Niobium Oxide Thin Films Grown on Flexible ITO-Coated PET Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Film Preparation
2.2. Characterization
2.2.1. Microstructural and Morphologic Characterization
2.2.2. Optical Characterization
2.2.3. Electrical Characterization
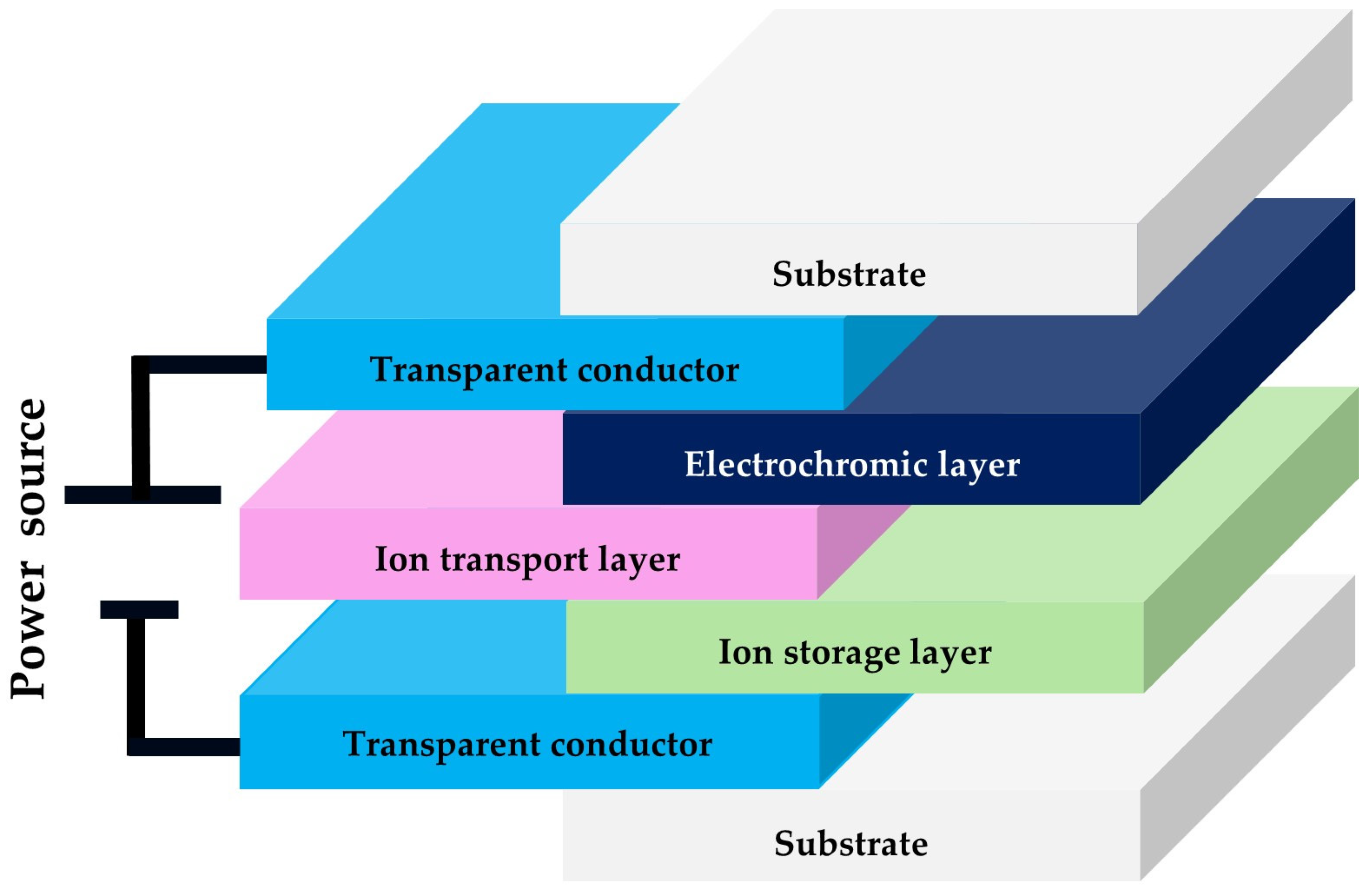
2.2.4. Electrochromic Characterization
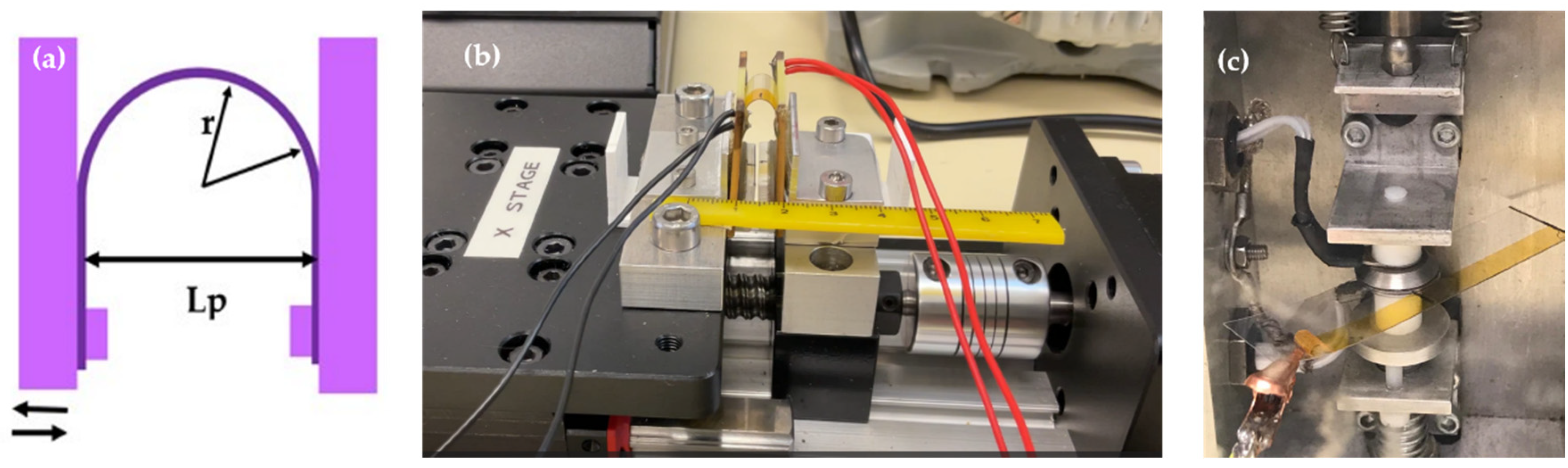
2.2.5. Mechanical Characterization
3. Results and Discussion
3.1. Microstructural and Morphologic Characterization
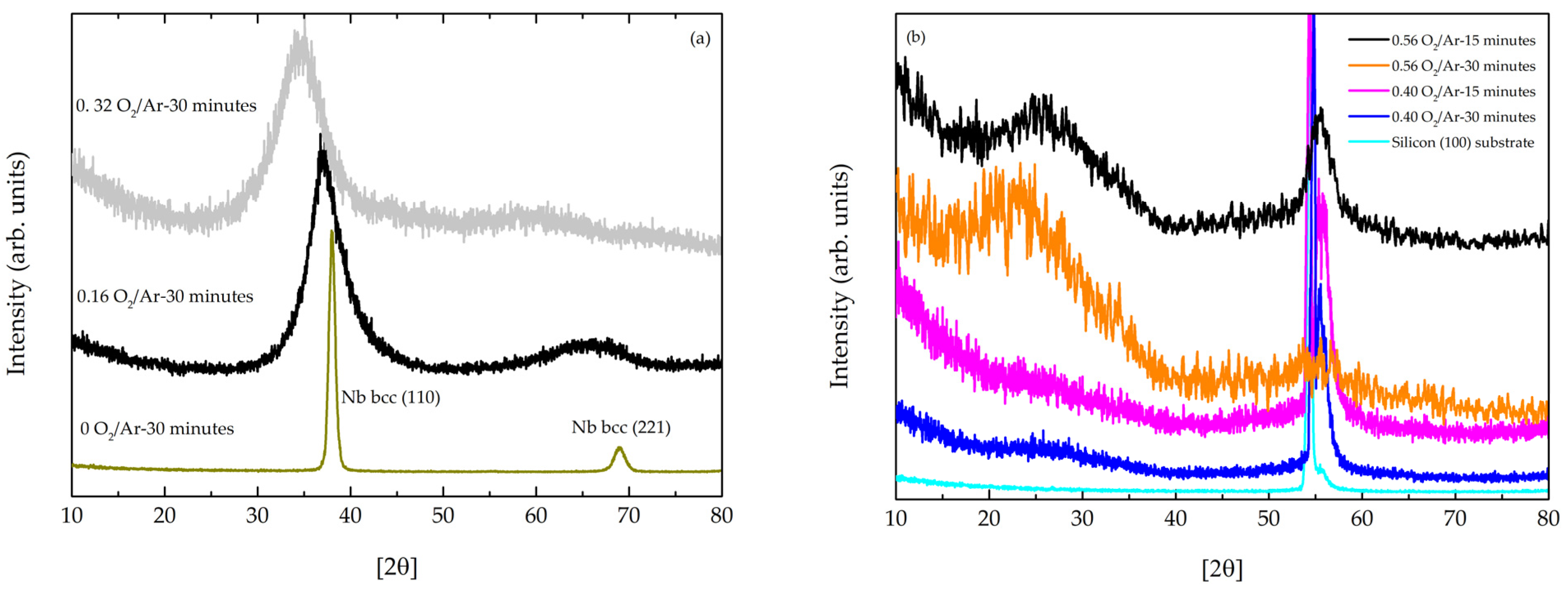
3.1.1. Structural Analysis
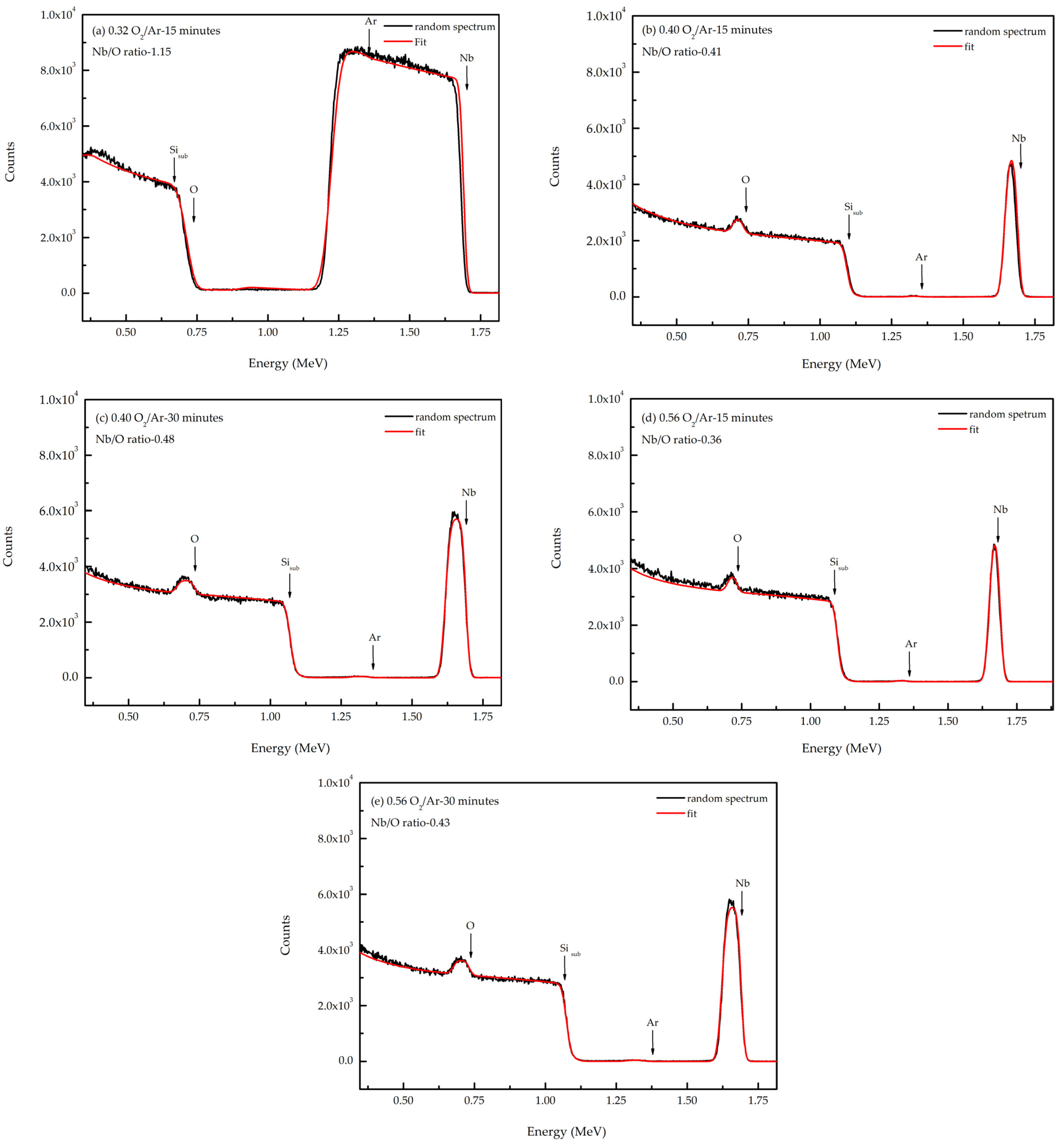
3.1.2. Composition of the Films
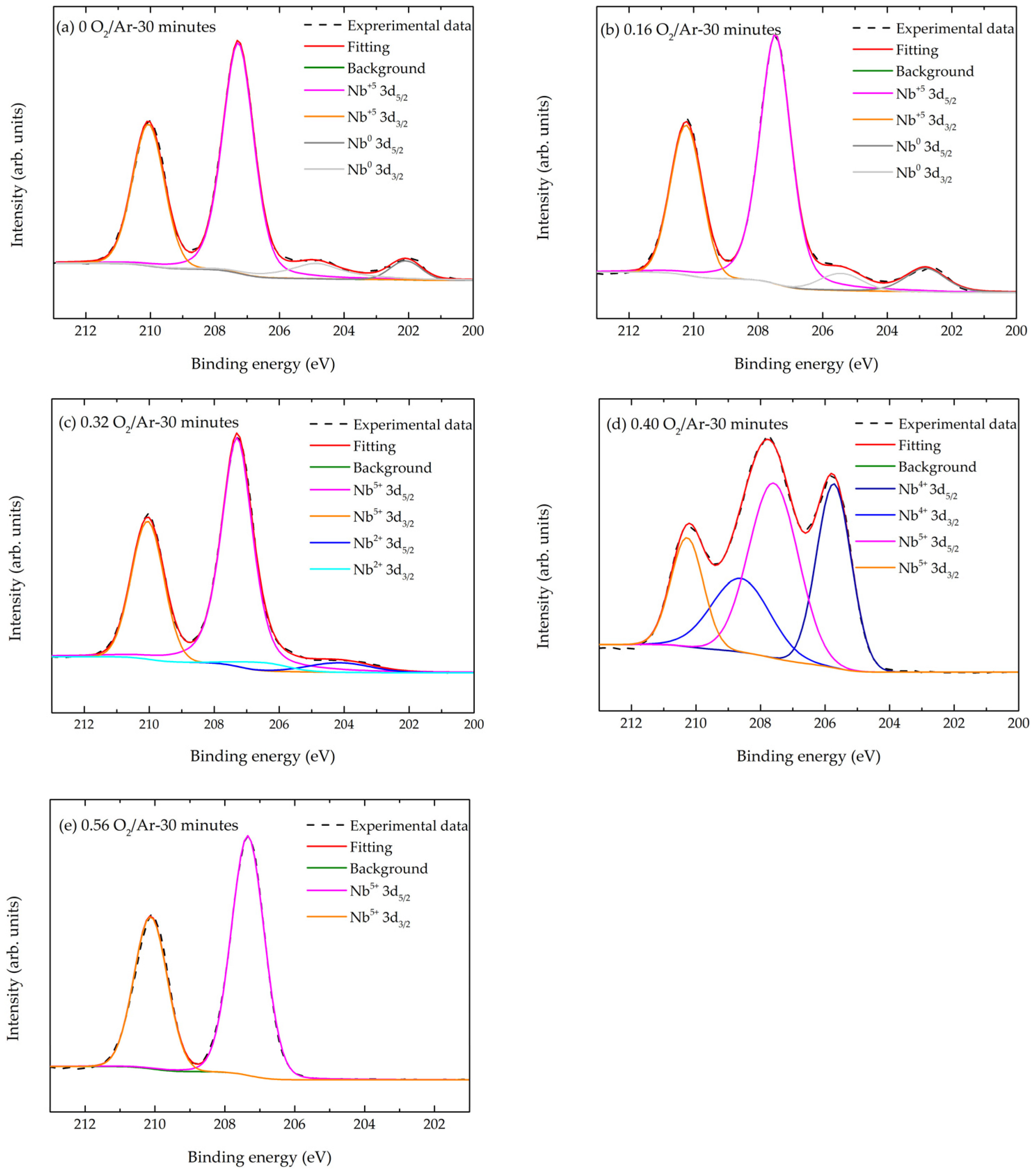
3.1.3. Surface Chemistry Analyses
3.1.4. Raman Analyses
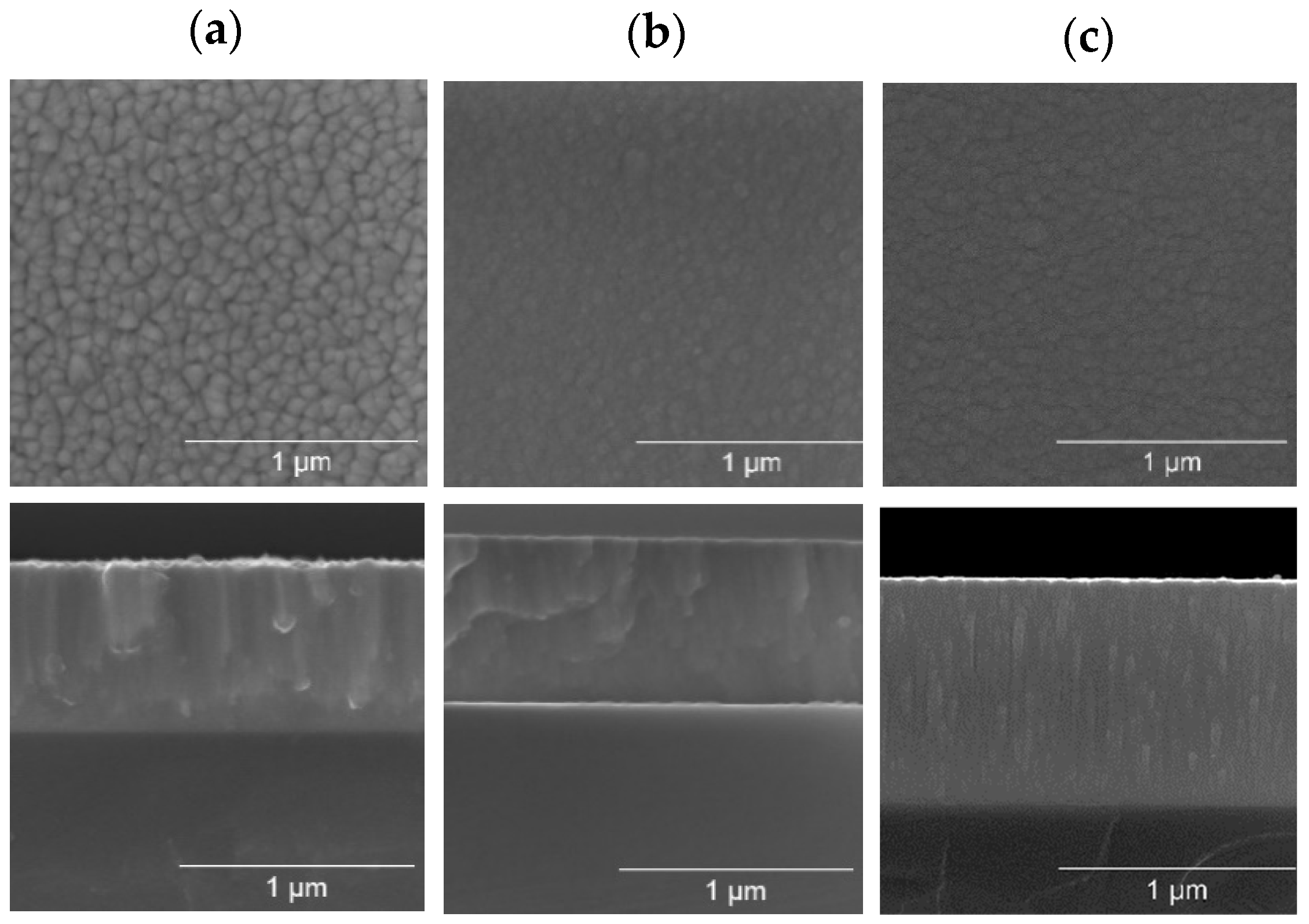
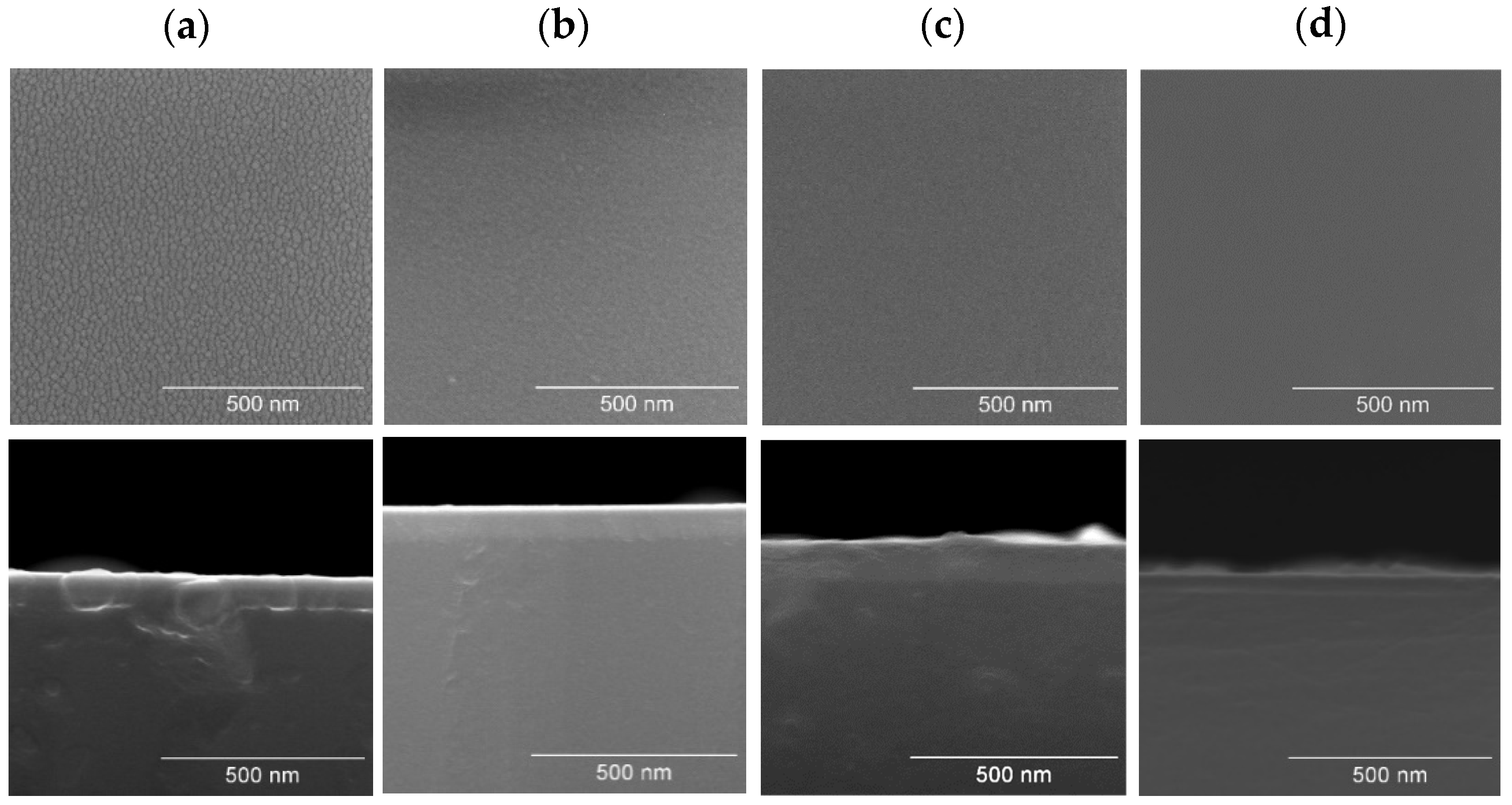
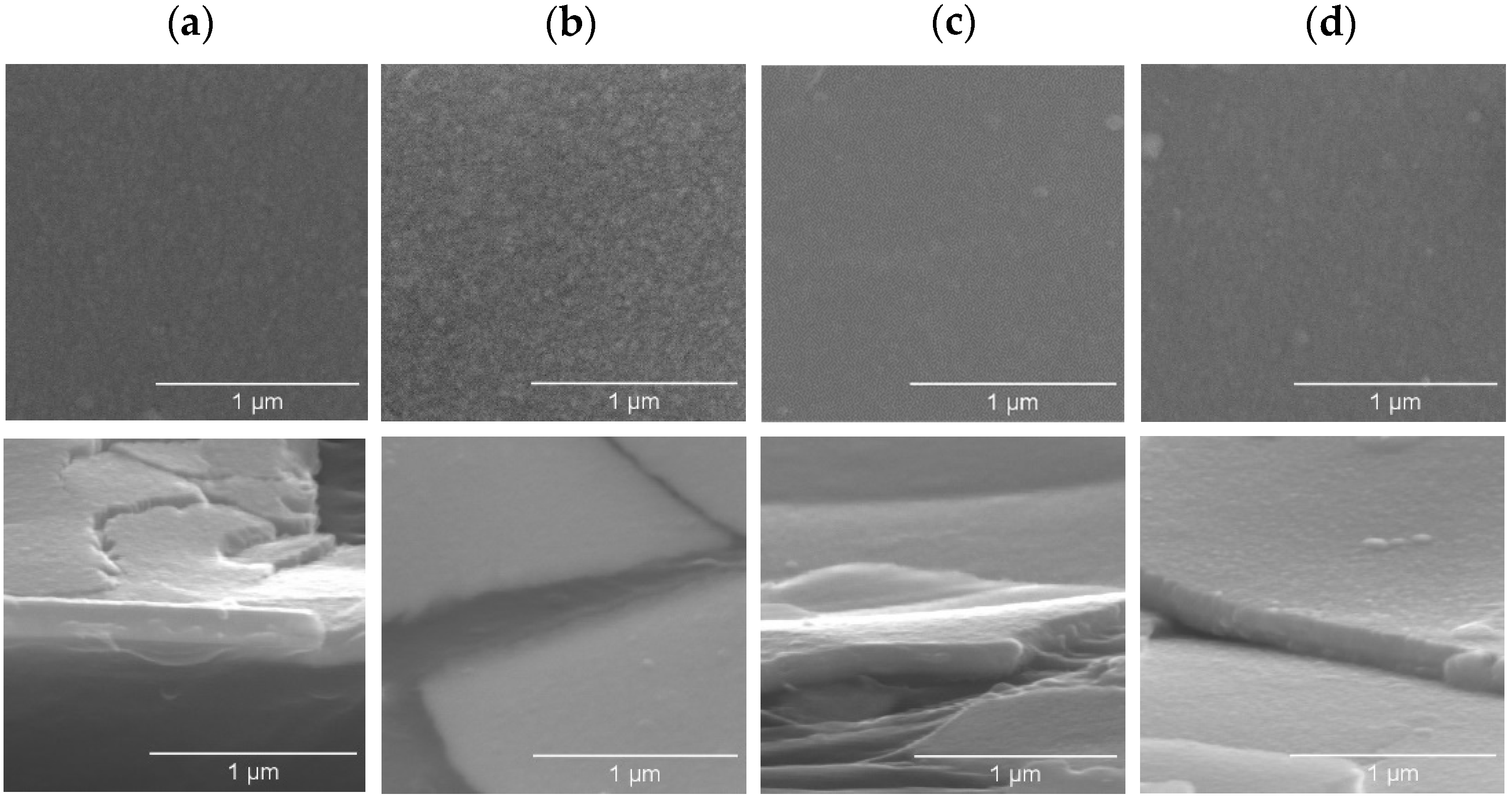
3.1.5. Morphological and Thin Growth Features
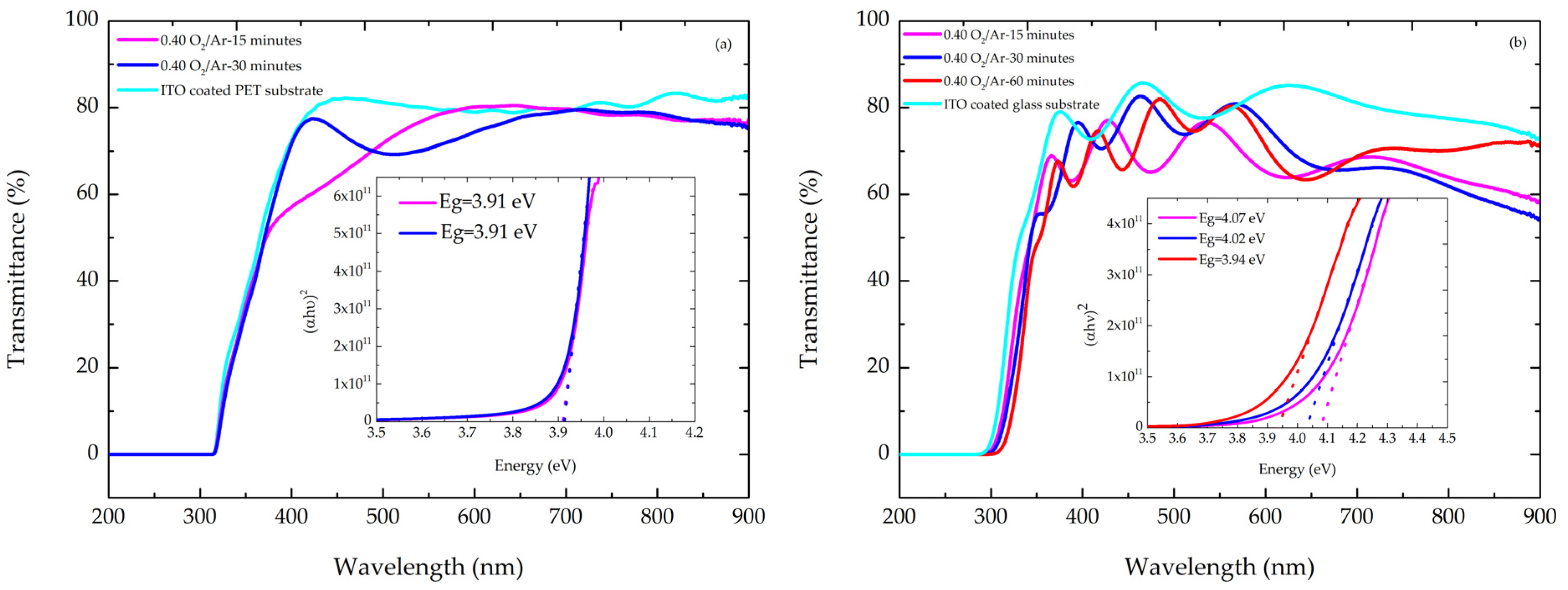
3.2. Optical Response of Thin Films
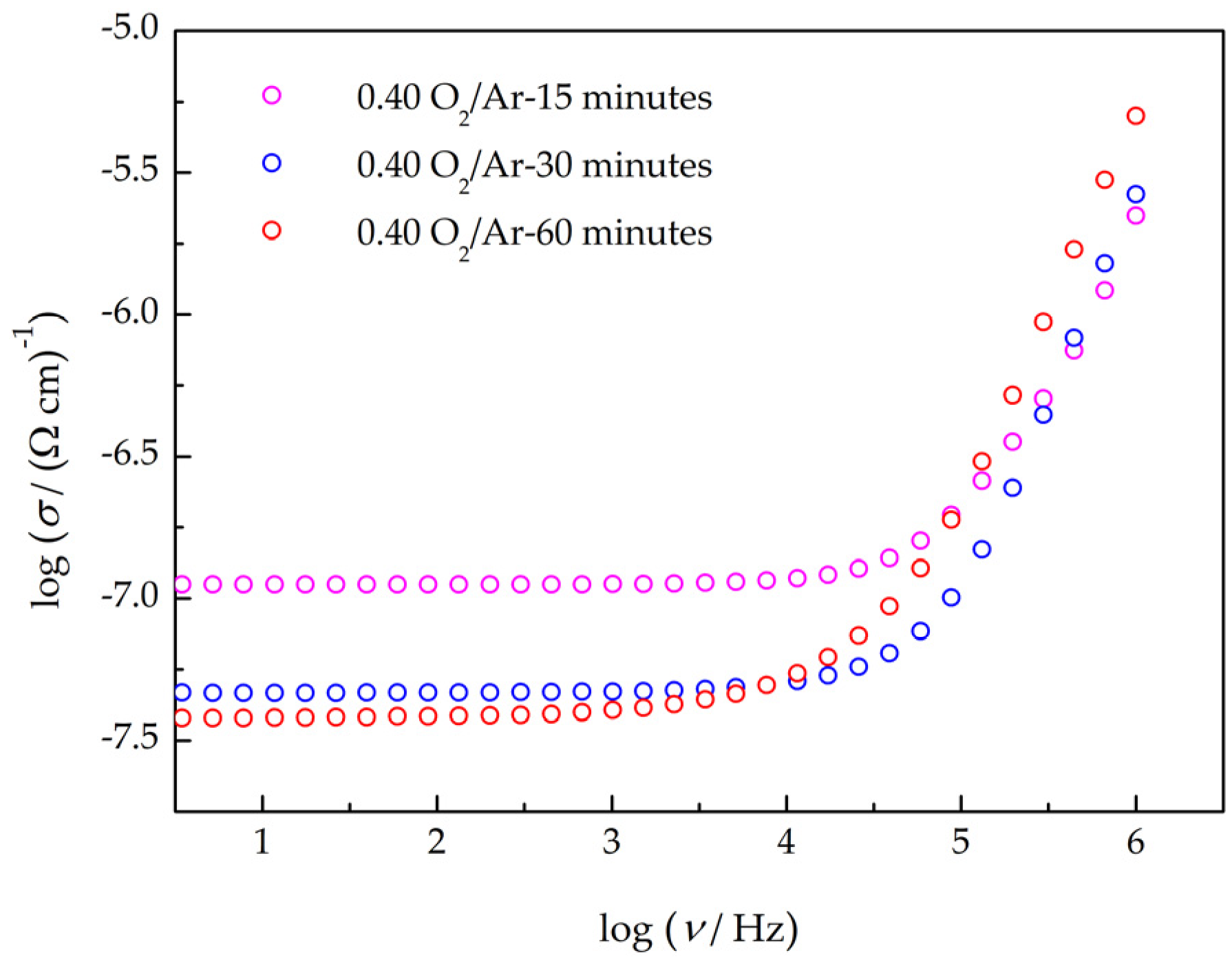
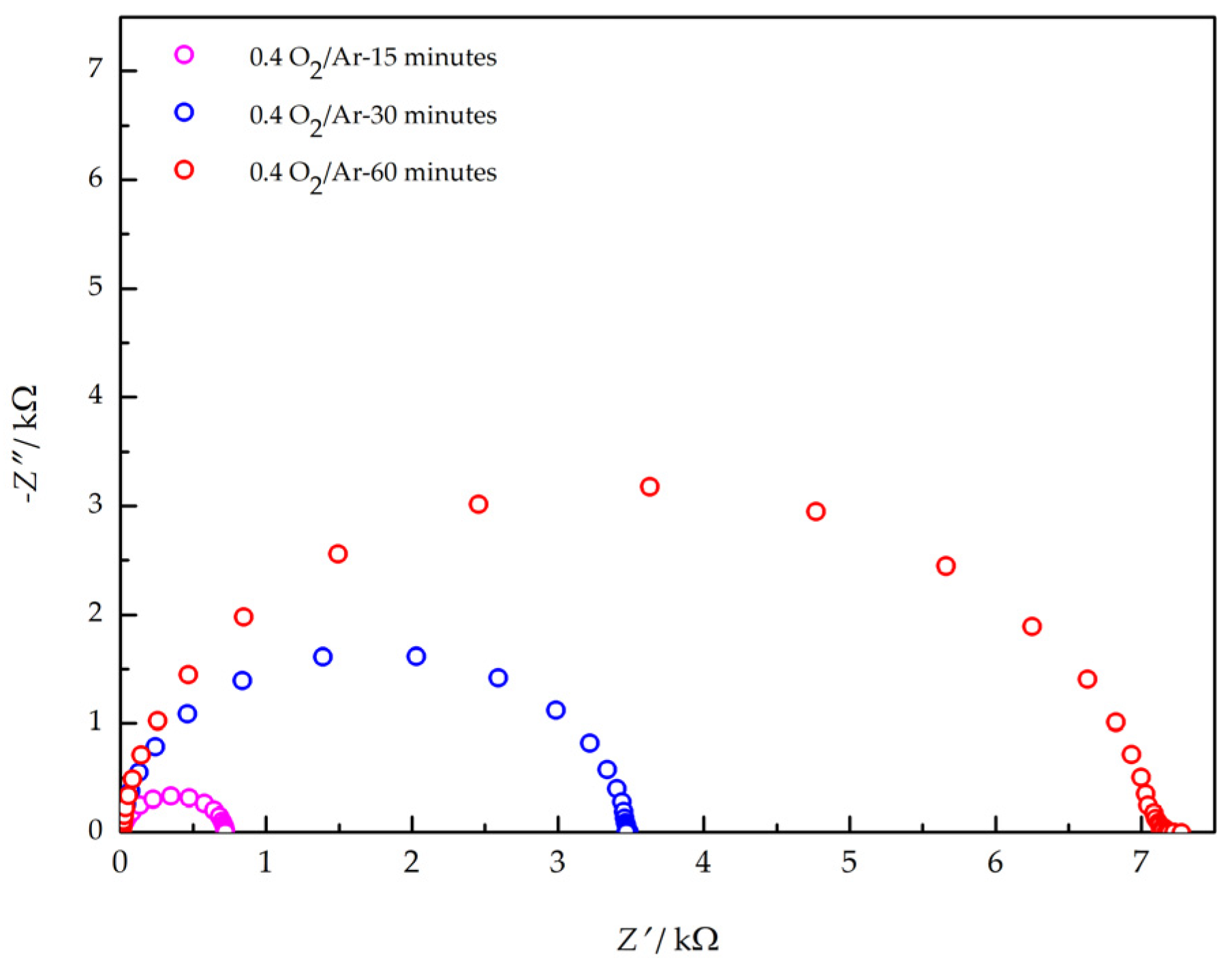
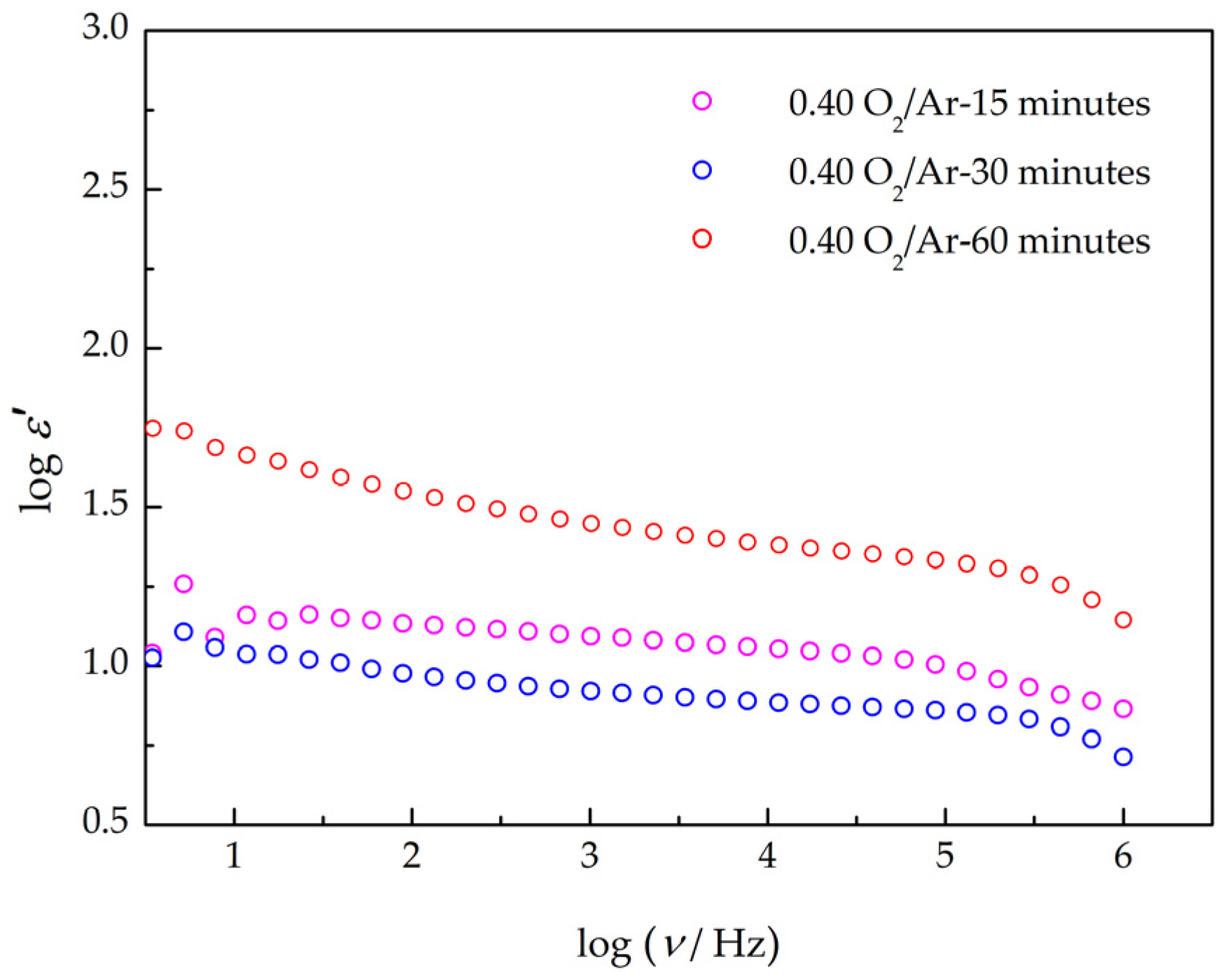
3.3. Electrical Characterization
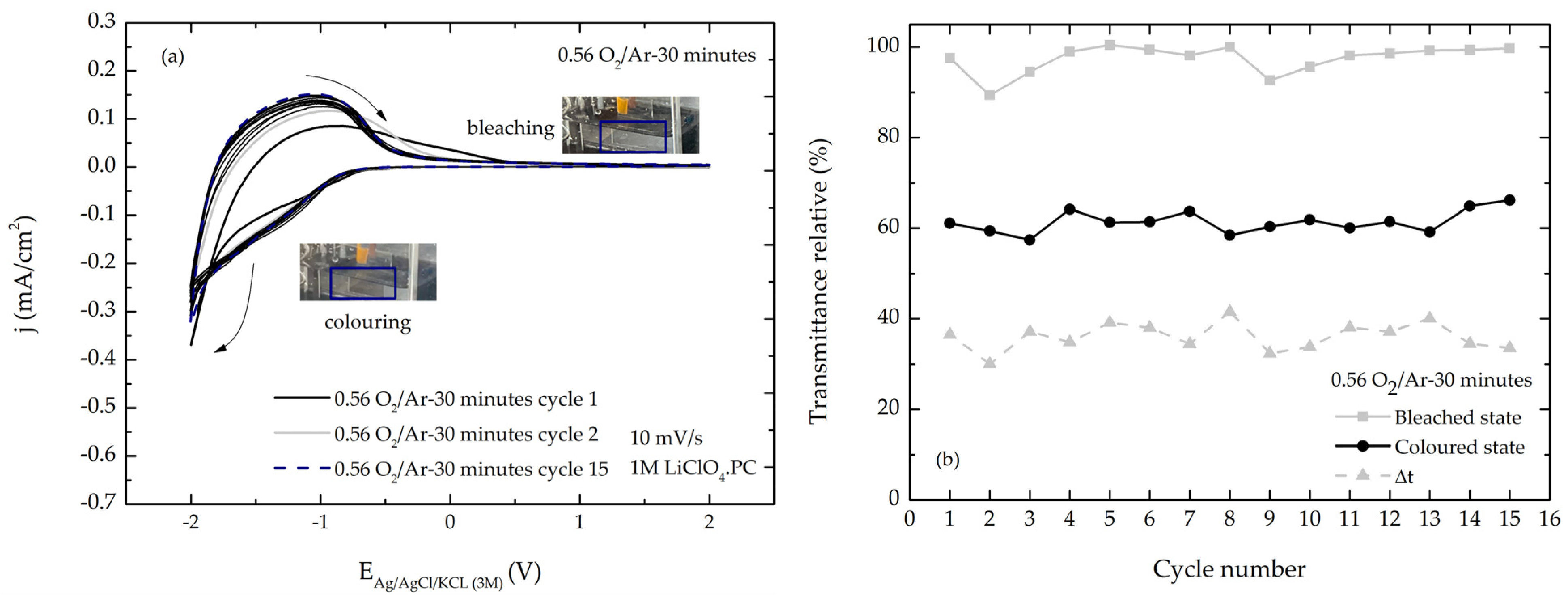
3.4. Electrochromic Characterization
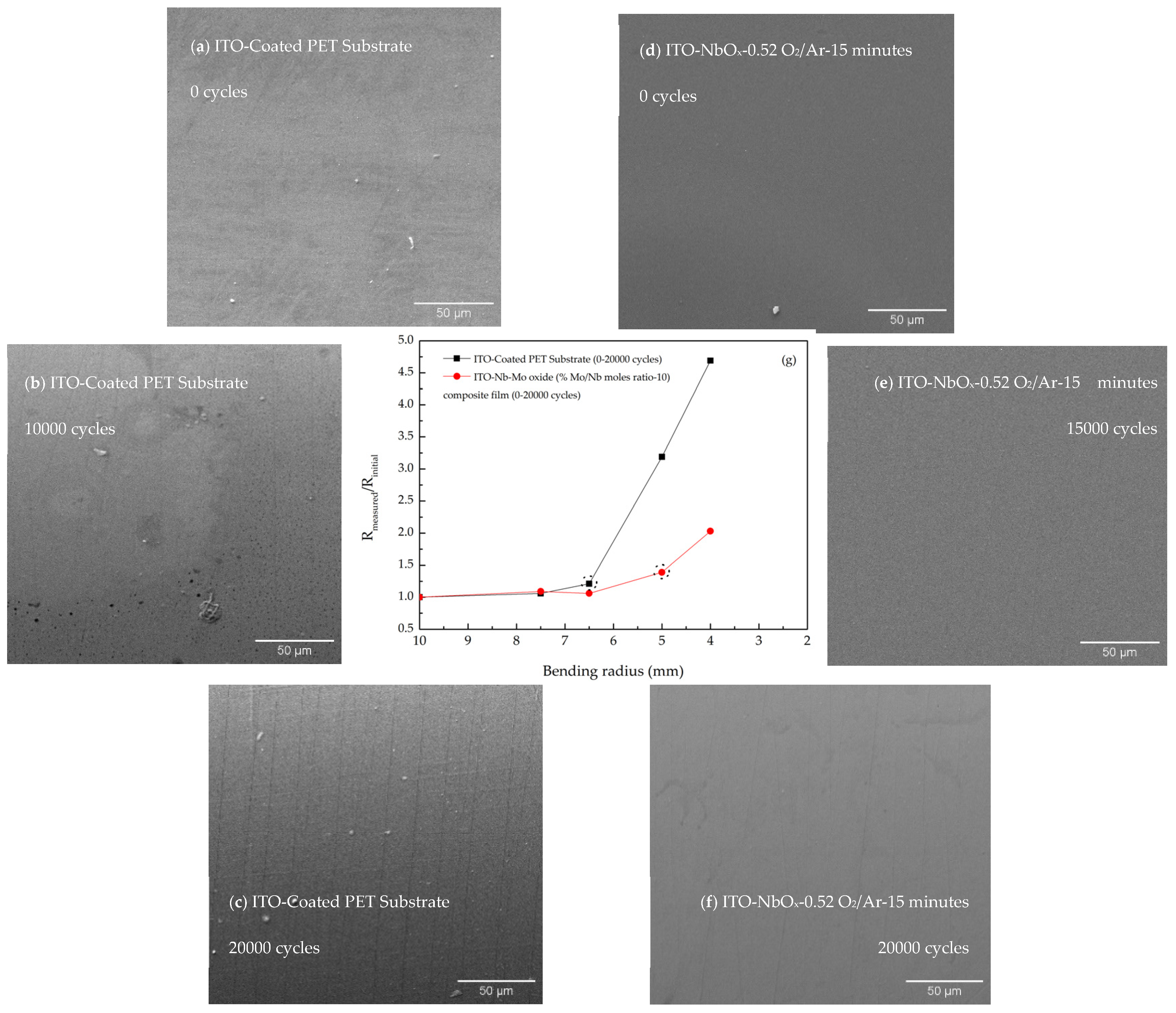
3.5. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norman, N. Metallic oxide phases of niobium and tantalum. J. Less Common Met. 1962, 4, 52–61. [Google Scholar] [CrossRef]
- Andersson, S.; Wadsley, A.D. Crystallographic shear and diffusion paths in certain higher oxides of niobium, tungsten, molybdenum and titanium. Nature 1966, 211, 581–583. [Google Scholar] [CrossRef]
- Kimura, S. Phase Equilibria in the System NbO2-Nb2O5: Phase Relations at 1300 and 1400 °C and Related Thermodynamic Treatment. J. Solid State Chem. 1973, 6, 438–449. [Google Scholar] [CrossRef]
- Gatehouse, B.M.; Wadsley, A.D. The Crystal Structure of the High Temperature form of Niobium Pentoxide. Acta Cryst. 1964, 17, 1545. [Google Scholar] [CrossRef]
- Lindau, I.; Spicer, W.E. Oxidation of Nb as studied by the uv-photoemission technique. J. Appl. Phys. 1974, 45, 3720–3725. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Le Viet, A.; Reddy, M.V.; Jose, R.; Chowdari, B.V.R. Nanostructured Nb2O5 polymorphs by electrospinning for rechargeable lithium batteries. J. Phys. Chem. C 2010, 114, 664–671. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graça, M.P.F. Niobium oxides and niobates physical properties: Review and prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Hulm, J.K.; Jones, C.K.; Hein, R.A.; Gibson, J.W. Superconductivity in the TiO and NbO Systems. J. Low Temp. Phys. 1972, 7, 291–307. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Adler, D. Mechanisms for Metal-Nonmetal Transitions in Transition-Metal Oxides and Sulfifes. Rev. Mod. Phys. 1968, 40, 714–736. [Google Scholar] [CrossRef]
- Bach, D. EELS Investigations of Stoichiometric Niobium Oxides and Niobium-Based Capacitors; Karlsruher Institut für Technologie: Karlsruhe, Germany, 2009. [Google Scholar] [CrossRef]
- Mozalev, A.; Vázquez, R.M.; Bittencourt, C.; Cossement, D.; Gispert-Guirado, F.; Llobet, E.; Habazaki, H. Formation-structure-properties of niobium-oxide nanocolumn arrays via self-organized anodization of sputter-deposited aluminum-on-niobium layers. J. Mater. Chem. C Mater. 2014, 2, 4847–4860. [Google Scholar] [CrossRef]
- Venkataraj, S.; Drese, R.; Liesch, C.; Kappertz, O.; Jayavel, R.; Wuttig, M. Temperature stability of sputtered niobium-oxide films. J. Appl. Phys. 2002, 91, 4863–4871. [Google Scholar] [CrossRef]
- Macek, B.O.M. Electrochromism of sol-gel derived niobium oxide films. Sol. Energy Mater. Sol. Cells 1998, 54, 121–130. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Zhao, Y.; Banis, M.N.; Sun, Q.; Adair, K.R.; Li, R.; Sham, T.K.; Sun, X. Atomic Layer Deposition of Lithium Niobium Oxides as Potential Solid-State Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.F.; Sari, H.M.K.; Li, X.; Wang, F.; Zhu, Y.R.; Hu, J.; Zhang, J.; Li, X. A review of niobium oxides based nanocomposites for lithium-ion batteries, sodium-ion batteries and supercapacitors. Nano Energy 2021, 85, 105955. [Google Scholar] [CrossRef]
- Yuan, T.; Soule, L.; Zhao, B.; Zou, J.; Yang, J.; Liu, M.; Zheng, S. Recent Advances in Titanium Niobium Oxide Anodes for High-Power Lithium-Ion Batteries. Energy Fuels 2020, 34, 13321–13334. [Google Scholar] [CrossRef]
- Lira-Cantu, M.; Krebs, F.C. Hybrid solar cells based on MEH-PPV and thin film semiconductor oxides (TiO2, Nb2O5, ZnO, CeO2 and CeO2-TiO2): Performance improvement during long-time irradiation. Sol. Energy Mater. Sol. Cells 2006, 90, 2076–2086. [Google Scholar] [CrossRef]
- Ok, M.R.; Ghosh, R.; Brennaman, M.K.; Lopez, R.; Meyer, T.J.; Samulski, E.T. Surface patterning of mesoporous niobium oxide films for solar energy conversion. ACS Appl. Mater. Interfaces 2013, 5, 3469–3474. [Google Scholar] [CrossRef]
- Lira-Cantu, M.; Norrman, K.; Andreasen, J.W.; Krebs, F.C. Oxygen release and exchange in niobium oxide MEHPPV hybrid solar cells. Chem. Mater. 2006, 18, 5684–5690. [Google Scholar] [CrossRef]
- Lim, E.; Kim, H.; Jo, C.; Chun, J.; Ku, K.; Kim, S.; Lee, H.I.; Nam, I.S.; Yoon, S.; Kang, K.; et al. Advanced hybrid supercapacitor based on a mesoporous niobium pentoxide/carbon as high-performance anode. ACS Nano 2014, 8, 8968–8978. [Google Scholar] [CrossRef]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef]
- Onozato, T.; Katase, T.; Yamamoto, A.; Katayama, S.; Matsushima, K.; Itagaki, N.; Yoshida, H.; Ohta, H. Optoelectronic properties of valence-state-controlled amorphous niobium oxide. J. Phys. Condens. Matter 2016, 28, 255001. [Google Scholar] [CrossRef] [PubMed]
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef]
- Ushikubo, T. Recent topics of research and development of catalysis by niobium and tantalum oxides. Catal. Today 2000, 57, 331–338. [Google Scholar] [CrossRef]
- Wachs, I.E.; Jehng, J.-M.; Deo, G.; Hu, H.; Arora, N. Redox properties of niobium oxide catalysts. Catal. Today 1996, 28, 199–205. [Google Scholar] [CrossRef]
- Gimon-Kinsel, M.E.; Balkus, K.J. Pulsed laser deposition of mesoporous niobium oxide thin films and application as chemical sensors. Microporous Mesoporous Mater. 1999, 28, 113–123. [Google Scholar] [CrossRef]
- Kurioka, N.; Watanabe, D.; Haneda, M.; Shimanouchi, T.; Mizushima, T.; Kakuta, N.; Ueno, A.; Hanaoka, T.; Sugi, Y. Preparation of Niobium Oxide Films as a Humidity Sensor. Catal. Today 1993, 16, 495–501. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Visai, L.; Khalil-Allafi, J. Progress in Niobium Oxide-Containing Coatings for Biomedical Applications: A Critical Review. ACS Omega 2022, 7, 9088–9107. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Olaya, J.J.; Ramírez, C.; Rodil, S.E. Biocompatibility of niobium coatings. Coatings 2011, 1, 72–87. [Google Scholar] [CrossRef]
- Mjejri, I.; Grocassan, R.; Rougier, A. Enhanced Coloration for Hybrid Niobium-Based Electrochromic Devices. ACS Appl. Energy Mater. 2018, 1, 4359–4366. [Google Scholar] [CrossRef]
- Reichman, B.; Bard, A.J. Electrochromism at Niobium Pentoxide Electrodes in Aqueous and Acetonitrile Solutions. J. Electrochem. Soc. 1980, 127, 241. [Google Scholar] [CrossRef]
- Yoshimura, K.; Miki, T.; Tanemura, S. Niobium Oxide Electrochromic Thin Films Prepared by Reactive DC Magnetron Sputtering. Jpn. J. Appl. Phys. 1995, 34, L1293. [Google Scholar] [CrossRef]
- Savinell, R.F.; Miki, T.; Tanemura, S. Electrochromic Properties of Niobium Oxide Thin Films Prepared by DC Magnetron Sputtering; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Orel, B. In Situ UV-Vis and Ex Situ IR spectroelectrochemical investigations of amorphous and crystalline electrochromic Nb2O5 films in charged/discharged states. J. Solid State Electrochem. 1998, 2, 221–236. [Google Scholar] [CrossRef]
- Marciel, A.; Graça, M.; Bastos, A.; Pereira, L.; Suresh Kumar, J.; Borges, J.; Vaz, F.; Peres, M.; Magalhães, S.; Lorenz, K.; et al. Molybdenum oxide thin films grown on flexible ito-coated pet substrates. Materials 2021, 14, 821. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Rosseinsky, D.R.; Monk, P.M.S. Electrochromic Materials and Devices; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials, 1995th ed.; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Zhao, Y.; Zhang, Z.; Lin, Y. Optical and dielectric properties of a nanostructured NbO2 thin film prepared by thermal oxidation. J. Phys. D Appl. Phys. 2004, 37, 3392–3395. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, J. Formation of niobium oxide nanowires by thermal oxidation. J. Ind. Eng. Chem. 2009, 15, 860–864. [Google Scholar] [CrossRef]
- Nico, C.; Rino, L.; Matos, M.; Monteiro, R.; Costa, F.M.; Monteiro, T.; Graça, M.P. NbO/Nb2O5 core-shells by thermal oxidation. J. Eur. Ceram Soc. 2013, 33, 3077–3083. [Google Scholar] [CrossRef]
- Murphy, N.R.; Moreno-Tarango, A.J.; Ramana, C.V.; Sun, L.; Jones, J.G.; Grant, J.T. Hybrid co-deposition of molybdenum doped niobium pentoxide (NbxMoyOz) thin films. J. Alloys Compd. 2016, 681, 350–358. [Google Scholar] [CrossRef]
- Foroughi-Abari, A.; Cadien, K.C. Growth, structure and properties of sputtered niobium oxide thin films. Thin Solid Films 2011, 519, 3068–3073. [Google Scholar] [CrossRef]
- Tanvir, M.T.; Aoki, Y.; Habazaki, H. Formation of porous niobium films by oblique angle deposition: Influence of substrate morphology. Thin Solid Films 2009, 517, 6711–6716. [Google Scholar] [CrossRef]
- Gao, H.; Wang, S.; Xu, D.; Wang, X.; Zhong, Q.; Zhong, Y.; Li, J.; Cao, W. Study of DC magnetron sputtered Nb films. Crystals 2022, 12, 31. [Google Scholar] [CrossRef]
- Lorenz, R.; O’Sullivan, M.; Fian, A.; Sprenger, D.; Lang, B.; Mitterer, C. Effects of bias pulse frequencies on reactively sputter deposited NbOx films. Thin Solid Films 2018, 660, 335–342. [Google Scholar] [CrossRef]
- Choi, S.; Kong, J.J.; Qin, Q.Z. Electrochemical and Electrochromic Properties of Niobium Oxide Thin Films Fabricated by Pulsed Laser Deposition. J. Electrochem. Soc. 1999, 146, 3914–3918. [Google Scholar]
- Grosse, V.; Pansow, C.; Steppke, A.; Schmidl, F.; Undisz, A.; Rettenmayr, M.; Grib, A.; Seidel, P. Pulsed laser deposition of niobium thin films for in-situ device fabrication and their superconducting properties. J. Phys. Conf. Ser. 2010, 234, 012015. [Google Scholar] [CrossRef]
- Fiz, R.; Appel, L.; Gutiérrez-Pardo, A.; Ramírez-Rico, J.; Mathur, S. Electrochemical Energy Storage Applications of CVD Grown Niobium Oxide Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 21423–21430. [Google Scholar] [CrossRef]
- Maruyama, T.; Kanagawa, T. Electrochromic Properties of Niobium Oxide Thin Films Prepared by Chemical Vapor Deposition. J. Electrochem. Soc. 1994, 141, 2868–2871. [Google Scholar] [CrossRef]
- Dabirian, A.; Kuzminykh, Y.; Wagner, E.; Benvenuti, G.; Rushworth, S.; Hoffmann, P. Evaluation of niobium dimethylamino-ethoxide for chemical vapour deposition of niobium oxide thin films. Thin Solid Films 2014, 571, 94–101. [Google Scholar] [CrossRef]
- Kukli, K.; Ritala, M.; Leskelä, M.; Lappalainen, R. Niobium Oxide Thin Films Grown by Atomic Layer Epitaxy. Chem. Vap. Depos. 1998, 4, 29–34. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.D.; Ahn, J.H.; Park, J.S. Compositional and electrical modulation of niobium oxide thin films deposited by plasma-enhanced atomic layer deposition. Ceram. Int. 2017, 43, 6580–6584. [Google Scholar] [CrossRef]
- Basuvalingam, S.B.; Macco, B.; Knoops, H.C.M.; Melskens, J.; Kessels, W.M.; Bol, A.A. Comparison of thermal and plasma-enhanced atomic layer deposition of niobium oxide thin films. J. Vac. Sci. Technol. A Vac. Surf. Films 2018, 36, 041503. [Google Scholar] [CrossRef]
- Aribia, A.; Sastre, J.; Chen, X.; Gilshtein, E.; Futscher, M.H.; Tiwari, A.N.; Romanyuk, Y.E. In Situ Lithiated ALD Niobium Oxide for Improved Long Term Cycling of Layered Oxide Cathodes: A Thin-Film Model Study. J. Electrochem. Soc. 2021, 168, 040513. [Google Scholar] [CrossRef]
- Leet, G.R.; Crayston, J.A. Studies on the electrochemical deposition of niobium oxide. J. Mater. Chem. 1996, 6, 187–192. [Google Scholar]
- Fomanyuk, S.S.; Krasnov, Y.S.; Kolbasov, G.Y.; Zaichenko, V.N. Electrochemical deposition of electrochromic niobium oxide films from an acidic solution of niobium peroxo complexes. Russ. J. Appl. Chem. 2013, 86, 644–647. [Google Scholar] [CrossRef]
- Mujawar, S.H.; Inamdar, A.I.; Patil, S.B.; Patil, P.S. Electrochromic properties of spray-deposited niobium oxide thin films. Solid State Ion. 2006, 177, 3333–3338. [Google Scholar] [CrossRef]
- Mujawar, S.H.; Inamdar, A.I.; Betty, C.A.; Ganesan, V.; Patil, P.S. Effect of post annealing treatment on electrochromic properties of spray deposited niobium oxide thin films. Electrochim. Acta 2007, 52, 4899–4906. [Google Scholar] [CrossRef]
- Romero, R.; Dalchiele, E.A.; Martín, F.; Leinen, D.; Ramos-Barrado, J.R. Electrochromic behaviour of Nb2O5 thin films with different morphologies obtained by spray pyrolysis. Sol. Energy Mater. Sol. Cells 2009, 93, 222–229. [Google Scholar] [CrossRef]
- Özer, N.; Chen, D.G.; Lampert, C.M. Preparation and properties of spin-coated Nb2O5 films by the sol-gel process for electrochromic applications. Thin Solid Films 1996, 277, 162–168. [Google Scholar] [CrossRef]
- Schmitt, M.; Heusing, S.; Aegerter, M.A.; Pawlicka, A.; Avellaneda, C. Electrochromic properties of Nb2O5 sol-gel coatings. Sol. Energy Mater. Sol. Cells 1998, 54, 9–17. [Google Scholar] [CrossRef]
- Özer, N.; Rubin, M.D.; Lampert, C.M. Optical and electrochemical characteristics of niobium oxide films prepared by sol-gel process and magnetron sputtering: A comparison. Sol. Energy Mater. Sol. Cells 1996, 40, 285–296. [Google Scholar] [CrossRef]
- Andoni, I.; Ziegler, J.M.; Jha, G.; Gadre, C.A.; Flores-Zuleta, H.; Dai, S.; Qiao, S.; Xu, M.; Chen, V.T.; Pan, X.; et al. Investigating the Degradation of Nb2O5 Thin Films across 10,000 Lithiation/Delithiation Cycles. ACS Appl. Energy Mater. 2021, 4, 6542–6552. [Google Scholar] [CrossRef]
- Granqvist, C.G. Recent progress in thermochromics and electrochromics: A brief survey. Thin Solid Films 2016, 614, 90–96. [Google Scholar] [CrossRef]
- Guo, J.; Liang, Y.; Zhang, S.; Ma, D.; Yang, T.; Zhang, W.; Li, H.; Cao, S.; Zou, B. Recent progress in improving strategies of metal oxide-based electrochromic smart window. Green Energy Resour. 2023, 1, 100007. [Google Scholar] [CrossRef]
- Rai, V.; Singh, R.S.; Blackwood, D.J.; Zhili, D. A Review on Recent Advances in Electrochromic Devices: A Material Approach. Adv. Eng. Mater. 2020, 22, 2000082. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Arvizu, M.A.; Pehlivan, I.B.; Qu, H.Y.; Wen, R.T.; Niklasson, G.A. Electrochromic materials and devices for energy efficiency and human comfort in buildings: A critical review. Electrochim. Acta 2017, 259, 1170–1182. [Google Scholar] [CrossRef]
- Livage, J.; Ganguli, D. Sol-gel electrochromic coatings and devices: A review. Sol. Energy Mater. Sol. Cells 2001, 68, 365–381. [Google Scholar] [CrossRef]
- Granqvist, C. Electrochromic Materials Out of a niche. Nat. Mater. 2006, 5, 89–90. [Google Scholar] [CrossRef]
- Granqvist, C.G. Transparent Conductive Electrodes for Electrochromic Devices: A Review. Appl. Phys. A 1993, 57, 19–24. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Lee, P.S. Next-Generation Multifunctional Electrochromic Devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef]
- Khemasiri, N.; Klamchuen, A.; Jessadaluk, S.; Rattanawarinchai, P.; Borklom, P.; Rangkasikorn, A.; Rahong, S.; Saekung, C.; Horprathum, M.; Chananonnawathorn, C.; et al. Systematic investigations on morphological properties of aluminum-doped zinc oxide transparent electrode prepared from pulsed laser deposition and its electrochromic application. Vacuum 2023, 209, 111797. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Q.; Dong, G.; He, Y.; Diao, X. Influence of thickness on the structure, electrical, optical and electrochromic properties of AZO thin films and their inorganic all-solid-state devices. Electrochim. Acta 2017, 258, 1336–1347. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Wang, J.; Chen, J.; Magdassi, S.; Lee, P.S. Highly Stable Transparent Conductive Silver Grid/PEDOT:PSS Electrodes for Integrated Bifunctional Flexible Electrochromic Supercapacitors. Adv. Energy Mater. 2016, 6, 1501882. [Google Scholar] [CrossRef]
- Han, J.; Yang, J.; Gao, W.; Bai, H. Ice-Templated, Large-Area Silver Nanowire Pattern for Flexible Transparent Electrode. Adv. Funct. Mater. 2021, 31, 2010155. [Google Scholar] [CrossRef]
- Che, B.; Zhou, D.; Li, H.; He, C.; Liu, E.; Lu, X. A highly bendable transparent electrode for organic electrochromic devices. Org. Electron. 2019, 66, 86–93. [Google Scholar] [CrossRef]
- Shen, K.Y.; Hu, C.W.; Chang, L.C.; Ho, K.C. A complementary electrochromic device based on carbon nanotubes/conducting polymers. Sol. Energy Mater. Sol. Cells 2012, 98, 294–299. [Google Scholar] [CrossRef]
- Costalin, M.; Mjejri, I.; Penin, N.; Viraphong, O.; Shanov, V.; Rougier, A. Films of directionally oriented carbon nanotubes as counter electrodes for electrochromic devices. J. Phys. Chem. Solids 2021, 154, 110035. [Google Scholar] [CrossRef]
- Yanagi, K.; Moriya, R.; Yomogida, Y.; Takenobu, T.; Naitoh, Y.; Ishida, T.; Kataura, H.; Matsuda, K.; Maniwa, Y. Electrochromic carbon electrodes: Controllable visible color changes in metallic single-wall carbon nanotubes. Adv. Mater. 2011, 23, 2811–2814. [Google Scholar] [CrossRef]
- Choi, D.S.; Han, S.H.; Kim, H.; Kang, S.H.; Kim, Y.; Yang, C.M.; Kim, T.Y.; Yoon, D.H.; Yang, W.S. Flexible electrochromic films based on CVD-graphene electrodes. Nanotechnology 2014, 25, 395702. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, L.; Xu, Y.; Qiu, T.; Zhi, L.; Shi, G. Polyaniline electrochromic devices with transparent graphene electrodes. Electrochim. Acta 2009, 55, 491–497. [Google Scholar] [CrossRef]
- Lin, F.; Bult, J.B.; Nanayakkara, S.; Dillon, A.C.; Richards, R.M.; Blackburn, J.L.; Engtrakul, C. Graphene as an efficient interfacial layer for electrochromic devices. ACS Appl. Mater. Interfaces 2015, 7, 11330–11336. [Google Scholar] [CrossRef]
- Ghosh, D.S.; Chen, T.L.; Pruneri, V. High figure-of-merit ultrathin metal transparent electrodes incorporating a conductive grid. Appl. Phys. Lett. 2010, 96, 041109. [Google Scholar] [CrossRef]
- Schneider, J.; Rohner, P.; Thureja, D.; Schmid, M.; Galliker, P.; Poulikakos, D. Electrohydrodynamic NanoDrip Printing of High Aspect Ratio Metal Grid Transparent Electrodes. Adv. Funct. Mater. 2016, 26, 833–840. [Google Scholar] [CrossRef]
- Bueno, P.R.; Faira, R.C.; Bulhões, L.O.S. EQCM study during lithium insertion/deinsertion processes in Nb2O5 films prepared by the polymeric precursor method. Solid State Ion. 2005, 176, 1175–1180. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic tungsten oxide films: Review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Avendaño, E.; Azens, A. Electrochromic coatings and devices: Survey of some recent advances. Thin Solid Films 2003, 442, 201–211. [Google Scholar] [CrossRef]
- Dong, D.; Dhanabalan, S.S. Peter Francis Mathew Elango, Mingjie Yang, Sumeet Walia, Sharath Sriram, and Madhu Bhaskaran, “Emerging applications of metal-oxide thin films for flexible and stretchable electronic devices”. Appl. Phys. Rev. 2023, 10, 031314. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Jung, Y.H.; Park, J.; Kang, Y.-C.; Kim, Y.I. Electrochromic Performance of NiOx Thin Film on Flexible PET/ITO Prepared by Nanocrystallite-Dispersion Sol. J. Korean Chem. Soc. 2021, 65, 125–132. [Google Scholar]
- Wang, H.; Barrett, M.; Duane, B.; Gu, J.; Zenhausern, F. Materials and processing of polymer-based electrochromic devices. Mater. Sci. Eng. B 2018, 228, 167–174. [Google Scholar] [CrossRef]
- Jensen, J.; Hösel, M.; Dyer, A.L.; Krebs, F.C. Development and manufacture of polymer-based electrochromic devices. Adv. Funct. Mater. 2015, 25, 2073–2090. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Y.M.; Cai, Y.; Yang, B.; Gu, C.; Zhang, S.X.A. Advances in nanomaterials for electrochromic devices. Chem. Soc. Rev. 2020, 49, 8687–8720. [Google Scholar] [CrossRef]
- Runnerstrom, E.L.; Llordés, A.; Lounis, S.D.; Milliron, D.J. Nanostructured electrochromic smart windows: Traditional materials and NIR-selective plasmonic nanocrystals. Chem. Commun. 2014, 50, 10555–10572. [Google Scholar] [CrossRef]
- Thakur, V.K.; Ding, G.; Ma, J.; Lee, P.S.; Lu, X. Hybrid materials and polymer electrolytes for electrochromic device applications. Adv. Mater. 2012, 24, 4071–4096. [Google Scholar] [CrossRef]
- Goodenough, J.B. Ceramic solid electrolytes. Solid State Ion. 1997, 94, 17–25. [Google Scholar] [CrossRef]
- Ito, Y.; Nohira, T. Non-Conventional Electrolytes for Electrochemical Applications. Electrochim. Acta 2000, 45, 2611–2622. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, R.; Chen, C.; Yee, W.A.; Kong, J.; Ding, G.; Lu, X. Non-volatile polymer electrolyte based on poly(propylene carbonate), Ionic liquid, and lithium perchlorate for electrochromic devices. J. Phys. Chem. B 2013, 117, 7783–7789. [Google Scholar] [CrossRef]
- Hallinan, D.T.; Balsara, N.P. Polymer electrolytes. Annu. Rev. Mater. Res. 2013, 43, 503–525. [Google Scholar] [CrossRef]
- Di Noto, V.; Lavina, S.; Giffin, G.A.; Negro, E.; Scrosati, B. Polymer electrolytes: Present, past and future. Electrochim. Acta 2011, 57, 4–13. [Google Scholar] [CrossRef]
- Ruan, Q.; Yao, M.; Yuan, D.; Dong, H.; Liu, J.; Yuan, X.; Fang, W.; Zhao, G.; Zhang, H. Ionic liquid crystal electrolytes: Fundamental, applications and prospects. Nano Energy 2023, 106, 108087. [Google Scholar] [CrossRef]
- Chai, S.; Xu, F.; Zhang, R.; Wang, X.; Zhai, L.; Li, X.; Qian, H.-J.; Wu, L.; Li, H. Hybrid Liquid-Crystalline Electrolytes with High-Temperature-Stable Channels for Anhydrous Proton Conduction. J. Am. Chem. Soc. 2021, 143, 21433–21442. [Google Scholar] [CrossRef]
- Eftekhari, A.; Kim, D.W. Sodium-ion batteries: New opportunities beyond energy storage by lithium. J. Power Sources 2018, 395, 336–348. [Google Scholar] [CrossRef]
- Fu, J.; Li, Z.; Zhou, X.; Guo, X. Ion transport in composite polymer electrolytes. Mater. Adv. 2022, 3, 3809–3819. [Google Scholar] [CrossRef]
- Huang, J.; Wu, K.; Xu, G.; Wu, M.; Dou, S.; Wu, C. Recent progress and strategic perspectives of inorganic solid electrolytes: Fundamentals, modifications, and applications in sodium metal batteries. Chem. Soc. Rev. 2023, 52, 4933–4995. [Google Scholar] [CrossRef]
- He, J.; You, L.; Tran, D.T.; Mei, J. Low-Temperature Thermally Annealed Niobium Oxide Thin Films as a Minimally Color Changing Ion Storage Layer in Solution-Processed Polymer Electrochromic Devices. ACS Appl. Mater. Interfaces 2019, 11, 4169–4177. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Chen, K.; Zemlyanov, D.Y.; You, L.; Mei, J. Stabilizing Hybrid Electrochromic Devices through Pairing Electrochromic Polymers with Minimally Color-Changing Ion-Storage Materials Having Closely Matched Electroactive Voltage Windows. ACS Appl. Mater. Interfaces 2021, 13, 5312–5318. [Google Scholar] [CrossRef]
- He, J.; Mukherjee, S.; Zhu, X.; You, L.; Boudouris, B.W.; Mei, J. Highly Transparent Crosslinkable Radical Copolymer Thin Film as the Ion Storage Layer in Organic Electrochromic Devices. ACS Appl. Mater. Interfaces 2018, 10, 18956–18963. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Zhang, Y.; Wei, Z. Integrated energy storage and electrochromic function in one flexible device: An energy storage smart window. Energy Env. Sci. 2012, 5, 8384–8389. [Google Scholar] [CrossRef]
- Nuroldayeva, G.; Balanay, M.P. Flexing the Spectrum: Advancements and Prospects of Flexible Electrochromic Materials. Polymers 2023, 15, 2924. [Google Scholar] [CrossRef]
- Hui, Z.; Zhang, L.; Ren, G.; Sun, G.; Yu, H.D.; Huang, W. Green Flexible Electronics: Natural Materials, Fabrication, and Applications. Adv. Mater. 2023, 35, 2211202. [Google Scholar] [CrossRef]
- Li, W.; Bai, T.; Fu, G.; Zhang, Q.; Liu, J.; Wang, H.; Sun, Y.; Yan, H. Progress and challenges in flexible electrochromic devices. Sol. Energy Mater. Sol. Cells 2022, 240, 111709. [Google Scholar] [CrossRef]
- Fang, H.; Zhao, Z.; Wu, W.; Wang, H. Progress in Flexible Electrochromic Devices. Wuji Cailiao Xuebao/J. Inorg. Mater. 2021, 36, 140–151. [Google Scholar] [CrossRef]
- Yun, T.G.; Park, M.; Kim, D.-H.; Kim, D.; Cheong, J.Y.; Bae, J.G.; Han, S.M.; Kim, I.-D. All-Transparent Stretchable Electrochromic Supercapacitor Wearable Patch Device. ACS Nano 2019, 13, 3141–3150. [Google Scholar] [CrossRef]
- Li, X.; Cai, K.; Gao, M.; Du, Y.; Shen, S. Recent advances in flexible thermoelectric films and devices. Nano Energy 2021, 89, 106309. [Google Scholar] [CrossRef]
- Nandihalli, N. Thermoelectric films and periodic structures and spin Seebeck effect systems: Facets of performance optimization. Mater. Today Energy 2022, 25, 100965. [Google Scholar] [CrossRef]
- Domingues, R.P.; Rodrigues, M.S.; Lopes, C.; Pedrosa, P.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Thin films composed of metal nanoparticles (Au, Ag, Cu) dispersed in AlN: The influence of composition and thermal annealing on the structure and plasmonic response. Thin Solid Films 2019, 676, 12–25. [Google Scholar] [CrossRef]
- Barradas, N.P.; Jeynes, C.; Webb, R.P. Simulated annealing analysis of Rutherford backscattering data. Appl. Phys. Lett. 1997, 71, 291–293. [Google Scholar] [CrossRef]
- Barradas, N.P.; Jeynes, C. Advanced physics and algorithms in the IBA DataFurnace. Nucl. Instrum. Methods Phys. Res. B 2008, 266, 1875–1879. [Google Scholar] [CrossRef]
- Grego, S.; Lewis, J.; Vick, E.; Temple, D. Development and evaluation of bend-testing techniques for flexible-display applications. J. Soc. Inf. Disp. 2005, 13, 575. [Google Scholar] [CrossRef]
- Li, X.; Cao, W.H.; Tao, X.F.; Ren, L.L.; Zhou, L.Q.; Xu, G.F. Structural and nanomechanical characterization of niobium films deposited by DC magnetron sputtering. Appl. Phys. A. Mater. Sci. Process 2016, 122, 1–6. [Google Scholar] [CrossRef]
- Kumar, T.S.; Kumar, S.R.; Rao, M.L.; Prakash, T.L. Preparation of Niobium Metal Powder by Two-Stage Magnesium Vapor Reduction of Niobium Pentoxide. J. Metall. 2013, 2013, 629341. [Google Scholar] [CrossRef]
- Halbritter, J. On the Oxidation and on the Superconductivity of Niobium. Appl. Phys. A 1987, 43, 1–28. [Google Scholar] [CrossRef]
- Coşkun, Ö.D.; Demirel, S. The optical and structural properties of amorphous Nb2O5 thin films prepared by RF magnetron sputtering. Appl. Surf. Sci. 2013, 277, 35–39. [Google Scholar] [CrossRef]
- Lee, C.-C.; Tien, C.-L.; Hsu, J.-C. Internal stress and optical properties of Nb2O5 thin films deposited by ion-beam sputtering. Appl. Opt. 2002, 41, 2043–2047. [Google Scholar] [CrossRef]
- Ye, R.; Ohta, K.; Baba, M. Electrochemical Properties of Amorphous Nb2O5 Thin Film and Its Application to Rechargeable Thin Film Lithium Ion Batteries. ECS Trans. 2016, 73, 49–55. [Google Scholar] [CrossRef]
- Coşkun, Ö.D.; Demirel, S.; Atak, G. The effects of heat treatment on optical, structural, electrochromic and bonding properties of Nb2O5 thin films. J. Alloys Compd. 2015, 648, 994–1004. [Google Scholar] [CrossRef]
- Karulkar, P.C.; Nordman, J.E. STUDY OF THIN Nb OXIDE FILMS. J. Vac. Sci. Technol. 1979, 17, 462–465. [Google Scholar] [CrossRef]
- King, B.R.; Patel, H.C.; Gulino, D.A.; Tatarchuk, B.J. Kinetic measurements of oxygen dissolution into niobium substrates: In situ x-ray photoelectron spectroscopy studies. Thin Solid Films 1990, 192, 351–369. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E.; Spectroscopy, H.O.X.-R.P. Eden Prairie, 1979th ed.; Perkin-Elmer Corporation: Waltham, MA, USA, 1979. [Google Scholar]
- Bharti, D.C.; Rhee, S.W. Dielectric properties and X-ray photoelectron spectroscopic studies of niobium oxide thin films prepared by direct liquid injection chemical vapor deposition method. Thin Solid Films 2013, 548, 195–201. [Google Scholar] [CrossRef]
- Darlinski, A.; Halbritter, J. Angle-resolved XPS Studies of Oxides at NbN, NbC, and Nb Surfaces. Surf. Interface Anal. 1987, 10, 223–237. [Google Scholar] [CrossRef]
- McConnell, A.A. Raman spectra of niobium oxides. Spectrochim. Acts 1976, 32, 1067–1076. [Google Scholar] [CrossRef]
- Jehng, J.-M.; Wachs, I.E. Niobium Oxide Solution Chemistry. J. Raman Spectrosc. 1991, 22, 83–89. [Google Scholar] [CrossRef]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun. Chem. 2019, 2, 129. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Saraiva, M.; Freire, F.N.A.; Valente, M.A.; Costa, L.C. Electrical analysis of niobium oxide thin films. Thin Solid Films 2015, 585, 95–99. [Google Scholar] [CrossRef]
- Jehng, J.-M.; Wachs, I.E. Structural Chemistry and Raman Spectra of Niobium Oxides. Am. Chem. Soc. 1991, 3, 100–107. [Google Scholar] [CrossRef]
- Goncharov, A.F.; Struzhkin, V.V. Raman spectroscopy of metals, high-temperature superconductors and related materials under high pressure. J. Raman Spectrosc. 2003, 34, 532–548. [Google Scholar] [CrossRef]
- Usha, N.; Sivakumar, R.; Sanjeeviraja, C.; Arivanandhan, M. Niobium pentoxide (Nb2O5) thin films: Rf Power and substrate temperature induced changes in physical properties. Optik 2015, 126, 1945–1950. [Google Scholar] [CrossRef]
- Chen, K.N.; Hsu, C.M.; Liu, J.; Liou, Y.C.; Yang, C.F. Investigation of antireflection Nb2O5 thin films by the sputtering method under different deposition parameters. Micromachines 2016, 7, 151. [Google Scholar] [CrossRef]
- Al-Baradi, A.M.; El-Nahass, M.M.; Hassanien, A.M.; Atta, A.A.; Alqahtani, M.S.; Aldawsari, A.O. Influence of RF sputtering power on structural and optical properties of Nb2O5 thin films. Optik 2018, 168, 853–863. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Lai, F.; Lin, L.; Huang, Z.; Gai, R.; Qu, Y. Effect of thickness on the structure, morphology and optical properties of sputter deposited Nb2O5 films. Appl. Surf. Sci. 2006, 253, 1801–1805. [Google Scholar] [CrossRef]
- Valdest, L.B. Resistivity Measurements on Germanium for Transistors. Proc. IRE 1954, 42, 420–427. [Google Scholar] [CrossRef]
- Smits, F.M. Measurement the of Sheet Resistivities Four-Point Probe. Bell Syst. Tech. J. 1958, 37, 711–718. [Google Scholar] [CrossRef]
- Sosniak, J. The deposition of niobium thin films by dc diode and substrate bias sputtering. J. Appl. Phys. 1968, 39, 4157–4163. [Google Scholar] [CrossRef]
- Fuschillo, N.; Annamalai, N.K. Dielectric properties of amorphous Nb2O5 thin films. Thin Solid Films 1975, 30, 145–154. [Google Scholar] [CrossRef]
- Fernández, J.A.; Schmelcher, P.; González-Férez, A.R. Preparation, Optical and Dielectric Properties of Vapor-Deposited Niobium Oxide Thin Films. J. Electrochem. Soc. 1969, 116, 234–239. [Google Scholar]
- Cho, N.H.; Kang, H.B.; Kim, Y.H. Dielectric characteristics and chemical structures of Nb2O5 thin films grown by solgel techniques. Ferroelectrics 1994, 152, 43–48. [Google Scholar] [CrossRef]
- Ong, G.K.; Cabezas, C.A.S.; Dominguez, M.N.; Skjærvø, S.L.; Heo, S.; Milliron, D.J. Electrochromic Niobium Oxide Nanorods. Chem. Mater. 2020, 32, 468–475. [Google Scholar] [CrossRef]
- Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Yao, D.D.; Rani, R.A.; O’Mullane, A.P.; Kalantar-Zadeh, K.; Ou, J.Z. High performance electrochromic devices based on anodized nanoporous Nb2O5. J. Phys. Chem. C 2014, 118, 476–481. [Google Scholar] [CrossRef]
- Pawlicka, A.; Atik, M.; Aegerter, M.A. Synthesis of Nb2O5 thin films for electrochromic devices. J. Mater. Sci. Lett. 1995, 14, 1568–1570. [Google Scholar] [CrossRef]






| Physical Properties | Nb [7,8,9] | NbO [7,8,9] | NbO2 [9,10,11,12,13] | Nb2O5 [9,11,14] |
|---|---|---|---|---|
| crystal system | cubic | cubic | tetragonal | depends on its polymorph, synthesis parameters and technique |
| space group | ||||
| density (g/cm3) at 20 °C | 8.57 | 7.30 | 5.90 | 4.47 |
| melting point (°C) | 2477 | 1937 | 1901 | 1500 |
| boiling point (°C) | 4744 | - | - | - |
| qualitative solubility | acid | - | - | hydrofluoric acid |
| electrical resistivity μΩ·cm at 25 °C | 15.2 | 21 | 107 | - |
| conductivity σ (S/cm) at 25 °C | ~7.5 × 104 | ~4.8 × 104 | ~10−4 | ~10−16–10−6 |
| relative atomic mass | 92.906 | 108.905 | 124.905 | 265.80 |
| NbOx Thin Film | Density | Thickness (nm) | +Density (g/cm3) | ++Nb/O Ratio |
|---|---|---|---|---|
| (1015 atm/cm2) | * | |||
| 0.32 O2/Ar-15 min | 3505 | 510 | 6.11 | 1.15 |
| 0.40 O2/Ar-60 min | 1454 | 198 | 4.57 | 0.49 |
| 0.40 O2/Ar-30 min | 693 | 75 | 4.72 | 0.48 |
| 0.40 O2/Ar-15 min | 463 | 60 | 4.45 | 0.41 |
| 0.56 O2/Ar-60 min | 1107 | 153 | 4.48 | 0.48 |
| 0.56 O2/Ar-30 min | 669 | 88 | 4.49 | 0.43 |
| 0.56 O2/Ar-15 min | 426 | 51 | 4.57 | 0.36 |
| NbOx Thin Film | Nb 3d Orbitals | Nb0(metal) | Nb2+ (NbO) | Nb4+ (NbO2) | Nb5+ (Nb2O5) |
|---|---|---|---|---|---|
| 0 O2/Ar-30 min | Nb 3d3/2 | 204.9 | - | - | 210.6 |
| Nb 3d5/2 | 202.1 | - | - | 207.1 | |
| 0.16 O2/Ar-30 min | Nb 3d3/2 | 205.5 | - | - | 210.2 |
| Nb 3d5/2 | 202.8 | - | - | 207.5 | |
| 0.32 O2/Ar-30 min | Nb 3d3/2 | - | 206.8 | - | 210.0 |
| Nb 3d5/2 | - | 204.0 | - | 207.3 | |
| 0.40 O2/Ar-30 min | Nb 3d3/2 | - | - | 208.6 | 210.3 |
| Nb 3d5/2 | - | - | 205.7 | 207.6 | |
| 0.56 O2/Ar-30 min | Nb 3d3/2 | - | - | - | 210.1 |
| Nb 3d5/2 | - | - | - | 207.3 | |
| From the literature [130,131,132,133] | Nb 3d3/2 | 205.0 204.7 205.1 | 206.8 206.6 207.0 | 208.8 208.6 208.8 | 210.0 209.9 210.2 |
| Nb 3d5/2 | 202.2 202.0 202.3 | 204.0 203.9 204.3 | 206.0 205.9 206.0 | 207.3 207.2 207.4 |
| Sample | Sq (nm) of NbOx Thin Films Grown on ITO-Coated PET Substrate | Sq (nm) of NbOx Thin Films Grown on ITO-Coated Glass Substrate |
|---|---|---|
| 0.40 O2/Ar-15 min | 9 | 10 |
| 0.40 O2/Ar-30 min | 6 | 7 |
| 0.40 O2/Ar-60 min | - | 4 |
| 0.56 O2/Ar-15 min | 3 | 7 |
| 0.56 O2/Ar-30 min | 3 | 3 |
| 0.56 O2/Ar-60 min | - | 5 |
| Sample | Sq (nm) | |
| ITO-coated PET substrate | 4 | |
| ITO-coated glass substrate | 2 | |
| Transmittance (%) (Thin Films Grown on ITO-Coated PET Substrate) | Bandgap (eV) (Thin Films Grown on ITO-Coated PET Substrate) | Transmittance (%) (Thin Films Grown on ITO-Coated Glass Substrate) | Bandgap (eV) (Thin Films Grown on ITO-Coated Glass Substrate) | |
|---|---|---|---|---|
| 0.40 O2/Ar-15 min | 81% | 3.91 | 78% | 4.07 |
| 0.40 O2/Ar-30 min | 80% | 3.91 | 77% | 4.02 |
| 0.40 O2/Ar-60 min | - | - | 83% | 3.94 |
| 0.56 O2/Ar-15 min | 81% | 3.91 | 77% | 4.05 |
| 0.56 O2/Ar-30 min | 80% | 3.90 | 83% | 4.04 |
| 0.56 O2/Ar-60 min | - | - | 82% | 3.98 |
| Sample | 0.40 O2/Ar 15 min | 0.40 O2/Ar 30 min | 0.56 O2/Ar 15 min | 0.56 O2/Ar 30 min | |
|---|---|---|---|---|---|
| Scan rate (mV/s) | 10 | 10 | 10 | 10 | |
| jpc (A/cm2) | 3.67 × 10−4 | 4.60 × 10−4 | 4.28 × 10−4 | 2.81 × 10−4 | |
| Vpc (VAg/AgCl/KCl (3M)) | −2.00 | −2.00 | −2.00 | −2.00 | |
| jpa (A/cm2) | 1.28 × 10−4 | 2.14 × 10−4 | 1.30 × 10−4 | 1.51 × 10−4 | |
| Vpc (VAg/AgCl/KCl (3M)) | −1.20 | −1.04 | −1.14 | −1.02 | |
| Diffusion coefficient (cm2s−1) | Dinsertion | 1.82 × 10−10 | 2.36 × 10−10 | 2.86 × 10−10 | 1.07 × 10−10 |
| Dextraction | 2.21 × 10−11 | 6.19 × 10−11 | 2.29 × 10−11 | 3.07 × 10−11 | |
| Intercalated charge (C/cm2) | 0.017 | 0.028 | 0.017 | 0.017 | |
| Deintercalated charge (C/cm2) | 0.016 | 0.026 | 0.014 | 0.016 | |
| Reversibility (%) | 96% | 95% | 82% | 89% | |
| Coloration efficiency at 630 nm (cm2/C) | 30 | 27 | 26 | 25 | |
| From the Literature | Technique of Growth | Substrate | Reversibility (%) | ΔT | Color Efficiency (CE) cm2/C | Tc (s) | Tb (s) |
|---|---|---|---|---|---|---|---|
| Nb2O5 [153] | RF sputtering | FTO coated glass | - | - | 47 | 13.3 | 7.5 |
| Nb2O5 [31] | Sol–gel/dip coating | ITO-coated glass | - | 33 | 26 | 2 | 3 |
| Nb2O5 [62] | Sol–gel/dip coating | ITO-coated glass | - | - | 22 | - | - |
| Nb2O5 [154] | Sol–gel/dip coating | ITO-coated glass | - | - | 15 | - | - |
| Nb2O5 [58] | Spray pyrolysis | FTO-coated glass | 85% | - | 13 | 3.7 | 4.7 |
| Nb2O5 [128] | RF sputtering | 1731 (high temperature glass) | - | 25.4 | 4.63 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marciel, A.; Bastos, A.; Pereira, L.; Jakka, S.K.; Borges, J.; Vaz, F.; Peres, M.; Lorenz, K.; Bafti, A.; Pavić, L.; et al. Niobium Oxide Thin Films Grown on Flexible ITO-Coated PET Substrates. Coatings 2024, 14, 1127. https://doi.org/10.3390/coatings14091127
Marciel A, Bastos A, Pereira L, Jakka SK, Borges J, Vaz F, Peres M, Lorenz K, Bafti A, Pavić L, et al. Niobium Oxide Thin Films Grown on Flexible ITO-Coated PET Substrates. Coatings. 2024; 14(9):1127. https://doi.org/10.3390/coatings14091127
Chicago/Turabian StyleMarciel, Alice, Alexandre Bastos, Luiz Pereira, Suresh Kumar Jakka, Joel Borges, Filipe Vaz, Marco Peres, Katharina Lorenz, Arijeta Bafti, Luka Pavić, and et al. 2024. "Niobium Oxide Thin Films Grown on Flexible ITO-Coated PET Substrates" Coatings 14, no. 9: 1127. https://doi.org/10.3390/coatings14091127
APA StyleMarciel, A., Bastos, A., Pereira, L., Jakka, S. K., Borges, J., Vaz, F., Peres, M., Lorenz, K., Bafti, A., Pavić, L., Silva, R., & Graça, M. (2024). Niobium Oxide Thin Films Grown on Flexible ITO-Coated PET Substrates. Coatings, 14(9), 1127. https://doi.org/10.3390/coatings14091127











