Research Progress on the Application of One-Step Fabrication Techniques for Iridium-Based Thin Films in the Oxygen Evolution Reaction
Abstract
1. Introduction
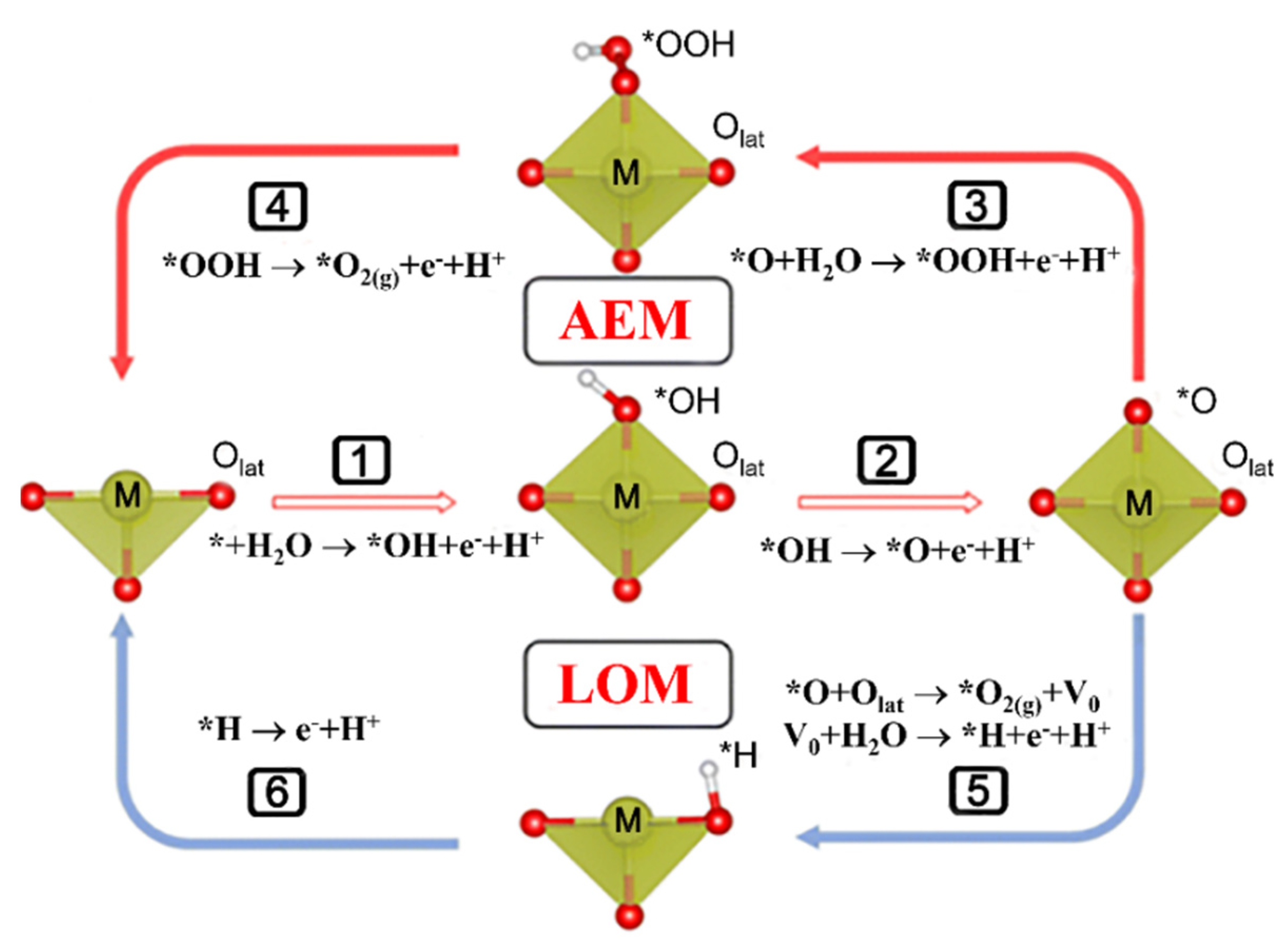
2. Catalytic Mechanism of Iridium Oxide in OER
3. One-Step Fabrication Techniques for Iridium-Based Thin Films
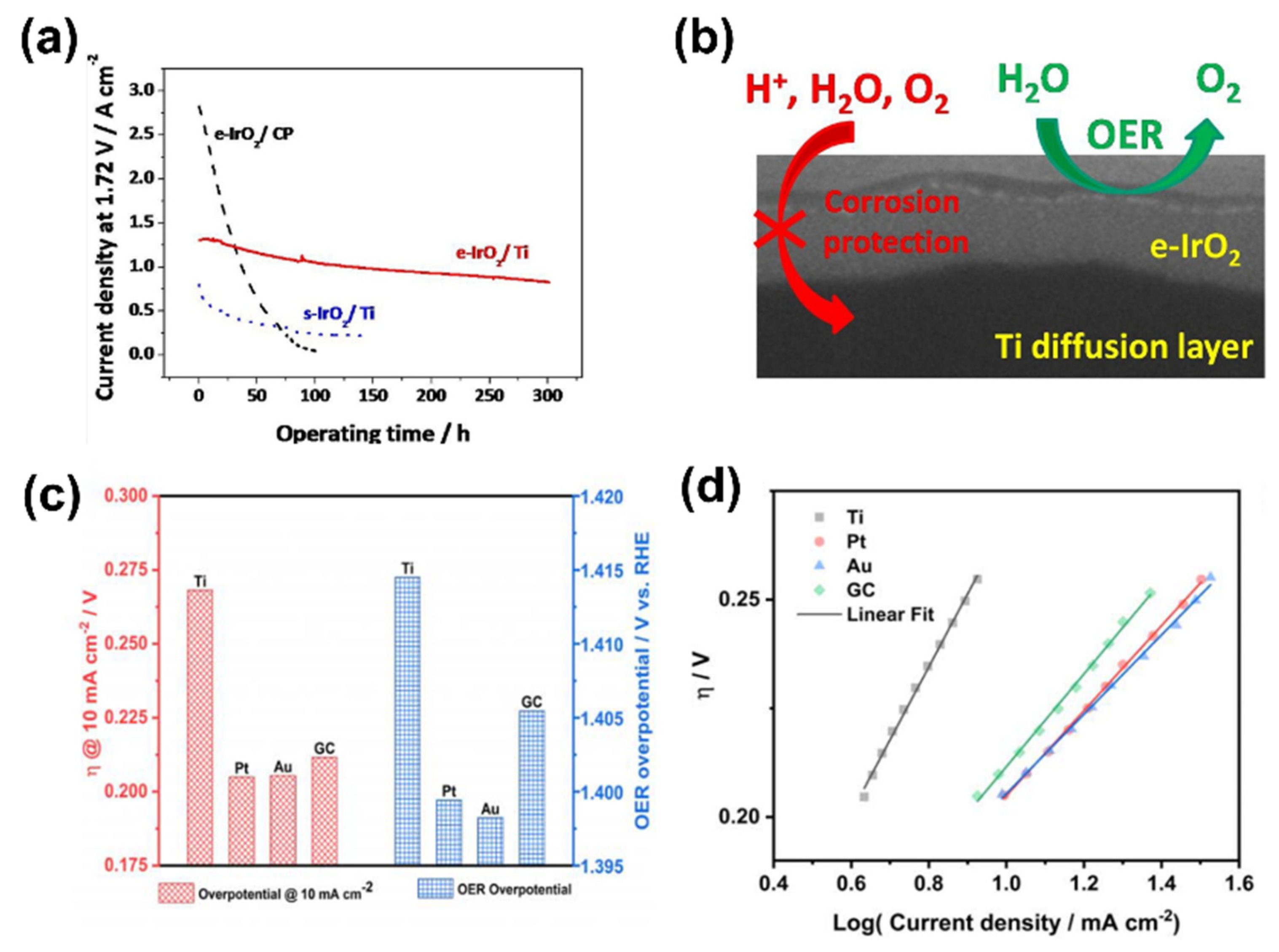
3.1. Electrochemical Deposition Method
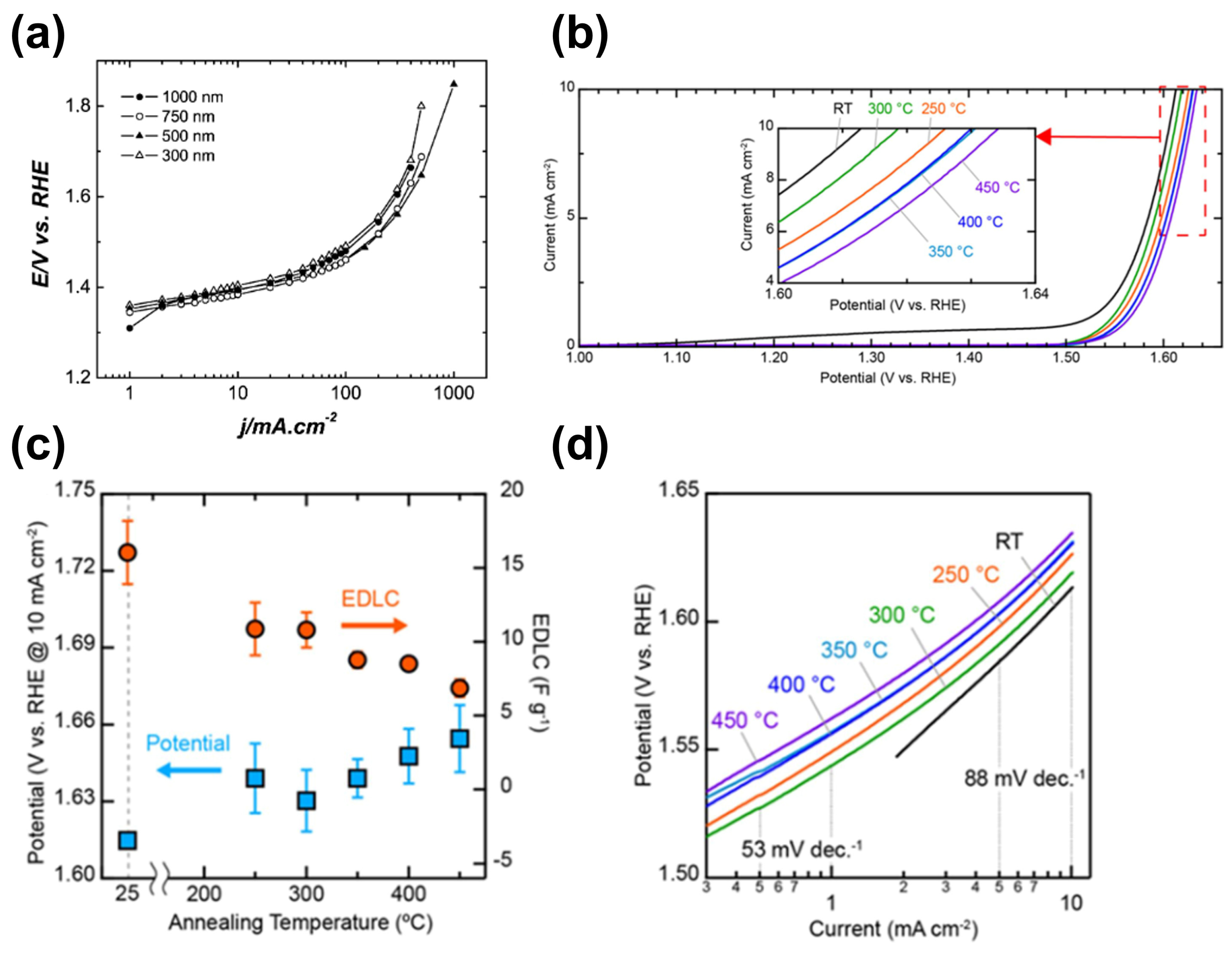
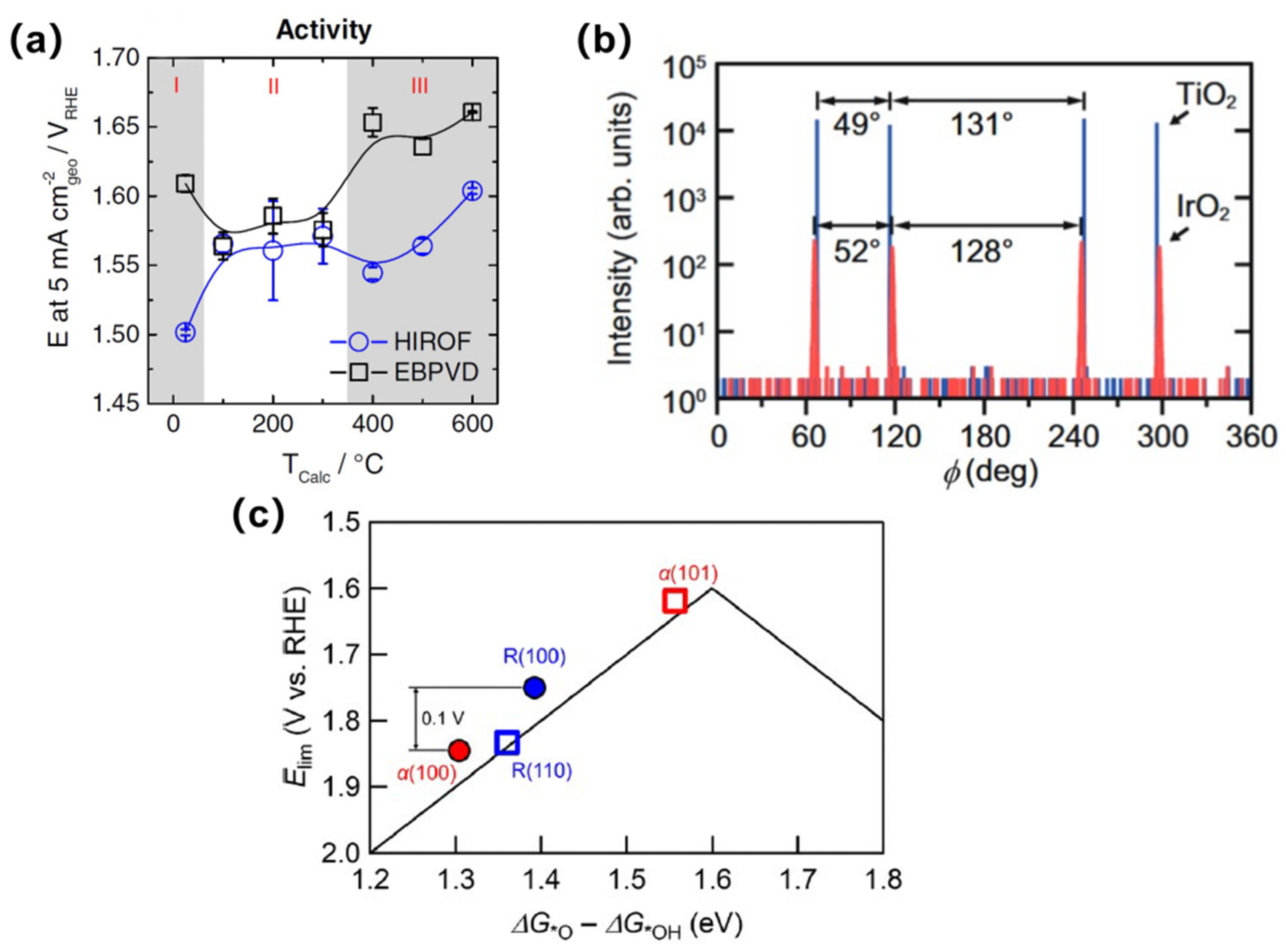
3.2. Physical Vapor Deposition Method
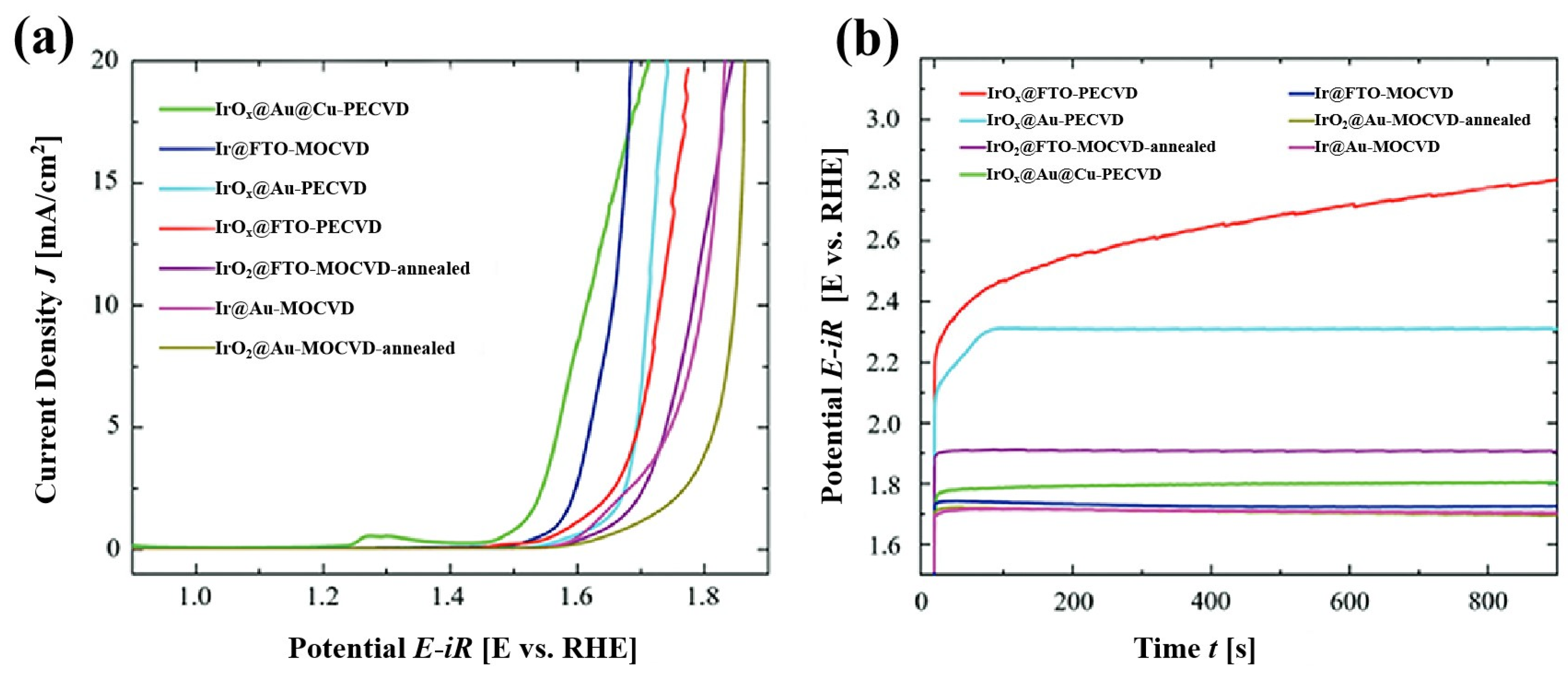
3.3. Chemical Vapor Deposition Method
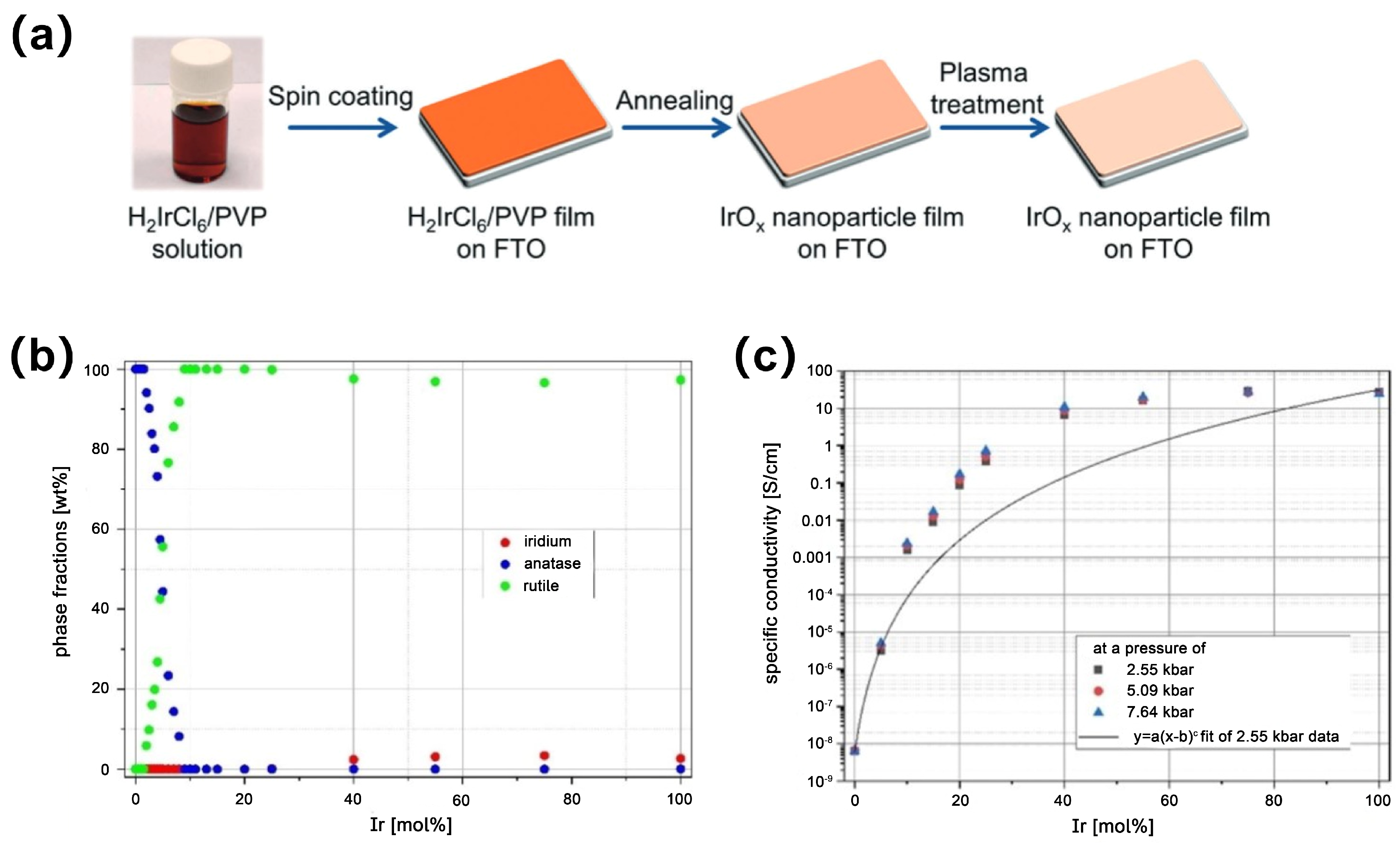
3.4. Sol–Gel Method
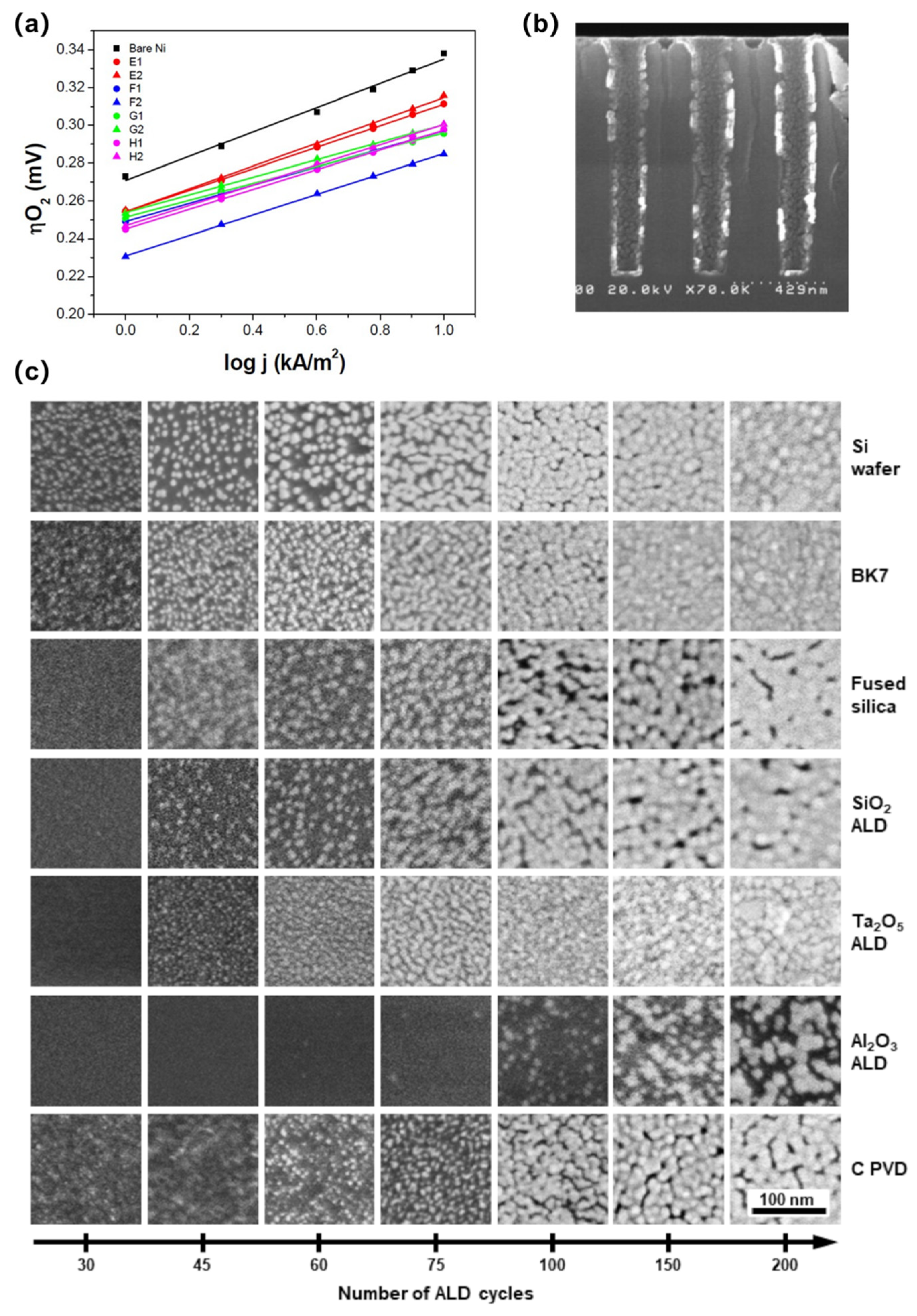
3.5. Other Methods
4. Future Prospects
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A Comprehensive Review on the Electrochemical Parameters and Recent Material Development of Electrochemical Water Splitting Electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, W.; Liu, J. MOF-Based/Derived Catalysts for Electrochemical Overall Water Splitting. J. Colloid Interface Sci. 2024, 661, 409–435. [Google Scholar] [CrossRef]
- Qian, Q.; Zhu, Y.; Ahmad, N.; Feng, Y.; Zhang, H.; Cheng, M.; Liu, H.; Xiao, C.; Zhang, G.; Xie, Y. Recent Advancements in Electrochemical Hydrogen Production via Hybrid Water Splitting. Adv. Mater. 2024, 36, 2306108. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.D.; Fowler, M.; Elkamel, A.; Almansoori, A.; Walker, S.B. Enabling Utility-Scale Electrical Energy Storage by a Power-to-Gas Energy Hub and Underground Storage of Hydrogen and Natural Gas. J. Nat. Gas Sci. Eng. 2016, 35, 1180–1199. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Rezazadeh, A. Hydrogen Fuel and Electricity Generation from a New Hybrid Energy System Based on Wind and Solar Energies and Alkaline Fuel Cell. Energy Rep. 2021, 7, 2594–2604. [Google Scholar] [CrossRef]
- Dong, H.; Guo, J.E.; Liu, J.; Meng, T.; Li, M.; Chen, X.; Li, N.; Alavi, H. Energy Generation and Storing Electrical Energy in an Energy Hybrid System Consisting of Solar Thermal Collector, Stirling Engine and Thermoelectric Generator. Sustain. Cities Soc. 2021, 75, 103357. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An Overview of Water Electrolysis Technologies for Green Hydrogen Production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Amini Horri, B.; Ozcan, H. Green Hydrogen Production by Water Electrolysis: Current Status and Challenges. Curr. Opin. Green Sustain. Chem. 2024, 47, 100932. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Liu, Y.; Wang, R.; Yang, Y.; Chen, J. A Critical Review of Research Progress for Metal Alloy Materials in Hydrogen Evolution and Oxygen Evolution Reaction. Environ. Sci. Pollut. Res. 2023, 30, 11302–11320. [Google Scholar] [CrossRef]
- Batchelor, T.A.A.; Pedersen, J.K.; Winther, S.H.; Castelli, I.E.; Jacobsen, K.W.; Rossmeisl, J. High-Entropy Alloys as a Discovery Platform for Electrocatalysis. Joule 2019, 3, 834–845. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, R. Advanced Transition Metal-Based OER Electrocatalysts: Current Status, Opportunities, and Challenges. Small 2021, 17, 2100129. [Google Scholar] [CrossRef]
- Choudhury, D.; Das, R.; Maurya, R.; Kumawat, H.; Neergat, M. Kinetics of the Oxygen Evolution Reaction (OER) on Amorphous and Crystalline Iridium Oxide Surfaces in Acidic Medium. Langmuir 2023, 39, 13748–13757. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Zhang, X.; Zhang, C.; Feng, S.; Zhang, W.; Guo, W.; Zheng, R.; Zheng, R.; Liu, P.; Li, Y.; et al. Lowering the Kinetic Barrier via Enhancing Electrophilicity of Surface Oxygen to Boost Acidic Oxygen Evolution Reaction. Matter 2024, 7, 1330–1343. [Google Scholar] [CrossRef]
- Naito, T.; Shinagawa, T.; Nishimoto, T.; Takanabe, K. Recent Advances in Understanding Oxygen Evolution Reaction Mechanisms over Iridium Oxide. Inorg. Chem. Front. 2021, 8, 2900–2917. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Shi, Z.; Wang, X.; Yang, J.; Xiao, M.; Ge, J.; Xing, W.; Liu, C. Recent Developments of Iridium-Based Catalysts for the Oxygen Evolution Reaction in Acidic Water Electrolysis. J. Mater. Chem. A 2022, 10, 13170–13189. [Google Scholar] [CrossRef]
- Ledendecker, M.; Geiger, S.; Hengge, K.; Lim, J.; Cherevko, S.; Mingers, A.M.; Göhl, D.; Fortunato, G.V.; Jalalpoor, D.; Schüth, F.; et al. Towards Maximized Utilization of Iridium for the Acidic Oxygen Evolution Reaction. Nano Res. 2019, 12, 2275–2280. [Google Scholar] [CrossRef]
- Chen, X.; Niu, K.; Xue, Z.; Liu, X.; Liu, B.; Zhang, B.; Zeng, H.; Lv, W.; Zhang, Y.; Wu, Y. Ultrafine Platinum Nanoparticles Supported on N,S-Codoped Porous Carbon Nanofibers as Efficient Multifunctional Materials for Noticeable Oxygen Reduction Reaction and Water Splitting Performance. Nanoscale Adv. 2022, 4, 1639–1648. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Zhang, G.; Shi, P.; Wang, A.-L. Rational Catalyst Design for Oxygen Evolution under Acidic Conditions: Strategies toward Enhanced Electrocatalytic Performance. J. Mater. Chem. A 2021, 9, 5890–5914. [Google Scholar] [CrossRef]
- Qin, D.-D.; Tang, Y.; Ma, G.; Qin, L.; Tao, C.-L.; Zhang, X.; Tang, Z. Molecular Metal Nanoclusters for ORR, HER and OER: Achievements, Opportunities and Challenges. Int. J. Hydrogen Energy 2021, 46, 25771–25781. [Google Scholar] [CrossRef]
- Cherevko, S.; Reier, T.; Zeradjanin, A.R.; Pawolek, Z.; Strasser, P.; Mayrhofer, K.J.J. Stability of Nanostructured Iridium Oxide Electrocatalysts during Oxygen Evolution Reaction in Acidic Environment. Electrochem. Commun. 2014, 48, 81–85. [Google Scholar] [CrossRef]
- Xie, Z.; Liang, X.; Kang, Z.; Zou, Y.; Wang, X.; Wu, Y.A.; King, G.; Liu, Q.; Huang, Y.; Zhao, X.; et al. High-Porosity, Layered Iridium Oxide as an Efficient, Durable Anode Catalyst for Water Splitting. CCS Chem. 2024. [Google Scholar] [CrossRef]
- Tachikawa, T.; Beniya, A.; Shigetoh, K.; Higashi, S. Relationship Between OER Activity and Annealing Temperature of Sputter-Deposited Flat IrO2 Thin Films. Catal. Lett. 2020, 150, 1976–1984. [Google Scholar] [CrossRef]
- Frisch, M.; Raza, M.H.; Ye, M.-Y.; Sachse, R.; Paul, B.; Gunder, R.; Pinna, N.; Kraehnert, R. ALD-Coated Mesoporous Iridium-Titanium Mixed Oxides: Maximizing Iridium Utilization for an Outstanding OER Performance. Adv. Mater. Interfaces 2022, 9, 2102035. [Google Scholar] [CrossRef]
- Matienzo, D.D.; Settipani, D.; Instuli, E.; Kallio, T. Active IrO2 and NiO Thin Films Prepared by Atomic Layer Deposition for Oxygen Evolution Reaction. Catalysts 2020, 10, 92. [Google Scholar] [CrossRef]
- Mei, B.; Seger, B.; Pedersen, T.; Malizia, M.; Hansen, O.; Chorkendorff, I.; Vesborg, P.C.K. Protection of P+-n-Si Photoanodes by Sputter-Deposited Ir/IrOx Thin Films. J. Phys. Chem. Lett. 2014, 5, 1948–1952. [Google Scholar] [CrossRef]
- Strickler, A.L.; Flores, R.A.; King, L.A.; Nørskov, J.K.; Bajdich, M.; Jaramillo, T.F. Systematic Investigation of Iridium-Based Bimetallic Thin Film Catalysts for the Oxygen Evolution Reaction in Acidic Media. ACS Appl. Mater. Interfaces 2019, 11, 34059–34066. [Google Scholar] [CrossRef]
- Özer, E.; Pawolek, Z.; Kühl, S.; Nong, H.N.; Paul, B.; Selve, S.; Spöri, C.; Bernitzky, C.; Strasser, P. Metallic Iridium Thin-Films as Model Catalysts for the Electrochemical Oxygen Evolution Reaction (OER)—Morphology and Activity. Surfaces 2018, 1, 151–164. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and Hydrogen Evolution Reactions on Ru, RuO2, Ir, and IrO2 Thin Film Electrodes in Acidic and Alkaline Electrolytes: A Comparative Study on Activity and Stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Buvat, G.; Eslamibidgoli, M.J.; Garbarino, S.; Eikerling, M.; Guay, D. OER Performances of Cationic Substituted (100)-Oriented IrO2 Thin Films: A Joint Experimental and Theoretical Study. ACS Appl. Energy Mater. 2020, 3, 5229–5237. [Google Scholar] [CrossRef]
- Ding, L.; Li, K.; Wang, W.; Xie, Z.; Yu, S.; Yu, H.; Cullen, D.A.; Keane, A.; Ayers, K.; Capuano, C.B.; et al. Amorphous Iridium Oxide-Integrated Anode Electrodes with Ultrahigh Material Utilization for Hydrogen Production at Industrial Current Densities. Nano-Micro Lett. 2024, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Petkucheva, E.; Borisov, G.; Lefterova, E.; Heiss, J.; Schnakenberg, U.; Slavcheva, E. Gold-Supported Magnetron Sputtered Ir Thin Films as OER Catalysts for Cost-Efficient Water Electrolysis. Int. J. Hydrogen Energy 2018, 43, 16905–16912. [Google Scholar] [CrossRef]
- Choe, S.; Lee, B.-S.; Cho, M.K.; Kim, H.-J.; Henkensmeier, D.; Yoo, S.J.; Kim, J.Y.; Lee, S.Y.; Park, H.S.; Jang, J.H. Electrodeposited IrO2/Ti Electrodes as Durable and Cost-Effective Anodes in High-Temperature Polymer-Membrane-Electrolyte Water Electrolyzers. Appl. Catal. B Environ. 2018, 226, 289–294. [Google Scholar] [CrossRef]
- Lee, K.; Flores, R.A.; Liu, Y.; Wang, B.Y.; Hikita, Y.; Sinclair, R.; Bajdich, M.; Hwang, H.Y. Epitaxial Stabilization and Oxygen Evolution Reaction Activity of Metastable Columbite Iridium Oxide. ACS Appl. Energy Mater. 2021, 4, 3074–3082. [Google Scholar] [CrossRef]
- Zhao, F.; Wen, B.; Niu, W.; Chen, Z.; Yan, C.; Selloni, A.; Tully, C.G.; Yang, X.; Koel, B.E. Increasing Iridium Oxide Activity for the Oxygen Evolution Reaction with Hafnium Modification. J. Am. Chem. Soc. 2021, 143, 15616–15623. [Google Scholar] [CrossRef]
- Hakimi, F.; Ghalkhani, M.; Rashchi, F.; Dolati, A. Pulse Electrodeposition Synthesis of Ti/PbO2-IrO2 Nano-Composite Electrode to Restrict the OER in the Zinc Electrowinning. J. Environ. Chem. Eng. 2024, 12, 111985. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, F.; Gong, J.; Wu, B.; Zhang, Z.; Chu, J. Platinum Nanoparticles Decorated IrO2 @MWCNT as an Improved Catalyst for Oxygen Evolution Reaction. ChemistrySelect 2021, 6, 7542–7550. [Google Scholar] [CrossRef]
- Liu, B.; Li, G.; Cai, X.; Wang, Y.; Zeng, Y.; Ren, Q.; Li, J. IrO2 Nanoparticles Supported on Submicrometer-Sized TiO2 as an Efficient and Stable Coating for Oxygen Evolution Reaction. Electrochim. Acta 2024, 493, 144392. [Google Scholar] [CrossRef]
- Yoo, P.; Woo, M.; Lee, H.I.; Kim, H.S.; Lim, D.-H. Fabrication of Well-Dispersed IrO2 Anchored on rGO Composite for High-Performance OER Electrocatalyst Application by Microwave-Assisted Method. Electrocatalysis 2023, 14, 891–900. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Liang, X.; Zhao, X.; Wang, X.; Jana, S.; Wu, Y.A.; Zou, Y.; Li, L.; Chen, H.; et al. Supported IrO2 Nanocatalyst with Multilayered Structure for Proton Exchange Membrane Water Electrolysis. Adv. Mater. 2024, 2407717. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, W.; Ma, M.; Fan, Z.; Yang, J.; Liao, F.; Shao, M. Lattice Strain Enhances the Activity of Ir–IrO2/C for Acidic Oxygen Evolution Reaction. ChemElectroChem 2022, 9, e202200732. [Google Scholar] [CrossRef]
- Joshi, P.; Yadav, R.; Hara, M.; Inoue, T.; Motoyama, Y.; Yoshimura, M. Contribution of BN-Co-Doped Reduced Graphene Oxide as a Catalyst Support on Activity of Iridium Oxide for Oxygen Evolution Reaction. J. Mater. Chem. A 2021. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Lv, N.; Zhang, Q.; Zhang, X.; Wei, Z.; Wang, Y.; Tang, H.; Pan, H. Ti-Doped SnO2 Supports IrO2 Electrocatalysts for the Oxygen Evolution Reaction (OER) in PEM Water Electrolysis. ACS Sustain. Chem. Eng. 2023, 11, 1121–1132. [Google Scholar] [CrossRef]
- Han, W.; Qian, Y.; Zhang, F.; He, Y.; Li, P.; Zhang, X. 1 Ultrasmall IrO2 Nanoparticles Anchored on 2 Hollow Co-Mo Multi-Oxide Heterostructure 3 Nanocages for Efficient Oxygen Evolution in 4 Acid. Chem. Eng. J. 2023, 473, 145353. [Google Scholar] [CrossRef]
- Cao, H.; Chen, M.; Wu, L.; Hou, G.; Tang, Y.; Zheng, G. Electrochemical Properties of IrO2 Active Anode with TNTs Interlayer for Oxygen Evolution. Appl. Surf. Sci. 2018, 428, 861–869. [Google Scholar] [CrossRef]
- Osaka, A.; Takatsuna, T.; Miura, Y. Iridium Oxide Films via Sol-Gel Processing. J. Non-Cryst. Solids 1994, 178, 313–319. [Google Scholar] [CrossRef]
- Reichert, D.; Stöwe, K. Sol-Gel-Syntheses and Structural as Well as Electrical Characterizations of Anatase- and Rutile-Type Solid Solutions in the System IrO2–TiO2. ChemistryOpen 2023, 12, e202300032. [Google Scholar] [CrossRef]
- Felix, C.; Bladergroen, B.J.; Linkov, V.; Pollet, B.G.; Pasupathi, S. Ex-Situ Electrochemical Characterization of IrO2 Synthesized by a Modified Adams Fusion Method for the Oxygen Evolution Reaction. Catalysts 2019, 9, 318. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, J.; Zeng, H.; Zeng, Y.; Wang, J.; Gu, L.; Hong, E.; Yang, M.; Fu, Q.; Chen, J.; et al. Electrochemical Preparation of Iridium Hydroxide Nanosheets with Ordered Honeycomb Structures for the Oxygen Evolution Reaction in Acid. ACS Appl. Energy Mater. 2022, 5, 6869–6877. [Google Scholar] [CrossRef]
- Hrbek, T.; Kúš, P.; Kosto, Y.; Rodríguez, M.G.; Matolínová, I. Magnetron-Sputtered Thin-Film Catalyst with Low-Ir-Ru Content for Water Electrolysis: Long-Term Stability and Degradation Analysis. J. Power Sources 2023, 556, 232375. [Google Scholar] [CrossRef]
- Slavcheva, E.; Radev, I.; Bliznakov, S.; Topalov, G.; Andreev, P.; Budevski, E. Sputtered Iridium Oxide Films as Electrocatalysts for Water Splitting via PEM Electrolysis. Electrochim. Acta 2007, 52, 3889–3894. [Google Scholar] [CrossRef]
- Parsons, G.N.; Clark, R.D. Area-Selective Deposition: Fundamentals, Applications, and Future Outlook. Chem. Mater. 2020, 32, 4920–4953. [Google Scholar] [CrossRef]
- Ben-Naim, M.; Palm, D.W.; Strickler, A.L.; Nielander, A.C.; Sanchez, J.; King, L.A.; Higgins, D.C.; Jaramillo, T.F. A Spin Coating Method to Deposit Iridium-Based Catalysts onto Silicon for Water Oxidation Photoanodes. ACS Appl. Mater. Interfaces 2020, 12, 5901–5908. [Google Scholar] [CrossRef]
- Reier, T.; Weidinger, I.; Hildebrandt, P.; Kraehnert, R.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction on Iridium Oxide Model Film Catalysts: Influence of Oxide Type and Catalyst Substrate Interactions. ECS Trans. 2013, 58, 39. [Google Scholar] [CrossRef]
- Chandra, D.; Sato, T.; Tanahashi, Y.; Takeuchi, R.; Yagi, M. Facile Fabrication and Nanostructure Control of Mesoporous Iridium Oxide Films for Efficient Electrocatalytic Water Oxidation. Energy 2019, 173, 278–289. [Google Scholar] [CrossRef]
- Gao, J.; Xu, C.-Q.; Hung, S.-F.; Liu, W.; Cai, W.; Zeng, Z.; Jia, C.; Chen, H.M.; Xiao, H.; Li, J.; et al. Breaking Long-Range Order in Iridium Oxide by Alkali Ion for Efficient Water Oxidation. J. Am. Chem. Soc. 2019, 141, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Kasian, O.; Geiger, S.; Li, T.; Grote, J.-P.; Schweinar, K.; Zhang, S.; Scheu, C.; Raabe, D.; Cherevko, S.; Gault, B.; et al. Degradation of Iridium Oxides via Oxygen Evolution from the Lattice: Correlating Atomic Scale Structure with Reaction Mechanisms. Energy Environ. Sci. 2019, 12, 3548–3555. [Google Scholar] [CrossRef]
- Wang, C.; Schechter, A.; Feng, L. Iridium-Based Catalysts for Oxygen Evolution Reaction in Acidic Media: Mechanism, Catalytic Promotion Effects and Recent Progress. Nano Res. Energy 2023, 2, e9120056. [Google Scholar] [CrossRef]
- Saveleva, V.A.; Wang, L.; Teschner, D.; Jones, T.; Gago, A.S.; Friedrich, K.A.; Zafeiratos, S.; Schlögl, R.; Savinova, E.R. Operando Evidence for a Universal Oxygen Evolution Mechanism on Thermal and Electrochemical Iridium Oxides. J. Phys. Chem. Lett. 2018, 9, 3154–3160. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, W.; Xu, Z.; Duan, Z. The Origin of High Electrochemical Stability of Iridium Oxides for Oxygen Evolution. J. Mater. Chem. A 2024, 12, 20317–20326. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Li, Q. Designing Active and Stable Ir-Based Catalysts for the Acidic Oxygen Evolution Reaction. Ind. Chem. Mater. 2023, 1, 299–311. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, H.; Xi, S.; Lee, W.S.V.; Xue, J. Understanding of Oxygen Redox in the Oxygen Evolution Reaction. Adv. Mater. 2022, 34, 2107956. [Google Scholar] [CrossRef]
- Xu, J.; Jin, H.; Lu, T.; Li, J.; Liu, Y.; Davey, K.; Zheng, Y.; Qiao, S.-Z. IrOx·nH2O with Lattice Water–Assisted Oxygen Exchange for High-Performance Proton Exchange Membrane Water Electrolyzers. Sci. Adv. 2023, 9, eadh1718. [Google Scholar] [CrossRef]
- Zhao, M.; Feng, J.; Li, Z.; Zou, Z. Lattice Water Fuels Acidic Water Oxidation. Sci. China Chem. 2024, 67, 2132–2133. [Google Scholar] [CrossRef]
- Schwarzacher, W. Electrodeposition: A Technology for the Future. Electrochem. Soc. Interface 2006, 15, 32–33. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Wu, X. A Study on the Anodic Electrodeposition of Iridium Oxide on Different Substrates. J. Electrochem. Soc. 2022, 169, 092503. [Google Scholar] [CrossRef]
- Lee, B.-S.; Ahn, S.H.; Park, H.-Y.; Choi, I.; Yoo, S.J.; Kim, H.-J.; Henkensmeier, D.; Kim, J.Y.; Park, S.; Nam, S.W.; et al. Development of Electrodeposited IrO2 Electrodes as Anodes in Polymer Electrolyte Membrane Water Electrolysis. Appl. Catal. B-Environ. 2015, 179, 285–291. [Google Scholar] [CrossRef]
- Jeong, H.-Y.; Oh, J.; Yi, G.S.; Park, H.-Y.; Cho, S.K.; Jang, J.H.; Yoo, S.J.; Park, H.S. High–Performance Water Electrolyzer with Minimum Platinum Group Metal Usage: Iron Nitride–Iridium Oxide Core–Shell Nanostructures for Stable and Efficient Oxygen Evolution Reaction. Appl. Catal. B Environ. 2023, 330, 122596. [Google Scholar] [CrossRef]
- Nga Ngo (Sarah Ngo), T.H.; Love, J.; O’Mullane, A.P. Investigating the Influence of Amorphous/Crystalline Interfaces on the Stability of IrO2 for the Oxygen Evolution Reaction in Acidic Electrolyte. ChemElectroChem 2023, 10, e202300438. [Google Scholar] [CrossRef]
- Cho, J.; Kim, K.-S.; Kim, S.; Shao, Y.; Kim, Y.-T.; Park, S. Substrate-Driven Catalyst Reducibility for Oxygen Evolution and Its Effect on the Operation of Proton Exchange Membrane Water Electrolyzers. Small Struct. 2024, 5, 2300276. [Google Scholar] [CrossRef]
- Abyaneh, M.Y.; Fleischmann, M. General Models for Surface Nucleation and Three-Dimensional Growth: The Effects of Concurrent Redox Reactions and of Diffusion. J. Electrochem. Soc. 1991, 138, 2491. [Google Scholar] [CrossRef]
- Reier, T.; Teschner, D.; Lunkenbein, T.; Bergmann, A.; Selve, S.; Kraehnert, R.; Schlögl, R.; Strasser, P. Electrocatalytic Oxygen Evolution on Iridium Oxide: Uncovering Catalyst-Substrate Interactions and Active Iridium Oxide Species. J. Electrochem. Soc. 2014, 161, F876. [Google Scholar] [CrossRef]
- Schneider, C.W.; Esposito, M.; Marozau, I.; Conder, K.; Doebeli, M.; Hu, Y.; Mallepell, M.; Wokaun, A.; Lippert, T. The Origin of Oxygen in Oxide Thin Films: Role of the Substrate. Appl. Phys. Lett. 2010, 97, 192107. [Google Scholar] [CrossRef]
- Oyshi, T.A.; Islam, M.T.; Al-Humaidi, J.Y.; Rahman, M.M.; Hasnat, M.A. Nanoarchitectonics for Optimization of a Ti/Au-IrOx Electrode for Enhanced Catalytic Performance Pertinent to Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2024, 64, 1011–1020. [Google Scholar] [CrossRef]
- Kumar, S.A.; Sahoo, S.; Laxminarayana, G.K.; Rout, C.S. Electrochemical Deposition for Cultivating Nano- and Microstructured Electroactive Materials for Supercapacitors: Recent Developments and Future Perspectives. Small 2024. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamath, P.V. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Petit, M.A.; Plichon, V. Anodic Electrodeposition of Iridium Oxide Films. J. Electroanal. Chem. 1998, 444, 247–252. [Google Scholar] [CrossRef]
- Schmitt, P.; Beladiya, V.; Felde, N.; Paul, P.; Otto, F.; Fritz, T.; Tünnermann, A.; Szeghalmi, A.V. Influence of Substrate Materials on Nucleation and Properties of Iridium Thin Films Grown by ALD. Coatings 2021, 11, 173. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Z. Iridium Coating: Processes, Properties and Application. Part I. Available online: https://www.ingentaconnect.com/content/matthey/jmtr/2017/00000061/00000001/art00004 (accessed on 3 August 2024).
- El Khakani, M.A.; Chaker, M.; Le Drogoff, B. Iridium Thin Films Deposited by Radio-Frequency Magnetron Sputtering. J. Vac. Sci. Technol. A 1998, 16, 885–888. [Google Scholar] [CrossRef]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. Effect of Sputtering Pressure on Pulsed-DC Sputtered Iridium Oxide Films. Sens. Actuators B Chem. 2009, 137, 370–378. [Google Scholar] [CrossRef]
- Geiger, S.; Kasian, O.; Shrestha, B.R.; Mingers, A.M.; Mayrhofer, K.J.J.; Cherevko, S. Activity and Stability of Electrochemically and Thermally Treated Iridium for the Oxygen Evolution Reaction. J. Electrochem. Soc. 2016, 163, F3132. [Google Scholar] [CrossRef]
- Buvat, G.; Eslamibidgoli, M.J.; Youssef, A.H.; Garbarino, S.; Ruediger, A.; Eikerling, M.; Guay, D. Effect of IrO6 Octahedron Distortion on the OER Activity at (100) IrO2 Thin Film. ACS Catal. 2020, 10, 806–817. [Google Scholar] [CrossRef]
- El Khakani, M.A.; Chaker, M. Reactive Pulsed Laser Deposition of Iridium Oxide Thin Films. Thin Solid Film. 1998, 335, 6–12. [Google Scholar] [CrossRef]
- Wilson, A.A.; Graziano, M.B.; Leff, A.C.; Hanrahan, B.; Baker, D.R.; Rivas, M.; Sanchez, B.; Parker, T.; Sunal, P. Growth Conditions and Mechanisms for IrOx Nano-Platelet Formation by Reactive Sputtering. J. Cryst. Growth 2022, 577, 126374. [Google Scholar] [CrossRef]
- Laguna-Marco, M.A.; Herrero-Albillos, J.; Aguirre, M.H.; Rueda-Jimenez, M.; Mikulska, I. Novel Ir1−xCoxO2 Thin Films: Growth and Characterization. J. Alloy. Compd. 2023, 968, 171975. [Google Scholar] [CrossRef]
- Slavcheva, E.P. Magnetron Sputtered Iridium Oxide as Anode Catalyst for Pem Hydrogen Generation. Maced. J. Chem. Chem. Eng. 2011, 30, 45–54. [Google Scholar] [CrossRef]
- Khoa, T.D.; Horii, S.; Horita, S. High Deposition Rate of Epitaxial (100) Iridium Film on (100)YSZ/1 0 0)Si Substrate by RF Sputtering Deposition. Thin Solid Film. 2002, 419, 88–94. [Google Scholar] [CrossRef]
- Kang, X.; Liu, J.; Tian, H.; Yang, B.; NuLi, Y.; Yang, C. Optimization and Electrochemical Characterization of RF-Sputtered Iridium Oxide Microelectrodes for Electrical Stimulation. J. Micromech. Microeng. 2014, 24, 025015. [Google Scholar] [CrossRef]
- Zenkin, S.; Gaydaychuk, A.; Linnik, S. Effects of Sputtering Gas on the Microstructure of Ir Thin Films Deposited by HiPIMS and Pulsed DC Sputtering. Surf. Coat. Technol. 2021, 412, 127038. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Z.; Lv, B.; Sun, B.; Wang, H.; He, P.; Wang, X.; Liang, X.; Jin, G. The New Iridium-Hafnium-Aluminum Alloy Thin Films with Excellent Mechanical Properties and Oxidation Resistance. Appl. Surf. Sci. 2024, 657, 159802. [Google Scholar] [CrossRef]
- Ohmori, T.; Go, H.; Nakayama, A.; Mametsuka, H.; Suzuki, E. Influence of Sputtering Parameters on Hydrogen Evolution Overvoltage in Sputter-Deposited Co–Mo Alloy Electrode. Mater. Lett. 2001, 47, 103–106. [Google Scholar] [CrossRef]
- Li, C.; Hsieh, J.-H. Parameters and Stability Analysis of Reactive Sputtering. Jpn. J. Appl. Phys. 2003, 42, 6653. [Google Scholar] [CrossRef]
- Slavcheva, E.; Schnakenberg, U.; Mokwa, W. Deposition of Sputtered Iridium Oxide—Influence of Oxygen Flow in the Reactor on the Film Properties. Appl. Surf. Sci. 2006, 253, 1964–1969. [Google Scholar] [CrossRef]
- Reinhold, E.; Faber, J. Large Area Electron Beam Physical Vapor Deposition (EB-PVD) and Plasma Activated Electron Beam (EB) Evaporation—Status and Prospects. Surf. Coat. Technol. 2011, 206, 1653–1659. [Google Scholar] [CrossRef]
- Matthews, L.R.; Parkhill, R.L.; Knobbe, E.T. Optical Characterization of Binary, Tertiary, and Quaternary II-VI Semiconductor Thin Films Prepared by Pulsed Excimer Laser Deposition. In Proceedings of the Excimer Lasers, Optics, and Applications, San Jose, CA, USA, 31 March 1997; SPIE: San Jose, CA, USA, 1997; Volume 2992, pp. 72–80. [Google Scholar]
- Hou, X.; Takahashi, R.; Yamamoto, T.; Lippmaa, M. Microstructure Analysis of IrO2 Thin Films. J. Cryst. Growth 2017, 462, 24–28. [Google Scholar] [CrossRef]
- Hampden-Smith, M.J.; Kodas, T.T. Chemical Vapor Deposition of Metals: Part 1. An Overview of CVD Processes. Chem. Vap. Depos. 1995, 1, 8–23. [Google Scholar] [CrossRef]
- Vasilyev, V.Y.; Morozova, N.B.; Basova, T.V.; Igumenov, I.K.; Hassan, A. Chemical Vapour Deposition of Ir-Based Coatings: Chemistry, Processes and Applications. RSC Adv. 2015, 5, 32034–32063. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Endle, J.P.; Smith, K.; Whaley, S.; Mahaffy, R.; Ekerdt, J.G.; White, J.M.; Hance, R.L. Iridium Film Growth with Indium Tris-Acetylacetonate: Oxygen and Substrate Effects. Thin Solid Film. 1999, 346, 100–107. [Google Scholar] [CrossRef]
- Guerrero, R.M.; Torres, M.Z.F.; Zuñiga, I.M.M.; Estrada, E.M.A.; Garcia, J.R.V. Preparation and Characterization of Nanocrystalline Noble Metal Films by MOCVD. In Proceedings of the Metastable, Mechanically Alloyed and Nanocrystalline Materials; Inoue, A., Ed.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2005; Volume 24–25, pp. 623–626. [Google Scholar]
- Zhang, F.; Barrowcliff, R.; Stecker, G.; Pan, W.; Wang, D.; Hsu, S.-T. Synthesis of Metallic Iridium Oxide Nanowires via Metal Organic Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2005, 44, L398. [Google Scholar] [CrossRef]
- Jürgensen, L.; Frank, M.; Pyeon, M.; Czympiel, L.; Mathur, S. Subvalent Iridium Precursors for Atom-Efficient Chemical Vapor Deposition of Ir and IrO2 Thin Films. Organometallics 2017, 36, 2331–2337. [Google Scholar] [CrossRef]
- Garcia, J.R.V.; Goto, T. Chemical Vapor Deposition of Iridium, Platinum, Rhodium and Palladium. Mater. Trans. 2003, 44, 1717–1728. [Google Scholar] [CrossRef]
- Jürgensen, L.; Frank, M.; Graf, D.; Gessner, I.; Fischer, T.; Welter, K.; Jägermann, W.; Mathur, S. Nanostructured IrOx Coatings for Efficient Oxygen Evolution Reactions in PV-EC Setup. Z. Für Phys. Chem. 2020, 234, 911–924. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Q.; Fan, X. New MOCVD Precursor for Iridium Thin Films Deposition. Mater. Lett. 2007, 61, 216–218. [Google Scholar] [CrossRef]
- Xu, C.; Baum, T.H.; Rheingold, A.L. New Precursors for Chemical Vapor Deposition of Iridium. Chem. Mater. 1998, 10, 2329–2331. [Google Scholar] [CrossRef]
- Maury, F.; Senocq, F. Iridium Coatings Grown by Metal–Organic Chemical Vapor Deposition in a Hot-Wall CVD Reactor. Surf. Coat. Technol. 2003, 163–164, 208–213. [Google Scholar] [CrossRef]
- Hernández-Pérez, M.A.; Vargas-García, J.R.; Romero-Serrano, J.A. Diagramas de fase CVD para la preparación de películas de iridio. Rev. De Metal. 2002, 38, 30–37. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Q.; Fan, X. A new precursor for metal organic chemical vapor deposition of iridium thin films. Rare Met. Mat. Eng. 2006, 35, 1129–1131. [Google Scholar]
- Dougherty, G.M.; Sands, T.D.; Pisano, A.P. Microfabrication Using One-Step LPCVD Porous Polysilicon Films. J. Microelectromechanical Syst. 2003, 12, 418–424. [Google Scholar] [CrossRef]
- Modreanu, M.; Tomozeiu, N.; Gartner, M.; Cosmin, P. Microstructural and Optical Properties of As-Deposited LPCVD Silicon Films. Thin Solid Film. 2001, 383, 254–257. [Google Scholar] [CrossRef]
- Chao, H.; Wu, Y.T.; Wang, W.-C. Optical Thin Films Using LPCVD and Thermal Reoxidation Techniques. In Proceedings of the Optical Thin Films V: New Developments, SPIE, San Diego, CA, USA, 1 October 1997; Volume 3133, pp. 226–229. [Google Scholar] [CrossRef]
- Gulleri, G.; Carpanese, C.; Cascarano, C.; Lodi, D.; Ninni, R.; Ottaviani, G. Deposition Temperature Determination of HDPCVD Silicon Dioxide Films. Microelectron. Eng. 2005, 82, 236–241. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, H.S.; Jang, J.; Lee, C.H. Field Emission from Nanostructure Carbon Films. Int. J. Mod. Phys. B 2002, 16, 983–987. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Li, Z.; Wang, S.; Huang, Y.; Peng, W. Non-Switching to Switching Transferring Mechanism Investigation for Ag/SiOx/p-Si Structure with SiOx Deposited by HWCVD. J. Phys. D Appl. Phys. 2018, 51, 165102. [Google Scholar] [CrossRef]
- Swain, B.P. The Structural Characterisation of HWCVD-Deposited Nanocrystalline Silicon Films. S. Afr. J. Sci. 2009, 105, 77–80. [Google Scholar] [CrossRef]
- Mungkalasiri, J.; Bedel, L.; Emieux, F.; Cara, A.V.-D.; Freney, J.; Maury, F.; Renaud, F.N.R. Antibacterial Properties of TiO2–Cu Composite Thin Films Grown by a One Step DLICVD Process. Surf. Coat. Technol. 2014, 242, 187–194. [Google Scholar] [CrossRef]
- Boisselier, G.; Maury, F.; Schuster, F. Growth of Chromium Carbide in a Hot Wall DLICVD Reactor. J. Nanosci. Nanotechnol. 2011, 11, 8289–8293. [Google Scholar] [CrossRef]
- Manole, C.C.; Maury, F.; Demetrescu, I. Patterned PPy Polymer and PPy/Ag Nanocomposites Thin Films by Photo-DLICVD. Phys. Procedia 2013, 46, 46–55. [Google Scholar] [CrossRef]
- Mineshige, A. Preparation of Dense Electrolyte Layer Using Dissociated Oxygen Electrochemical Vapor Deposition Technique. Solid State Ion. 2004, 175, 483–485. [Google Scholar] [CrossRef]
- Rosestolato, D.; Neodo, S.; Ferro, S.; Battaglin, G.; Rigato, V.; Battisti, A.D. A Comparison between Structural and Electrochemical Properties of Iridium Oxide-Based Electrocatalysts Prepared by Sol-Gel and Reactive Sputtering Deposition. J. Electrochem. Soc. 2014, 161, E151. [Google Scholar] [CrossRef]
- Reksten, A.; Moradi, F.; Seland, F.; Sunde, S. Iridium-Ruthenium Mixed Oxides for Oxygen Evolution Reaction Prepared by Pechini Synthesis. ECS Trans. 2014, 58, 39. [Google Scholar] [CrossRef]
- Osman, J.R.; Crayston, J.A.; Pratt, A.; Richens, D.T. RuO2–TiO2 Mixed Oxides Prepared from the Hydrolysis of the Metal Alkoxides. Mater. Chem. Phys. 2008, 110, 256–262. [Google Scholar] [CrossRef]
- Green, L.M.; Meek, D.W. Synthesis, Characterization and Reactivity of Alkoxide and Hydroxide Complexes of Rhodium(I) and Iridium(I). Organometallics 1989, 8, 659–666. [Google Scholar] [CrossRef]
- Makote, R.; Collinson, M.M. Template Recognition in Inorganic–Organic Hybrid Films Prepared by the Sol–Gel Process. Chem. Mater. 1998, 10, 2440–2445. [Google Scholar] [CrossRef]
- Luo, Q.; Nie, F.; Long, X.; Qiao, Z.; Yang, G.; Ma, Y. Preparation of Nano-Fe2O3 by CO2-Supercritical-Process-Assisted Sol-Gel Method. Curr. Nanosci. 2014, 10, 722–729. [Google Scholar] [CrossRef]
- Guan, H.; Ke, Q.; Lv, C.; Zeng, N.; Hu, C.; Wang, S.; Ge, X.; Cai, J. Amorphous Iridium Oxide Nanoparticle Films Prepared by Low-Temperature Annealing and Plasma Treatment as Highly Efficient Oxygen Evolution Electrocatalysts. Chem. Lett. 2020, 49, 705–708. [Google Scholar] [CrossRef]
- Yagi, M.; Tomita, E.; Sakita, S.; Kuwabara, T.; Nagai, K. Self-Assembly of Active IrO2 Colloid Catalyst on an ITO Electrode for Efficient Electrochemical Water Oxidation. J. Phys. Chem. B 2005, 109, 21489–21491. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.; Qian, X.; Zhao, S.; Liang, Q.; Chen, Y.E.; Liu, M.; Su, B.-J.; Wu, K.-H.; Qu, L.; Duan, L.; et al. Self-Assembly of Ir-Based Nanosheets with Ordered Interlayer Space for Enhanced Electrocatalytic Water Oxidation. J. Am. Chem. Soc. 2022, 144, 2208–2217. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, Y.; Chen, C.; Ni, P.; Kong, B.; Song, Z.; Lu, Y.; Niu, L. Amorphous Cobalt Boride Nanosheets Directly Grown on Nickel Foam: Controllable Alternately Dipping Deposition for Efficient Oxygen Evolution. ChemElectroChem 2019, 6, 3684–3689. [Google Scholar] [CrossRef]
- Lee, J.; Kim, I.; Park, S. Boosting Stability and Activity of Oxygen Evolution Catalyst in Acidic Medium: Bimetallic Ir–Fe Oxides on Reduced Graphene Oxide Prepared through Ultrasonic Spray Pyrolysis. ChemCatChem 2019, 11, 2615–2623. [Google Scholar] [CrossRef]
- Mahmoud, S.A.; Al-Shomar, S.M.; Akl, A.A. Electrical Characteristics and Nanocrystalline Formation of Sprayed Iridium Oxide Thin Films. Adv. Condens. Matter Phys. 2010, 2010, 518209. [Google Scholar] [CrossRef]
- Park, N.-Y.; Kim, M.; Kim, Y.-H.; Ramesh, R.; Nandi, D.K.; Tsugawa, T.; Shigetomi, T.; Suzuki, K.; Harada, R.; Kim, M.; et al. Atomic Layer Deposition of Iridium Using a Tricarbonyl Cyclopropenyl Precursor and Oxygen. Chem. Mater. 2022, 34, 1533–1543. [Google Scholar] [CrossRef]
- Cloward, I.N.; Liu, T.; Rose, J.; Jurado, T.; Bonn, A.G.; Chambers, M.B.; Pitman, C.L.; Ter Horst, M.A.; Miller, A.J.M. Catalyst Self-Assembly Accelerates Bimetallic Light-Driven Electrocatalytic H2 Evolution in Water. Nat. Chem. 2024, 16, 709–716. [Google Scholar] [CrossRef] [PubMed]


| Materials | Overpotential (mV) | Iridium Loading Amount (mg cm−2) | Tafel Slope (mV dec−1) | Stability Test Duration | Ref. |
|---|---|---|---|---|---|
| IrO2/Ti | - | 0.100 | 53 | - | [23] |
| IrTiOx | 353 | 0.007 | 55 | 50 h | [24] |
| IrO2/NiO | 285–316 | 0.160–0.270 | 45–60 | - | [25] |
| IrOx/Ir/p+-n-Si | 112 | - | 51–55 | 18 h | [26] |
| IrCrOx/FTO | 430 | - | 59 | - | [27] |
| Ir/Ti | 330 | - | 30–40 | 20 h | [28] |
| IrO2/Ni | 320 | - | 45 | - | [29] |
| IrO2(100)/NiO | 310–320 | 54–55 | - | [30] | |
| IrOx/TTLGDL | - | 0.075–0.340 | - | 80 h | [31] |
| Au-Ir/CP | 418–708 | - | 90–134 | - | [32] |
| IrO2/Ti | - | 0.4 | - | 300 h | [33] |
| α-IrO2/YSZ | 300 | - | 250 | - | [34] |
| IrHfxOy | 370 | - | 50–66 | 6 h | [35] |
| p-L-IrO2 | 270 | 0.56 | 42.3 | 2300 h | [22] |
| Materials | Overpotential (mV) | Iridium Loading Amount (mg cm−2) | Tafel Slope (mV dec−1) | Stability Test Duration | Ref. |
|---|---|---|---|---|---|
| PbO2-IrO2 | 471 | - | 467 | - | [36] |
| Pt/IrO2@MWCNT | 270 | 85.1 | - | [37] | |
| Ti/SMST/IrO2 | 350 | 0.857 | 61 | 443 h | [38] |
| MW-IrO2/rGO | 251 | 0.25 | 34.7 | - | [39] |
| IrO2@TaOx@TaB | 279 | 0.26 | 50 | 1500 h | [40] |
| Ir-IrO2/C-3 | 264 | - | 63.3 | 42 h | [41] |
| IrO2-BN-rGO | 300 | 0.140 | 65.2 | - | [42] |
| IrO2-c-BN | 400 | 0.140 | 61.0 | - | [42] |
| IrO2/TSO | 263 | 0.131 | 51 | 200 h | [43] |
| IrO2@Co3O4-CoMoO4 | 236 | - | 46.5 | 6 h | [44] |
| IrO2/TNTs/Ti | 400 | 0.514 | - | 550 h | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhu, J.; Cai, H.; Tong, Z.; Wang, X.; Wei, Y.; Wang, X.; Hu, C.; Zhao, X.; Zhang, X. Research Progress on the Application of One-Step Fabrication Techniques for Iridium-Based Thin Films in the Oxygen Evolution Reaction. Coatings 2024, 14, 1147. https://doi.org/10.3390/coatings14091147
Li W, Zhu J, Cai H, Tong Z, Wang X, Wei Y, Wang X, Hu C, Zhao X, Zhang X. Research Progress on the Application of One-Step Fabrication Techniques for Iridium-Based Thin Films in the Oxygen Evolution Reaction. Coatings. 2024; 14(9):1147. https://doi.org/10.3390/coatings14091147
Chicago/Turabian StyleLi, Wenting, Junyu Zhu, Hongzhong Cai, Zhongqiu Tong, Xian Wang, Yan Wei, Xingqiang Wang, Changyi Hu, Xingdong Zhao, and Xuxiang Zhang. 2024. "Research Progress on the Application of One-Step Fabrication Techniques for Iridium-Based Thin Films in the Oxygen Evolution Reaction" Coatings 14, no. 9: 1147. https://doi.org/10.3390/coatings14091147
APA StyleLi, W., Zhu, J., Cai, H., Tong, Z., Wang, X., Wei, Y., Wang, X., Hu, C., Zhao, X., & Zhang, X. (2024). Research Progress on the Application of One-Step Fabrication Techniques for Iridium-Based Thin Films in the Oxygen Evolution Reaction. Coatings, 14(9), 1147. https://doi.org/10.3390/coatings14091147






