Fatty Imidazolines as a Green Corrosion Inhibitor of Bronze Exposed to Acid Rain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Testing Material
2.2. Synthesis of Fatty Imidazolines (2-(2-Alkyl-4,5-dihydro-1H-imidazoline-1-yl) Ethanol
2.3. Electrochemical Tests
2.4. Analysis of Oxides on the Surface
2.5. Analysis of Surface Wettability
3. Results and Discussion
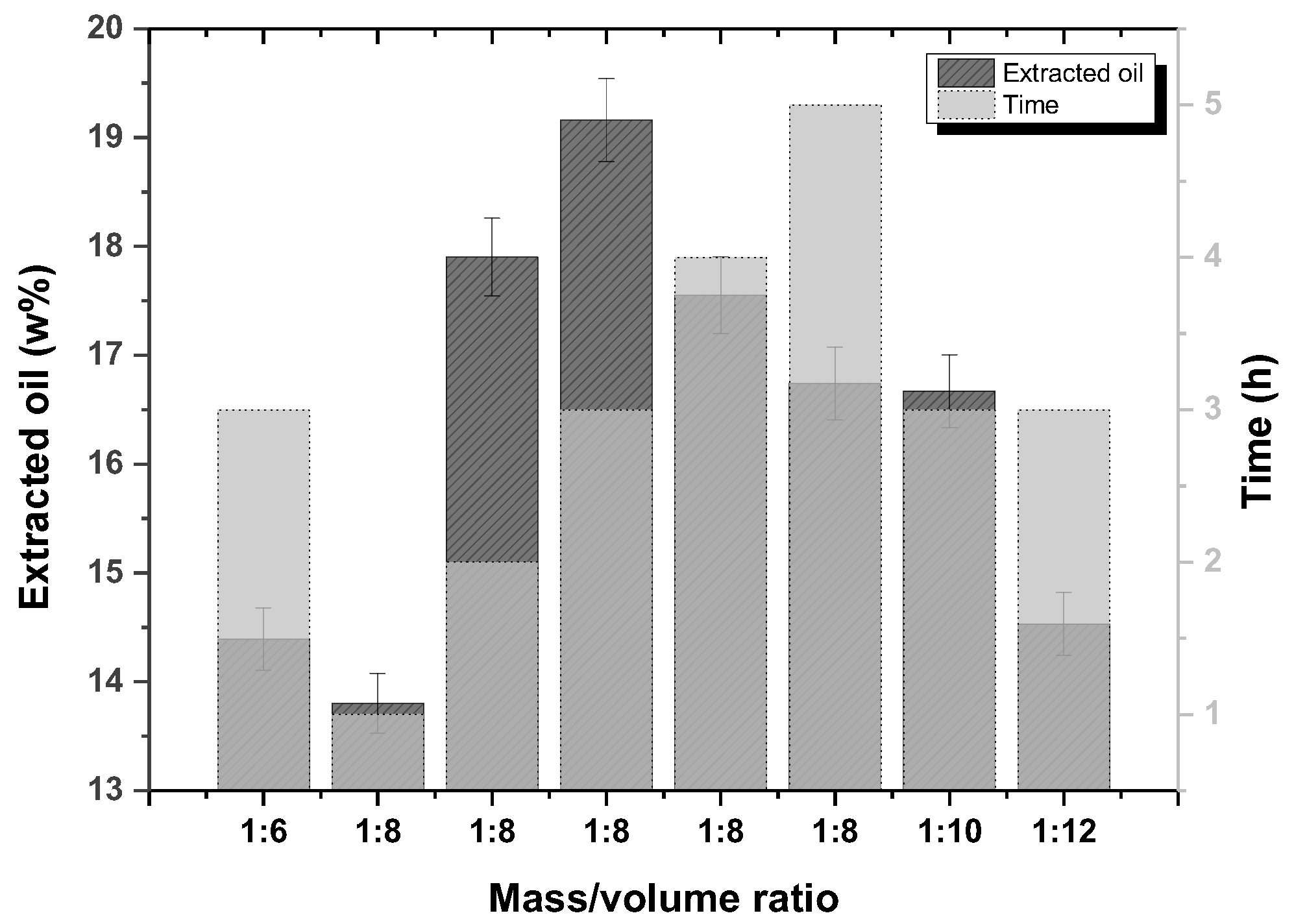
3.1. Oil Extraction from Rice Bran
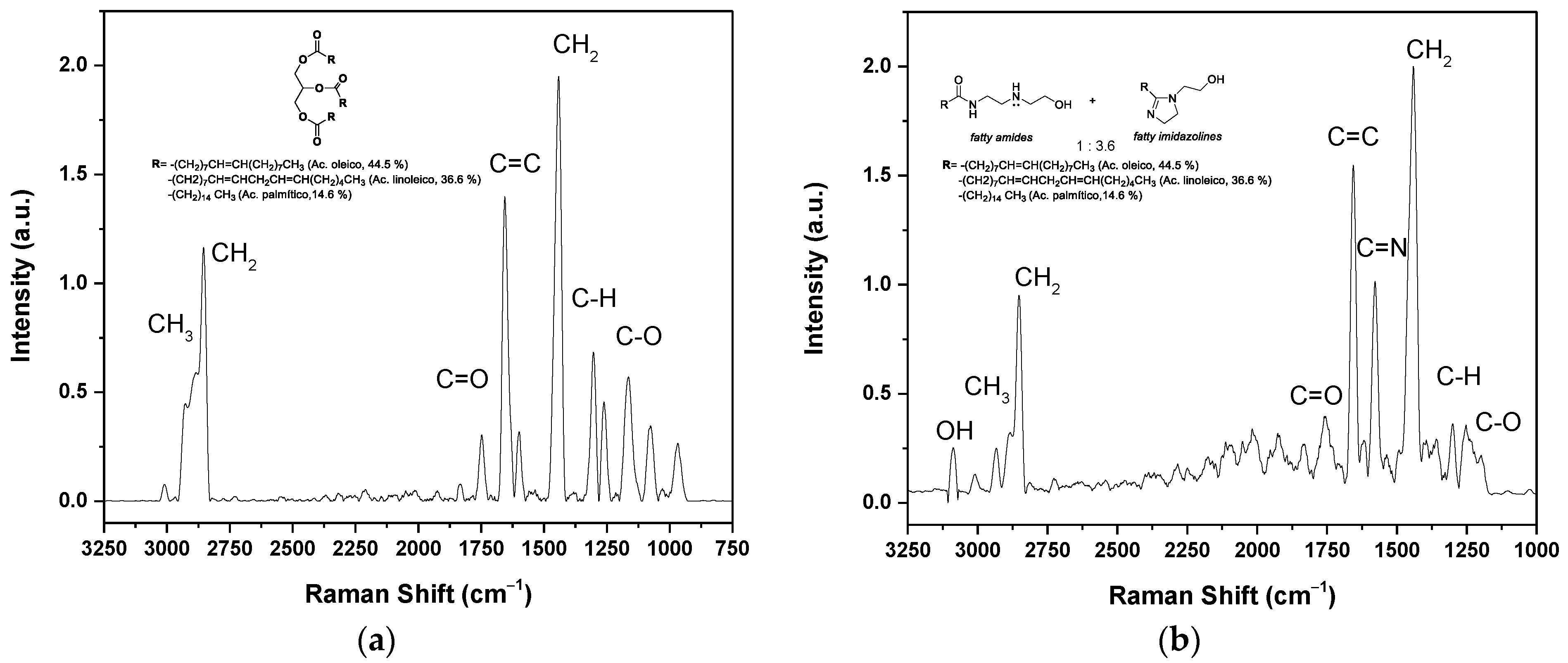
3.2. Chemical Analysis of the Oil and Green Corrosion Inhibitor
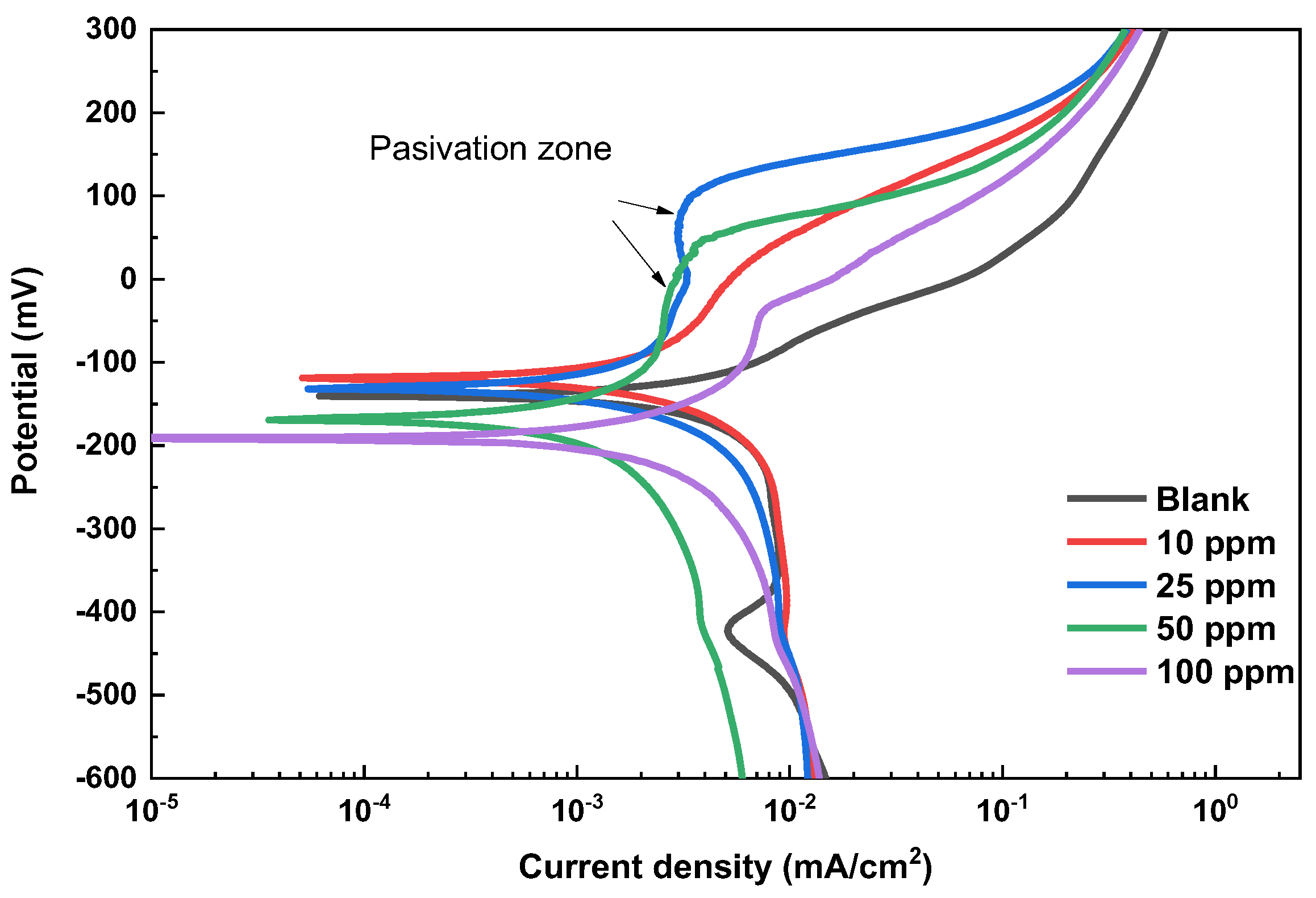
3.3. Polarization Curves
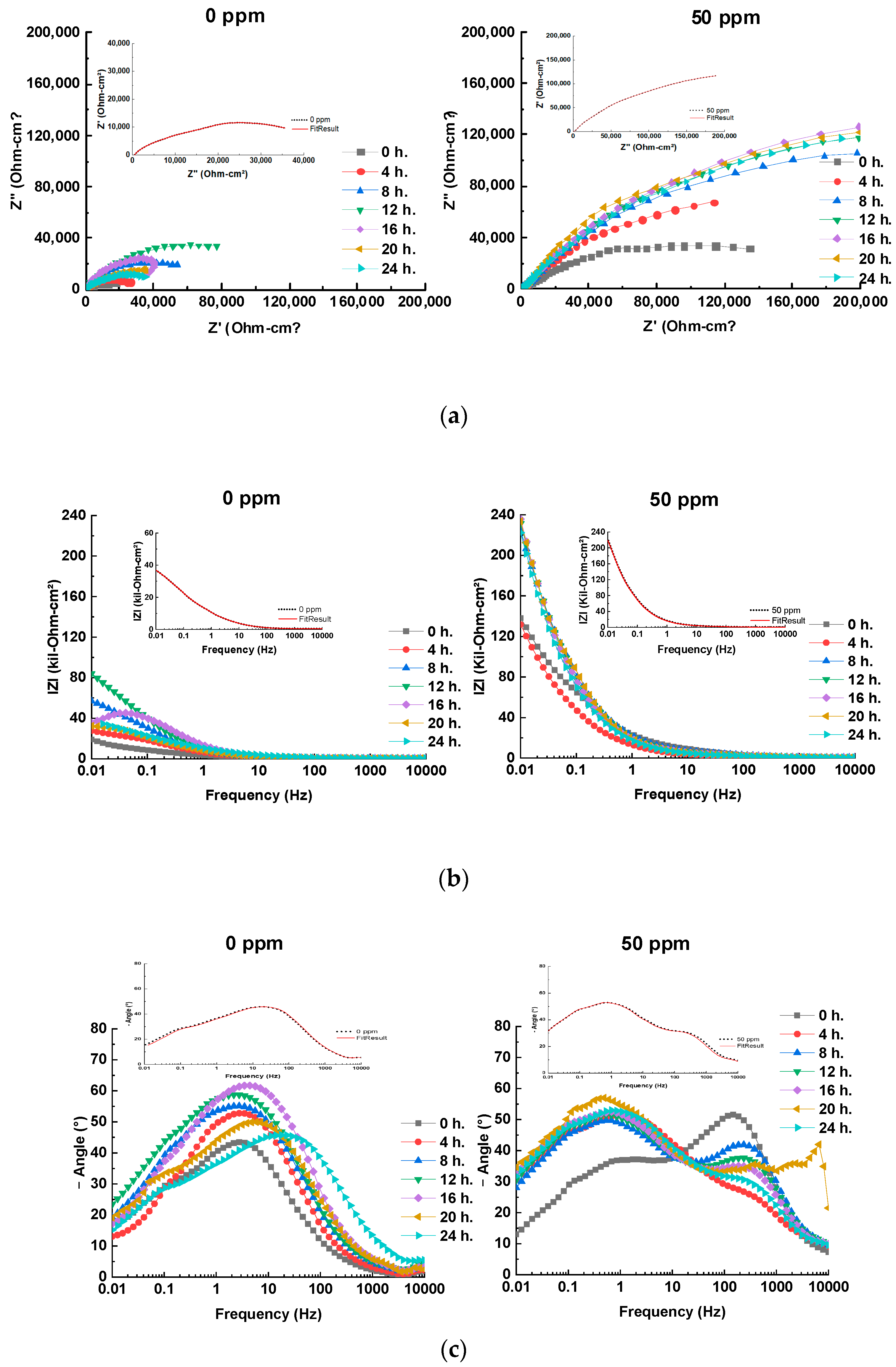
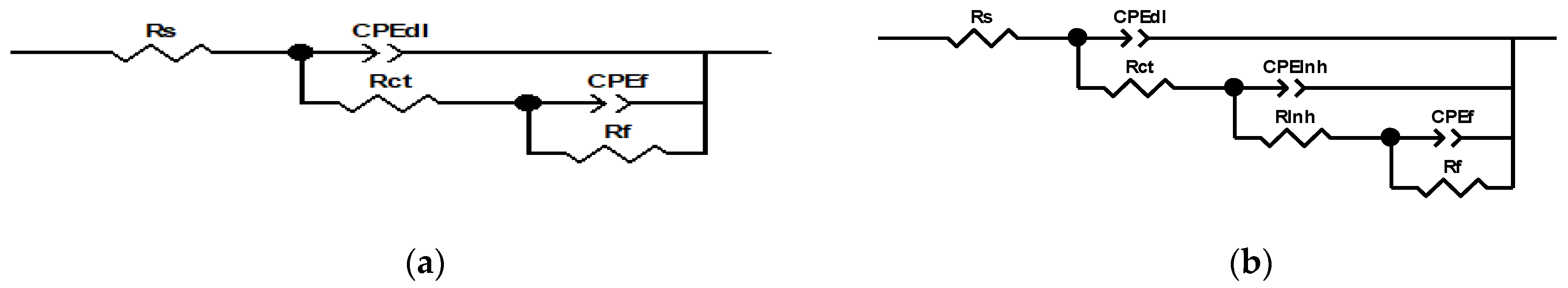
3.4. EIS Results and Equivalent Circuit Models
3.5. Scanning Electron Microscopy
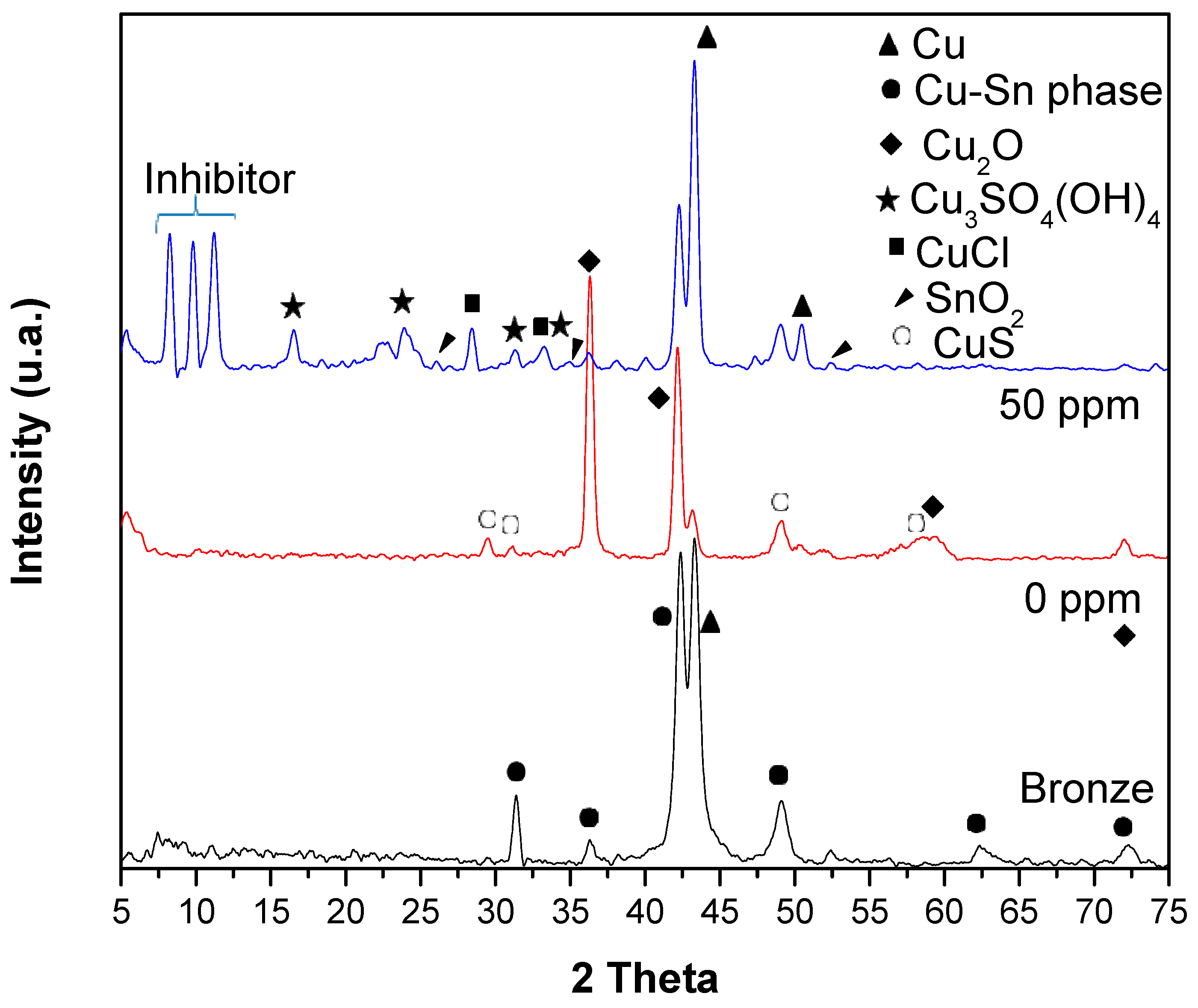
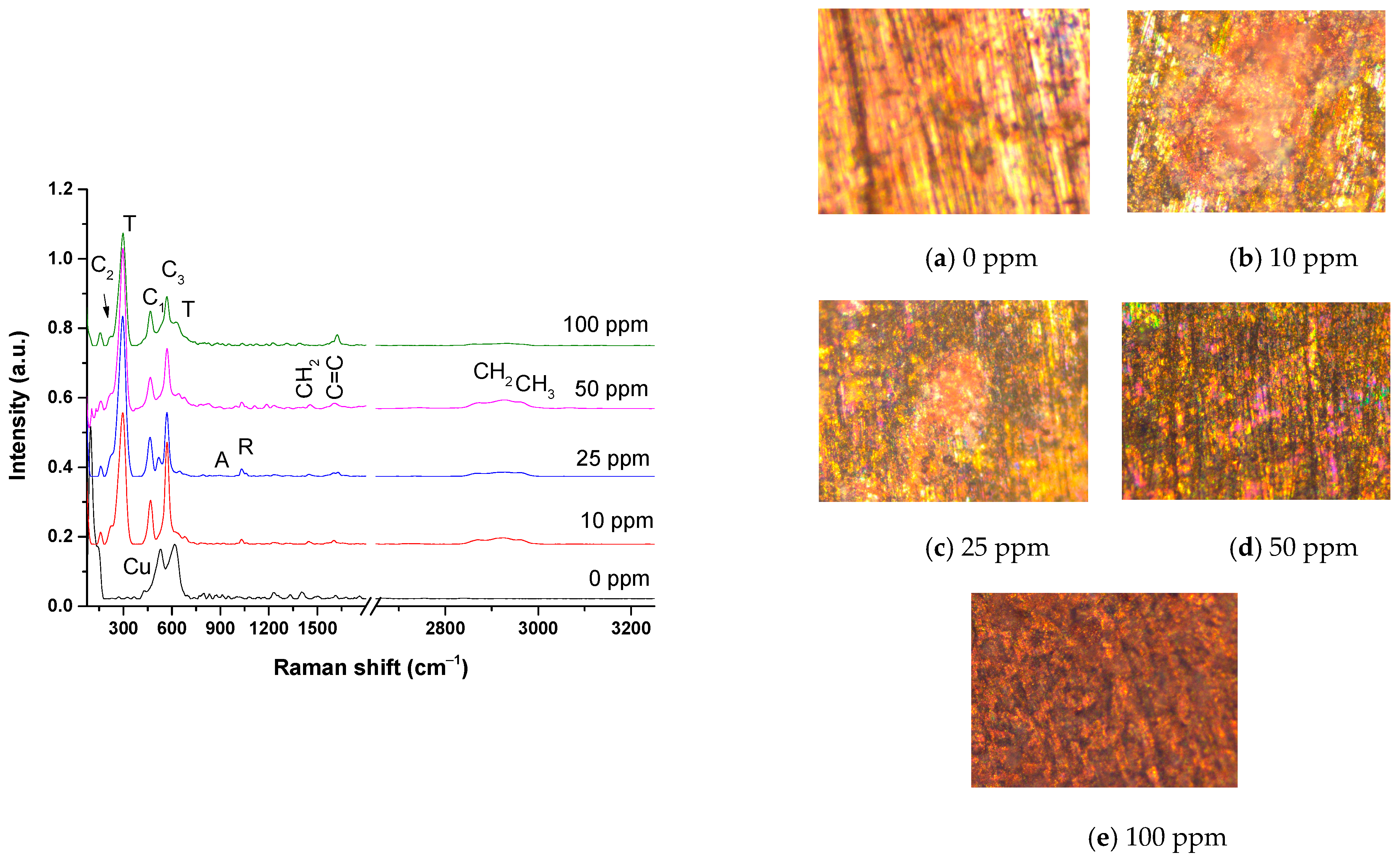
3.6. XRD and Raman Spectroscopy Analysis
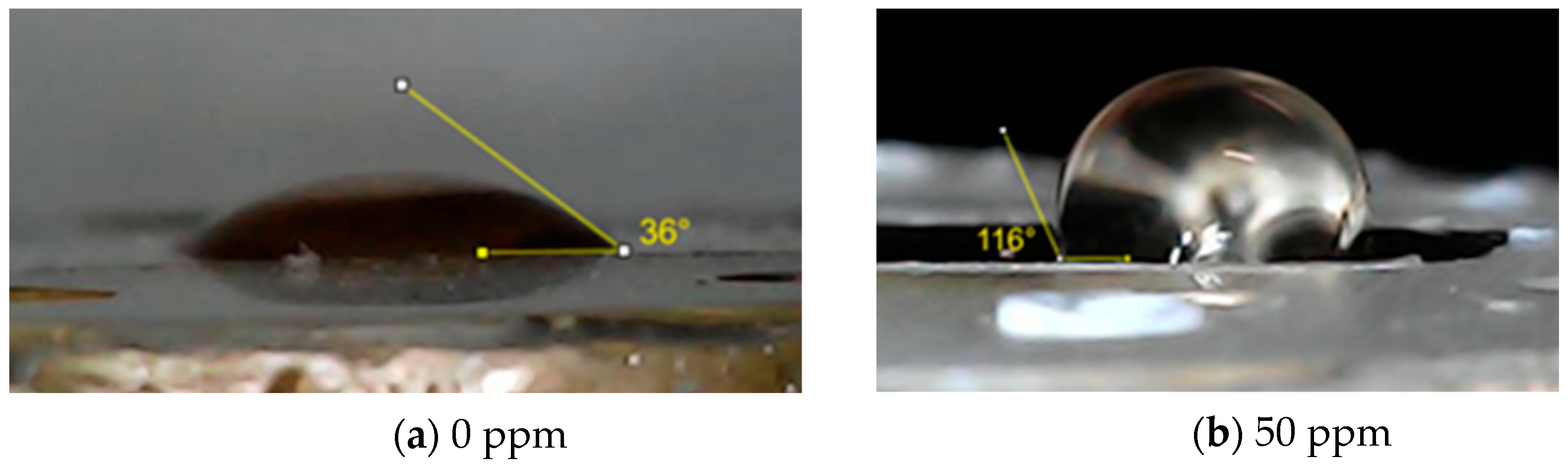
3.7. Surface Wettability
3.8. Adsorption Isotherm
- qe is the amount of inhibitor adsorbed per unit mass of the adsorbent (mg/g or mmol/g).
- Ce is the equilibrium concentration of the inhibitor in the solution (mg/L or mmol/L).
- Kf is the Freundlich constant related to the adsorption capacity.
- 1/n is the Freundlich exponent that indicates the intensity of adsorption.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Z.; Zhang, J.; Xiao, Z.; Lu, T.; Ren, X.; Wei, H. Effects of acid rain on plant growth: A meta-analysis. J. Environ. Manag. 2021, 297, 113213. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.S. Acid Rain. In Introduction to Chemistry; LibreTexts, Ed.; California State University: Long Beach, CA, USA, 2024; pp. 724–727. [Google Scholar]
- Lal, N. Effects of acid rain on plant growth and development. e-J. Sci. Technol. 2016, 11, 85–101. [Google Scholar]
- Van Roy, W.; Van Roozendael, B.; Vigin, L.; Van Nieuwenhove, A.; Scheldeman, K.; Merveille, J.-B.; Weigelt, A.; Mellqvist, J.; Van Vliet, J.; van Dinther, D.; et al. International maritime regulation decreases sulfur dioxide but increases nitrogen oxide emissions in the North and Baltic Sea. Commun. Earth Environ. 2023, 4, 391. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, C.; Chen, L.; Huang, G.; Lamarque, J.-F.; Nie, J.; Yang, J.; Hu, K.; Liu, P.; Wang, J.; et al. Sulfur emissions from consumption by developed and developing countries produce comparable climate impacts. Nat. Geosci. 2022, 15, 184–189. [Google Scholar] [CrossRef]
- Sosa-Echeverría, R.; Bravo-Álvarez, H.; Alarcón-Jiménez, A.L.; Torres-Barrera, M.D.C.; Jaimes-Palomera, M.; Sánchez-Álvarez, P.; Granados-Hernández, E. Acid rain in a Mexican site on the coast of the Gulf of Mexico. Atmosphere 2018, 31, 317–330. [Google Scholar] [CrossRef]
- Sosa-Echeverría, R.; Alarcón-Jimémez, A.L.; Torres-Barrera, M.D.C.; Jaimes-Palomera, M.; Retama-Hernández, A.; Sánchez-Álvarez, P.; Granados Hernández, E.; Bravo-Álvarez, H. Spatial and temporal variation of acid rain in the Mexico City Metropolitan Zone. Atmosphere 2019, 32, 55–69. [Google Scholar] [CrossRef]
- Jaramillo, J.V. Los 100 Sitios y Monumentos más Importantes del Centro Histórico de la Ciudad de México, CDMX: Matesis Asociados, S de R. L. Mi. 2012. Available online: http://centro.paot.org.mx/documentos/gdf/sitios_monumentos.pdf (accessed on 1 January 2024).
- Rahmouni, K.; Takenouti, H.; Hajjaji, N.; Srhiri, A.; Robbiola, L. Protection of ancient and historic bronzes by triazole derivatives. Electrochim. Acta 2009, 54, 5206–5215. [Google Scholar] [CrossRef]
- Serghini-Idrissi, M.; Bernard, M.; Harrif, F.; Joiret, S.; Rahmouni, K.; Srhiri, A.; Takenouti, H.; Vivier, V.; Ziani, M. Electrochemical and spectroscopic characterizations of patinas formed on an archaeological bronze coin. Electrochim. Acta 2005, 50, 4699–4709. [Google Scholar] [CrossRef]
- Li, W.; Hu, L.; Zhang, S.; Hou, B. Effects of two fungicides on the corrosion resistance of copper in 3.5% NaCl solution under various conditions. Corros. Sci. 2011, 53, 735–745. [Google Scholar] [CrossRef]
- Velazquez-Torres, N.; Martinez, H.; Porcayo-Calderon, J.; Vazquez-Velez, E.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. Use of an amide-type corrosion inhibitor synthesized from the coffee bagasse oil on the corrosion of Cu in NaCl. Green Chem. Lett. Rev. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Pillard, D.; Cornell, J.S.; DuFresne, D.L.; Hernandez, M.T. Toxicity of Benzotriazole and Benzotriazole Derivatives to Three Aquatic Species. Water Res. 2001, 35, 557–560. [Google Scholar] [CrossRef]
- MGil, M.J.; Soto, A.M.; Usma, J.I.; Gutiérrez, O.D. Contaminantes emergentes en aguas, efectos y posibles cambios. Prod. + Limpia 2012, 7, 52–73. [Google Scholar]
- Tasic, Z.Z.; Mihajlovic, M.B.P.; Simonovic, A.T.; Radovanovic, M.B.; Antonijevic, M.M. Ibuprofen as a corrosion inhibitor for copper in synthetic acid rain solution. Sci. Rep. 2019, 9, 14710. [Google Scholar] [CrossRef] [PubMed]
- Montaser, A.A.; El-Mahdy, M.S.; Mahmoud, E.E.E.; Fouda, A.S. Recycling of expired ciprofloxacin in synthetic acid rain (SAR) solution as a green corrosion inhibitor for copper: A theoretical and experimental evaluation. J. Appl. Electrochem. 2024, 54, 439–456. [Google Scholar] [CrossRef]
- Gardić, V.; Tasić, Ž.Z.; Mihajlović, M.B.P.; Radovanović, M.B.; Antonijević, M.M. Corrosion Behavior of the Cu24Zn5Al Alloy in Sodium Sulfate Solution in the Presence of 1-Phenyl-5-mercaptotetrazole. Metals 2023, 13, 1863. [Google Scholar] [CrossRef]
- Abdel-Karim, A.M.; El-Shamy, A.M. A Review on Green Corrosion Inhibitors for Protection of Archeological Metal Artifacts. J. Bio-Tribo-Corros. 2022, 8, 35. [Google Scholar] [CrossRef]
- Reyes-Dorantes, E.; Zúñiga-Díaz, J.; Quinto-Hernández, A.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.; Pedraza-Basulto, G.; Martínez-Gomez, L. Rice Bran as Source for the Synthesis of Imidazoline-type Inhibitors: Synthesis and Corrosion Performance. Int. J. Electrochem. Sci. 2018, 13, 101–118. [Google Scholar] [CrossRef]
- Sotelo, O.; Henao, J.; Vazquez-Velez, E.; Poblano-Salas, C.A.; Martinez-Valencia, H. Corrosion Inhibition Studies of Synthesized Oleic Sources-Based Green Inhibitors from Agro-Industrial Waste. In Handbook of Research on Corrosion Science and Engineering; IGI Global: Hershey, PA, USA, 2023; pp. 362–382. [Google Scholar]
- Cruz-Zabalegui, A.; Vazquez-Velez, E.; Galicia-Aguilar, G.; Casales-Diaz, M.; Lopez-Sesenes, R.; Gonzalez-Rodriguez, J.; Martinez-Gomez, L. Use of a non-ionic Gemini-surfactant synthesized from the wasted avocado oil as a CO2-corrosion inhibitor for X-52 steel. Ind. Crop. Prod. 2019, 133, 203–211. [Google Scholar] [CrossRef]
- Sanchez-Salazar, E.; Vazquez-Velez, E.; Uruchurtu, J.; Porcayo-Calderon, J.; Casales, M.; Rosales-Cadena, I.; Lopes-Cecenes, R.; Gonzalez-Rodriguez, J.G. Use of a Gemini-Surfactant Synthesized from the Mango Seed Oil as a CO2-Corrosion Inhibitor for X-120 Steel. Materials 2021, 15, 4206. [Google Scholar] [CrossRef]
- Cárdenas, D.; Olaya, J.; Herrera, L.K. Estudio de la Resistencia a la Corrosión en Recubrimientos de Bronce al aluminio Proxon 21071® producidos con la técnica de proyección Térmica por Llama. Rev. Colomb. Mater. 2014, 5, 170–176. [Google Scholar] [CrossRef]
- Zúñiga-Diaz, J.; Reyes-Dorantes, E.; Quinto-Hernandez, A.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. Oil Extraction from “Morelos Rice” Bran: Kinetics and Raw Oil Stability. J. Chem. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Schönemann, A.E.; Edwards, H.G.M. Raman and FTIR microspectroscopic study of the alteration of Chinese tung oil and related drying oils during ageing. Anal. Bioanal. Chem. 2011, 400, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Porcayo-Calderon, J.; Regla, I.; Vazquez-Velez, E.; de la Escalera, L.M.M.; Canto, J.; Casales-Diaz, M. Effect of the Unsaturation of the Hydrocarbon Chain of Fatty-Amides on the CO2 Corrosion of Carbon Steel Using EIS and Real-Time Corrosion Measurement. J. Spectrosc. 2015, 2015, 184140. [Google Scholar] [CrossRef]
- Amin, M.A.; Arida, H.; Kandemirli, F.; Saracoglu, M.; Arslan, T.; Basaran, M.A. Monitoring corrosion and corrosion control of iron in HCl by non-ionic surfactants of the TRITON-X series—Part III. Immersion time effects and theoretical studies. Corros. Sci. 2011, 53, 1895–1909. [Google Scholar] [CrossRef]
- Marušić, K.; Ćurković, H.O.; Takenouti, H. Corrosion Inhibition of Bronze and Its Patina Exposed to Acid Rain. J. Electrochem. Soc. 2013, 160, C356–C363. [Google Scholar] [CrossRef]
- Simonović, A.T.; Tasić, Z.; Radovanović, M.B.; Mihajlović, M.B.P.; Antonijević, M.M. Influence of 5-Chlorobenzotriazole on Inhibition of Copper Corrosion in Acid Rain Solution. ACS Omega 2020, 5, 12832–12841. [Google Scholar] [CrossRef]
- Hassairi, H.; Bousselmi, L.; Khosrof, S.; Triki, E. Evaluation of the inhibitive effect of benzotriazole on archeological bronze in acidic medium. Appl. Phys. A 2013, 113, 923–931. [Google Scholar] [CrossRef]
- Elachouri, M.; Hajji, M.S.; Kertit, S.; Essassi, E.M.; Salem, M.; Coudert, R. Coudert, Some surfactants in the series of 2-(alkyldimethylammonio) alkanol bromides as inhibitors of the corrosion of iron in acid chloride solution. Corros. Sci. 1995, 37, 381–389. [Google Scholar] [CrossRef]
- Kwon, H. Corrosion Behaviors of Artificial Chloride Patina for Studying Bronze Sculpture Corrosion in Marine Environments. Coatings 2023, 13, 1630. [Google Scholar] [CrossRef]
- Bernardi, E.; Chiavari, C.; Lenza, B.; Martini, C.; Morselli, L.; Ospitali, F.; Robbiola, L. The atmospheric corrosion of quaternary bronzes: The leaching action of acid rain. Corros. Sci. 2009, 51, 159–170. [Google Scholar] [CrossRef]
- Gianni, L.; Cavallini, M.; Natali, S.; Adriaens, A. Wet and Dry Accelerated Aging Tests in a Spray Chamber to Understand the Effects of Acid Rain Frequencies on Bronze Corrosion. Int. J. Electrochem. Sci. 2013, 8, 1822–1838. [Google Scholar] [CrossRef]
- Kwon, H.; Cho, N. Corrosion Behaviors of Outdoor Bronze Sculptures in an Urban–Industrial Environment: Corrosion Experiment on Artificial Sulfide Patina. Metals 2023, 13, 1101. [Google Scholar] [CrossRef]
- Juškėnas, R.; Mockus, Z.; Kanapeckaitė, S.; Stalnionis, G.; Survila, A. XRD studies of the phase composition of the electrodeposited copper-rich Cu–Sn alloys. Electrochim. Acta 2006, 52, 928–935. [Google Scholar] [CrossRef]
- De Oliveira, F.J.R.; Lago, D.C.B.; Senna, L.F.; De Miranda, L.R.M.; D’elia, E. Study of patina formation on bronze specimens. Mater. Chem. Phys. 2009, 115, 761–770. [Google Scholar] [CrossRef]
- Graedel, T.E.; Nassau, K.; Franey, J.P. Copper patinas formed in the atmosphere—I. Introduction. Corros. Sci. 1987, 27, 639–657. [Google Scholar] [CrossRef]
- Di Carlo, G.; Giuliani, C.; Riccucci, C.; Pascucci, M.; Messina, E.; Fierro, G.; Lavorgna, M.; Ingo, G.M. Artificial patina formation onto copper-based alloys: Chloride and sulphate induced corrosion processes. Appl. Surf. Sci. 2017, 421, 120–127. [Google Scholar] [CrossRef]
- FitzGerald, K.P.; Nairn, J.; Skennerton, G.; Atrens, A. Atmospheric corrosion of copper and the colour, structure and composition of natural patinas on copper. Corros. Sci. 2006, 48, 2480–2509. [Google Scholar] [CrossRef]
- Milošev, I.; Kovačević, N.; Kovač, J.; Kokalj, A. The roles of mercapto, benzene and methyl groups in the corrosion inhibition of imidazoles on copper: I. Experimental characterization. Corros. Sci. 2015, 98, 107–118. [Google Scholar] [CrossRef]
- Privitera, A.; Corbascio, A.; Calcani, G.; Della Ventura, G.; Ricci, M.A.; Sodo, A. Raman approach to the forensic study of bronze patinas. J. Archaeol. Sci. Rep. 2021, 39, 103115. [Google Scholar] [CrossRef]
- Kosec, T.; Ropret, P.; Legat, A. Raman investigation of artificial patinas on recent bronze—Part II: Urban rain exposure. J. Raman Spectrosc. 2012, 43, 1587–1595. [Google Scholar] [CrossRef]
- Hayez, V.; Costa, V.; Guillaume, J.; Terryn, H.; Hubin, A. Micro Raman spectroscopy used for the study of corrosion products on copper alloys: Study of the chemical composition of artificial patinas used for restoration purposes. Analyst 2005, 130, 550–556. [Google Scholar] [CrossRef]
- Sharygin, L.M.; Vovk, S.M. Raman spectroscopy studies of structural changes in hydrated titanium and tin dioxide gels under drying. J. Appl. Spectrosc. 1997, 64, 283–286. [Google Scholar] [CrossRef]
- Said, K.A.M.; Ismail, N.Z.; Jama’In, R.L.; Alipah, N.A.M.; Sutan, N.M.; Gadung, G.G.; Baini, R.; Zauzi, N.S.A. Application of Freundlich and Temkin Isotherm to Study the Removal of Pb(II) Via Adsorption on Activated Carbon Equipped Polysulfone Membrane. Int. J. Eng. Technol. 2018, 7, 91–93. [Google Scholar] [CrossRef]
- Akinbulumo, O.A.; Odejobi, O.J.; Odekanle, E.L. Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results Mater. 2020, 5, 100074. [Google Scholar] [CrossRef]


| Element | Al | Cu | Fe | Ni | P | Pb | Si | Sn | Zn |
|---|---|---|---|---|---|---|---|---|---|
| SAE 62 | 0.07 | 87.59 | 0.14 | 0.60 | 0.26 | 0.38 | 0.28 | 10.1 | 0.33 |
| Ions | Ca2+ | Cl− | Mg2+ | SO42− | NH4+ | NO3− | K+ |
|---|---|---|---|---|---|---|---|
| mgL−1 | 0.264 | 0.339 | 0.066 | 0.066 | 0.319 | 0.319 | 0.075 |
| Inhibitor Concentration | Ecorr (mV) vs. Ag/AgCl E | Icorr (µAcm−2) | βc (mV/Dec) | βa (mV/Dec) | Inhibitor Efficiency (%) | Surface Coverage (Θ) | Vcorr (mm/year) |
|---|---|---|---|---|---|---|---|
| 0 ppm | −140.69 | 5.84 | −169 | 128 | -- | -- | 0.044 |
| 10 ppm | −117.80 | 1.88 | −198 | 179 | 68% | 0.68 | 0.014 |
| 25 ppm | −131.96 | 1.38 | −209 | 175 | 76% | 0.76 | 0.010 |
| 50 ppm | −168.86 | 0.61 | −228 | 158 | 90% | 0.90 | 0.004 |
| 100 ppm | −190.23 | 1.71 | −230 | 183 | 71% | 0.71 | 0.013 |
| Cinh (ppm) | Rs (Ω×cm2) | CPEdl (F/cm2) | Rct (Ω×cm2) | CPEInh (F/cm2) | RInh (Ω×cm2) | CPEf (F/cm2) | Rf (Ω×cm2) | Rp (Ω×cm2) | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm | 457 | 8.79 × 10−6 | 5897 | -- | -- | 3.98 × 10−5 | 56,292 | 62,646 | 1.14 × 10−3 |
| 10 ppm | 503 | 3.12 × 10−6 | 9924 | 4.47 × 10−5 | 71,437 | 2.41 × 10−7 | 19,873 | 101,737 | 6.52 × 10−4 |
| 25 ppm | 533 | 2.46 × 10−6 | 12,136 | 3.48 × 10−5 | 148,532 | 1.09 × 10−3 | 60,289 | 221,490 | 5.40 × 10−4 |
| 50 ppm | 574 | 2.01 × 10−6 | 15,367 | 2.98 × 10−5 | 181,297 | 2.83 × 10−5 | 182,413 | 379,651 | 2.55 × 10−3 |
| 100 ppm | 521 | 1.82 × 10−6 | 14,289 | 2.54 × 10−5 | 159,874 | 3.47 × 10−4 | 151,629 | 326,313 | 6.26 × 10−4 |
| Cinh (ppm) | Rs (Ω×cm2) | CPEdl (F/cm2) | Rct (Ω×cm2) | CPEInh (F/cm2) | RInh (Ω×cm2) | CPEf (F/cm2) | Rf (Ω×cm2) | Rp (Ω×cm2) | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm | 457 | 8.79 × 10−6 | 5897 | -- | -- | 3.98 × 10−5 | 56,292 | 62,646 | 1.14 × 10−3 |
| 50 ppm | |||||||||
| 0 h | 374 | 6.11 × 10−6 | 9573 | 1.97 × 10−5 | 104,497 | 4.01 × 10−5 | 96,043 | 210,487 | 2.58 × 10−3 |
| 4 h | 487 | 5.95 × 10−6 | 11,150 | 2.29 × 10−5 | 132,668 | 3.92 × 10−5 | 126,440 | 270,745 | 2.52 × 10−3 |
| 8 h | 552 | 4.70 × 10−6 | 15,824 | 2.55 × 10−5 | 152,811 | 3.80 × 10−5 | 164,330 | 333,517 | 2.56 × 10−3 |
| 12 h | 590 | 3.68 × 10−6 | 17,538 | 2.88 × 10−5 | 179,273 | 2.85 × 10−5 | 180,523 | 377,924 | 2.51 × 10−3 |
| 16 h | 615 | 1.40 × 10−6 | 22,432 | 3.38 × 10−5 | 240,916 | 2.50 × 10−5 | 195,445 | 459,408 | 2.50 × 10−3 |
| 20 h | 604 | 1.91 × 10−6 | 19,758 | 3.02 × 10−5 | 223,555 | 2.72 × 10−5 | 188,536 | 432,453 | 2.53 × 10−3 |
| 24 h | 574 | 2.01 × 10−6 | 15,367 | 2.98 × 10−5 | 181,297 | 2.83 × 10−5 | 182,413 | 379,651 | 2.55 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Aguirre, I.D.; Torres-Islas, A.; Vázquez-Vélez, E.; Martínez, H.; del Pozo-Mares, A.; Cotero-Villegas, A.M. Fatty Imidazolines as a Green Corrosion Inhibitor of Bronze Exposed to Acid Rain. Coatings 2024, 14, 1152. https://doi.org/10.3390/coatings14091152
Vázquez-Aguirre ID, Torres-Islas A, Vázquez-Vélez E, Martínez H, del Pozo-Mares A, Cotero-Villegas AM. Fatty Imidazolines as a Green Corrosion Inhibitor of Bronze Exposed to Acid Rain. Coatings. 2024; 14(9):1152. https://doi.org/10.3390/coatings14091152
Chicago/Turabian StyleVázquez-Aguirre, Ian Didiere, Alvaro Torres-Islas, Edna Vázquez-Vélez, Horacio Martínez, Adrián del Pozo-Mares, and Ave María Cotero-Villegas. 2024. "Fatty Imidazolines as a Green Corrosion Inhibitor of Bronze Exposed to Acid Rain" Coatings 14, no. 9: 1152. https://doi.org/10.3390/coatings14091152
APA StyleVázquez-Aguirre, I. D., Torres-Islas, A., Vázquez-Vélez, E., Martínez, H., del Pozo-Mares, A., & Cotero-Villegas, A. M. (2024). Fatty Imidazolines as a Green Corrosion Inhibitor of Bronze Exposed to Acid Rain. Coatings, 14(9), 1152. https://doi.org/10.3390/coatings14091152








