Laser-Melted Wc/Ni-Based Coating Remelting Study on Q235 Steel Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Equipment
2.3. Test Method
3. Results and Discussion
3.1. Coating Macro-Morphology
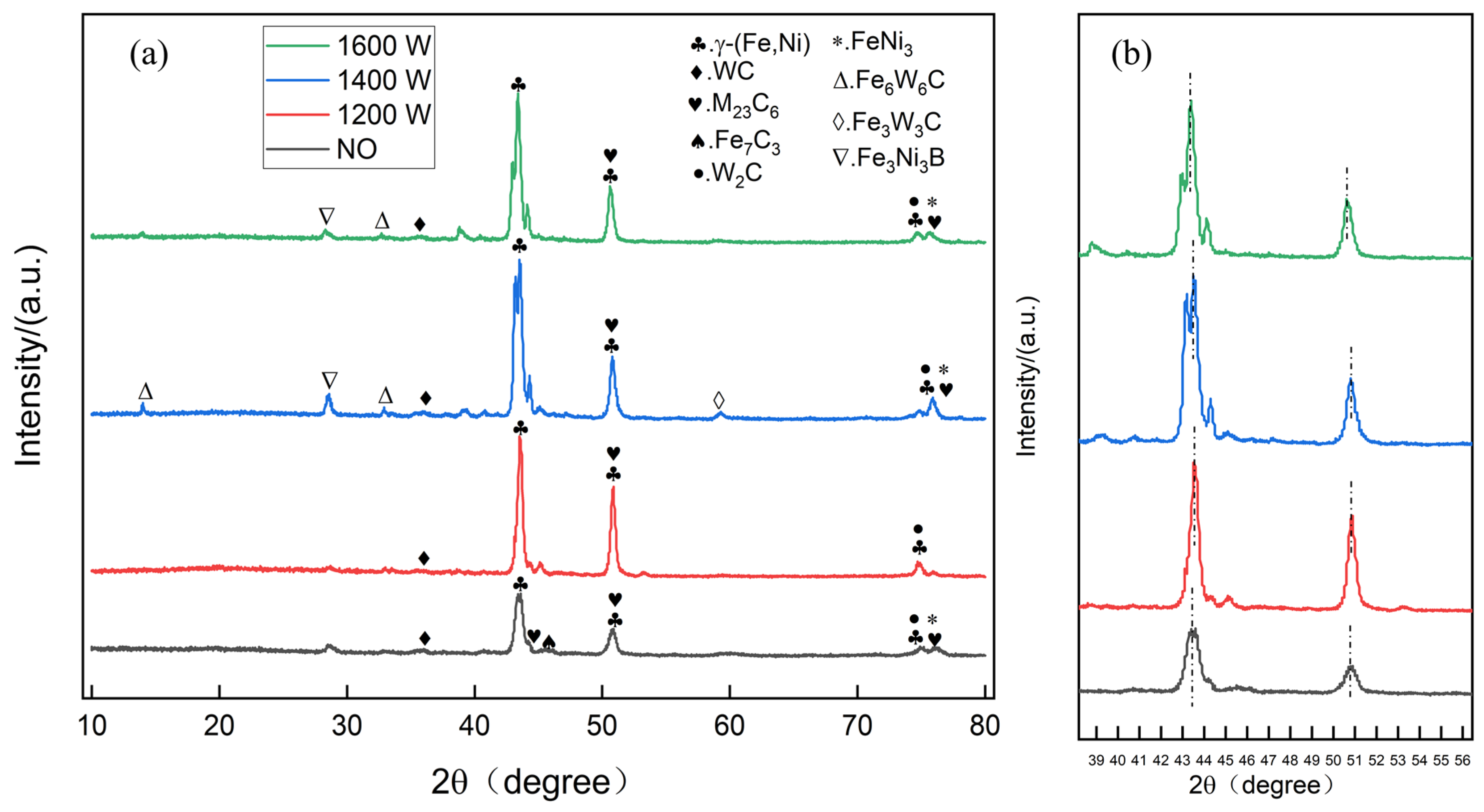
3.2. Physical Phase Analysis of the Coating
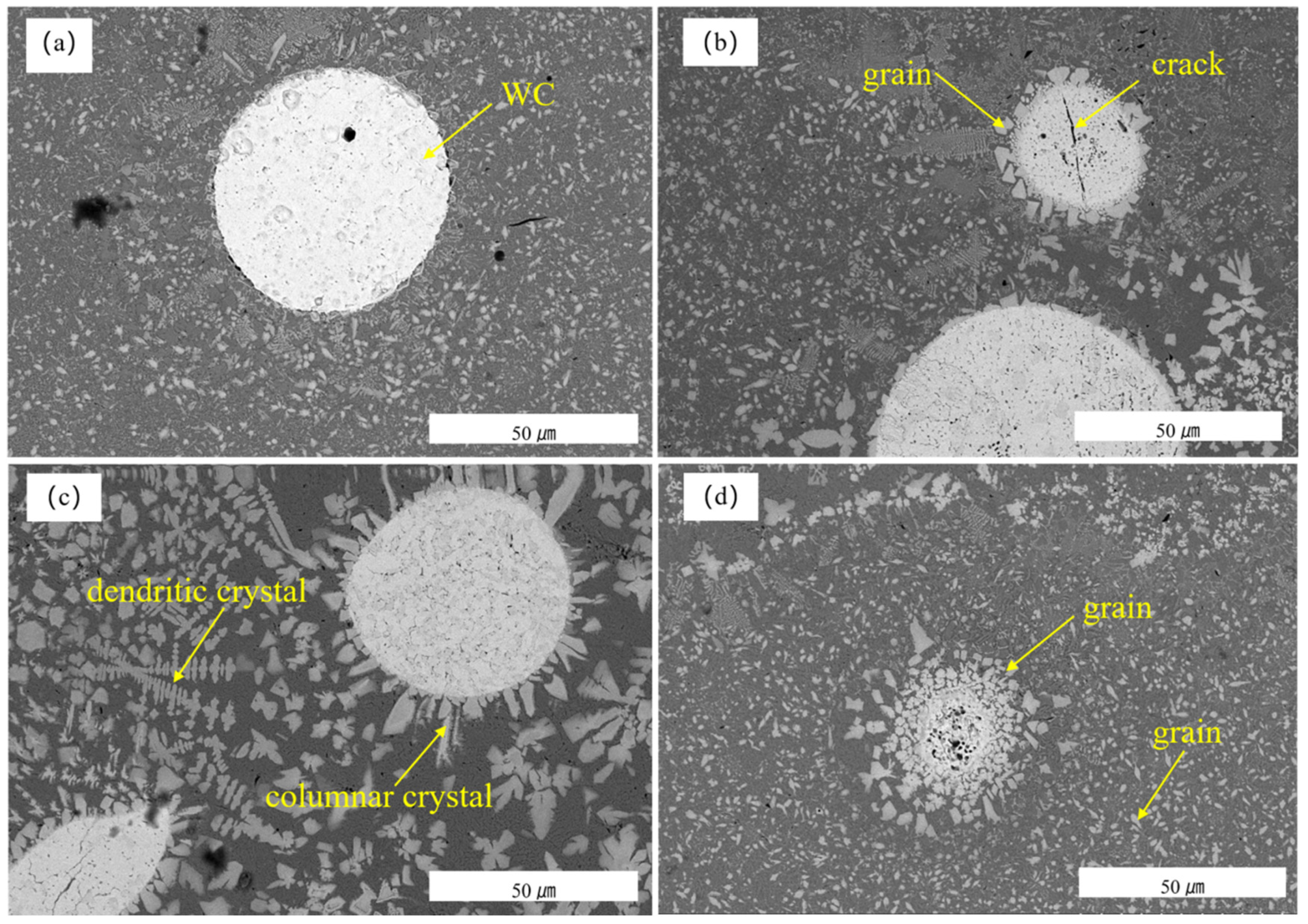
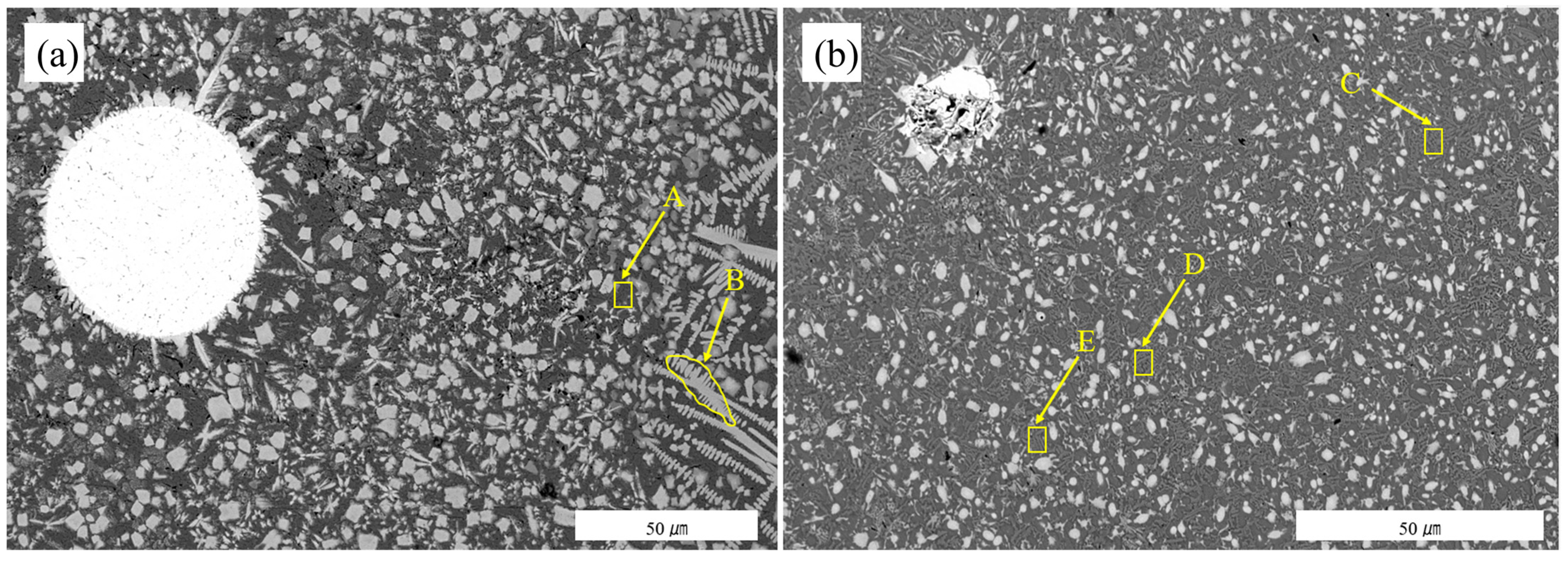
3.3. Scanning Microscopy Analysis
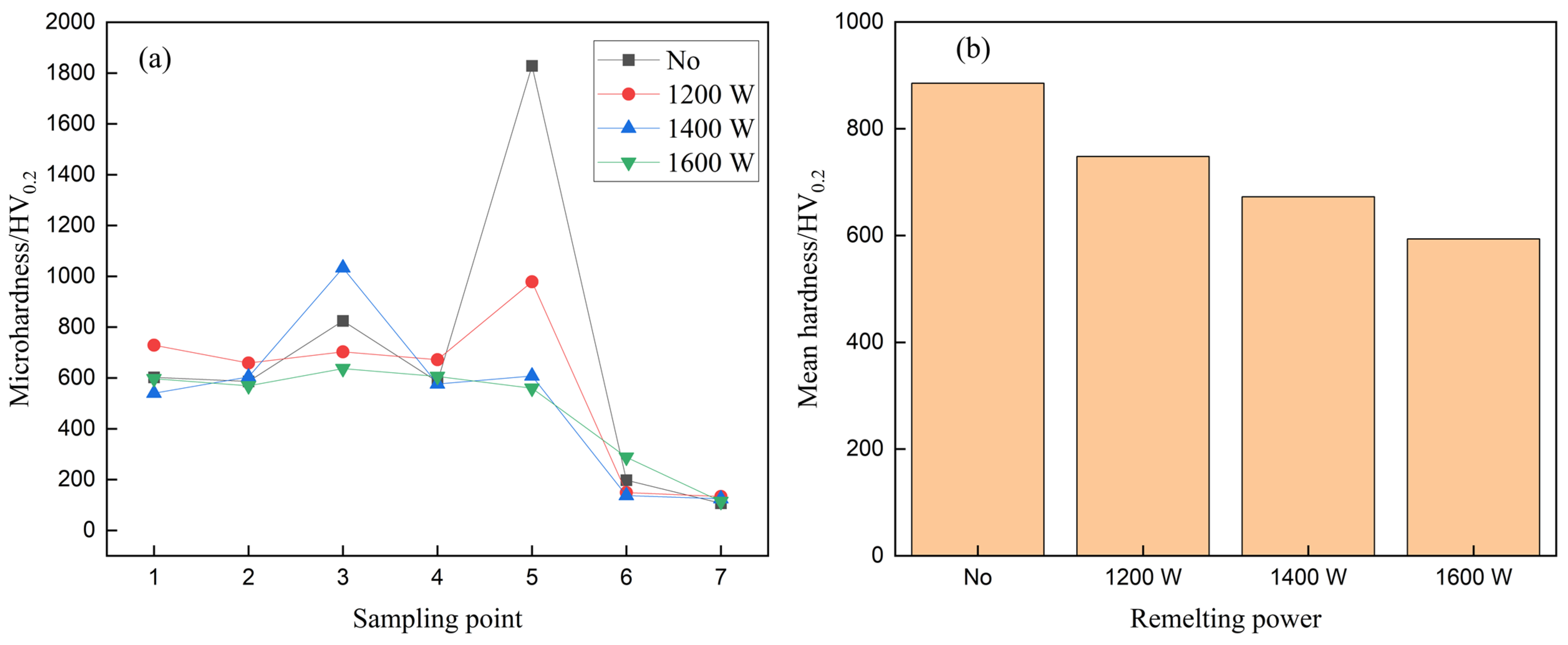
3.4. Hardness Analysis
3.5. Analysis of Corrosion Resistance of Fused Cladding Layer before and after Remelting
4. Conclusions
- Remelting treatments affect the macroscopic morphology, microstructure, and coating properties of WC/Ni-based fused cladding layers at different laser powers. Experiments showed that the remelting of coatings at different laser powers did not always have a positive effect on the coating properties. In the coatings, the main phases include (Ni, Fe) solid solutions, WC/W2C ceramic particles, and MC carbides. When remelted at powers greater than 1400 W, new phases, Fe6W6C and Fe3W3C, were formed in the coating.
- When the laser power was 1200 W, the dissolution of ceramic particles in the WC remelted layer did not change significantly relative to the non-remelted coating. When the laser power was 1400 W, the ceramic particles in the WC coating were partially dissolved, the (Ni, Fe) dendrites and MC carbides increased, and the internal uniformity of the coating began to increase. The surface hardness of the fused coating was lower than that of the other remelted layers. When the laser power was 1600 W, the WC particles decreased and more solid solution was generated, which decreased the microhardness but increased the smoothness of the coating surface.
- When the laser power was 1600 W, the self-corrosion potential of 0.0284 V was the largest relative to the other remelted layers, and the self-corrosion current density of 2.397 × 10−5 A/cm2 was the smallest relative to the other remelted layers, so the corrosion resistance was the best.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Liao, Z.Y.; Zhang, L.L.; Cai, M.W.; Wei, X.S.; Shen, J. A review on coatings deposited by extreme high-speed laser cladding: Processes, materials, and properties. Opt. Laser Technol. 2023, 164, 109472. [Google Scholar] [CrossRef]
- Wan, M.Q.; Shi, J.; Lei, L.; Cui, Z.Y.; Wang, H.L.; Wang, X. A comparative study of the microstructure, mechanical properties and corrosion resistance of Ni-or Fe-based composite coatings by laser cladding. J. Mater. Eng. Perform. 2018, 27, 2844–2854. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Jiang, G.; Tang, S.; Wu, J.; Wang, J. Effect of WC on microstructure, molten pool and properties of Ni based coatings by multi-layer laser cladding. Opt. Laser Technol. 2024, 174, 110612. [Google Scholar] [CrossRef]
- Chen, G.D.; Liu, X.B.; Zhang, F.Z.; Liu, Q.S.; Ou, H.Z.; Zhang, S.H. Fabrication and tribological properties of laser cladding WC-Cu/Co-based composite coatings. Surf. Coat. Technol. 2023, 472, 129930. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, Z.G.; Dai, S.; Zhao, X.R.; Yi, J. Microstructure and properties of laser cladding Co-based alloy coatings. Adv. Mater. Res. 2011, 295, 1665–1668. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Lu, B.; Tan, N.; Cai, L.; Yong, Q. Effect of WC content on microstructure and properties of high-speed laser cladding Ni-based coating. Opt. Laser Technol. 2022, 155, 108449. [Google Scholar] [CrossRef]
- Lei, J.; Shi, C.; Zhou, S.; Gu, Z.; Zhang, L.-C. Enhanced corrosion and wear resistance properties of carbon fiber reinforced Ni-based composite coating by laser cladding. Surf. Coat. Technol. 2018, 334, 274–285. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.; Deng, G.; Han, C.; Zhou, H.; Tan, C.; Long, Y.; Liu, Z.; Zhang, M.; Yang, C.; et al. Crack inhibition and mechanical property enhancement of a CM247LC alloy fabricated by laser powder bed fusion through remelting strategy. Mater. Charact. 2024, 214, 114073. [Google Scholar] [CrossRef]
- Zeng, Z.; Shang, J.; Lin, D. Effect of laser remelting power on the microstructure and properties of vanadizing layer on AISI 52100 steel pin shaft. Heliyon 2024, 10, e06729. [Google Scholar] [CrossRef]
- Gurappa, I. Characterization of different materials for corrosion resistance under simulated body fluid conditions. Mater. Charact. 2002, 49, 73–79. [Google Scholar] [CrossRef]
- Chang, W.; Gao, G.; Zhang, H.; Chen, H.; Yi, M.; Zhang, J.; Chen, Z.; Xu, C. Microstructure and properties of graphene reinforced co-based composite coating by laser cladding. Surf. Coat. Technol. 2023, 453, 129139. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Yao, F.; Zheng, F. Effect of laser remelting on internal defects and properties of Ni-based coatings reinforced by multi-channel WC in situ. Mater. Today Commun. 2024, 38, 108375. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Yao, F.; Li, X. Effect of laser remelting on the organization and properties of WC/Ni-based coatings generated in situ by laser cladding. J. Manuf. Process. 2023, 102, 501–512. [Google Scholar] [CrossRef]
- Li, K.; Li, D.; Liu, D.; Pei, G.; Sun, L. Microstructure evolution and mechanical properties of multiple-layer laser cladding coating of 308L stainless steel. Appl. Surf. Sci. 2015, 340, 143–150. [Google Scholar] [CrossRef]
- Blake, A.J.; Cole, J.M.; Evans, J.S.; Main, P.; Parsons, S.; Watkin, D.J. Crystal Structure Analysis: Principles and Practice; OUP Oxford: Oxford, UK, 2009; Volume 13. [Google Scholar]
- Weng, F.; Yu, H.; Chen, C.; Liu, J.; Zhao, L.; Dai, J.; Zhao, Z. Effect of process parameters on the microstructure evolution and wear property of the laser cladding coatings on Ti-6Al-4V alloy. J. Alloys Compd. 2017, 692, 989–996. [Google Scholar] [CrossRef]
- Al-Qawabah, S.M.; Zaid, A.I. Different methods for grain refinement of materials. Int. J. Sci. Eng. Res. 2016, 7, 1133–1140. [Google Scholar]
- Ortiz, A.L.; Tian, J.W.; Villegas, J.C.; Shaw, L.L.; Liaw, P.K. Interrogation of the microstructure and residual stress of a nickel-base alloy subjected to surface severe plastic deformation. Acta Mater. 2008, 56, 413–426. [Google Scholar] [CrossRef]
- Shen, X.; Ma, S.; He, X.; Zhang, J.; Ma, X.; Yin, Y.; Pan, S.; Wei, F. Quality enhancement of crack-free laser-cladded NiCrSiBC-WC coating by in-situ temperature field assistance (ITFA) and post-laser remelting (PLR). Surf. Coat. Technol. 2024, 484, 130873. [Google Scholar] [CrossRef]
- Chen, C.; Feng, A.; Wei, Y.; Wang, Y.; Pan, X.; Song, X. Effects of WC particles on microstructure and wear behavior of laser cladding Ni60 composite coatings. Opt. Laser Technol. 2023, 163, 109425. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, B.; Liu, X.; Zeng, X. Investigation on the Ni60-WC composite coatings deposited by extreme-high-speed laser-induction hybrid cladding technology: Forming characteristics, microstructure and wear behaviors. Surf. Coat. Technol. 2023, 473, 130033. [Google Scholar] [CrossRef]
- Chen, C.; Feng, A.; Wei, Y.; Wang, Y.; Pan, X.; Song, X. Effect of multiple laser shock peening on the microstructure and properties of laser cladding nano-WC/Ni60 composite coatings. Opt. Laser Technol. 2023, 167, 109719. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Wang, S.; Duan, D.; Li, F.; Qiao, Y.; Liu, Y.; Liu, X. The effect of WC content on the bonding strength and mechanical properties of WC/Ni60 coatings of brake disc. Opt. Laser Technol. 2022, 149, 107822. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Wang, F.; Deng, P.; Lu, Q.; Zhang, Y.; Li, S.; Ma, P.; Li, W.; Wang, Y. Microstructure and wear behaviors of WC-Ni coatings fabricated by laser cladding under high frequency micro-vibration. Appl. Surf. Sci. 2019, 485, 513–519. [Google Scholar] [CrossRef]
- Jian, Y.; Liu, Y.; Qi, H.; He, P.; Huang, G.; Huang, Z. Effects of scanning speed on the microstructure, hardness and corrosion properties of high-speed laser cladding Fe-based stainless coatings. J. Mater. Res. Technol. 2024, 29, 3380–3392. [Google Scholar] [CrossRef]
- Zhang, Z.; Kovacevic, R. Laser cladding of iron-based erosion resistant metal matrix composites. J. Manuf. Process. 2019, 38, 63–75. [Google Scholar] [CrossRef]
- Tan, N.; Hu, Z.; Zhou, Y.; Li, Y.; Lu, B.; Hu, D.; Liu, Y.; Li, Q. Effect of WC particle size on the microstructure and tribological properties of high-speed laser cladding Ni/WC composite coatings. Mater. Today Commun. 2024, 39, 109066. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Cheng, C.; Liang, F.; Li, K. Effect of process parameters on microstructure and properties of laser cladding Ni60 + 30% WC coating on Q235 steel. Materials 2023, 16, 7070. [Google Scholar] [CrossRef]
- Shengfeng, Z.; Gaoqin, D.; Haizhong, Z. Characteristics on structure and properties of WC-Ni60A coatings by laser cladding and laser-induction hybrid cladding. J. Mech. Eng. 2012, 48, 113–118. [Google Scholar]
- Luo, F.; Yang, T.; Zhao, Y.; Xiong, Z.; Huang, J. Effect of W Content on Microstructure and Properties of Laser Cladding CoCrFeNi HEA Coating. Coatings 2023, 13, 1301. [Google Scholar] [CrossRef]
- He, B. Effect of WC content on microstructure and properties of WC/316L laser coatings. Ferroelectrics 2023, 608, 1–2. [Google Scholar] [CrossRef]
- Munagala, V.N.; Wasekar, N.P.; Bathini, L.; Ramakrishna, L.; Sundararajan, G. Deciphering the role of W content, triple junctions, and heat treatment on the corrosion performance of Ni–W alloy coatings used for automotive applications. Mater. Chem. Phys. 2023, 308, 128305. [Google Scholar] [CrossRef]
- Tam, P.L. Tailoring of Transition Metal Silicides as Protective Thin Films on Austenitic Stainless Steel; Chalmers Tekniska Hogskola: Gothenburg, Sweden, 2011. [Google Scholar]
- Wu, R.; Han, Z.; Gao, B.; Zhang, Z.; Wu, P.; Shuang, Y.; Chen, H.; Liu, B. Effect of Pre-Strain on Microstructure and Corrosion Resistance of 840 Nickel Base Alloy Steel. SSRN 2024. Available online: https://ssrn.com/abstract=4629171 (accessed on 1 March 2024).
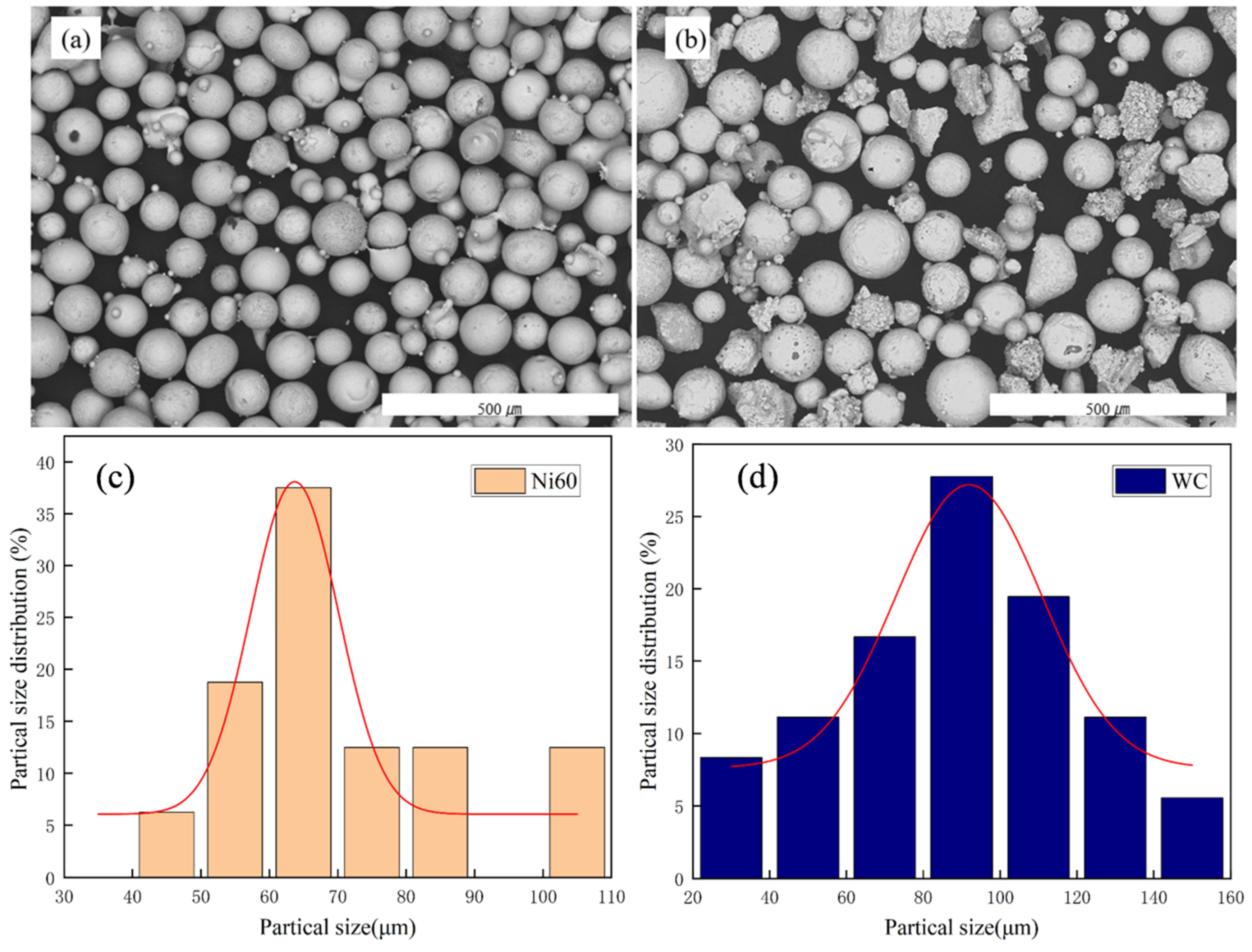



| Element | C | Cr | B | Mn | Si | Fe | P | S | Ni |
|---|---|---|---|---|---|---|---|---|---|
| Ni60 | 0.8–1.2 | 14–16 | 3–3.5 | - | 3.5–4.0 | 14–15 | 0.02 | 0.02 | Bal |
| Q235 | 0.22 | - | - | 0.3–0.7 | 0.35 | Bal | 0.045 | 0.05 | - |
| Area | C | O | F | Si | Cr | Fe | Ni | W |
|---|---|---|---|---|---|---|---|---|
| A | 1.99 | 1.25 | 4.07 | 4.59 | 9.90 | 24.92 | 50.31 | 2.96 |
| B | 2.85 | 2.08 | 3.00 | 6.31 | 14.76 | 15.23 | 41.45 | 14.32 |
| Area | C | O | F | Si | Cr | Fe | Ni | W |
|---|---|---|---|---|---|---|---|---|
| C | 2.67 | 2.03 | 2.81 | 2.71 | 11.34 | 37.86 | 34.38 | 6.20 |
| D | 3.78 | 1.84 | 4.45 | 1.87 | 11.23 | 37.51 | 32.99 | 6.32 |
| E | 3.68 | 0.44 | 4.01 | 2.65 | 9.48 | 36.33 | 35.29 | 8.13 |
| Sample | Ecorr/V | I/(A/cm2) |
|---|---|---|
| NO | −0.0392 | 8.108 × 10−4 |
| 1200 W | −0.0322 | 4.880 × 10−4 |
| 1400 W | −0.0669 | 5.364 × 10−5 |
| 1600 W | 0.0284 | 2.397 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Chen, J.; Huang, J.; Shi, W.; Wang, Q.; An, F.; Wu, J. Laser-Melted Wc/Ni-Based Coating Remelting Study on Q235 Steel Surface. Coatings 2024, 14, 1172. https://doi.org/10.3390/coatings14091172
Wu X, Chen J, Huang J, Shi W, Wang Q, An F, Wu J. Laser-Melted Wc/Ni-Based Coating Remelting Study on Q235 Steel Surface. Coatings. 2024; 14(9):1172. https://doi.org/10.3390/coatings14091172
Chicago/Turabian StyleWu, Xianglin, Junhao Chen, Jiang Huang, Wenqing Shi, Qingheng Wang, Fenju An, and Jingquan Wu. 2024. "Laser-Melted Wc/Ni-Based Coating Remelting Study on Q235 Steel Surface" Coatings 14, no. 9: 1172. https://doi.org/10.3390/coatings14091172






