Expanded Illite Filler in UV-Curable Polymer Electrolytes for All-Solid-State Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
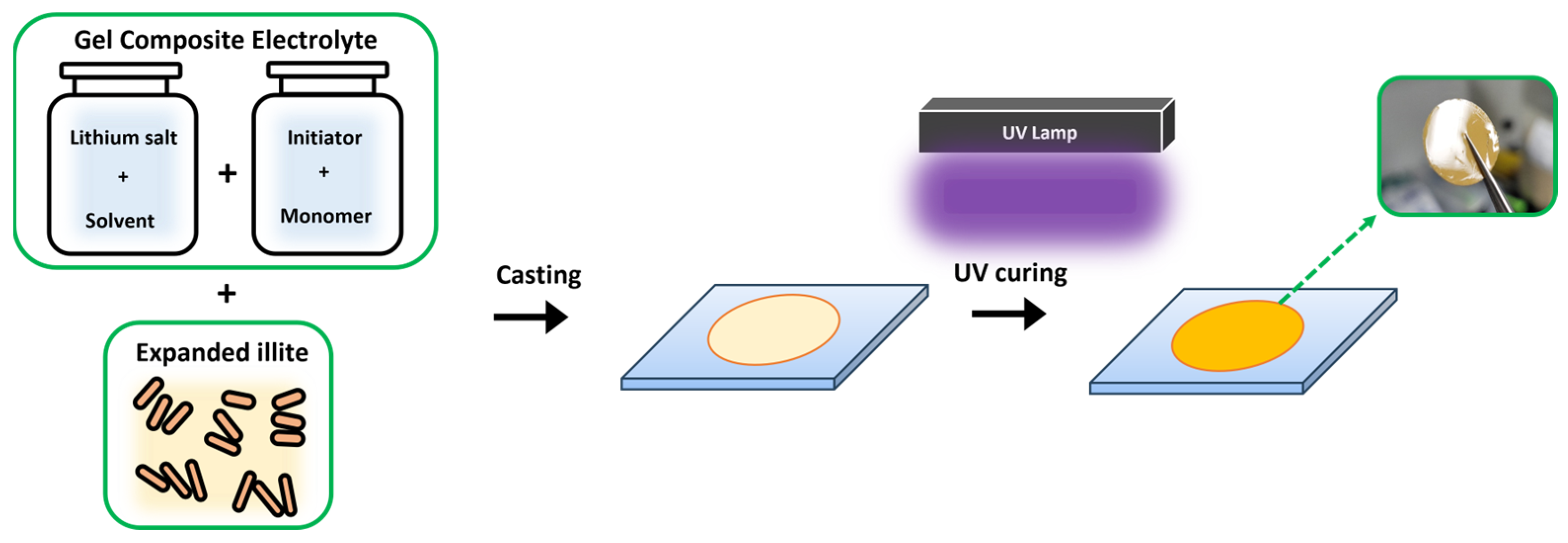
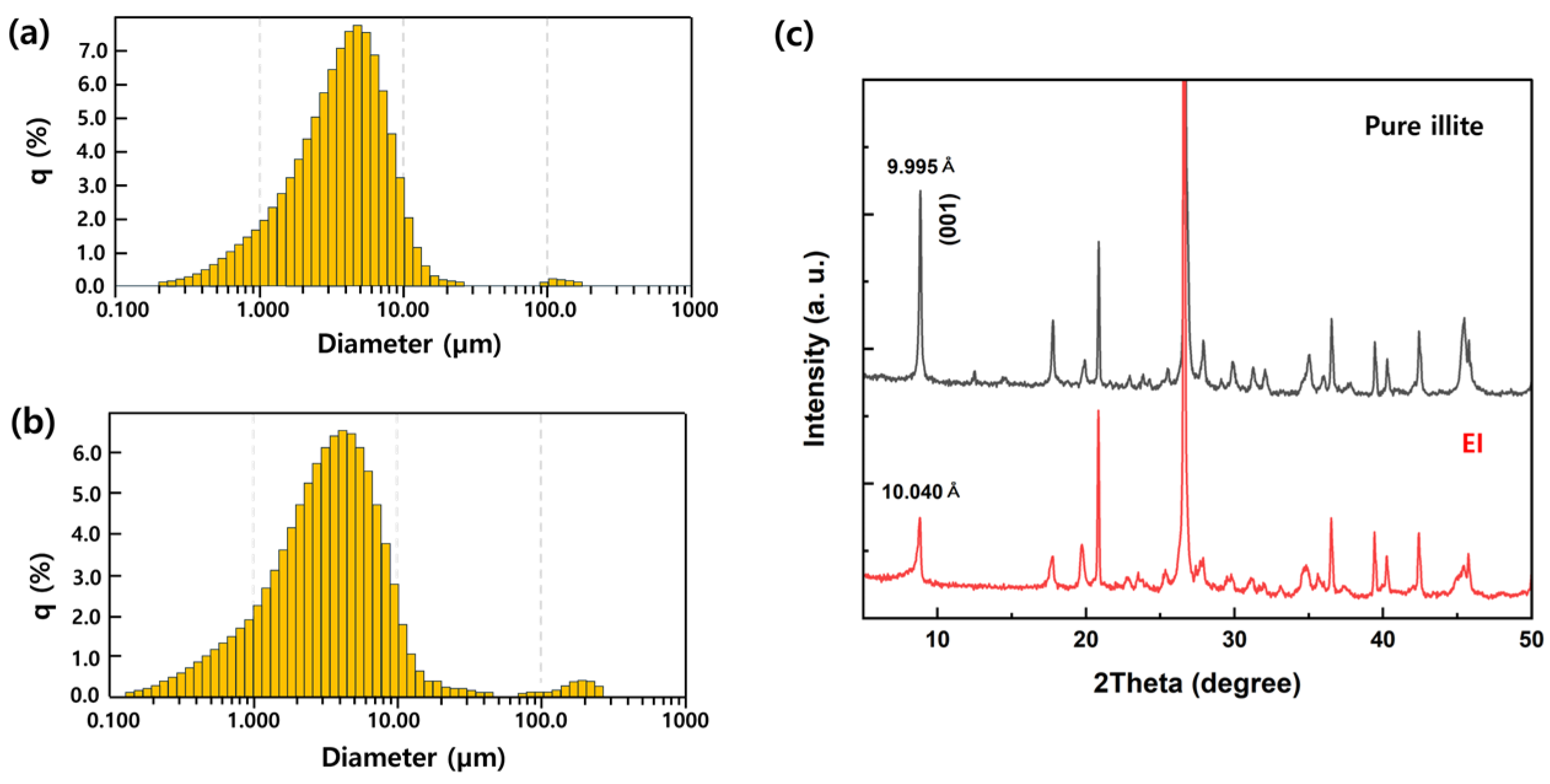
2.1. Preparation of the EI
2.2. Synthesis of the PBSSE
2.3. Materials and Electrochemical Characterization
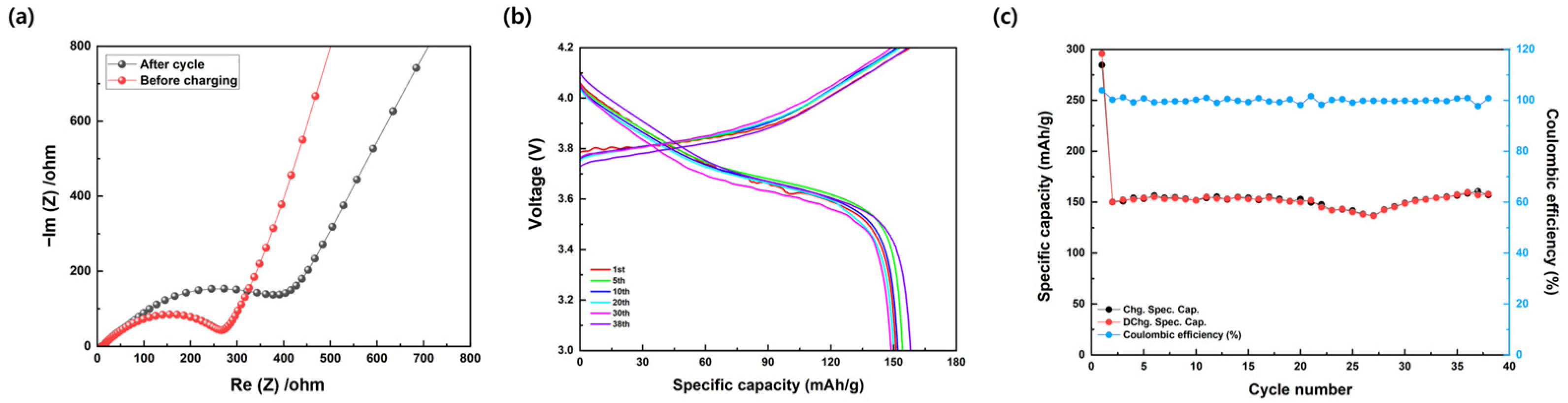
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Sanguesa, J.A.; Torres-Sanz, V.; Garrido, P.; Martinez, F.J.; Marquez-Barja, J.M. A Review on Electric Vehicles: Technologies and Challenges. Smart Cities 2021, 4, 372–404. [Google Scholar] [CrossRef]
- Blomgren, G.E. The development and future of lithium ion batteries. J. Electrochem. Soc. 2016, 164, A5019. [Google Scholar] [CrossRef]
- Hesse, H.C.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-Ion Battery Storage for the Grid—A Review of Stationary Battery Storage System Design Tailored for Applications in Modern Power Grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef]
- Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Un-Noor, F.; Padmanaban, S.; Mihet-Popa, L.; Mollah, M.N.; Hossain, E. A Comprehensive Study of Key Electric Vehicle (EV) Components, Technologies, Challenges, Impacts, and Future Direction of Development. Energies 2017, 10, 1217. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, K.; Wang, H.; Lu, L.; Gao, B.; Wang, Y.; Li, Y. Experimental and Modeling Study of Arc Fault Induced Thermal Runaway in Prismatic Lithium-Ion Batteries. Batteries 2024, 10, 269. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Rahmani, A.; Dibaj, M.; Akrami, M. Recent Advancements in Battery Thermal Management Systems for Enhanced Performance of Li-Ion Batteries: A Comprehensive Review. Batteries 2024, 10, 265. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- Chombo, P.V.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhao, C.-Z.; Yao, Y.-X.; Liu, H.; Zhang, Q. Recent advances in energy chemistry between solid-state electrolyte and safe lithium-metal anodes. Chem 2019, 5, 74–96. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, H.; Hasa, I.; Passerini, S. Challenges and strategies for high-energy aqueous electrolyte rechargeable batteries. Angew. Chem. Int. Ed. 2021, 60, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, N.L.; Scheiman, M.A. Surface potentials of aqueous electrolyte solutions. J. Phys. Chem. 1968, 72, 74–78. [Google Scholar] [CrossRef]
- Wu, F.; Wu, B.; Mu, Y.; Zhou, B.; Zhang, G.; Zeng, L. Metal-Organic Framework-Based Materials in Aqueous Zinc-Ion Batteries. Int. J. Mol. Sci. 2023, 24, 6041. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Hao, J.; Du, J.; Wu, C.; Ma, Z.; Qin, W. Mini-Review on the Regulation of Electrolyte Solvation Structure for Aqueous Zinc Ion Batteries. Batteries 2023, 9, 73. [Google Scholar] [CrossRef]
- Reis, S.; Grosso, R.; Kosctiuk, J.; Franchetti, M.; Oliveira, F.; Souza, A.; Gonin, C.; Freitas, H.; Monteiro, R.; Parreira, L.; et al. Effect of Zr4+ on Lithium-Ion Conductivity of Garnet-Type Li5+xLa3(Nb2−xZrx)O12 Solid Electrolytes. Batteries 2023, 9, 137. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. Challenges in speeding up solid-state battery development. Nat. Energy 2023, 8, 230–240. [Google Scholar] [CrossRef]
- Sung, J.; Heo, J.; Kim, D.-H.; Jo, S.; Ha, Y.-C.; Kim, D.; Ahn, S.; Park, J.-W. Recent advances in all-solid-state batteries for commercialization. Mater. Chem. Front. 2024, 8, 1861–1887. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.-Y.; He, Z.-J.; Li, Y.-J.; Mao, J.; Dai, K.-H.; Yan, C.; Zheng, J.-C. An advance review of solid-state battery: Challenges, progress and prospects. Sustain. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Yu, S.; Schmidt, R.D.; Garcia-Mendez, R.; Herbert, E.; Dudney, N.J.; Wolfenstine, J.B.; Sakamoto, J.; Siegel, D.J. Elastic properties of the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 2016, 28, 197–206. [Google Scholar] [CrossRef]
- Yu, S.; Siegel, D.J. Grain boundary contributions to Li-ion transport in the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 2017, 29, 9639–9647. [Google Scholar] [CrossRef]
- Liang, C.C. Conduction characteristics of the lithium iodide-aluminum oxide solid electrolytes. J. Electrochem. Soc. 1973, 120, 1289–1292. [Google Scholar] [CrossRef]
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide solid electrolytes for lithium battery applications. Adv. Energy Mater. 2018, 8, 1800933. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef]
- Wu, F.; Fitzhugh, W.; Ye, L.; Ning, J.; Li, X. Advanced sulfide solid electrolyte by core-shell structural design. Nat. Commun. 2018, 9, 4037. [Google Scholar] [CrossRef]
- Fan, X.; Zhong, C.; Liu, J.; Ding, J.; Deng, Y.; Han, X.; Zhang, L.; Hu, W.; Wilkinson, D.P.; Zhang, J. Opportunities of flexible and portable electrochemical devices for energy storage: Expanding the spotlight onto semi-solid/solid electrolytes. Chem. Rev. 2022, 122, 17155–17239. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Xu, X.; Yan, B.; Wu, T.; Lu, L. Polymer-Based Solid-State Electrolytes for High-Energy-Density Lithium-Ion Batteries–Review. Adv. Energy Mater. 2023, 13, 2301746. [Google Scholar] [CrossRef]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in solid polymer electrolytes for lithium-ion batteries and beyond. Small 2022, 18, 2103617. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Anothumakkool, B.; Kurungot, S.; Winter, M.; Nair, J.R. In situ polymerization process: An essential design tool for lithium polymer batteries. Energy Environ. Sci. 2021, 14, 2708–2788. [Google Scholar] [CrossRef]
- Laouchedi, D.; Bezzazi, B.; Aribi, C. Elaboration and characterization of composite material based on epoxy resin and clay fillers. J. Appl. Res. Technol. 2017, 15, 190–204. [Google Scholar] [CrossRef]
- Hashemifard, S.; Ismail, A.; Matsuura, T. Effects of montmorillonite nano-clay fillers on PEI mixed matrix membrane for CO2 removal. Chem. Eng. J. 2011, 170, 316–325. [Google Scholar] [CrossRef]
- Dong, H.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Clay nanosheets as charged filler materials for high-performance and fouling-resistant thin film nanocomposite membranes. J. Membr. Sci. 2015, 494, 92–103. [Google Scholar] [CrossRef]
- Pluta, M.; Paul, M.A.; Alexandre, M.; Dubois, P. Plasticized polylactide/clay nanocomposites. I. The role of filler content and its surface organo-modification on the physico-chemical properties. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 299–311. [Google Scholar] [CrossRef]
- Ray, S.; Easteal, A.J. Advances in Polymer-Filler Composites: Macro to Nano. Mater. Manuf. Process. 2007, 22, 741–749. [Google Scholar] [CrossRef]
- Zhen, R.; Chi, Q.; Wang, X.; Yang, K.; Jiang, Y.; Li, F.; Xue, B. Crystallinity, ion conductivity, and thermal and mechanical properties of poly(ethylene oxide)–illite nanocomposites with exfoliated illite as a filler. J. Appl. Polym. Sci. 2016, 13, 44226. [Google Scholar] [CrossRef]
- Zhen, R.; Jiang, Y.S.; Li, F.F.; Xue, B. A study on the intercalation and exfoliation of illite. Res. Chem. Intermed. 2017, 43, 679–692. [Google Scholar] [CrossRef]
- Ashurov, M.S.; Bakhia, T.; Saidzhonov, B.M.; Klimonsky, S.O. Preparation of inverse photonic crystals by ETPTA photopolymerization method and their optical properties. J. Phys. Conf. Ser. 2020, 1461, 012009. [Google Scholar] [CrossRef]




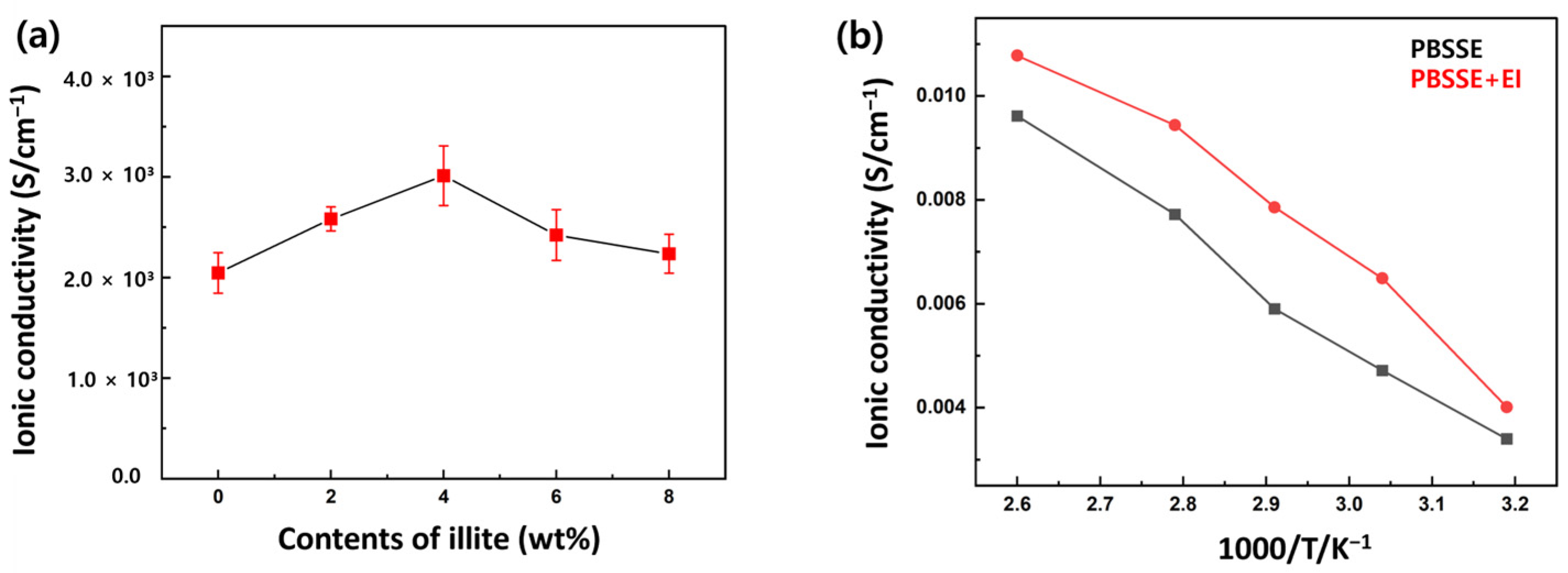
| Ionic Conductivity (S cm−1) | ||
|---|---|---|
| PBSSE without EI | PBSSE with 4 wt% of EI | |
| 2.60 | 9.61 × 10−3 | 1.08 × 10−2 |
| 2.79 | 7.72 × 10−3 | 9.44 × 10−3 |
| 2.91 | 5.90 × 10−3 | 7.85 × 10−3 |
| 3.04 | 4.71 × 10−3 | 6.49 × 10−3 |
| 3.19 | 3.40 × 10−3 | 4.01 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.; Ahn, S.; You, S.; Kim, J.-k.; Kim, D.; Kim, H.; Kim, H.-I.; Park, J. Expanded Illite Filler in UV-Curable Polymer Electrolytes for All-Solid-State Li-Ion Batteries. Coatings 2024, 14, 1158. https://doi.org/10.3390/coatings14091158
Bae M, Ahn S, You S, Kim J-k, Kim D, Kim H, Kim H-I, Park J. Expanded Illite Filler in UV-Curable Polymer Electrolytes for All-Solid-State Li-Ion Batteries. Coatings. 2024; 14(9):1158. https://doi.org/10.3390/coatings14091158
Chicago/Turabian StyleBae, Minseong, Seongki Ahn, Sunkyung You, Jae-kwang Kim, Daewon Kim, Hanjoo Kim, Hong-Il Kim, and Jinjoo Park. 2024. "Expanded Illite Filler in UV-Curable Polymer Electrolytes for All-Solid-State Li-Ion Batteries" Coatings 14, no. 9: 1158. https://doi.org/10.3390/coatings14091158
APA StyleBae, M., Ahn, S., You, S., Kim, J.-k., Kim, D., Kim, H., Kim, H.-I., & Park, J. (2024). Expanded Illite Filler in UV-Curable Polymer Electrolytes for All-Solid-State Li-Ion Batteries. Coatings, 14(9), 1158. https://doi.org/10.3390/coatings14091158






