abstract
:1. Introduction
2. Experimental
2.1. Materials
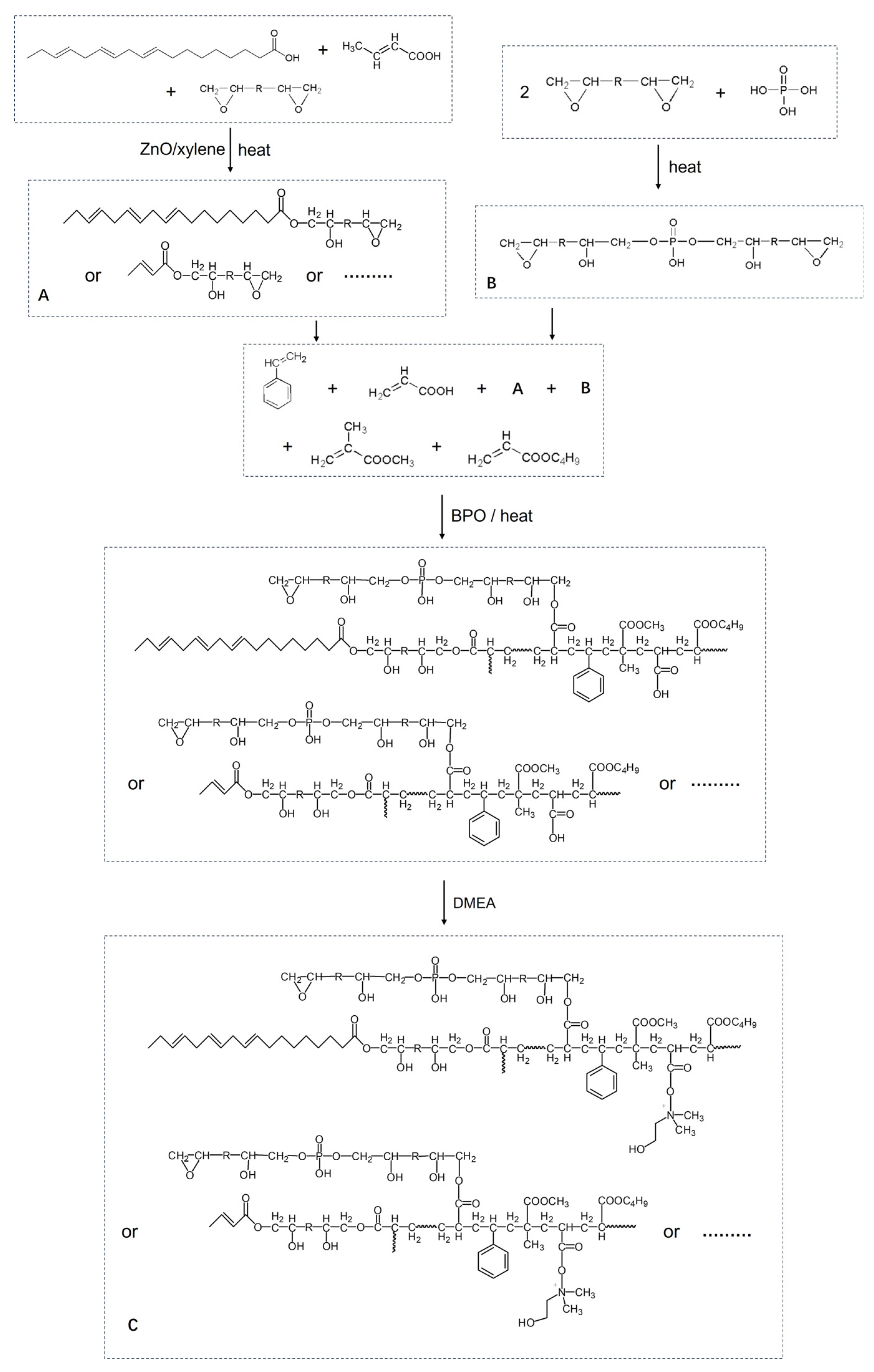
2.2. Preparation of the Epoxy Ester Resin A
2.3. Preparation of the Epoxy Phosphate Resin B
2.4. Preparation of the Acrylic-Modified Epoxy Phosphate Resin C
2.5. Preparation of Waterborne Epoxy Coating D
2.6. Performance Test of Waterborne Epoxy Coating
3. Results and Discussion
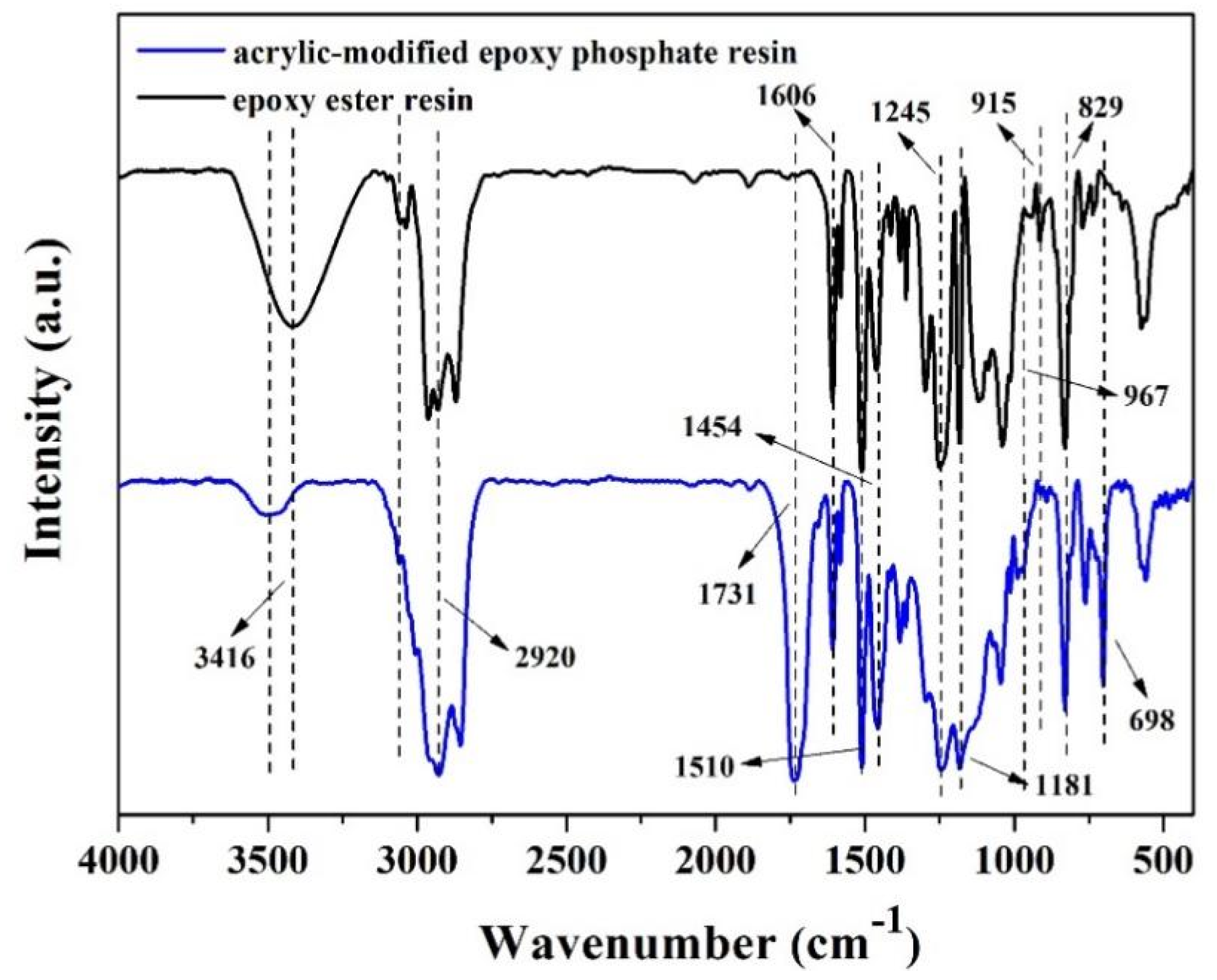
3.1. FT-IR Analysis of Epoxy Ester Resin and Acrylic-Modified Epoxy Phosphate Resin
3.2. Selection of Epoxy Resin Type
3.3. Selection of Fatty Acid Type
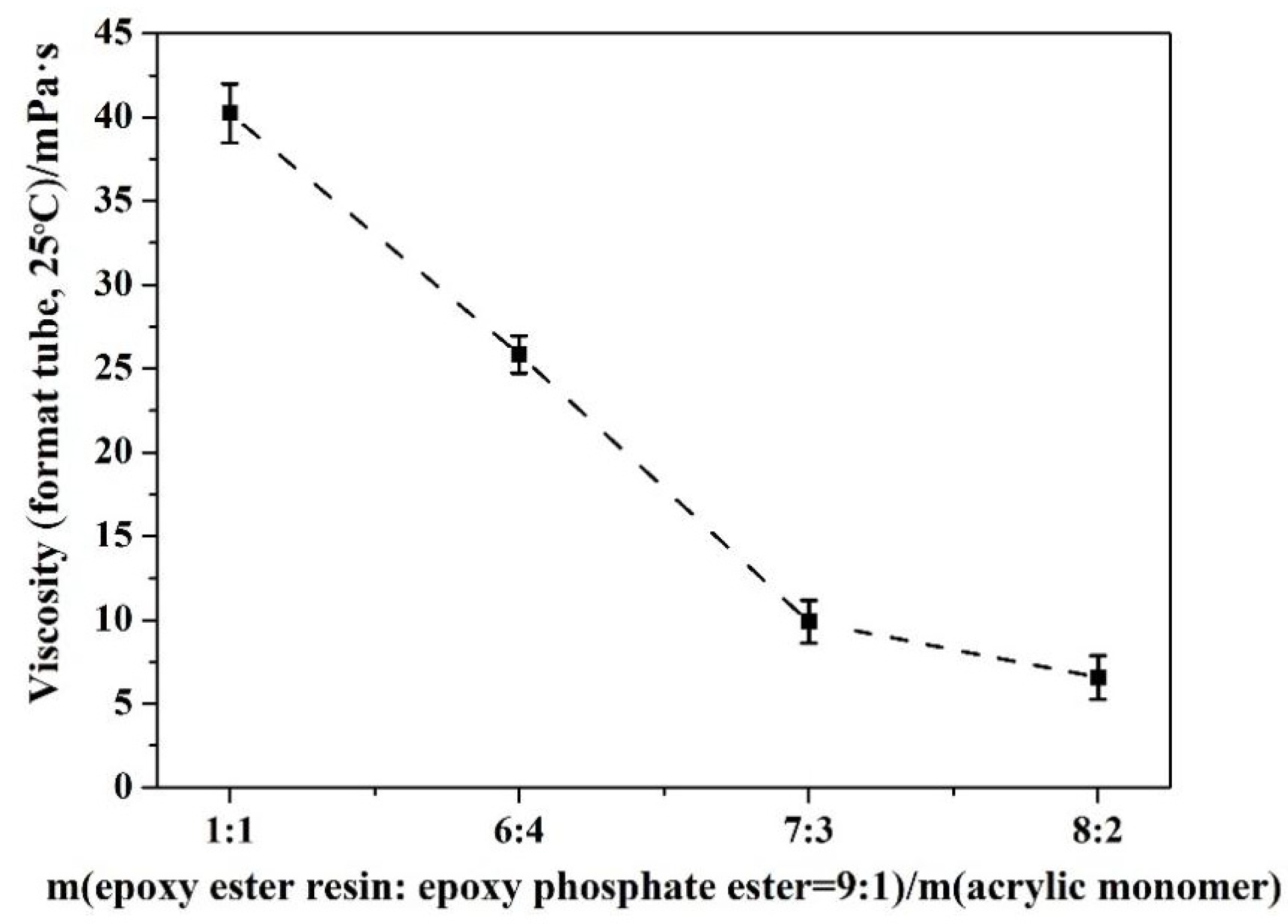
3.4. Influence of Ratio and Dosage of Base Resin
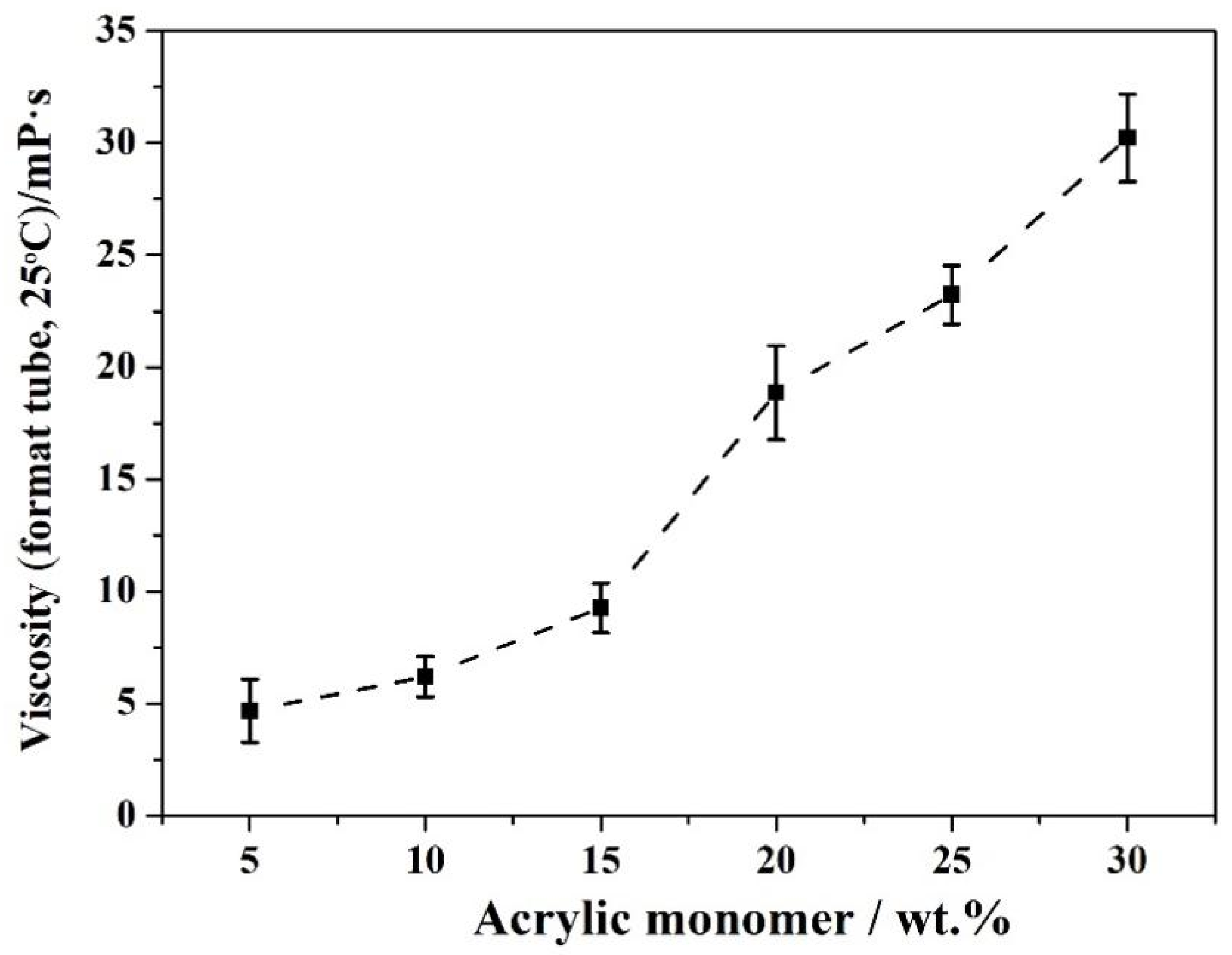
3.5. Effect of the Amount of Acrylic Monomer on the Properties of Resin C
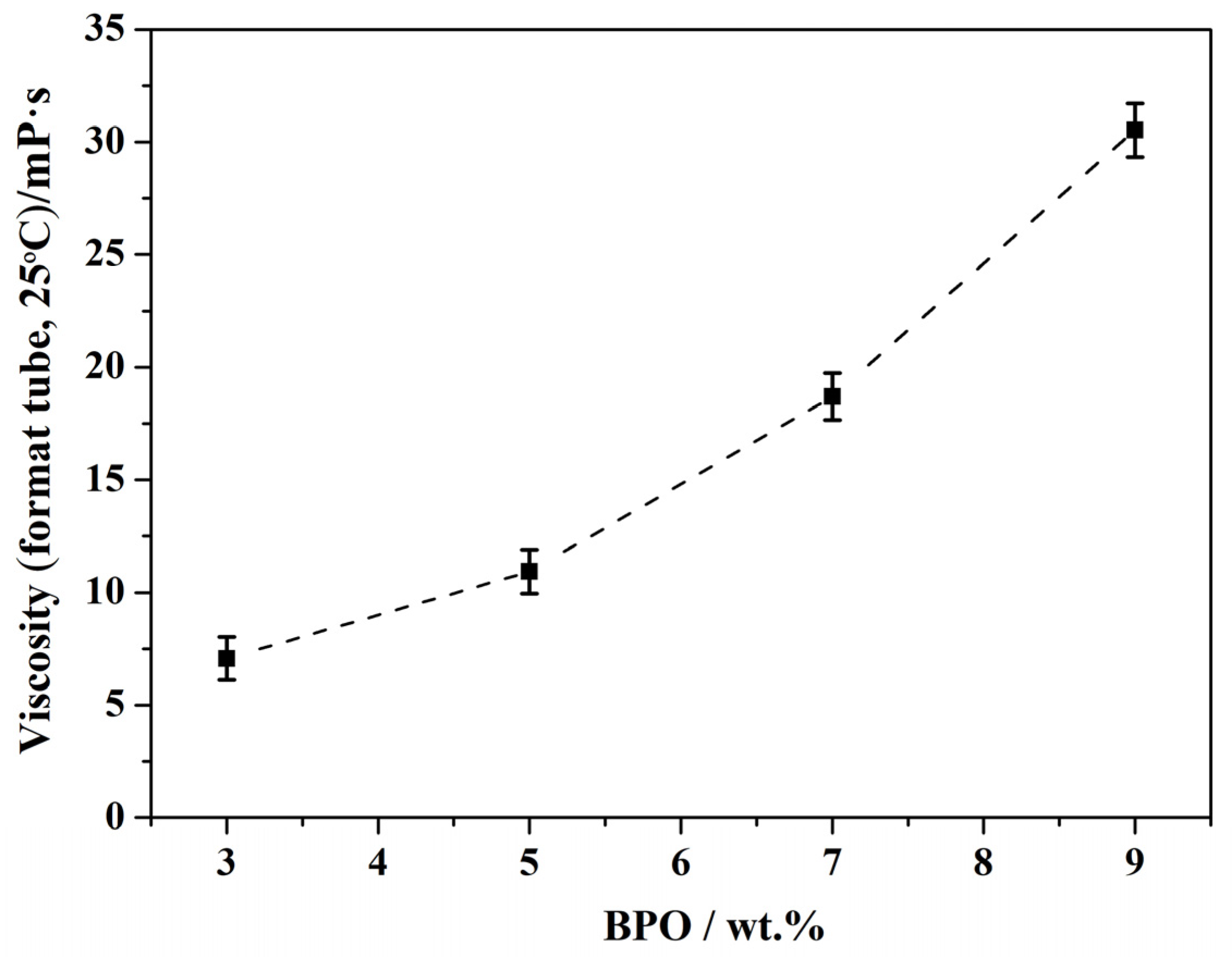
3.6. Effect of BPO Dosage on the Properties of Resin C
3.7. The Effect of Reaction Temperature of Acrylic Acid Monomer on Resin Properties
3.8. Effect of Reaction Time of Acrylic Acid Monomer on Resin Properties
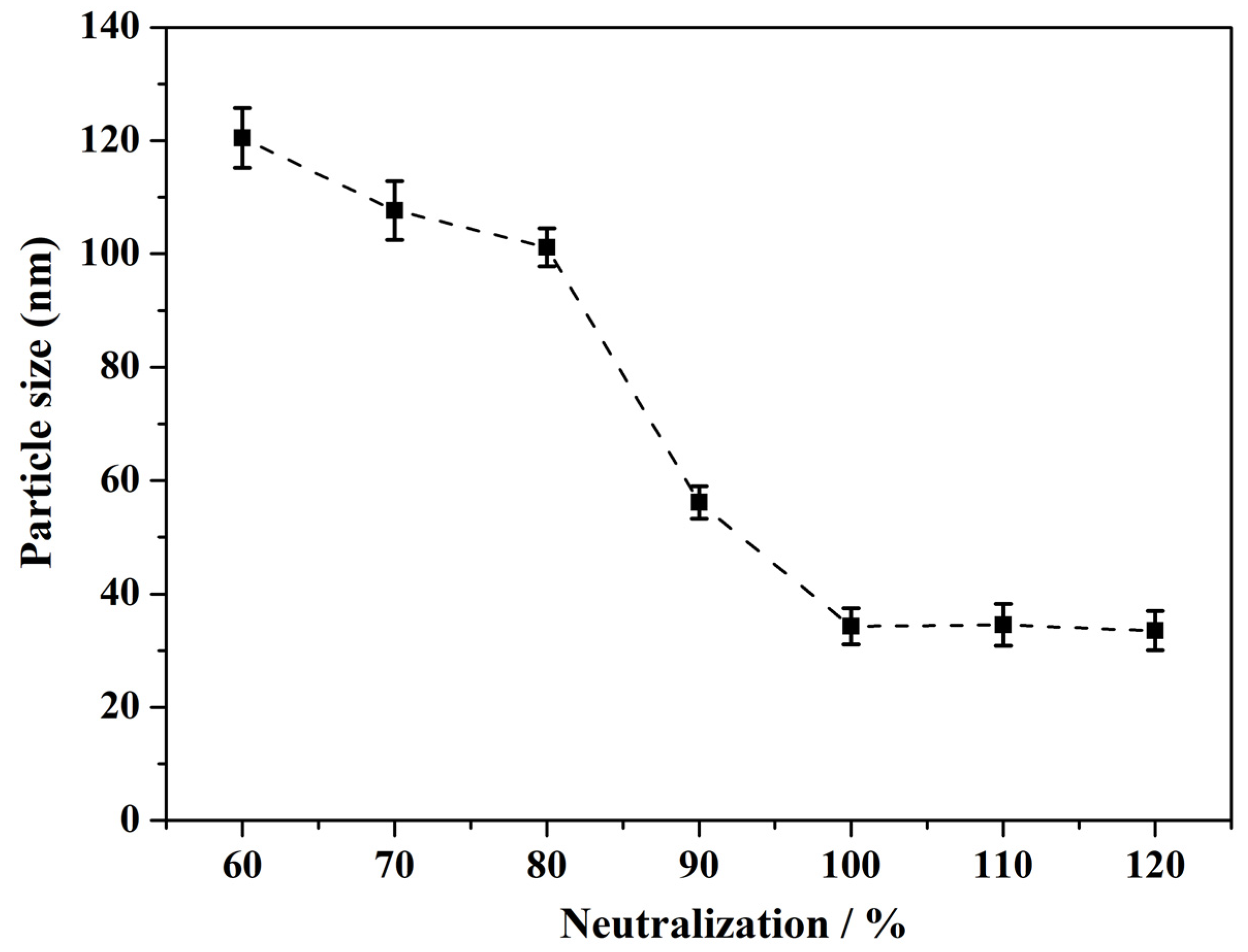
3.9. Effect of the Neutralizing Agent on the Modified Resin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Athawale, V.D.; Nimbalkar, R.V. Waterborne Coatings Based on Renewable Oil Resources: An Overview. J. Am. Oil Chem. Soc. 2011, 88, 159–185. [Google Scholar] [CrossRef]
- Chek, Y.W.; Ang, D.T.C. Progress of bio-based coatings in waterborne system: Synthesis routes and monomers from renewable resources. Prog. Org. Coat. 2024, 188, 108190. [Google Scholar] [CrossRef]
- Wei, J.H.; Shen, T.; Cao, W.K.; Jiang, L.; Hee, Y.; Li, W.H. Colorable photothermal-induced self-repairing anti-corrosion coating based on confined solid-liquid transition. J. Mater. Sci. Technol. 2024, 197, 227–237. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Pleshkova, A.I.; Piatkova, M.; Nadaraia, K.V.; Imshinetskiy, I.M.; Belov, E.A.; Suchkov, S.N.; Sinebryukhov, S.L.; Gnedenkov, S.V. PTFE-Containing Coating Obtained on Ti by Spraying and PEO Pretreatment. Coatings 2023, 13, 1249. [Google Scholar] [CrossRef]
- Tu, K.; Wu, J.; Zhao, X.; Zhang, L.F.; Zhang, Z.H. Repair Strategy for Epoxy Anticorrosive Coatings on Steel Structures. Materials 2023, 16, 6257. [Google Scholar] [CrossRef] [PubMed]
- Deyab, M.A.; El-Shamy, O.A.A.; Alghamdi, M.M.; El-Zahhar, A.A. Impact of Co3O4 nanoparticles on epoxy’s mechanical and corrosion-resistance properties for carbon steel in seawater. Sci. Rep. 2024, 14, 3535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, C.L.; Song, L.; Zeng, R.C.; Li, S.Q.; Cui, H.Z. Fabrication of the Superhydrophobic Surface on Magnesium Alloy and Its Corrosion Resistance. J. Mater. Sci. Technol. 2015, 31, 1139–1143. [Google Scholar] [CrossRef]
- Zhou, Y.R.; Effendy, S.; Zhu, J.; Petr, M.T.; Cwalina, C.D.; Bazant, M.Z.; Yildiz, B.; Li, J.; Short, M.P. Coupled effect of water absorption and ion transport in hydrated latex anti-corrosion coatings. J. Coat. Technol. Res. 2023, 20, 187–200. [Google Scholar] [CrossRef]
- Ai, D.; Mo, R.B.; Wang, H.H.; Lai, Y.B.; Jiang, X.; Zhang, X.Y. Preparation of waterborne epoxy dispersion and its application in 2K waterborne epoxy coatings. Prog. Org. Coat. 2019, 136, 105258. [Google Scholar] [CrossRef]
- Sung, L.P.; Gu, X.H.; Clerici, C.; Hu, H.; Loizou, E.; Ho, D.L. Effect of Pigment Dispersion on Optical Properties and Durability of a TiO2 Pigmented Epoxy Coating. Polym. Degrad. Perform. 2009, 1004, 276–286. [Google Scholar]
- Li, L.; Feng, M.; Zhu, J.T. Synthesis of hydrophilic epoxy-functionalized films by UV-initiated copolymerization. Trends Dept. Ind. 2013, 785, 892–895. [Google Scholar] [CrossRef]
- Bayliss, N.; Schmidt, B.V.K.J. Hydrophilic polymers: Current trends and visions for the future. Prog. Polym. Sci. 2023, 147, 101753. [Google Scholar] [CrossRef]
- Sun, W.F.; Chern, W.K.; Chan, J.C.Y.; Chen, Z. A Reactive Molecular Dynamics Study on Crosslinked Epoxy Resin Decomposition under High Electric Field and Thermal Aging Conditions. Polymer 2023, 15, 765. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Han, B.; Xu, Y.J.; Liu, X.; Zhao, C.H.; Xu, J. Conjugated polymer coating enabled light-resistant black phosphorus with enhanced stability. Nanoscale Adv. 2021, 3, 5650–5655. [Google Scholar] [CrossRef]
- Galliano, F.; Landolt, D. Evaluation of corrosion protection properties of additives for waterborne epoxy coatings on steel. Prog. Org. Coat. 2002, 44, 217–225. [Google Scholar] [CrossRef]
- Kinsley, P.C.; Zeng, A.P.; Hedlund Orbeck, J.K.; Debow, S.; Zander, Z.B.; Heaney, P.J.; Hamers, R.J. Reactivity passivation of red phosphorus with thin plasma-deposited carbon coating. Appl. Surf. Sci. 2022, 587, 152791. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, T.; Zhang, X.; Xin, Z.X.; Deng, X.; Prakashan, K. Mechanically stable superhydrophobic polymer films by a simple hot press lamination and peeling process. RSC Adv. 2016, 6, 12530–12531. [Google Scholar] [CrossRef]
- Gu, L.; Ding, J.H.; Liu, S.; Yu, H.B. Incorporation of reactive corrosion inhibitor in waterborne acrylic polyurethane coatings and evaluation of its corrosion performance. Chin. J. Chem. Phys. 2016, 29, 271–278. [Google Scholar] [CrossRef]
- Zhong, Z.Q.; Yu, Q.; Yao, H.; Wu, W.T.; Feng, W.L.; Yu, L.; Xu, Z.Y. Study of the styrene-acrylic emulsion modified by hydroxyl-phosphate ester and its stoving varnish. Prog. Org. Coat. 2013, 76, 858–862. [Google Scholar] [CrossRef]
- Han, B.; Li, S.; Chang, D.; Li, J.; Li, T.S.; Yang, S.H.; Chen, Y.M.; Zhang, H.L. Efficient phenyl phosphate ester extractant synthesis and solvent extraction performance evaluation for transition metals. Sep. Purif. Technol. 2024, 336, 126251. [Google Scholar] [CrossRef]
- Shen, C.H.; Kong, G.J.; Wang, J.; Zhang, X. Synthesis and characterization of high temperature proton exchange membrane from isocyanatopropyltriethoxysilane and hydroxyethane diphosphonic acid. Int. J. Hydrogen Energy 2015, 40, 363–372. [Google Scholar] [CrossRef]
- Tian, L.; Li, X.; Wang, L.R.; Zhang, Y.B.; Cui, J.F.; Guo, J.H.; Yang, B.P. Synthesis and characterization of an efficient flame retardant based on aromatic ring and phosphate ester for epoxy resin. Polym. Eng. Sci. 2019, 59, E406–E413. [Google Scholar] [CrossRef]
- Pang, X.L.; Li, Y.; Wu, X.F.; Zhang, B.M.; Hao, M.; Zhu, Y.; Zhang, Y.; Qin, C.J.; Zhan, H.M.; Qin, C.L. Phosphate ester functionalized ffuorene-benzothiadiazole alternating copolymer/hydroxylated g-C3N4 heterojunctions for efffcient hydrogen evolution under visible-light irradiation. J. Colloid Interface Sci. 2023, 652, 1405–1416. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yong, T.; Yang, J.X.; Zhang, A.J.; Niu, B.B. Preparation and study on properties of water acrylic modified epoxy alcohol acid resin. Coat. Ind. 2021, 51, 36–41. [Google Scholar]
- Ding, J.H.; Liu, S.; Gu, L. The corrosion resistance of epoxy phosphate/aqueous epoxy coating. Surf. Engr. 2015, 28, 126–131. [Google Scholar]
- Kim, W.G.; Lee, J.Y. Cure kinetics of methacrylate-type resin that include cyclohexane moiety. Polymer 2003, 44, 6303–6309. [Google Scholar] [CrossRef]
- Sami, V.; Ilkka, S.; Tuomo, S. Controlled partial neutralization of amphoteric ion exchange resin for improved metals separation. React. Funct. Polym. 2013, 73, 647–652. [Google Scholar]


| Test Item | Indicator Standards | Detection Results |
|---|---|---|
| Character | 1. transparent viscous liquid 2. no mechanical impurities | 1. transparent viscous liquid 2. no mechanical impurities |
| Color (Fe-Co), ≤ | 12# | 12# |
| Acid value/mg KOH/g | 9.0–14.0 | 9.5 |
| Solid/% | 64.0 ± 2 | 64.5 |
| Fineness/μm, ≤ | 20 | 20 |
| Test Item | Indicator Standards | Detection Results |
|---|---|---|
| Character | 1. transparent viscous liquid 2. no mechanical impurities | 1. transparent viscous liquid 2. no mechanical impurities |
| Color (Fe-Co), ≤ | 9# | 9# |
| Acid value/mg KOH/g | 9.0–12.0 | 10.6 |
| Solid/% | 60.0 ± 2 | 60.3 |
| Fineness/μm, ≤ | 20 | 20 |
| Test Item | Indicator Standards | Detection Results |
|---|---|---|
| Character | 1. transparent viscous liquid 2. permissive light fluorescence | 1. transparent viscous liquid 2. permissive light fluorescence |
| Color (Fe-Co), ≤ | 12# | 12# |
| Solid/% | 50 ± 2 | 50.1 |
| Fineness/μm, ≤ | 20 | 20 |
| pH value | 8.0–9.0 | 8.5 |
| Ingredients | Percentage/wt.% |
|---|---|
| acrylic-modified epoxy phosphate resin | 45.0–55.0 |
| dimethylethanolamine | 1.0–1.3 |
| triethanolamine | 0.5–0.8 |
| distilled water | 21.7–25.7 |
| dispersant | 3.0–3.5 |
| defoamer | 0.2–0.3 |
| carbon black | 3.0–3.5 |
| zinc phosphate | 7.0–8.0 |
| aluminum tripolyphosphate | 3.5–4.0 |
| barium sulfate | 10.0–15.0 |
| mica powder | 3.5–4.0 |
| waterborne bentonite | 0.2–0.3 |
| modified fumed nanosilica | 1.5–2.0 |
| triethanolamine | 0.3–0.5 |
| substrate wetting agent | 0.1–0.2 |
| waterborne anti-flash rust agent | 0.3–0.4 |
| waterborne drier | 1.3–1.6 |
| waterborne thickener | 0.1–0.2 |
| deionized water | 2.6–3.6 |
| Test Item | Indicator Standards | Detection Results |
|---|---|---|
| state in container | normal | normal |
| film appearance | normal | normal |
| no volatile matter content/%, ≥ | 40 | 48 |
| drying time/h, ≤ | - | - |
| surface drying time | 4 | 1 |
| actual drying | 24 | 12 |
| bending test/mm, ≤ | 3 | 1 |
| impact resistance/cm, ≥ | 40 | 50 |
| grid test/grade, ≤ | 1 | 1 |
| storage stability, (50 ± 2°C, 14 d) | normal | normal |
| volatile organic compound content, (g/L), ≤ | 200 | 134 |
| resistance to flash rust | normal | normal |
| water resistance (240 h) | no foaming, no peeling, no rust, no cracking | correspondency |
| salt spray resistance (300 h) | no foaming, no peeling, no rust, no cracking | correspondency |
| Test Item | Indicator Standards | Detection Results |
|---|---|---|
| adhesive force (pulling method)/MPa, ≥ | 3 | 6.5 |
| water resistance (120 h) | no foaming, no peeling, no rust, no cracking | correspondency |
| acid resistance (50 g/L H2SO4, 96 h) | no foaming, no peeling, no rust, no cracking | correspondency |
| alkali resistance (50 g/L NaOH, 96 h) | no foaming, no peeling, no rust, no cracking | correspondency |
| oil resistance (3# regular paint, 96 h) | no foaming, no peeling, no rust, no cracking | correspondency |
| continuous condensation experiment (240 h) | no foaming, no peeling, no rust, no cracking | correspondency |
| resistant to neutral salt spray (480 h) | no foaming, no peeling, no rust, no cracking | correspondency |
| resistance to artificial weathering (800 h) | discoloration ≤ 3, pulverization ≤ 1, cracking ≤ 1, foaming ≤ 1, rust = 1 | correspondency |
| adhesive force (pulling method, after salt spray test)/Mpa, ≥ | 2 | 4.2 |
| Epoxy Resin Type | Reaction Time | Epoxy Ester Viscosity, Appearance, Dryness |
|---|---|---|
| E-12 | 2~3 h | large viscosity, light brown, poor drying |
| E-20 | 2~3 h | large viscosity, light brown, good drying |
| E-51 | ≤1 h | large viscosity, light brown, good drying |
| M(Epoxy Ester Resin/Epoxy Phosphate Ester = 9:1)/M(Acrylic Monomer) | Water Dispersibility | Storage Stability | Viscosity (Mean Value, Format Tube, 25 °C)/mPa·s |
|---|---|---|---|
| 5:5 | normal | no separation transparency | 40.2 |
| 6:4 | good | no separation transparency | 25.9 |
| 7:3 | good | no separation translucency | 10.0 |
| 8:2 | normal | no separation translucency | 6.6 |
| wacrylic monomer/% | Water Dispersibility | Viscosity (Mean Value, Format Tube, 25 °C)/mPa·s |
|---|---|---|
| 5 | no transparency no separation | 4.7 |
| 10 | translucency no separation | 6.2 |
| 15 | transparency no separation | 9.3 |
| 20 | transparency no separation | 18.9 |
| 25 | transparency slight separation | 23.2 |
| 30 | translucency slight separation | 30.2 |
| WBPO/% | Water Dispersibility | Stability | Viscosity (Mean Value, Format Tube, 25 °C)/mPa·s |
|---|---|---|---|
| 3 | normal | no separation translucency | 7.1 |
| 5 | normal | no separation transparency | 10.9 |
| 7 | good | no separation transparency | 18.7 |
| 9 | good | no separation translucency | 30.5 |
| Reaction Temperature/°C | Water Dispersibility | Stability | Viscosity (Mean Value, Format Tube, 25 °C)/mPa·s |
|---|---|---|---|
| 75 | normal | no separation translucency | 7.7 |
| 95 | good | no separation transparency | 10.1 |
| 115 | good | no separation transparency | 18.1 |
| 135 | normal | no separation translucency | 32.3 |
| Reaction Time/h | Water Dispersibility | Stability | Viscosity (Mean Value, Format Tube, 25 °C)/mPa·s |
|---|---|---|---|
| 3.0 | normal | no separation translucency | 7.6 |
| 3.5 | good | no separation translucency | 10.9 |
| 4.0 | good | no separation transparency | 18.8 |
| 4.5 | good | no separation translucency | 27.3 |
| Neutralization/% | Mean Particle Size/nm | Dispersion State | Stability/d |
|---|---|---|---|
| 60 | 120.5 | no transparency, having particulate matter | / |
| 70 | 107.7 | no transparency, having particulate matter | / |
| 80 | 101.2 | no transparency, having particulate matter | ≤18 |
| 90 | 56.1 | transparent, no particulate matter | ≤24 |
| 100 | 34.3 | transparent, no particulate matter | ≥36 |
| 110 | 34.6 | transparent, no particulate matter | ≥36 |
| 120 | 33.5 | transparent, no particulate matter, strong smell, yellowish appearance | ≤30 |
| Neutralization temperature/°C | 35–45 | 50–70 | 70–90 |
| Particle size distribution index | 1.036 | 0.932 | 0.987 |
| Dispersibility of resin | poor dispersibility | good dispersibility | general dispersion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, F.; Yong, T.; Cao, T.; Shi, F.; Sun, X.; Zhang, J. Preparation and Properties of Waterborne Acrylic-Modified Epoxy Phosphate Resin and Its Coating. Coatings 2024, 14, 1129. https://doi.org/10.3390/coatings14091129
Xiao F, Yong T, Cao T, Shi F, Sun X, Zhang J. Preparation and Properties of Waterborne Acrylic-Modified Epoxy Phosphate Resin and Its Coating. Coatings. 2024; 14(9):1129. https://doi.org/10.3390/coatings14091129
Chicago/Turabian StyleXiao, Fei, Tao Yong, Tianlong Cao, Fangyuan Shi, Xuejun Sun, and Jin Zhang. 2024. "Preparation and Properties of Waterborne Acrylic-Modified Epoxy Phosphate Resin and Its Coating" Coatings 14, no. 9: 1129. https://doi.org/10.3390/coatings14091129






