A Narrative Review: Modification of Bio-Based Wood Adhesive for Performance Improvement
Abstract
:1. Introduction
2. Improvement of Bond Strength Performance
2.1. Protein-Based Adhesives
2.2. Lignin-Based Adhesives
2.3. Tannin-Based Adhesives
2.4. Polysaccharide-Based Adhesives
3. Improvement of Water Resistance
3.1. Protein-Based Adhesives
3.2. Lignin-Based Adhesives
3.3. Tannin-Based Adhesives
4. Antimicrobial Performance
4.1. Protein-Based Adhesives
4.2. Tannin-Based Adhesives
5. Conclusions
- (1)
- Bio-based adhesives offer a diverse array of raw material origins and possess the advantages of being renewable and degradable. Their development and application can address the problems of organic volatile emissions and reliance on fossil resources inherent in synthetic resin adhesives. The primary challenge lies in overcoming the bonding performance, water resistance, and mold resistance of bio-based adhesives during their use.
- (2)
- This paper presents a review of recent developments in the functional modification of bio-based adhesives. It provides a solution to the issues of formaldehyde release and excessive reliance on fossil resources for synthetic resin-based adhesives. The modification of bio-based adhesives leads to significant improvement in bonding performance, waterproofing performance, and mold resistance, which could be achieved by constructing multiple network structures, adding chemical reagents, and introducing functional groups. However, some of these modification methods have limitations, such as the corrosive nature of the modifying reagents, the high price of the modifying reagents, and the complexity of the modification steps. More research and development can be conducted in the future on the topic of modification methods for bio-based adhesives that can solve these problems.
- (3)
- The functional modification of bio-based adhesive could significantly expand the utilization scope of this adhesive in various areas. It can be employed as an alternative to traditional aldehyde-based resin adhesive. Furthermore, some of these bio-based adhesives have been employed in wood-based composite preparation, thereby facilitating the development of eco-friendly material manufacture. However, in the wood processing area, bio-based adhesives still have some problems, such as high viscosity and poor stability, which urgently need to be solved to improve their comprehensive performances. Fortunately, many researchers have an interest in the research of bio-based adhesives, which could be used in various fields. This will promote the green development of the wood industry.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent progress in ultra-low formaldehyde emitting adhesive systems and formaldehyde scavengers in wood-based panels: A review. Wood Mater. Sci. Eng. 2023, 18, 763–782. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Wang, Z.; Zhang, S.; Li, J. Biomimetic water-in-oil water/pMDI emulsion as an excellent ecofriendly adhesive for bonding wood-based composites. J. Hazard. Mater. 2020, 396, 122722. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Soy and cottonseed protein blends as wood adhesives. Ind. Crops Prod. 2016, 85, 324–330. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Xu, X.; Feng, H.; Hu, K.; Su, Y.; Zhou, S.; Zhu, J.; Weng, G.; Ma, S. Green and facile method for valorization of lignin to high-performance degradable thermosets. Green Chem. 2022, 24, 9659–9667. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Renders, T.; Lauwaert, J.; De Roo, T.; De Clercq, J.; Verberckmoes, A. Biobased Resins Using Lignin and Glyoxal. ACS Sustain. Chem. Eng. 2020, 8, 18789–18809. [Google Scholar] [CrossRef]
- Yang, G.; Gong, Z.; Luo, X.; Chen, L.; Shuai, L. Bonding wood with uncondensed lignins as adhesives. Nature 2023, 621, 511–515. [Google Scholar] [CrossRef]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef]
- Zhao, M.; Jing, J.; Zhu, Y.; Yang, X.; Wang, X.; Wang, Z. Preparation and performance of lignin-phenol-formaldehyde adhesives. Int. J. Adhes. Adhes. 2016, 64, 163–167. [Google Scholar] [CrossRef]
- Mahrdt, E.; Pinkl, S.; Schmidberger, C.; van Herwijnen, H.W.G.; Veigel, S.; Gindl-Altmutter, W. Effect of addition of microfibrillated cellulose to urea-formaldehyde on selected adhesive characteristics and distribution in particle board. Cellulose 2016, 23, 571–580. [Google Scholar] [CrossRef]
- Arias, A.; González-Rodríguez, S.; Barros, M.V.; Salvador, R.; de Francisco, A.C.; Piekarski, C.M.; Moreira, M.T. Recent developments in bio-based adhesives from renewable natural resources. J. Clean. Prod. 2021, 314, 127892. [Google Scholar] [CrossRef]
- Islam, M.N.; Rahman, F.; Das, A.K.; Hiziroglu, S. An overview of different types and potential of bio-based adhesives used for wood products. Int. J. Adhes. Adhes. 2022, 112, 102992. [Google Scholar] [CrossRef]
- Jorda, J.; Cesprini, E.; Barbu, M.-C.; Tondi, G.; Zanetti, M.; Král, P. Quebracho tannin bio-based adhesives for plywood. Polymers 2022, 14, 2257. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mekala, S.; Kravchenko, O.G.; Yilmaz, T.; Yuan, D.; Yue, L.; Gross, R.A.; Manas-Zloczower, I. Design and formulation of a completely biobased epoxy structural adhesive. ACS Sustain. Chem. Eng. 2019, 7, 16382–16391. [Google Scholar] [CrossRef]
- Li, T.; Zhang, B.; Jiang, S.; Zhou, X.; Du, G.; Wu, Z.; Cao, M.; Yang, L. Novel Highly Branched Polymer Wood Adhesive Resin. Acs Sustain. Chem. Eng. 2020, 8, 5209–5216. [Google Scholar] [CrossRef]
- Wang, X.; Fang, J.; Zhu, W.; Zhong, C.; Ye, D.; Zhu, M.; Lu, X.; Zhao, Y.; Ren, F. Bioinspired Highly Anisotropic, Ultrastrong and Stiff, and Osteoconductive Mineralized Wood Hydrogel Composites for Bone Repair. Adv. Funct. Mater. 2021, 31, 2010068. [Google Scholar] [CrossRef]
- Luo, Z.; Bian, F.; Cao, X.; Shang, L.; Zhao, Y.; Bi, Y. Bio-inspired self-adhesive microparticles with melanin nanoparticles integration for wound closure and healing. Nano Today 2024, 54, 102138. [Google Scholar] [CrossRef]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-based adhesives and evaluation for wood composites application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef]
- Vnucec, D.; Mikuljan, M.; Kutnar, A.; Sernek, M.; Gorsek, A. Influence of process parameters on the bonding performance of wood adhesive based on thermally modified soy proteins. Eur. J. Wood Wood Prod. 2016, 74, 553–561. [Google Scholar] [CrossRef]
- Averina, E.; Konnerth, J.; van Herwijnen, H.W.G. Protein-based glyoxal-polyethyleneimine-crosslinked adhesives for wood bonding. J. Adhes. 2023, 99, 363–378. [Google Scholar] [CrossRef]
- Podlena, M.; Bohm, M.; Saloni, D.; Velarde, G.; Salas, C. Tuning the Adhesive Properties of Soy Protein Wood Adhesives with Different Coadjutant Polymers, Nanocellulose and Lignin. Polymers 2021, 13, 1972. [Google Scholar] [CrossRef]
- Vnučec, D.; Kutnar, A.; Goršek, A. Soy-based adhesives for wood-bonding–a review. J. Adhes. Sci. Technol. 2017, 31, 910–931. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zhang, F.; Lyu, Y.; Li, X.; Li, K.; Li, J. Spider silk-inspired high-performance soybean meal-based adhesive reinforced by greenly produced chitosan-functionalized boron nitride nanosheets. Chem. Eng. J. 2022, 438, 135442. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, R.; Xu, Y.; Chen, M.; Zhang, J.; Gao, Q.; Li, J. Developing a stable high-performance soybean meal-based adhesive using a simple high-pressure homogenization technology. J. Clean. Prod. 2020, 256, 120336. [Google Scholar] [CrossRef]
- Pang, H.; Zhao, S.; Mo, L.; Wang, Z.; Zhang, W.; Huang, A.; Zhang, S.; Li, J. Mussel-inspired bio-based water-resistant soy adhesives with low-cost dopamine analogue-modified silkworm silk Fiber. J. Appl. Polym. Sci. 2020, 137, 48785. [Google Scholar] [CrossRef]
- Pang, H.; Zhao, S.; Qin, T.; Zhang, S.; Li, J. High-performance soy protein isolate-based film synergistically enhanced by waterborne epoxy and mussel-inspired poly (dopamine)-decorated silk fiber. Polymers 2019, 11, 1536. [Google Scholar] [CrossRef]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Tahir, P.M.; Halis, R. Lignin-based copolymer adhesives for composite wood panels—A review. Int. J. Adhes. Adhes. 2019, 95, 102408. [Google Scholar] [CrossRef]
- Karthaeuser, J.; Biziks, V.; Mai, C.; Militz, H. Lignin and Lignin-Derived Compounds for Wood Applications—A Review. Molecules 2021, 26, 2533. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, T.; Hao, C.; Wang, L.; Han, J.; Liu, H.; Zhang, J. Preparation of a lignin-based vitrimer material and its potential use for recoverable adhesives. Green Chem. 2018, 20, 2995–3000. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, H.; Chen, X.; Liu, T.; Zheng, Y.; Dai, L.; Si, C. Green and ultrastrong polyphenol lignin-based vitrimer adhesive with photothermal conversion property, wide temperature adaptability, and solvent resistance. Chem. Eng. J. 2023, 477, 147216. [Google Scholar] [CrossRef]
- Gong, X.; Liu, T.; Yu, S.; Meng, Y.; Lu, J.; Cheng, Y.; Wang, H. The preparation and performance of a novel lignin-based adhesive without formaldehyde. Ind. Crops Prod. 2020, 153, 112593. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Zhou, X.; Liang, J.; Du, G.; Wu, Z. Lignin-based adhesive crosslinked by furfuryl alcohol–glyoxal and epoxy resins. Nord. Pulp Pap. Res. J. 2019, 34, 228–238. [Google Scholar] [CrossRef]
- Liu, T.; Yang, Y.; Yan, L.; Lin, B.; Dai, L.; Huang, Z.; Si, C. Custom-designed polyphenol lignin for the enhancement of poly (vinyl alcohol)-based wood adhesive. Int. J. Biol. Macromol. 2024, 258, 129132. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Zhang, S.; Gao, Q.; Xia, C.; Zhang, W.; Li, J. Depolymerization and characterization of Acacia mangium tannin for the preparation of mussel-inspired fast-curing tannin-based phenolic resins. Chem. Eng. J. 2019, 370, 420–431. [Google Scholar] [CrossRef]
- Chen, Y.; Lyu, Y.; Yuan, X.; Ji, X.; Zhang, F.; Li, X.; Li, J.; Zhan, X.; Li, J. A biomimetic adhesive with high adhesion strength and toughness comprising soybean meal, chitosan, and condensed tannin-functionalized boron nitride nanosheets. Int. J. Biol. Macromol. 2022, 219, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Oktay, S.; Kızılcan, N.; Bengü, B. Environment-friendly cornstarch and tannin-based wood adhesives for interior particleboard production as an alternative to formaldehyde-based wood adhesives. Pigment Resin Technol. 2024, 53, 173–182. [Google Scholar] [CrossRef]
- Dewantoro, A.I.; Lubis, M.A.R.; Nurliasari, D.; Mardawati, E. Emerging technologies on developing high-performance and environmentally friendly carbohydrate-based adhesives for wood bonding. Int. J. Adhes. Adhes. 2024, 134, 103801. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Starch-based adhesives for wood/wood composite bonding. Open J. Polym. Chem. 2017, 7, 19–32. [Google Scholar] [CrossRef]
- Maulana, M.I.; Lubis, M.A.R.; Febrianto, F.; Hua, L.S.; Iswanto, A.H.; Antov, P.; Kristak, L.; Mardawati, E.; Sari, R.K.; Zaini, L.H. Environmentally friendly starch-based adhesives for bonding high-performance wood composites: A review. Forests 2022, 13, 1614. [Google Scholar] [CrossRef]
- Grylewicz, A.; Spychaj, T.; Zdanowicz, M. Thermoplastic starch/wood biocomposites processed with deep eutectic solvents. Compos. Part A Appl. Sci. Manuf. 2019, 121, 517–524. [Google Scholar] [CrossRef]
- De Carvalho, A.; Curvelo, A.A.d.S.; Agnelli, J.A.M. Wood pulp reinforced thermoplastic starch composites. Int. J. Polymer. Mater. 2002, 51, 647–660. [Google Scholar] [CrossRef]
- Zhou, G.W.; Zhang, H.S.; Su, Z.P.; Zhang, X.Q.; Zhou, H.A.; Yu, L.; Chen, C.J.; Wang, X.H. A Biodegradable, Waterproof, and Thermally Processable Cellulosic Bioplastic Enabled by Dynamic Covalent Modification. Adv. Mater. 2023, 35, 10. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future opportunities for bio-based adhesives–advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef]
- Pradyawong, S.; Qi, G.; Li, N.; Sun, X.S.; Wang, D. Adhesion properties of soy protein adhesives enhanced by biomass lignin. Int. J. Adhes. Adhes. 2017, 75, 66–73. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Y.; Bian, Y.; Gao, Q.; Shi, S.Q.; Cao, J.; Zhang, Q.; Li, J. A novel universal strategy for fabricating soybean protein adhesive with excellent adhesion and anti-mildew performances. Chem. Eng. J. 2023, 452, 139359. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Y.; Li, J.; Luo, J.; Shi, S.Q.; Li, J.; Gao, Q.; Mao, A. Research progress of soybean protein adhesive: A review. J. Renew. Mater 2022, 10, 2519–2541. [Google Scholar] [CrossRef]
- Zhao, S.J.; Wang, Z.; Pang, H.W.; Li, Z.; Zhang, W.; Zhang, S.F.; Li, J.Z.; Li, L. Designing Biomimetic Microphase-Separated Motifs to Construct Mechanically Robust Plant Protein Resin with Improved Water-Resistant Performance. Macromol. Mater. Eng. 2020, 305, 1900462. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.; Zhang, W.; Qi, C.; Zhang, S.; Li, J. Bio-inspired cellulose nanofiber-reinforced soy protein resin adhesives with dopamine-induced codeposition of “water-resistant” interphases. Appl. Surf. Sci. 2019, 478, 441–450. [Google Scholar] [CrossRef]
- Averina, E.; Konnerth, J.; van Herwijnen, H.W. Protein Adhesives: Investigation of Factors Affecting Wet Strength of Alkaline Treated Proteins Crosslinked with Glyoxal. Polymers 2022, 14, 4351. [Google Scholar] [CrossRef]
- Pang, H.; Wang, Y.; Chang, Z.; Xia, C.; Han, C.; Liu, H.; Li, J.; Zhang, S.; Cai, L.; Huang, Z. Soy meal adhesive with high strength and water resistance via carboxymethylated wood fiber-induced crosslinking. Cellulose 2021, 28, 3569–3584. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, H.; Yan, L.; Yang, H.; Dai, L.; Si, C. Lignin-Based Encapsulation of Liquid Metal Particles for Flexible and High-Efficiently Recyclable Electronics. Adv. Funct. Mater. 2024, 34, 2310653. [Google Scholar] [CrossRef]
- Siahkamari, M.; Emmanuel, S.; Hodge, D.B.; Nejad, M. Lignin-glyoxal: A fully biobased formaldehyde-free wood adhesive for interior engineered wood products. ACS Sustain. Chem. Eng. 2022, 10, 3430–3441. [Google Scholar] [CrossRef]
- Huo, M.; Chen, J.; Jin, C.; Huo, S.; Liu, G.; Kong, Z. Preparation, characterization, and application of waterborne lignin-based epoxy resin as eco-friendly wood adhesive. Int. J. Biol. Macromol. 2024, 259, 129327. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhu, X.; Peng, H.; Chen, J.; Zhang, F.; Zhao, Q. Facile preparation of lignin-based underwater adhesives with improved performances. ACS Sustain. Chem. Eng. 2019, 7, 4508–4514. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Zhang, H.; Chen, T.; Sun, G.; Han, Y.; Li, J. Double-interpenetrating-network lignin-based epoxy resin adhesives for resistance to extreme environment. Biomacromolecules 2022, 23, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, V.; Mamo, G.; Gustafsson, P.-J.; Hatti-Kaul, R. Production and properties of adhesives formulated from laccase modified Kraft lignin. Ind. Crops Prod. 2013, 45, 343–348. [Google Scholar] [CrossRef]
- Li, J.; Lyu, Y.; Li, C.; Zhang, F.; Li, K.; Li, X.; Li, J.; Kim, K.H. Development of strong, tough and flame-retardant phenolic resins by using Acacia mangium tannin-functionalized graphene nanoplatelets. Int. J. Biol. Macromol. 2023, 227, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. “Green” bio-thermoset resins derived from soy protein isolate and condensed tannins. Ind. Crops Prod. 2017, 108, 363–370. [Google Scholar] [CrossRef]
- Ghahri, S.; Pizzi, A. Improving soy-based adhesives for wood particleboard by tannins addition. Wood Sci. Technol. 2018, 52, 261–279. [Google Scholar] [CrossRef]
- Aladejana, J.T.; Zhang, F.; Zeng, G.; Li, K.; Dong, Y.; Li, X.; Li, J. Dual crosslinked soybean protein adhesives with high strength, mold resistance, and extended shelf-life via dynamic covalent bonds. Eur. Polym. J. 2023, 194, 112150. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Park, B.-D.; Kim, Y.-S.; Yun, J.; Shin, H.C. Visual inspection of surface mold growth on medium-density fiberboard bonded with oxidized starch adhesives. Wood Mater. Sci. Eng. 2023, 18, 819–826. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, S.; Pang, J.; Gao, Z. Plywood with soy protein—Acrylate Hybrid Adhesive. In Proceedings of the 3rd International Conference on Biotechnology, Chemical and Materials Engineering (CBCME 2013), Hong Kong, China, 12–13 December 2013; pp. 108–111. [Google Scholar]
- Zhou, Y.; Zeng, G.; Zhang, F.; Li, K.; Li, X.; Luo, J.; Li, J.; Li, J. Design of tough, strong and recyclable plant protein-based adhesive via dynamic covalent crosslinking chemistry. Chem. Eng. J. 2023, 460, 141774. [Google Scholar] [CrossRef]
- Aladejana, J.T.; Zeng, G.; Zhang, F.; Li, K.; Li, X.; Dong, Y.; Li, J. Tough, waterproof, and mildew-resistant fully biobased soybean protein adhesives enhanced by furfuryl alcohol with dynamic covalent linkages. Ind. Crop. Prod. 2023, 198, 116759. [Google Scholar] [CrossRef]
- Gu, W.; Li, F.; Liu, X.; Gao, Q.; Gong, S.; Li, J.; Shi, S.Q. Borate chemistry inspired by cell walls converts soy protein into high-strength, antibacterial, flame-retardant adhesive. Green Chem. 2020, 22, 1319–1328. [Google Scholar] [CrossRef]
- Li, K.; Geng, X.; Simonsen, J.; Karchesy, J. Novel wood adhesives from condensed tannins and polyethylenimine. Int. J. Adh. Adh. 2004, 24, 327–333. [Google Scholar] [CrossRef]
- Efhamisisi, D.; Thevenon, M.-F.; Hamzeh, Y.; Karimi, A.-N.; Pizzi, A.; Pourtahmasi, K. Induced tannin adhesive by boric acid addition and its effect on bonding quality and biological performance of poplar plywood. ACS Sustain. Chem. Eng. 2016, 4, 2734–2740. [Google Scholar] [CrossRef]
- Jin, S.; Song, X.; Li, K.; Xia, C.; Li, J. A mussel-inspired strategy toward antimicrobial and bacterially anti-adhesive soy protein surface. Polym. Compos. 2020, 41, 633–644. [Google Scholar] [CrossRef]
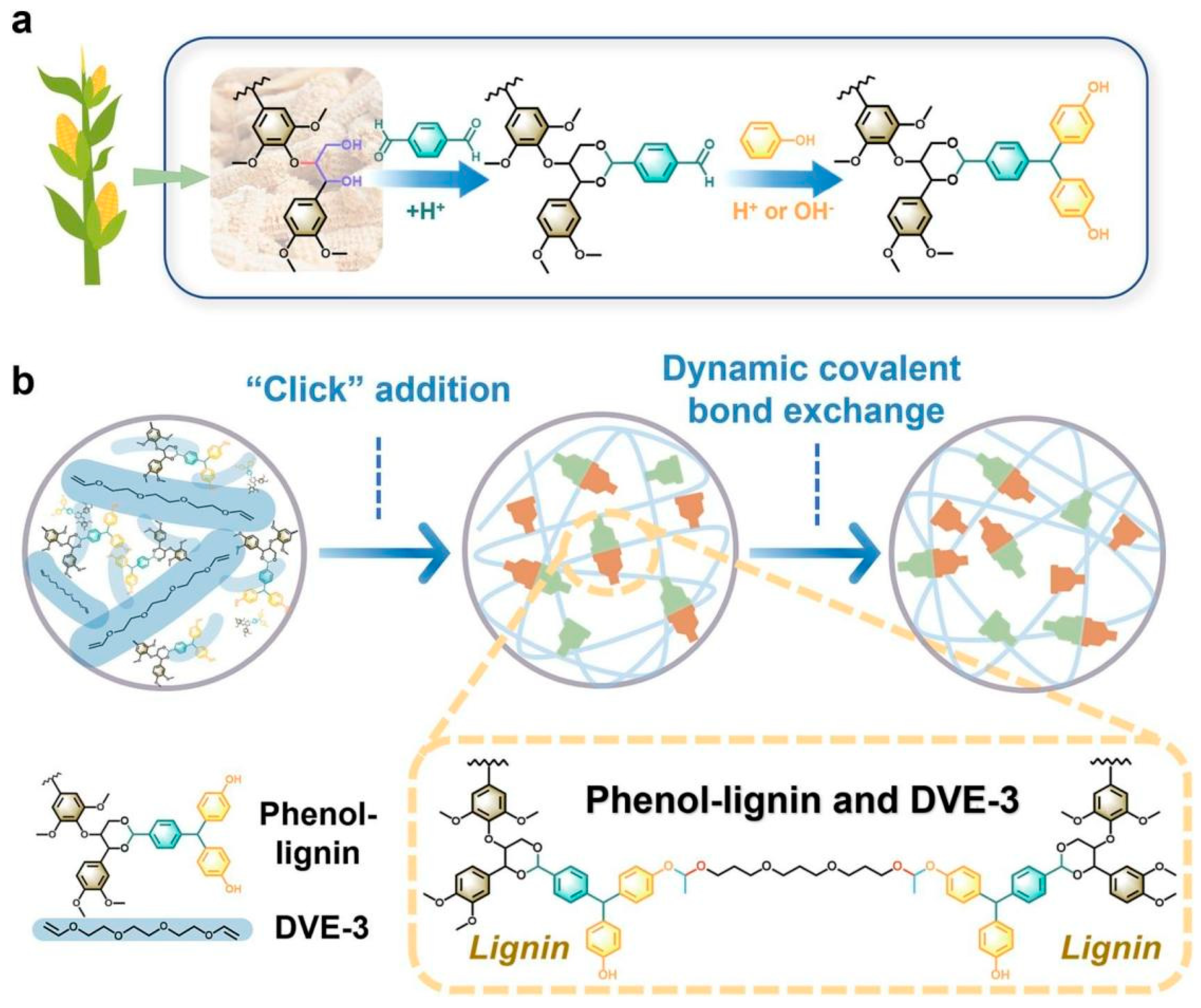
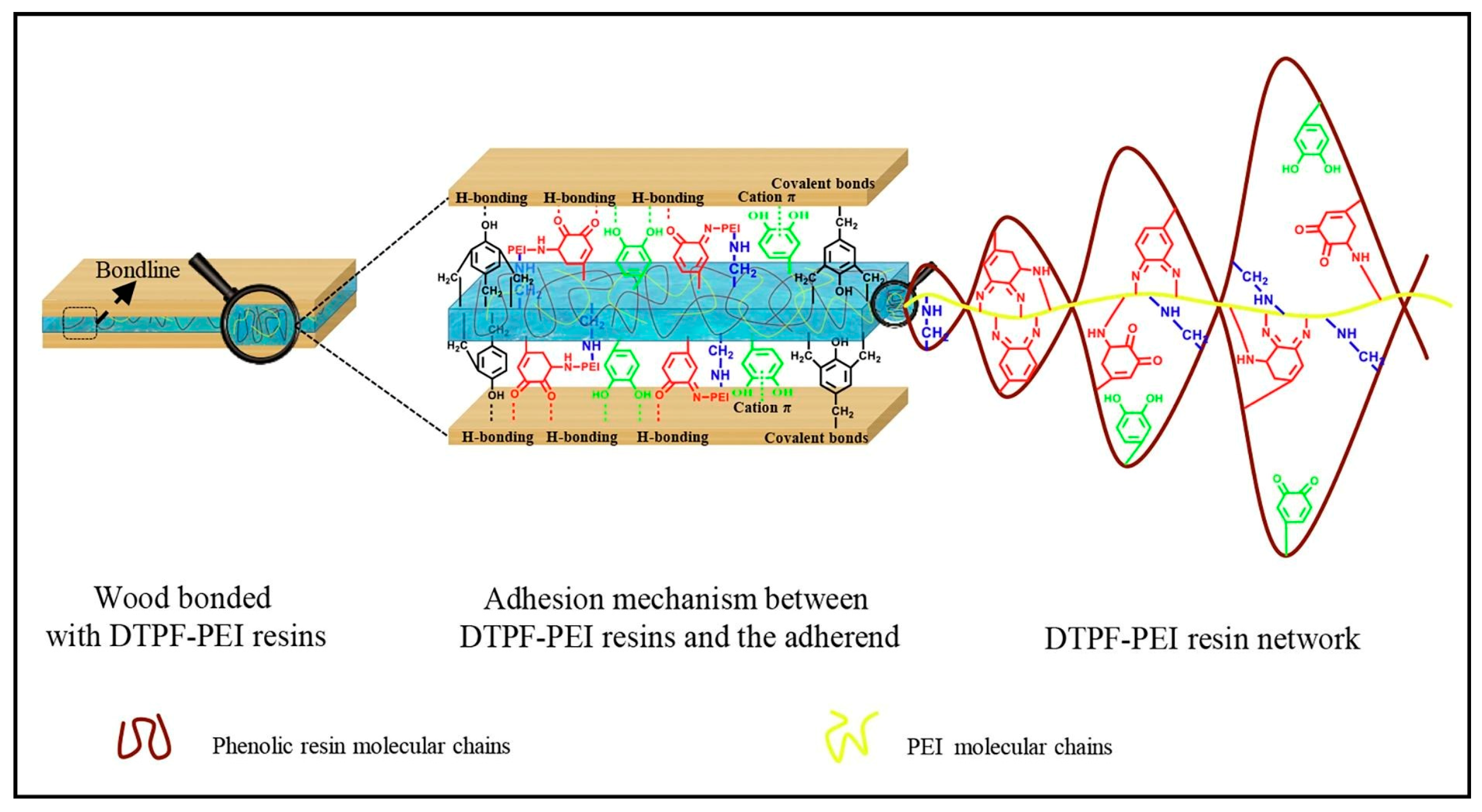
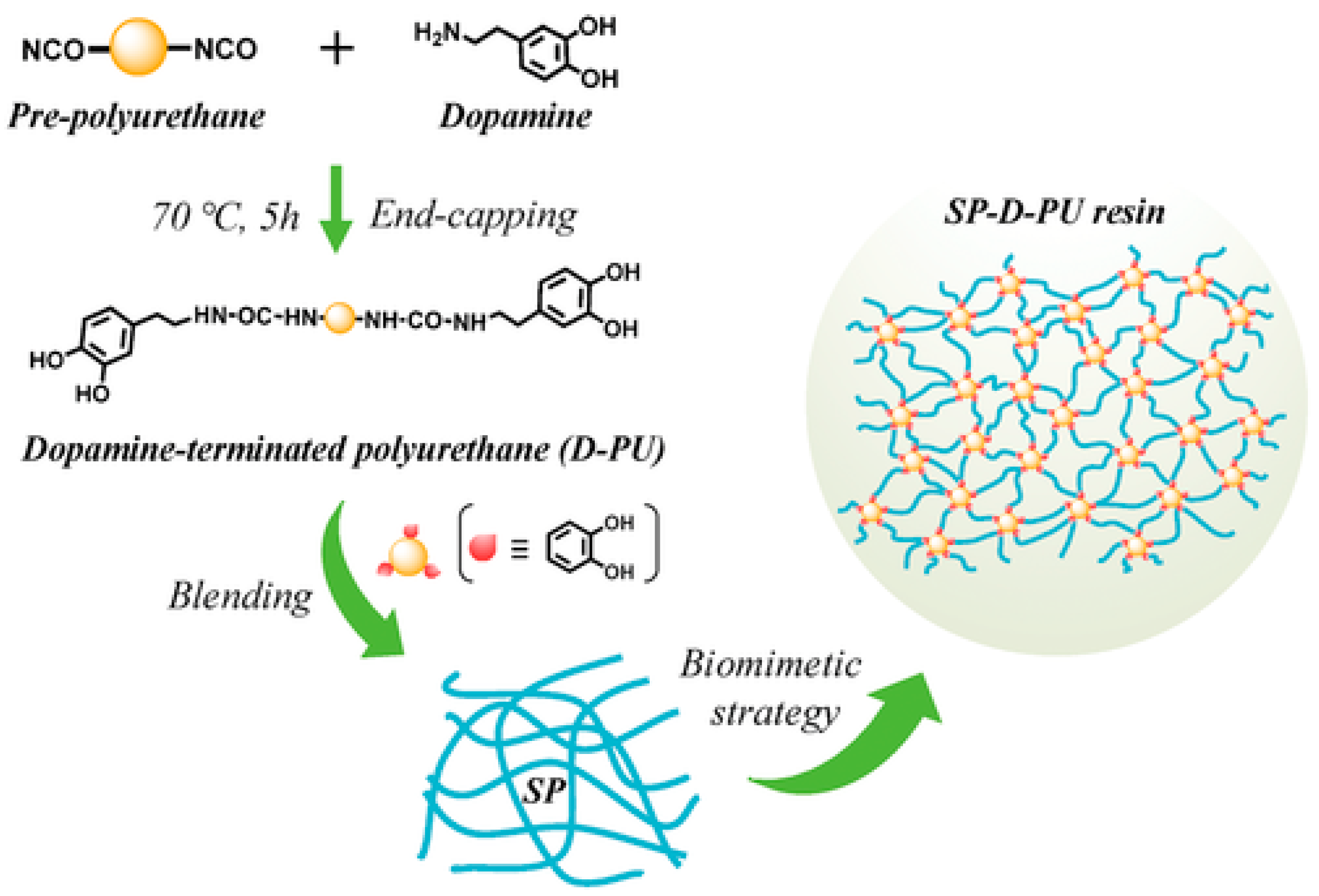
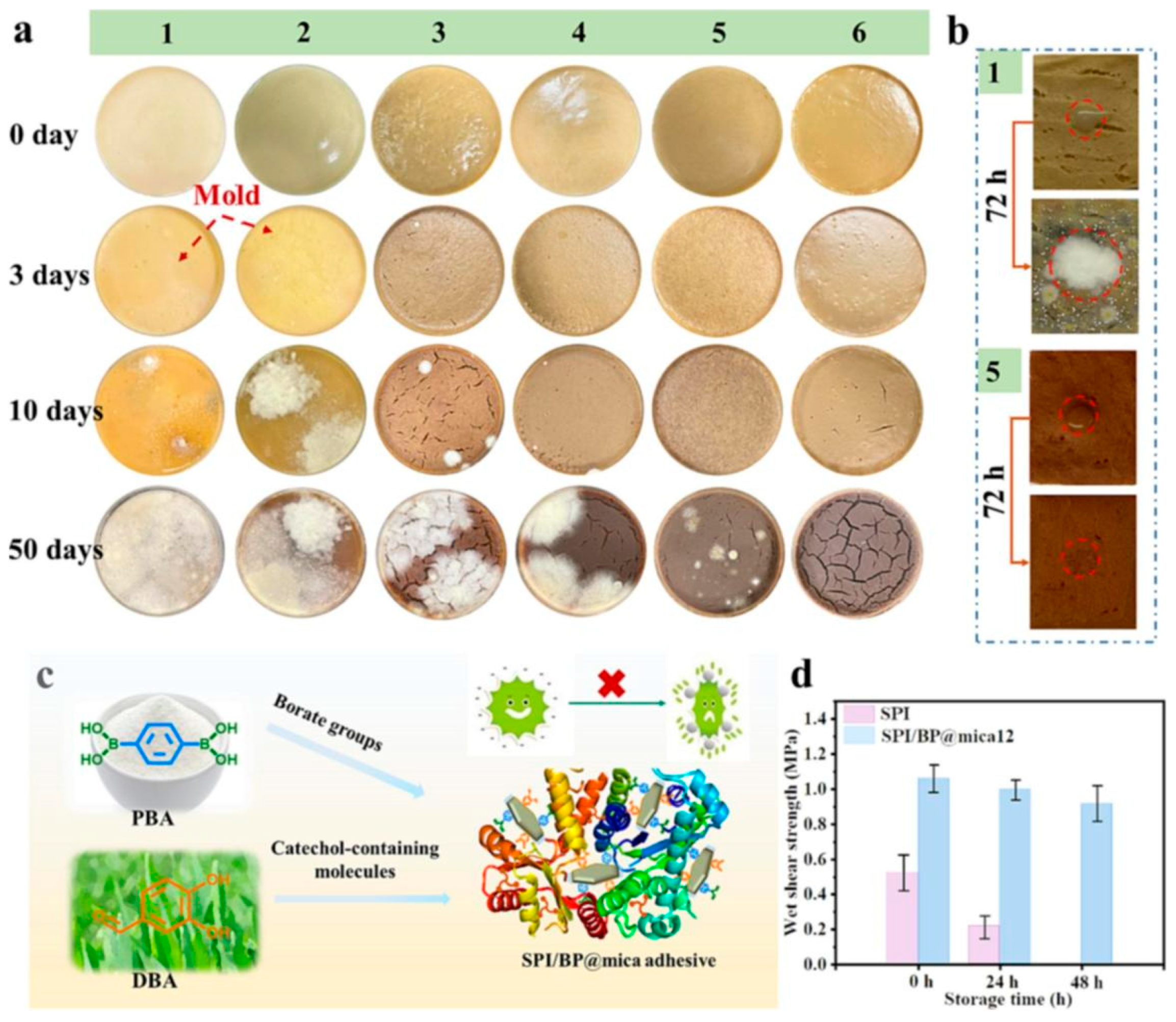
| Adhesives | Modification Methods | Test Methods | Substrates | Bond strength (MPa) | Ref. |
|---|---|---|---|---|---|
| Protein-based | Dual network by cationic interactions | Three-ply GB/T 17657-2013 | Poplar | 1.50 | [24] |
| Increasing intermolecular interactions | Three-ply GB/T 17657-2013 | Poplar | 1.96 | [22] | |
| High-pressure homogenization to process | Three-ply GB/T 17657-2013 | Poplar | 1.03 | [23] | |
| Lignin-based | Phenol modification and combination of DVE-3 | Two-ply Not mentioned | Walnut lumber | 12.87 | [29] |
| Ghaldolization and phenolization | Two-ply Not mentioned | Walnut lumber | 6.27 | [32] | |
| Phenol-modified and cross-linking reactions | Two-ply GB/T 14732-2017 | Not mentioned | 6.27 | [30] | |
| Tannin-based | Depolymerized tannins combine with PEI | Three-ply Not mentioned | Poplar | 1.44 | [33] |
| Condense tannin- functionalized boronnitride | Three-ply GB/T 17657–2013 | Poplar | 1.67 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Chen, Y.; Li, R.; Jiang, J.; Wang, X. A Narrative Review: Modification of Bio-Based Wood Adhesive for Performance Improvement. Coatings 2024, 14, 1153. https://doi.org/10.3390/coatings14091153
Yu C, Chen Y, Li R, Jiang J, Wang X. A Narrative Review: Modification of Bio-Based Wood Adhesive for Performance Improvement. Coatings. 2024; 14(9):1153. https://doi.org/10.3390/coatings14091153
Chicago/Turabian StyleYu, Caizhi, Yi Chen, Renjie Li, Jun Jiang, and Xiang Wang. 2024. "A Narrative Review: Modification of Bio-Based Wood Adhesive for Performance Improvement" Coatings 14, no. 9: 1153. https://doi.org/10.3390/coatings14091153








