Abstract
In this work, a series of fluorinated microemulsions were synthesized using thermally decomposable N-dodecyl-N,N-dimethylamine N-oxide (LDAO) as surfactant. Then, polybutylene terephthalate nonwoven fabrics (PBT) were coated with microemulsion and heat-treated. Superhydrophobic and oil-repellent modified PBT with WCA (water contact angle) of about 152°, a sliding angle of about 2.1°, and oil repellency grade of 8 were prepared. The effect of surfactants on the surface wettability of hydrophobic materials was analyzed by TG-DTA, XPS, and WCA tests. The results show that surfactants decrease the WCA of hydrophobic materials, but LDAO can eliminate this effect by heat treatment. The anti-corrosion and permeability of LDAO coatings were compared with those of conventional fluorinated coatings through degradation and anti-permeability tests. It was shown that the LDAO fluorinated superhydrophobic coating is more resistant to corrosion by chemical solutions and significantly improves the impermeability of porous materials. Anti-fouling and self-cleaning tests showed excellent anti-fouling and self-cleaning properties on several common substrate surfaces modified with LDAO fluorinated microemulsions. It is expected that these new LDAO fluorinated microemulsions have promising applications in the preparation of corrosion-resistant surfaces and impermeable structures.
1. Introduction
Materials commonly used in life, such as fabrics, paper, wood, and building materials, are easily wetted and penetrated by water due to their wettability and porosity, causing corrosion of the materials [1,2,3,4,5]. Bio-inspired superhydrophobic surfaces with excellent wettability resistance can effectively improve the corrosion and permeation resistance of materials [6,7,8,9,10,11,12]. The wettability of a material can be characterized by the CA (contact angle) and RA (roll angle). When the CA is less than 90°, it is a hydrophilic surface. When the CA is greater than 90°, it is a hydrophobic surface, and especially when the CA is greater than 151° and RA is less than 10°, it is a superhydrophobic surface. Currently, methods for artificially fabricating superhydrophobic surfaces include coating [13,14,15], template-based [16], electrospinning [17], etching [18], and electrochemical deposition methods [19]. In the case of most substrates, the coating method is the most suitable solution due to its low cost, simple process control, and applicability to a variety of complex structures. The coating method is a process in which compounds with low surface energy are dispersed by using water or organic solvents and then coated onto the surface of materials [20,21,22,23]. Low surface energy materials include fluorinated compounds, silane compounds, and long chain alkane compounds. Among them, fluorinated compounds coatings have not only excellent hydrophobicity, but also oil-repellent properties for a wider range of applications. However, Perfluorooctane Sulfonate (PFOS), Perfluorooctanoic Acid (PFOA), and perfluorinated compounds with a perfluoroalkyl chain length ≥ 8 are potentially harmful to the environment and human health. Therefore, 2,3,4,5,5,5,5-hexafluoro-2,4-bis(trifluoromethyl)pentyl methacrylate (DFHMA), which has a short and safe chain, was selected for this study. Fluorinated copolymer emulsions prepared with short-chain fluorine-containing monomers have attracted the most attention because they are environmentally friendly, effective, and easy to produce on a large scale [24,25,26,27,28]. However, due to the difficult emulsification of fluorine-containing monomers, a large number of surfactants are required in the preparation of fluorinated emulsions. These surfactants will remain in the fluorinated superhydrophobic coating and adversely affect the stability of the superhydrophobic coating. The contact of water with a rough superhydrophobic surface would form air pockets in the microtextured gaps of the interface, as described in the Cassie model [29]. When submerged, a solid–air–water interface exists between the superhydrophobic coated surface and water. As time increases, trapped air is lost to the water environment and the contact area between the water and the coating increases. The direct contact of water with the coating will induce the migration of the hydrophilic groups of the surfactant in the coating to the surface. The enrichment of hydrophilic groups on the surface causes the superhydrophobic coating to transition from dewetted (Cassie) to wetted (Wenzel) state. In the wetted state, the hydrophobicity of the coating is significantly weakened and even becomes hydrophilic. As a result, fluorinated superhydrophobic coatings containing surfactants have a short longevity (the time until transition from dewetted to wetted state) [30]. Superhydrophobic surfaces and porous structures fabricated with coatings containing surfactants are not resistant to corrosion and penetration of solutions.
To obtain stable corrosion-resistant superhydrophobic coatings, many attempts have been carried out, such as adding crosslinked surfactants and polymerizable surfactants [28,31,32]. Yin et al. [28] used fluorinated emulsions containing macromolecular surfactants with -SO3 to prepare superhydrophobic coatings and eliminate hydrophilic groups in the coatings by crosslinking with methyl etherified melamine. However, the crosslinking approach does not ensure that the -SO3 in the coating is completely eliminated, and new hydrophilic groups -NH and -NH2 may be introduced due to insufficient etherification of the methylated melamine used. The hydrophilic groups in the coating cannot be completely eliminated and the long-term durability of superhydrophobic coatings is uncertain. Hence, a suitable mechanism needs to be selected to completely eliminate surfactants from fluorinated superhydrophobic coatings in order to improve the corrosion resistance and lifetime of the coating.
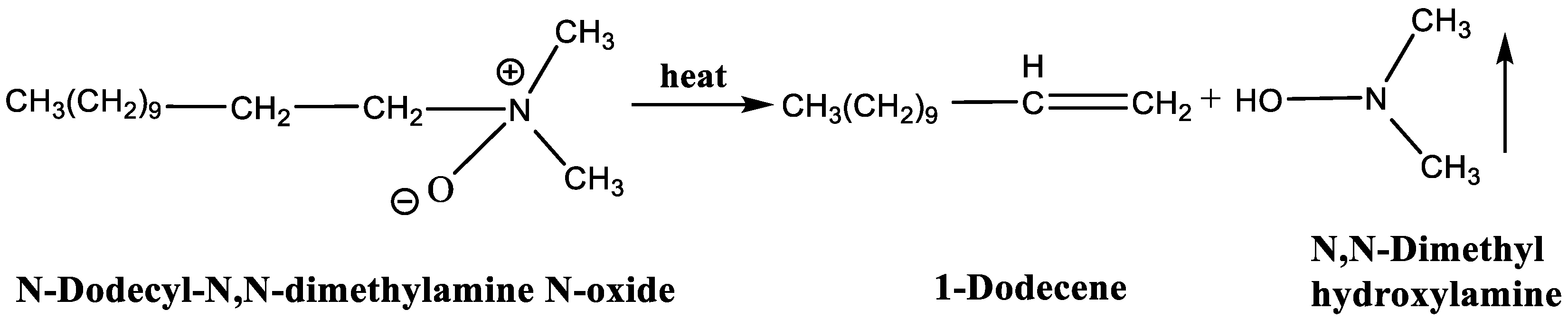
LDAO is an amphiphilic surfactant, cationic in acidic conditions and nonionic in neutral or alkaline media [33,34]. It is composed of long-chain alkanes as hydrophobic groups and amine oxide as hydrophilic groups. When heated above 100 °C, LDAO undergoes a Cope elimination reaction, decomposing into 1-dodecene and N, N-Dimethyl hydroxylamine [35], as shown in Figure 1. The N, N-Dimethyl hydroxylamine transformed by the hydrophilic group is volatile and the final residue is hydrophobic 1-Dodecene. Therefore, superhydrophobic coatings without residual surfactants can be obtained by coating with the fluorinated emulsions containing LDAO and heat-treating the substrates.
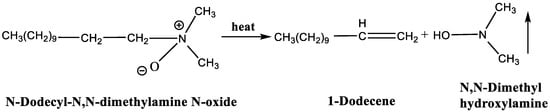
Figure 1.
Thermal decomposition of N-Dodecyl-N,N-dimethylamine N-oxide.
In this work, we provided an effective method to simply fabricate corrosion-resistant superhydrophobic surfaces and impermeable structures using synthesized fluorinated microemulsions containing the thermally decomposable surfactant LDAO via dip-coating. PBT nonwoven fabrics with high porosity, a certain degree of hydrophobicity, and harsh application environments (when used as medical gas-permeable hydrophobic membranes, the surface needs to be immersed in an aqueous solution for a long period of time) were chosen as the substrates. It was suitable for studying the wettability of hydrophobic surfaces coated with surfactants, the corrosion resistance of superhydrophobic coatings, and the permeation resistance of porous structures. The results of this study demonstrated the decomposition and volatilization of LDAO during heat treatment. The effect of the surfactant LDAO on the wettability of hydrophobic surfaces could be eliminated by heat treatment compared to conventional surfactants. Superhydrophobic coatings prepared by fluorinated microemulsions containing LDAO were more resistant to corrosion from water, salt solutions, acid solutions, and alkaline solutions. By applying the microemulsions to other substrates, corrosion-resistant, anti-fouling, and self-cleaning surfaces were also obtained. Moreover, porous structures modified with the microemulsions were significantly more impermeable and the pores were less likely to be clogged by water.
2. Experiments
2.1. Materials
PBT melt-blown nonwoven fabrics (PBT fabrics) with a 4 µm average fiber diameter and 0.3 μm average pore size were provided by Guangzhou Sanli Nonwoven Co., Ltd. (Guangzhou, China). 2,3,4,5,5,5-hexafluoro-2,4-bis(trifluoromethyl)pentyl methacrylate (DFHMA, 97%) and N-dodecyl-N,N-dimethylamine N-oxide (LDAO, 35% in H2O) were supplied by the Shanghai Yihong New Material Technology Co., Ltd. (Shanghai, China). The monomers of stearyl methacrylate (SMA, 96%) and methyl methacrylate (MMA, 97%) were obtained from Shanghai Yongzheng Chemical Co., Ltd. (Shanghai, China). Hydrochloric acid solution (1.015 mol/L) was purchased from Sinopharm Chemical Reagent Co., Ltd., China. (Shanghai, China). The other reagents were purchased in their analytical grade and used as received, including sodium dodecylbenzene sulfonate (SDBS, 90%), ethanol (99%), 4,4′-azobis (4-cyanovaleric acid), sodium chloride (99.5%), diethylamine, D-glucose (anhydrous), acetic acid (99.5%), and oil repellency standard test liquids. Deionized water (DI) was used throughout the emulsion synthesis and coating treatment process.
2.2. Fabrication of Emulsions and Superhydrophobic Fabrics
The fluorinated emulsions were prepared with concentrations of LDAO from 0.5% to 1%, 2%, 3%, and 4% (namely, E0.5, E1, E2, E3, and E4, respectively). First, different amounts of LDAO were dissolved in 135.3 g dilute acid aqueous solution (pH = 2, adjusted with hydrochloric acid solution) to prepare the desired concentration of surfactant solution. A 13.5 g measure of DFHMA, 6.8 g of SMA, and 13.5 g of MMA were introduced into a 250 mL flask and mixed with vigorous stirring for 30 min. Then, the mixed monomers were added to the LDAO solution and mixed for 3 min using a homogenizer. Finally, 4,4′-azobis (4-cyanovaleric acid)/absolute ethanol solution (0.05 g/2 g) was added and the reaction was carried out at 80 °C with constant stirring for 4 h to obtain the fluorinated emulsions. The product of P(DFHMA-co-SMA-co-MMA) (PFSA) was prepared by repeated precipitation and washing of the emulsion with ethanol and deionized water, and then dried under vacuum at 40 °C for further characterization. In addition, the emulsion ES was prepared with common surfactant SDBS and used as a control group for the superhydrophobic coating corrosion resistance test.
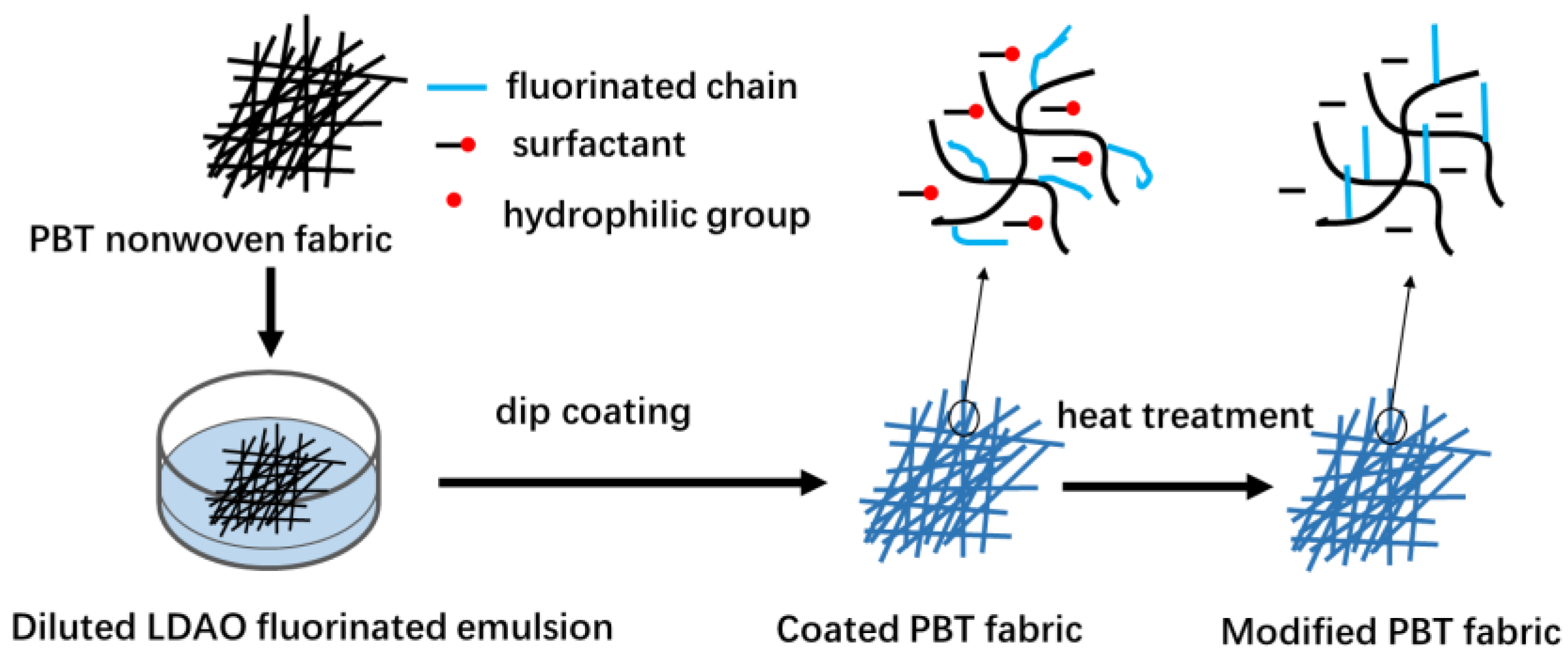
The fabrication process of superhydrophobic PBT fabric is shown in Figure 2. Firstly, an impregnating solution was prepared by adding 8% concentration of fluorinated acrylic emulsion to an ethanol/water mixture (13/87, g/g). Then, the PBT fabrics were immersed in the impregnating solution for 30 s. Finally, the fabrics were dried at 60 °C for 30 min and heat-treated at 170 °C for 10 min in an oven. PBT-E0.5, PBT-E1, PBT-E2, PBT-E3, PBT-E4, and PBT-ES were modified PBT fabrics coated with E0.5, E1, E2, E3, E4, and ES, respectively. PBT-E4a was fabric coated with E4 but not heat-treated at 170 °C. In addition, a series of superhydrophobic coatings were prepared on the surface of several materials susceptible to water corrosion, such as glass fiber paper, wool nonwoven mats, and wood substrate.
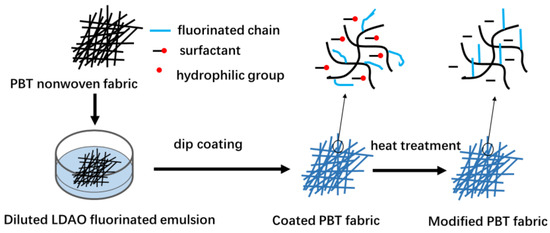
Figure 2.
The fabrication process of the superhydrophobic PBT fabrics.
2.3. Emulsion Characterizations
The molecular structure of PFSA was characterized by Fourier transform infrared spectroscopy (FTIR, BRUCKER, EQUINOX55, Billerica, MA, USA) and proton nuclear magnetic resonance (1H NMR, BRUCKER, Avance III 400 MHz, Billerica, MA, USA) using CDCl3 as solvent. The particle size of the fluorinated emulsions was measured by a laser particle size analyzer (PSDA, Beckman Coulter, LS 230, Pasadena, CA, USA). The monomer conversion of the emulsions was characterized by gravimetric analysis.
2.4. Surface Composition and Wetting Properties
The surface chemical composition of PBT fabrics before and after coating treatment was characterized by X-ray photoelectron spectroscopy (XPS, Thermo Scientific, ESCALAB 250Xi, Waltham, MA, USA) at an electron take-off angle of 60° with respect to the fiber sample plane. Scanning electron microscopy (SEM, ZEISS Gemini SEM 300, Oberkochen, Germany) was used to study the surface morphologies of fabrics. The wettability was analyzed using an optical contact angle meter (Dataphysics OCA20, Beijing, China) by measuring the contact of water droplets with volumes of 5 µL at ambient temperature. Each sample was tested 5 times for WCA at different positions and the average value was used. The oil repellency was tested according to AATCC test method 118-2002.
The effect of surfactants on the wettability of PBT fabrics was investigated as follows. Firstly, the fabric PBT-AO-1 was prepared by dip-coating PBT with a 4% aqueous solution of LDAO and drying at 60 °C for 10 min. The fabric PBT-AO-2 was prepared by heat-treating PBT-AO-1 at 170 °C for 10 min. The fabric PBT-SDBS was prepared by the same process as for PBT-AO-2 except that the surfactant was SDBS. Then, the WCAs of pristine PBT fabrics, PBT-AO-1, PBT-AO-2, and PBT-SDBS were measured using an optical contact angle meter (Dataphysics OCA20, Beijing, China).
2.5. Thermodynamic Analysis
The thermodynamic characteristics of LDAO were analyzed by a thermogravimetric analyzer (TGA, TA, Q200, New Castle County, DE, USA). Approximately 2 mg of sample was heated from 30 °C to 600 °C at a heating rate of 10 °C/min under an air atmosphere. The chemical structure of LDAO before and after heat treatment at 170 °C was characterized by Fourier transform infrared spectroscopy (FTIR, BRUCKER, EQUINOX55, Billerica, MA, USA).
2.6. Degradation Tests
Corrosion of PBT was mainly caused by the degradation of cellulose. The water absorption of cellulose changes significantly before and after degradation, so the degradation tests of PBT could be carried out through water absorption experiments. The water absorption test procedure was as follows. The fabrics were immersed in deionized water, saline, aqueous glucose solution (10%), aqueous acetic acid solution (1%), and aqueous diethylamine solution (1%) and tested for water absorption with time. Before the immersion experiments, the fabrics were dried. The dry weight of the fabric was measured using standard weighing balance. Then, the fabrics were completely immersed in the solution to be tested (fabrics were placed horizontally and the height of the upper surface from the liquid surface was 10 cm). On the first day, the wet weights of the fabrics immersed for 2 h, 4 h, and 8 h were measured. After that, the wet weight was recorded at 1-day intervals until day 7. The surfaces of fabrics were wiped with a dry cloth when recording the wet weight of the fabrics. Each sample was tested 3 times and the average value was taken as the test result. The water absorption was calculated according to Equation (1):
where ω is the water absorption, mwet is the wet weight of fabrics, and mdry is the dry weight of fabrics. In addition, the immersion times of the fabric surface being wetted were recorded during the immersion experiments.
2.7. Impermeability Test
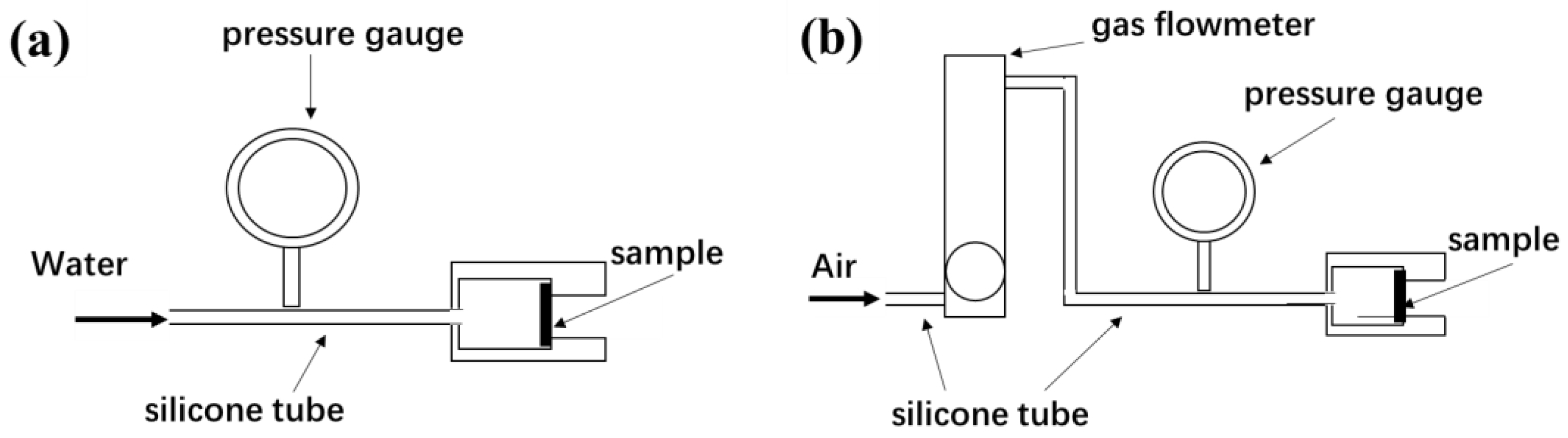
Osmotic pressure (ΔP) of the fabrics was tested according to Chinese pharmaceutical industry standard YY 0770.2-2009 [36], and ΔP value was used to characterize the impermeability of the porous materials. The schematic diagram for measuring the ΔP value is shown in Figure 3a. The fabric is fixed in the fixture and the water pressure on one side of the fabric is gradually increased until water droplets appear on the other side of the fabric, at which point the pressure value is the ΔP value.
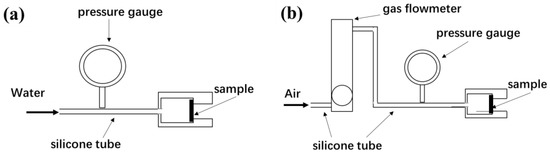
Figure 3.
The schematic diagram for measuring ΔP value (a) and air flux (b).
In order to investigate the effect of water permeation on the fabric pores, air flux values of the fabrics were tested and the air flux reduction rate of fabrics after the ΔP value test was calculated. The schematic diagram for measuring air flux value is shown in Figure 3b. The fabric is fixed in the fixture and the air flow value of the fabric at an air entry pressure of 10 kPa is recorded. The air flux reduction rate was calculated according to Equation (2):
where η (%) is the air flux reduction rate, Q1 is the air flux value of fabric before the ΔP value test, and Q1 is the air flux value of fabric after the ΔP value test.
3. Results and Discussion
3.1. Characterization of the Fluorinated Emulsions
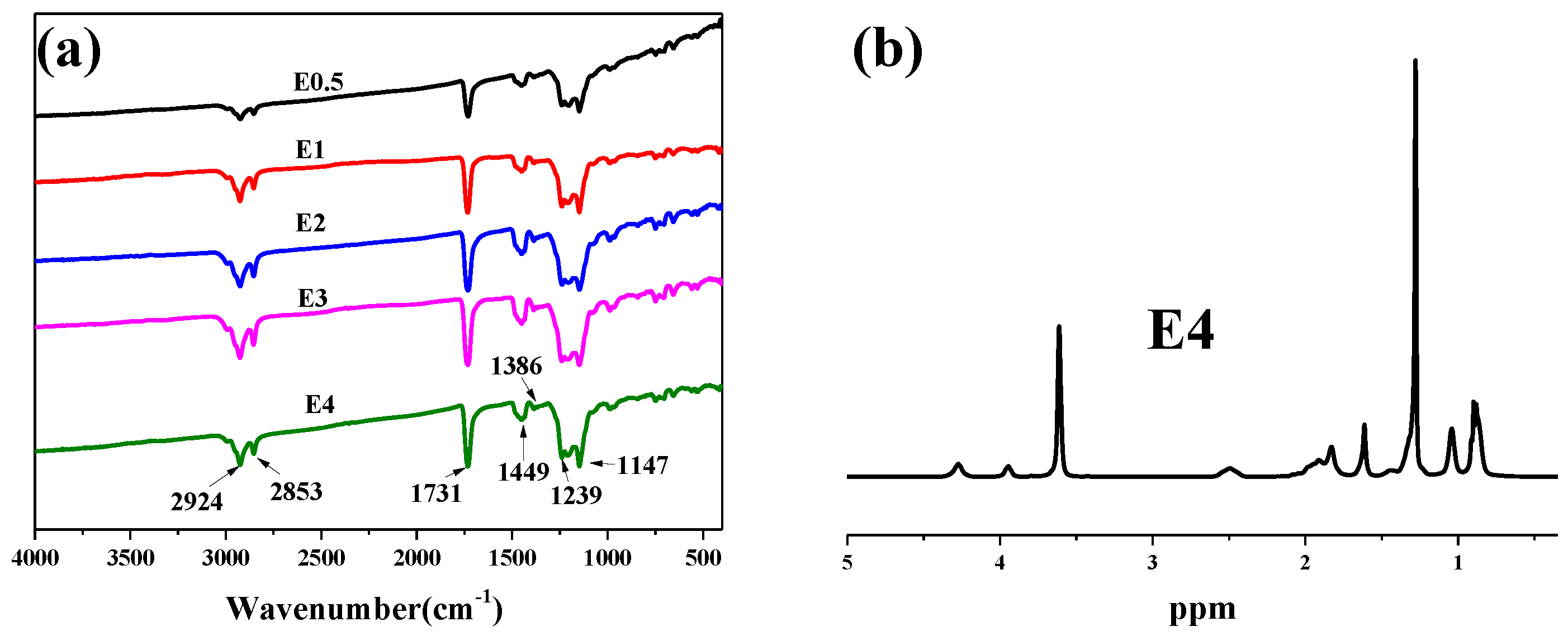
The chemical structure of fluorinated polyacrylate, PFSA, was confirmed by FTIR and 1H NMR spectroscopy. As shown in Figure 4a, the products prepared, including E0.5, E1, E2, E3, and E4 were typical fluorinated acrylate copolymer spectra [28]. The vibration bands at 2924 cm−1 and 2853 cm−1 are assigned to the C-H stretching of methyl and methylene groups in the main chain. The characteristic absorption peaks of the -CH3 groups are exhibited at 1449 cm−1 and 1386 cm−1. The strong stretching vibration appearing at 1731 cm−1 can be attributed to C=O in the esters. In addition, in all prepared fluorinated polyacrylates, distinctive characteristic peaks of -CF3 and -CF groups appeared at 1239 cm−1 and 1147 cm−1, respectively. Figure 4b shows the 1H-NMR spectrum of E0.5. The characteristic peaks of 4.3 ppm, 3.9 ppm, 3.6 ppm, and 1.7 ppm are assigned to -CH2 in DFHMA, -CH2 groups in SMA, -CH3 in MMA, and -(CH2)15 in SMA, respectively. The FTIR and 1H-NMR results demonstrate that DFHMA, SMA, and MMA were effectively polymerized.
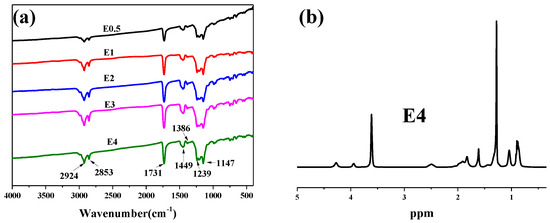
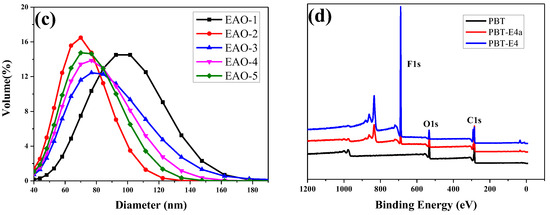
Figure 4.
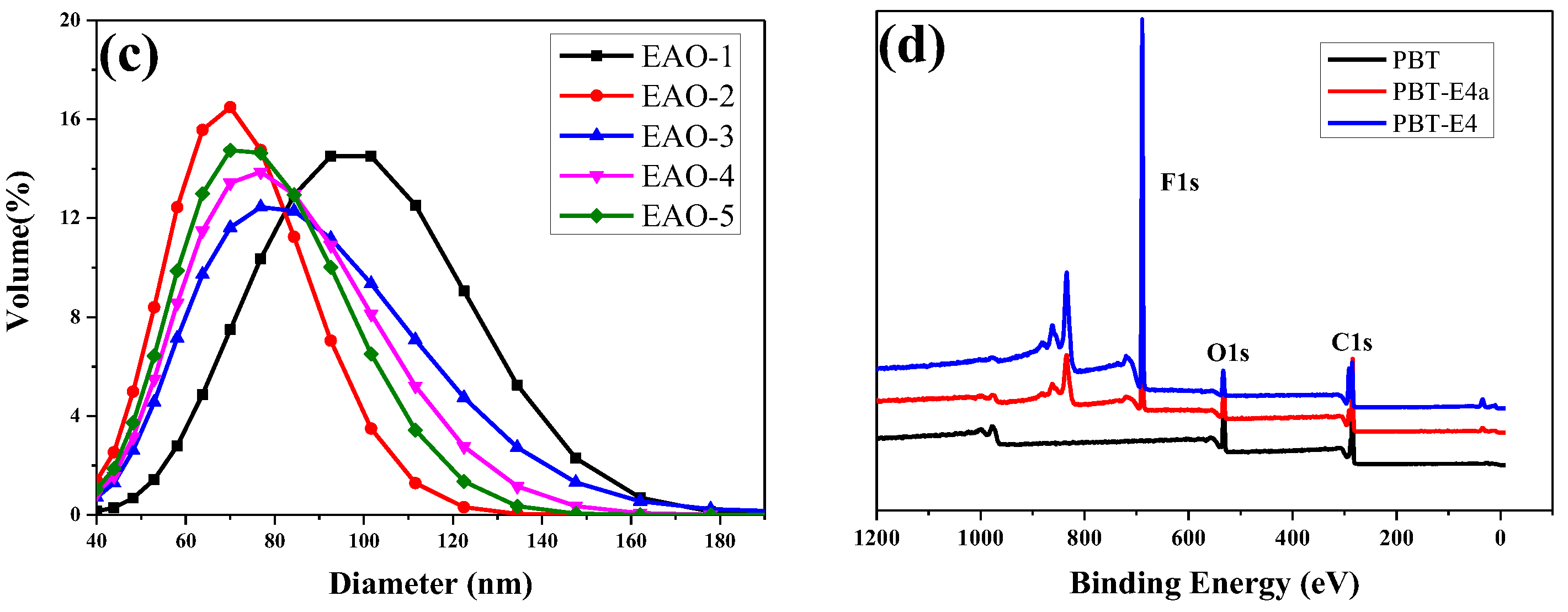
FTIR (a) and 1H-NMR (b) spectra of fluorinated emulsions after dehydration. (c) The laser particle size graphs of fluorinated emulsions. (d) XPS spectra of PBT, PBT-E4a, and PBT-E4.
The monomer conversions of fluorinated emulsions were analyzed by gravimetric analysis. As shown in Table 1, the monomer conversion of the synthesized PFSA was 82.18% when the concentration of surfactant LDAO was 0.5%. The low monomer conversion will lead to waste of monomers and instability of emulsion polymerization. However, as the surfactant concentration increases, the monomer conversion increased and approached 100%, indicating that LDAO has effective emulsification properties for fluorinated monomers. Figure 4c and Table 1 show that microemulsions with average particle size less than 100 nm were successfully synthesized. Microemulsions had a smaller particle size compared to normal emulsions [37]. The smaller particle size provided the microemulsions with better coating properties. Therefore, the amphiphilic surfactant LDAO effectively emulsified fluorinated acrylic monomers under acidic conditions. When the concentration of surfactant LDAO was appropriate, fluorinated microemulsions with small average particle size and high monomer conversion rate could be prepared.

Table 1.
The characterization of prepared fluorinated emulsions.
3.2. The Performance of Coated PBT Fabrics
PBT fabrics were chosen as modified substrates due to their work in corrosive solutions for long periods of time when used as filtration media (e.g., medical gas-permeable hydrophobic membrane). In addition, the surface hydrophobicity, high porosity, and excellent mechanical strength of PBT fabrics make them suitable for the preparation of highly impermeable porous materials.
3.2.1. The Surface Composition of Fabrics
The surface chemical compositions of pristine PBT fabrics (PBT), PBT-E4a (fabrics modified with E4 and not heat-treated with 170 °C), and PBT-E4 (fabrics modified with E4 and heat-treated with 170 °C) were characterized by XPS, as shown in Figure 4d and Table 2. The F content on the surface of PBT, PBT-E4a, and PBT-E4 was 0%, 35.23%, and 54.19%, respectively. This indicated that the fluorinated polymers were successfully coated onto the fabrics. The F content of PBT-E4 was higher than that of PBT-E4a due to the preferential enrichment of fluorinated groups in the coating at the film–air interface as a result of heat treatment at 170 °C [28]. The enrichment of fluorinated groups could further reduce the surface energy of the fabric. The N content on the surface of PBT, PBT-E4a, and PBT-E4 was 0%, 0.08%, and 0%, respectively. The N element on the surface of PBT-E4a was the result of surfactant LDAO residue in the coating. LDAO decomposed into 1-Dodecene and N, N-Dimethyl hydroxylamine under the heat treatment condition above 100 °C, and N, N-Dimethyl hydroxylamine was volatilized during the heat treatment. Therefore, the N content of PBT-E4 was 0%.

Table 2.
Chemical composition of fabrics.
3.2.2. The Topology and Wettability of Fabrics
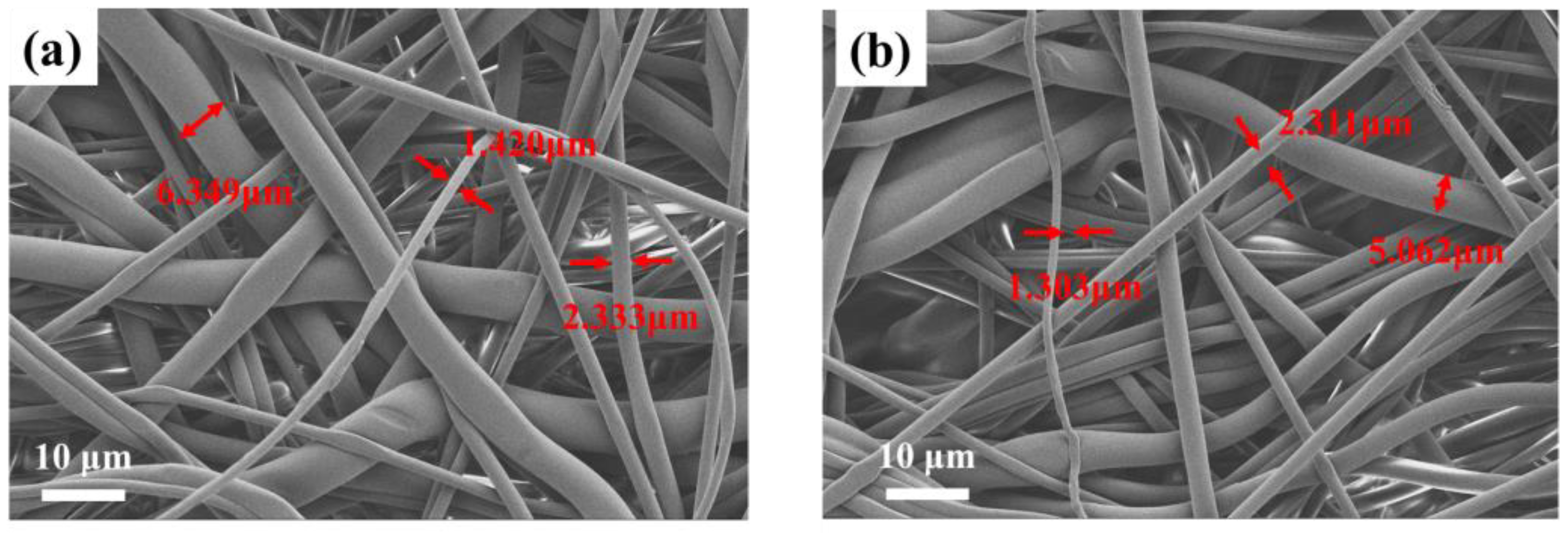
The morphological information of pristine PBT fabrics and PBT-E4 was investigated by SEM and is illustrated in Figure 5. The microscopic morphology of the coated fabrics did not change significantly, with fiber diameters ranging from 1.6 µm to 7 µm, which was similar to that of the pristine PBT fabrics. This indicates that the coating treatment did not alter the microscopic morphology of the fabrics.
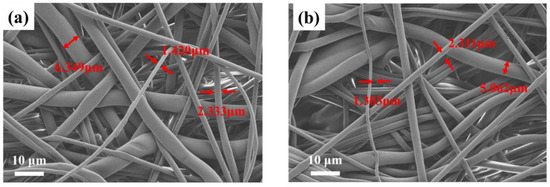
Figure 5.
SEM of PBT (a) and PBT-E4 (b).
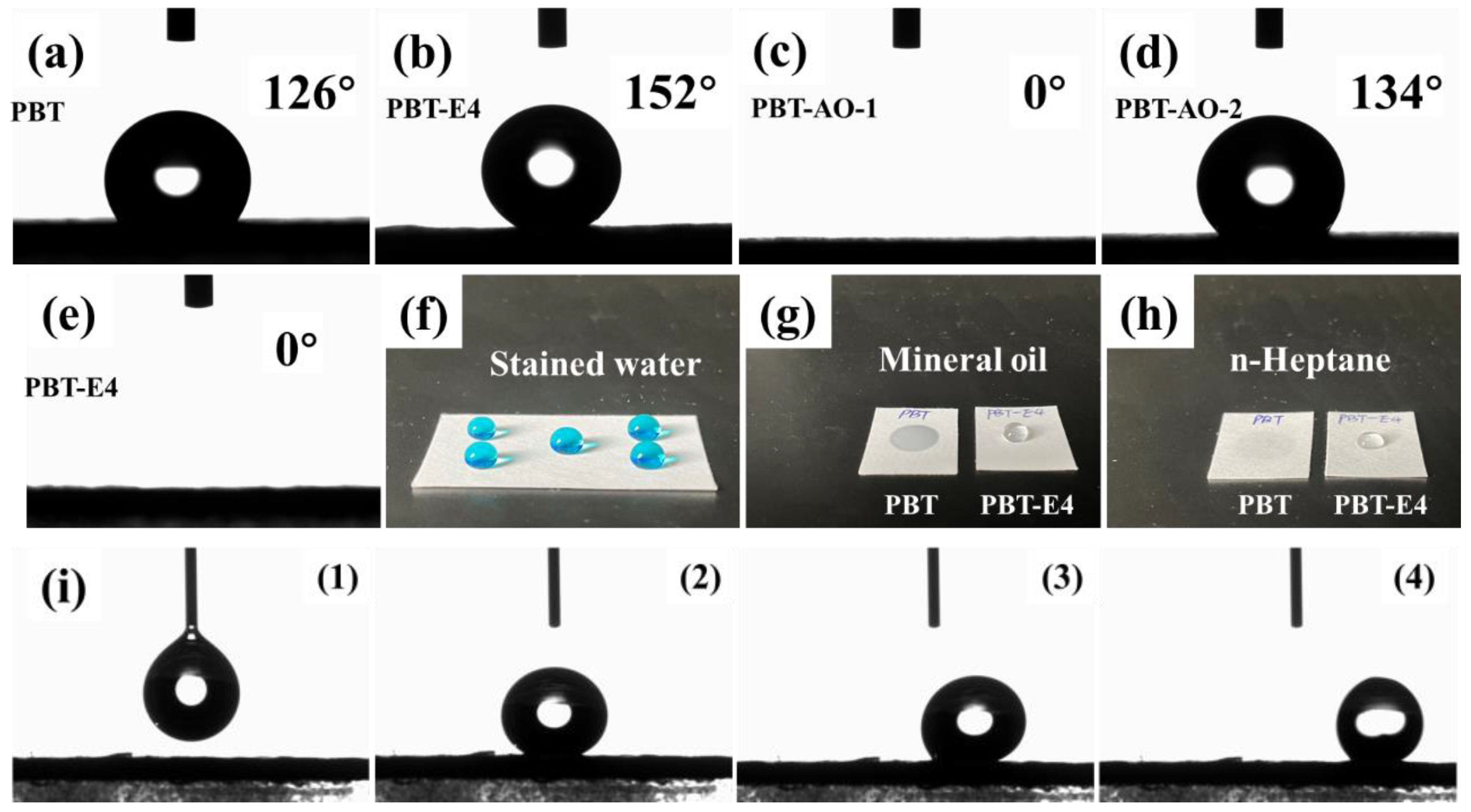
Figure 6 shows the wettability of water on the surface of PBT and PBT-E4. As can be seen in Figure 6a, PBT was strongly hydrophobic with a WCA of 126 ± 1.5°. As shown in Figure 6b,f, after modification, PBT-E4 reached a superhydrophobic state with a WCA of 152 ± 1.1° (stained with methyl blue). The surface sliding angle of PBT-E4 was 2.1 ± 0.6°, as shown in Figure 6i. The hydrophobicity of PBT-E4 was superior to the reported superhydrophobic glass fiber mats coated with fluorinated emulsions [28]. The oil repellency of the fabrics was tested according to AATCC test method 118-2002 (Oil Repellency: Hydrocarbon Resistance Test). The principle of the test is as follows. Drops of standard test liquids, consisting of a selected series of hydrocarbons with varying surfaces and observed for wetting, wicking, and contact angle. The oil repellency grade is the highest numbered test liquid which does not wet the fabric surface. Drops of standard test liquids numbered 0 to 8 were placed on the fabric surface and it was observed whether they were wetted within 30 ± 2 s. As shown in Figure 6g, when the mineral oil with number 0 was applied to the surface of PBT, permeation occurred immediately. The results showed that pristine PBT fabrics were lipophilic and the oil repellency grade was 0. This was due to the large number of alkane chains in PBT. When applying the test liquid dropped on the surface of PBT-E4 and gradually increasing the numbering of the test liquid until the n-heptane with numbering 8, penetration still did not occur, as seen in Figure 6h. It was proved that the coating improved the oil repellency grade of PBT fabrics from 0 to 8. The excellent oil repellency made the coated fabrics less susceptible to contamination by oily substances during use.
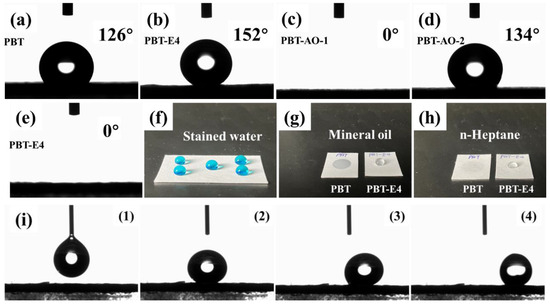
Figure 6.
Wettability test for fabrics. (a–e) Static contact angle of PBT (a), PBT-E4 (b), PBT-AO-1 (c), PBT-AO-2 (d), and PBT-SDBS (e). (f) Static photo of stained water droplets on the PBT-E4. (g,h) The droplets of mineral oil (g) and n-heptane (h) on the surface of PBT and PBT-E4. (i) From (1) to (4) are motion pictures of PBT-E4 slide angle test.
3.3. Effects of Surfactants on the Wettability of Hydrophobic Surfaces
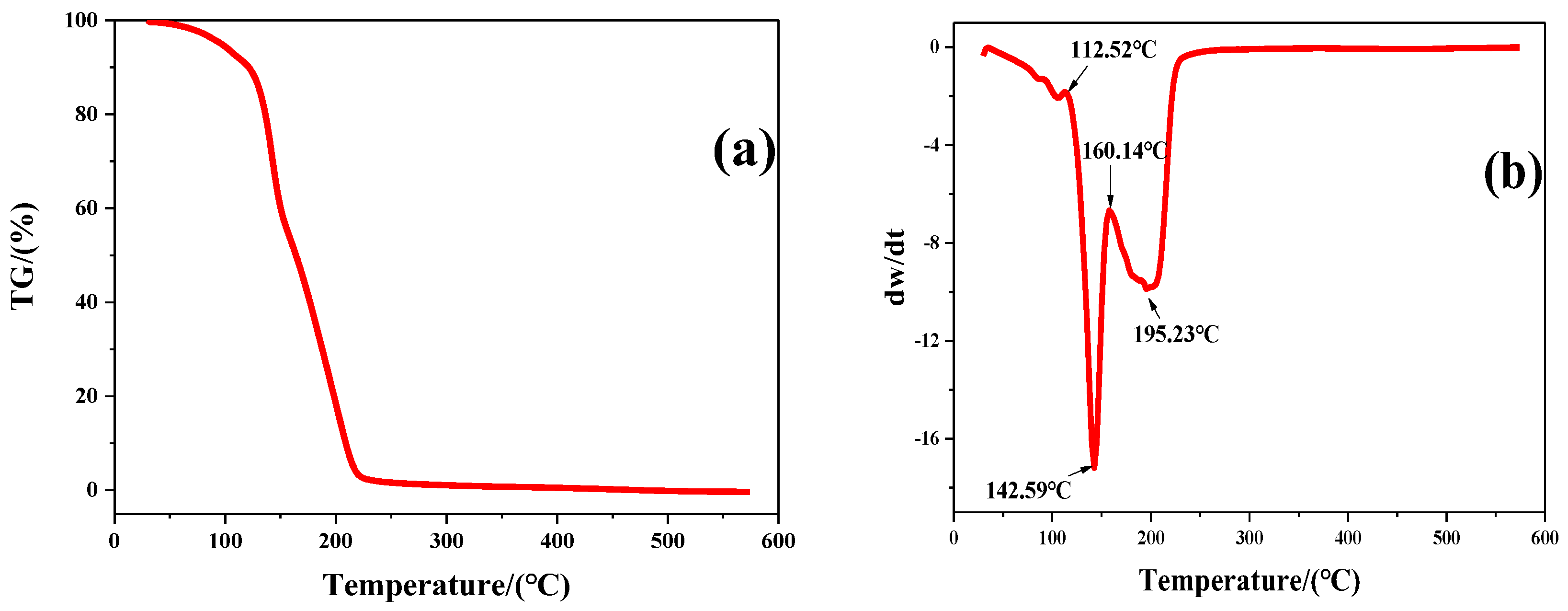
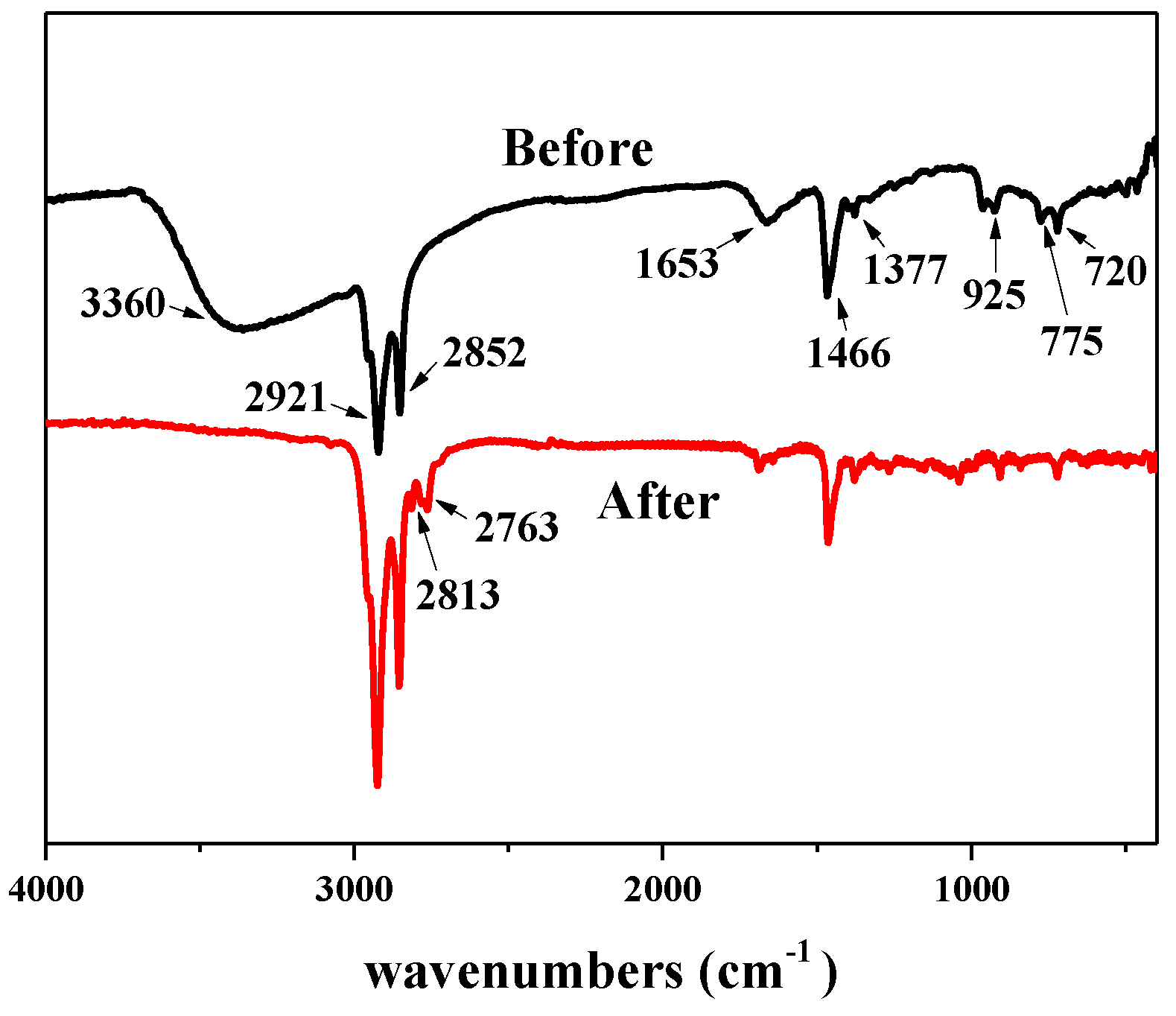
The thermodynamic properties of the surfactant LDAO were investigated by TGA and the corresponding curves are included in Figure 7. When LDAO was heated from 30 °C to 600 °C, there were two peaks of weight loss on the curve. The first weight loss occurred at 112.52 °C and peaked at 142.59 °C. This was caused by volatilization of N, N-Dimethyl hydroxylamine. N, N-Dimethyl hydroxylamine was one of the Cope elimination reaction products of LDAO, as shown in Figure 1. The second weight loss occurred at 160.14 °C and peaked at 195.23 °C. This was due to the decomposition and volatilization of 1-dodecene, another Cope elimination decomposition product of LDAO. The chemical structures of LDAO and its residue after heat treatment at 170 °C for 10 min were characterized by FTIR, as shown in Figure 8. The spectrum of LDAO was consistent with the study [33]. The vibrational bands at 3360 cm−1, 2921 cm−1, and 2852 cm−1 were assigned to the stretching of -OH, -CH3, and -CH2-, respectively. The bending vibration at 1466 cm−1 and 1377 cm−1 belonged to the -CH3 and -CH2 deformation. The characteristic peaks of LDAO at 1653 cm−1 and 932 cm−1 were the hydrogen bond and the stretching vibrational of N-O. After heat treatment, the characteristic peaks belonging to LDAO at 1653 cm−1 and 932 cm−1 disappeared, as shown in Figure 8. The results further confirmed that the hydrophilic group in LDAO was decomposed and volatilized after heat treatment.
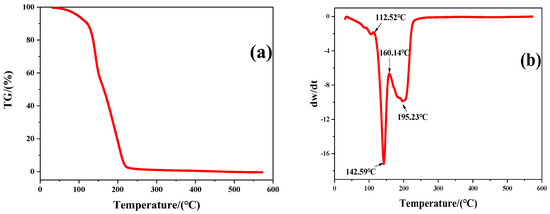
Figure 7.
TGA (a) and DTG (b) curves of N-dodecyl-N,N-dimethylamine N-oxide (LDAO).
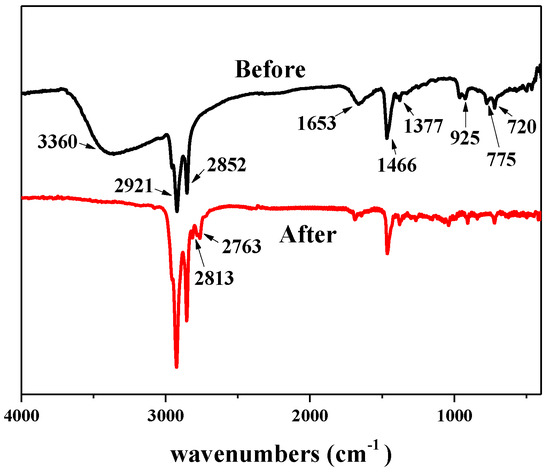
Figure 8.
FTIR spectra of N-dodecyl-N,N-dimethylamine N-oxide (LDAO) before and after thermal treatment.
In order to investigate the effect of LDAO on the wettability of fabric substrates, the surface WCA of pristine PBT, PBT-AO-1 (fabrics modified with LDAO solution and not heat-treated at 170 °C), PBT-AO-2 (fabrics modified with LDAO solution and heat-treated with 170 °C), and PBT-SDBS (fabrics modified with SDBS solution and heat-treated with 170 °C) was characterized. The results are shown in Figure 6. As shown in Figure 6a, PBT had a WCA of 126 ± 1.5° and was strongly hydrophobic. This was due to the large number of hydrophobic alkane chains on the surface of PBT. As shown in Figure 6c,e, PBT-AO-1 and PBT-SDBS were strongly hydrophilic with a WCA of 0°. This indicated that the surface wettability of the fabrics changed after coating with the surfactant solution. This change was caused by the hydrophilic groups on the surfactant. These hydrophilic groups had a strong ability to adsorb water, which could lead to a significant increase in the wettability of the fabric surface. As shown in Figure 6d, the WCA of PBT-AO-2 was 134 ± 0.8°. This indicated that PBT-AO-1 recovered strong hydrophobicity after heat treatment at 170 °C. This was due to the fact that the surfactant LDAO on the surface of PBT-AO-1 decomposed during heat treatment, and the hydrophilic groups were volatilized as hydroxylamine. Moreover, the WCA of PBT-AO-2 was slightly higher than that of PBT because the hydrophobicity of the fabrics was enhanced by residual long-chain alkanes after LDAO decomposition. In contrast, fabrics coated with SDBS solution were strongly hydrophilic even after heat treatment. This was due to the high thermodynamic stability of the hydrophilic groups on SDBS. The above experiments showed that surfactants convert the wettability of the material surface from hydrophobicity to hydrophilicity, but the surfactant LDAO eliminates this effect by heat treatment.
3.4. Corrosion Resistance Property
Superhydrophobic materials are often subjected to corrosion from solutions during application. In the case of superhydrophobic PBT fabrics, for example, when used as a medical gas-permeable hydrophobic membrane, one side of the surface is permanently immersed in saline, glucose solutions, and antibiotic solutions (hydrophilic amino and carboxyl groups are the main corrosive agents). Therefore, in this work, immersion experiments were carried out with deionized water, physiological saline (0.9% NaCl solution), 10% glucose solution, 1% acetic acid solution (pH = 2.42), and 1% diethylamine solution (pH = 12.83) to study the corrosion resistance of the coatings.
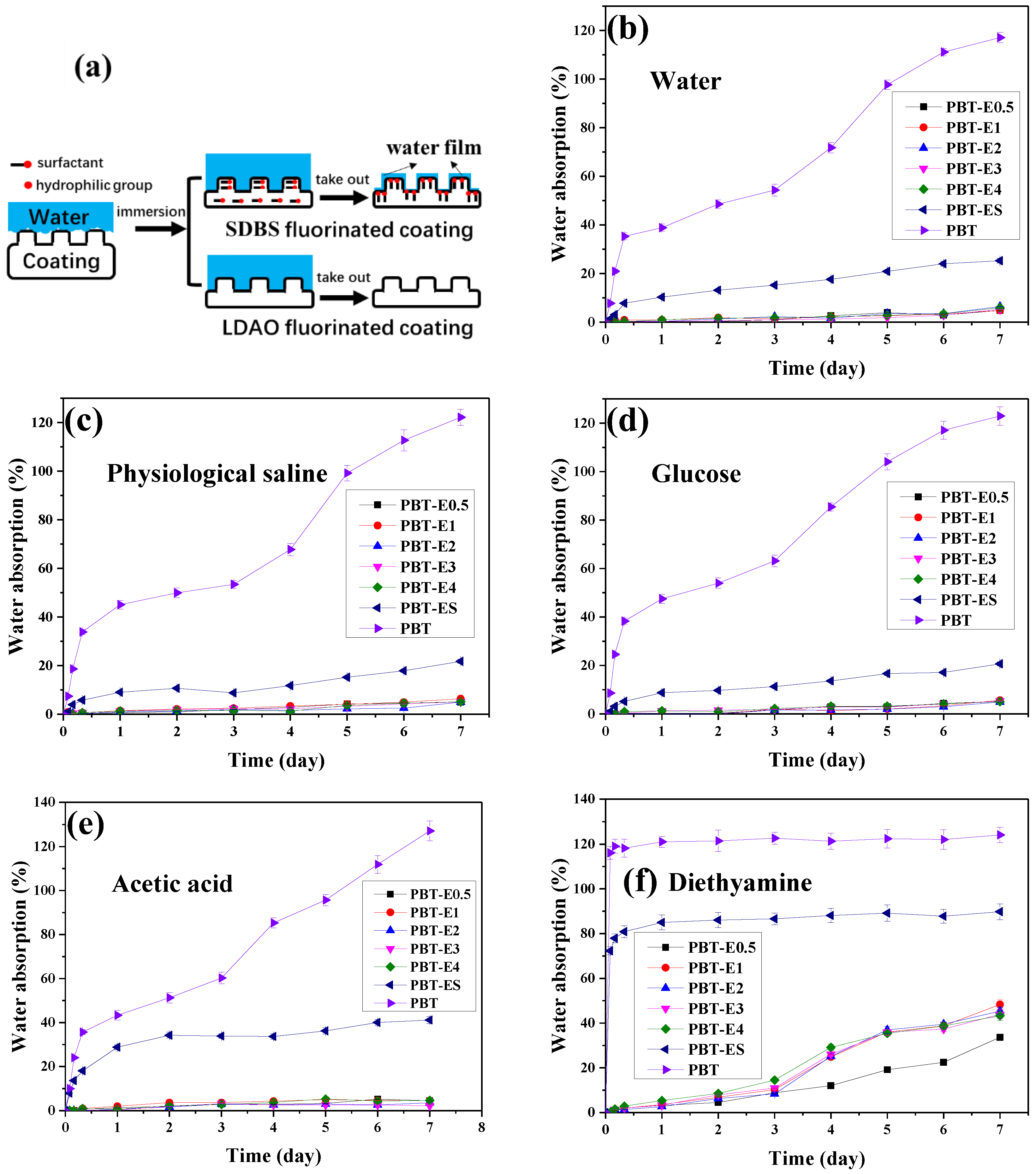
As seen in Figure 9b–d, the water absorption curves of the fabrics were similar in the immersion experiments with deionized water, saline, and glucose solutions. During the first 8 h, the water absorption of the pristine PBT fabrics in the three solutions increased rapidly, reaching 34.94%, 33.87%, and 38.34%, respectively. Then, with the increase in immersion time, it finally reached 117.63%, 122.58%, and 123% at 7 days, respectively. The 7-day water absorptions of PBT-EL (PBT-E0.5, PBT-E1, PBT-E2, PBT-E3, and PBT-E4, collectively referred to as PBT-EL) and PBT-ES coated with fluorinated emulsions was approximately 5% and 20%, respectively. They were all significantly lower than the pristine PBT fabrics. This was due to the strong hydrophobicity of fluorinated coatings, which slowed down the adsorption of water on the fabric surfaces. The water absorption of PBT-EL is lower than that of reported superhydrophobic fabrics [38,39,40,41,42]. In addition, the time at which the fabric surfaces were completely wetted in the three solution immersion experiments is recorded in Table 3. The surfaces of pristine PBT fabrics and PBT-ES were completely wetted after immersion in the three solutions for 2 h. In contrast, the surfaces of PBT-EL fabrics were not wetted in any of the three solution immersion experiments. These experimental phenomena described above were related to the change in wettability of the hydrophobic coating on the surface of the materials during the immersion process, as shown in Figure 9a. At the beginning of the immersion experiment, the hydrophobic material cannot be wetted directly, but is in a Casey state. Air pockets are formed in the microtextured interstices of the interface between water and the rough surface of the material. As the immersion experiment continues, the air in the air pockets is gradually expelled by water pressure. Water completely fills into the rough surface structures and the liquid–solid interface reaches the Wenzel wetting model state [9,30,43,44,45,46]. The enlarged interface causes a large number of hydrophilic groups on the material surface to migrate to the interface under the induction of water. These hydrophilic groups will enhance the adsorption ability of the material to water. When the interface is enriched with hydrophilic groups, the hydrophobic coating adsorbs a thin water film and transforms into a hydrophilic coating. Although the surfaces of PBT and PBT-ES are highly hydrophobic, a large number of hydrophilic groups such as -OH and -SO3 groups remain. This increases the risk of surface water film formation on fabrics in immersion experiments. Therefore, they have higher water absorption and earlier wetting time. The low water absorption of PBT-EL fabrics was due to the fact that the hydrophilic group N-O in the coatings were volatilized during heat treatment, which greatly reduced the water adsorption capacity of the coatings.
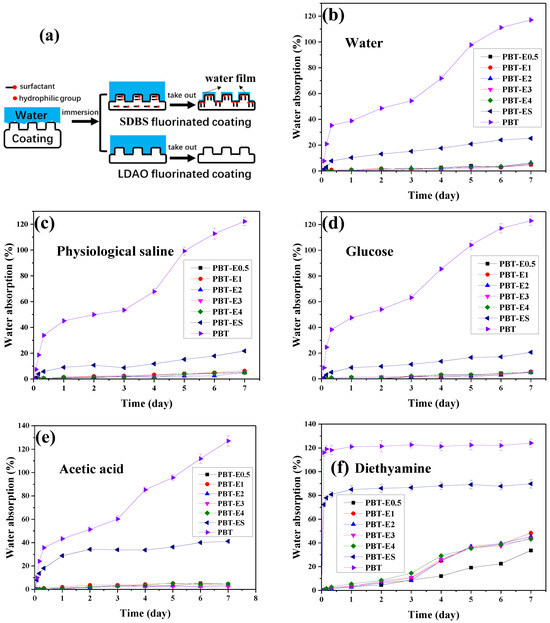
Figure 9.
(a) The wetting process of superhydrophobic coatings in immersion experiments. (b–f) The water absorption curves of fabrics in deionized water, physiological saline, 10% glucose solution, 1% acetic acid solution, and 1% diethylamine solution immersion experiments.

Table 3.
The ΔP values, η (%), and wetting time of immersion experiments.
In the immersion experiments with acetic acid solution, the water absorption curves and values of the pristine PBT fabrics and PBT-EL were similar to those of their immersion experiments with deionized water, as shown in Figure 9e. The 7-day water absorption of PBT-ES was 40.95%, which was higher than that of its deionized water immersion experiment. This was caused by the hydrolysis of the ester bond in the fluorinated acrylate coating in acidic solution, which generated hydrophilic hydroxyl and carboxyl groups. Fluorinated coatings on PBT-ES contained a large number of residual hydrophilic -SO3 groups, which were easier to wet. This increases the risk of hydrolysis of the ester bonds in the coating. In contrast, the coating on PBT-EL fabrics had no hydrophilic groups and was less susceptible to wetting by water, which effectively mitigates corrosion of the coating by acid solutions. As a result, PBT-EL fabrics had much lower water absorption in acid solutions than PBT-ES.
In the immersion experiments with alkali solutions of ethylenediamine, the water absorption curves of the pristine PBT fabrics and PBT-ES showed significant differences from their immersion experiments with other solutions, as shown in Figure 9f and Table 3. After 2 h of immersion, the surfaces of both fabrics were wetted and nearly saturated with water absorption of 116.01% and 72.26%, respectively. In contrast, the PBT-EL fabrics showed good corrosion resistance in the alkali solution immersion experiments. The PBT-EL fabrics exhibited surface wetting and a significant increase in water absorption only on day 3. Subsequently, it slowly increased and the water absorption at 7 days was 33.76–48.04%, which was significantly lower than those of the pristine PBT fabrics and PBT-ES. The water absorption of the fabrics in the alkali solution immersion experiments differed greatly from that in the other immersion experiments, which was related to the intense hydrolysis of the ester bonds in the fabrics and their coatings. The pristine PBT fabrics were composed of polybutylene terephthalate and contained a large number of ester bonds on the surface. When immersed in an alkali solution, these ester bonds were rapidly hydrolyzed, causing the fabrics to rapidly saturate with water absorption. PBT-ES was susceptible to wetting due to the hydrophilic -SO3 in their coatings. This exposed the ester bonds in the coating to alkali solutions. The ester bonds underwent intense hydrolysis in the alkali solution, generating a large number of hydroxyl and carboxyl groups. This caused the water absorption of the PBT-ES to increase rapidly and reach saturation values. PBT-EL fabrics were less susceptible to wetting because no hydrophilic groups were retained in the coating. As a result, the liquid–solid interface was small at the beginning of the immersion experiment, allowing fewer ester bonds to be exposed to the solution and hydrolysis to occur slowly. The surfaces of PBT-EL fabrics were not completely wetted until the third day of the immersion experiment.
As shown by the immersion experiments, fabric substrates coated with fluorinated emulsions exhibited enhanced resistance to corrosion induced by water, salt solutions, acid solutions, and alkali solutions. However, the corrosion resistance of the coating was compromised in the presence of hydrophilic surfactants within the superhydrophobic coating. After eliminating the hydrophilic groups in the surfactant through thermal decomposition, the corrosion resistance of superhydrophobic coatings was enhanced.
3.5. Anti-Fouling and Self-Cleaning Properties
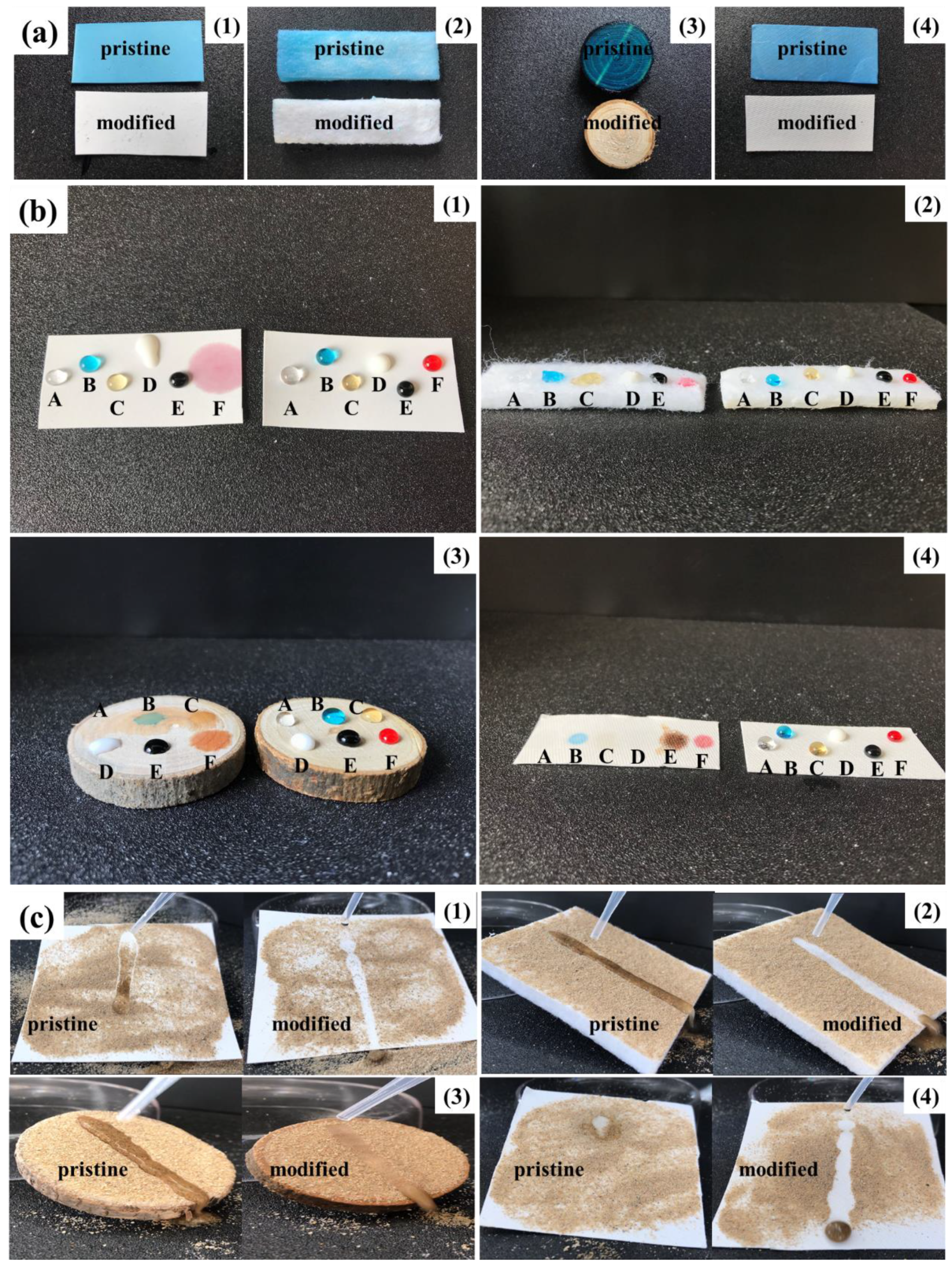
Anti-corrosion and longevity superhydrophobic coatings can be obtained by coating PBT fabrics with fluorinated microemulsions containing LDAO. In order to investigate the surface protection effect of the prepared fluorinated microemulsions on other materials, several substrates susceptible to solution corrosion, such as wool nonwoven mats, wood panels, and glass fiber paper, were selected. These materials were modified with microemulsions, and the anti-corrosion, anti-fouling, and self-cleaning properties of the coatings were studied. The pristine and modified PBT fabrics, wool nonwoven mats, wood panels, and glass fiber paper were immersed in methyl blue stained water at room temperature for 48 h. Figure 10a demonstrates that the surfaces of the four pristine materials turned blue due to wetting, while the surface of the modified materials still retained their original color. Several contaminating liquids, such as vinegar, stained water, coffee, milk, soy sauce, and stained oil (n-hexadecane), were dropped onto the coated substrates. As shown in Figure 10b, all liquids maintained a spherical shape on the four modified substrates. The self-cleaning properties of the modified substrates were evaluated by spreading fine on the surface of the substrates and dropping water. Figure 10c shows that once the water dropped on the surface of the modified substrates, it rolled off quickly, carrying away the sand particles and leaving a clear path on the sample surface. Droplets on the surface of superhydrophobic substrates are in a Cassie–Baxter state. This means that the adhesion and penetration of the droplets to substrates is extremely low. Therefore, the substrates are not contaminated by the droplets and the water droplets effectively attract and carry away the sand from the surface. The above results indicate that fluorinated microemulsions containing LDAO can be applied to other materials to fabricate superhydrophobic coatings with anti-corrosion, anti-fouling, and self-cleaning properties.
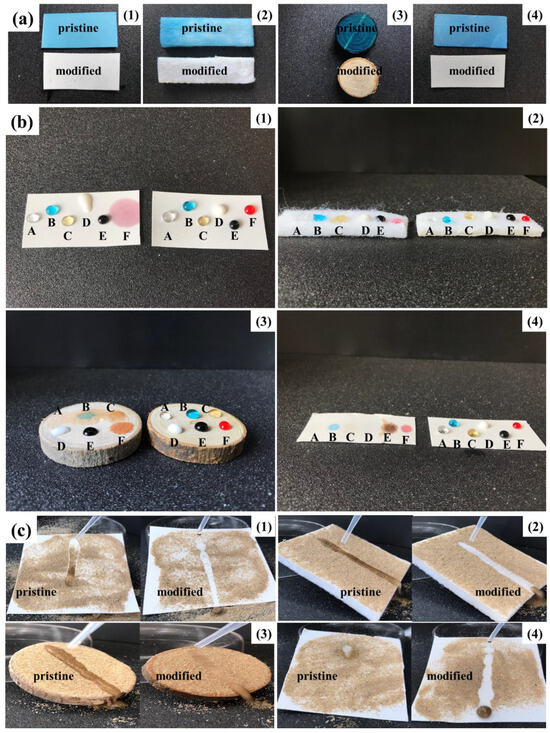
Figure 10.
(a) Comparison between the pristine and modified PBT fabrics (1), wool nonwoven mats (2), wood panels (3), and glass fiber paper (4) after 48 h immersion in methyl blue stained water. (b) Photographs of the diverse liquid droplets on the pristine and modified PBT fabrics, wool nonwoven mats, wood panels, and glass fiber paper. Liquids on each substrate were (A) vinegar, (B) stained water, (C) coffee, (D) milk, (E) soy sauce, (F) stained oil. (c) Self-cleaning test of the pristine and modified PBT fabrics, wool nonwoven mats, wood panels, and glass fiber paper.
3.6. Impermeability
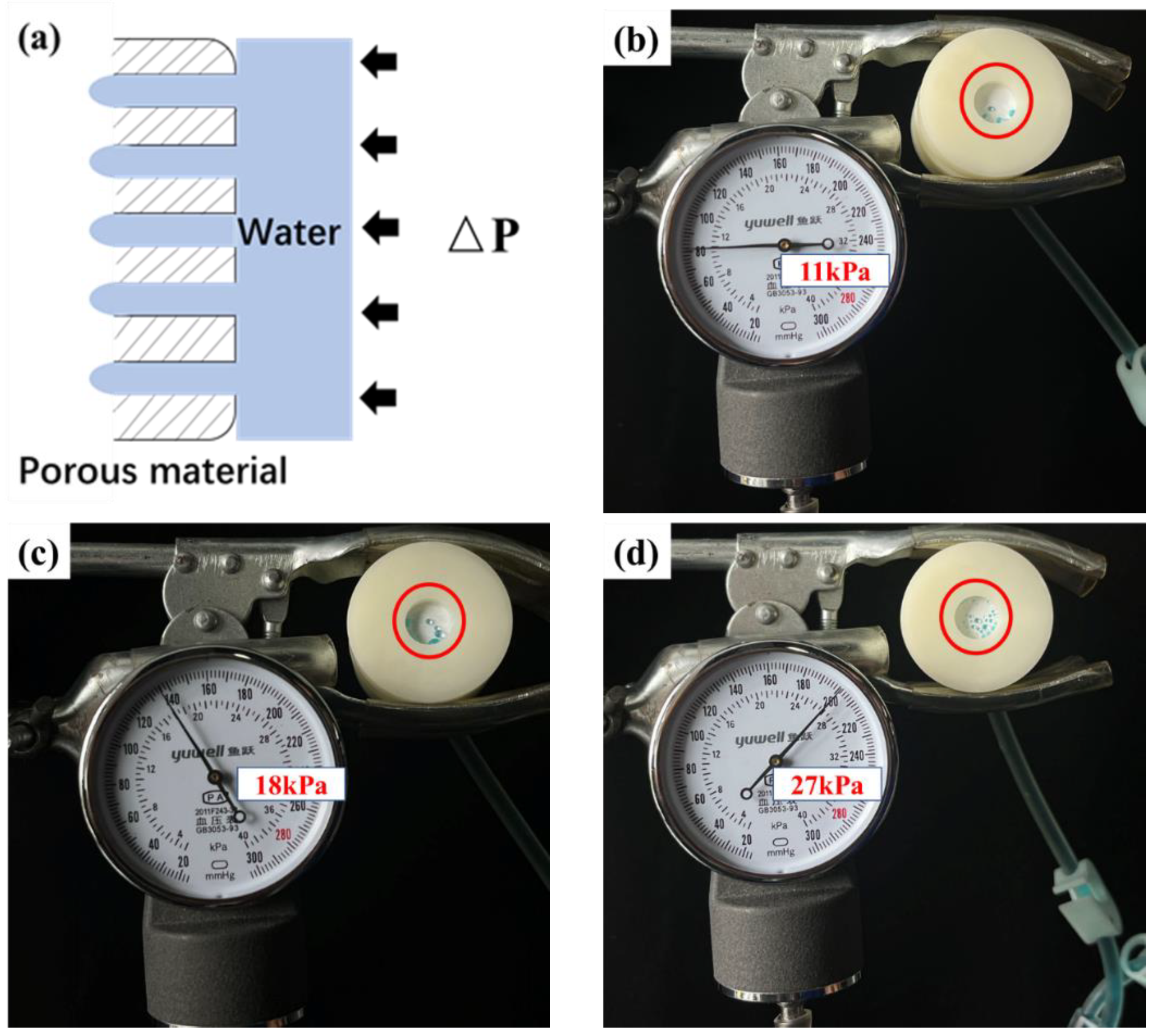
The permeability of a porous material is related to its osmotic pressure (ΔP), and permeation occurs only when the applied pressure drop exceeds ΔP value, as shown in Figure 11a. Therefore, the ΔP value can be used to describe the impermeability of porous materials. The ΔP values of the fabrics were tested according to the principle shown in Figure 3a. To observe osmosis, the water was stained with methyl blue. The results are shown in Table 3 and Figure 11. The ΔP value of the pristine PBT fabric was 11 kPa, demonstrating some resistance to water penetration. This was related to the strong hydrophobicity of PBT. The more hydrophobic the porous material, the higher the ΔP value [43,47]. The ΔP values for PBT-E0.5, PBT-E1, PBT-E2, PBT-E3, PBT-E4, and PBT-ES were 27, 26, 26, 25, 27, and 18, respectively, which were all much greater than those of the pristine PBT fabrics. This was due to the low surface energy of the fluorinated coating, which significantly increased the hydrophobicity of the fabric. Higher hydrophobicity resulted in a higher ΔP value of the fabric. In addition, fabrics coated with fluorinated microemulsions containing LDAO were more impermeable. This was relevant to the content of hydrophilic groups in the hydrophobic coating. Fluorinated coatings containing SDBS had a high amount of -SO3 and were more easily wetted. Therefore, PBT-ES was slightly less impermeable.
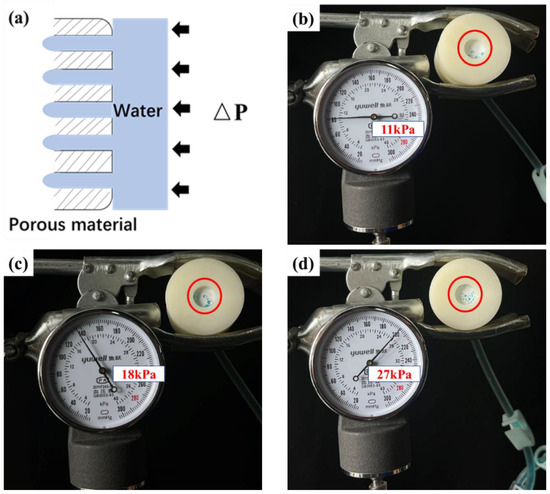
Figure 11.
(a) The schematic diagram of the permeation mechanism. (b–d) The ΔP value testing of PBT (b), PBT-ES (c), and PBT-E4 (d).
To investigate the effect of water permeation on the fabric pore channels, the air flux reduction rate of the fabrics before and after the ΔP value measurement was tested. The test principle is shown in Figure 3b. The air flux reduction rate for pristine PBT fabric was 100%, as shown in Table 3. This indicated that after the permeation occurred, the pores of the fabrics were completely blocked by water and air could not pass through from inside the fabrics. The air flux reduction rates of 0%, 0%, 2.5%, 15%, 16.67%, and 67.65% for PBT-E0.5, PBT-E1, PBT-E2, PBT-E3, PBT-E4, and PBT-ES, respectively, were all lower than those of the pristine PBT fabrics. This was due to the fact that the fluorinated coatings reduced the ability of the fabric pores to adsorb water and reduced the blockage of the pores by water. When the applied pressure drop exceeded the ΔP value, permeation occurred and the pore surface was in a wet state. However, after the pressure was removed, the wet state of the pore surface coating changed to a non-wetting state due to strong hydrophobicity, and the water film adhering to the pore surface became small water droplets [47]. The stronger the hydrophobicity of the coating, the weaker its ability to adsorb water droplets. Therefore, the easier it was to expel the water droplets remaining in the pore channels during the air flux test. PBT-E0.5, PBT-E1, PBT-E2, PBT-E3, and PBT-E4 showed significantly higher air flux reduction rates than that of PBT-ES. This was due to the presence of a large amount of hydrophilic group -SO3 introduced by the surfactant SDBS in the ES coating. In the wetted state, -SO3 migrates to the liquid–solid interface and enhances the pore adsorption capacity of water, leading to an increase in the air flux reduction rate of the fabrics. In addition, the difference between the air flux reduction rates of PBT-E0.5, PBT-E1, PBT-E2, PBT-E3, and PBT-E4 could be attributed to the presence of excessive LDAO in the coatings of PBT-E3 and PBT-E4.
As a result, the hydrophobicity of the porous materials was improved after coating with fluorinated microemulsion, and the impermeability was enhanced. However, when hydrophilic surfactants remain in the fluorinated coating, the pores of the material become clogged with infiltrated water after infiltration occurs. Fluorinated coatings containing LDAO could be heat-treated to eliminate the effect of hydrophilic groups. Porous materials modified with it were more impermeable and had a lower risk of retention of infiltrated water in the pores.
4. Conclusions
We demonstrated a facile route to fabricate corrosion-resistant surfaces and impermeable structures using fluorinated microemulsions containing thermally decomposable surfactant LDAO. It was found that LDAO could effectively emulsify fluorinated acrylic monomers under acidic conditions. The obtained microemulsion exhibited high monomer conversions as high as 96.28% at LDAO concentrations above 1%. After coating with the microemulsions and being heat-treated, the modified PBT fabrics showed remarkable hydrophobicity and oil repellency with a WCA of about 152°, a slide angle of only about 2.1°, and an oil repellency grade of 8. Hydrophobic pristine PBT fabrics were coated with surfactant solutions and the results showed that the surfactant changed the wettability of the materials. The surface of the materials changed from hydrophobic to hydrophilic. When the coated surfactant was LDAO, this effect could be eliminated by heat treatment. This was due to the fact that the hydrophilic groups of LDAO would decompose and volatilize above 112.52 °C, which could be observed from the thermal decomposition curves of LDAO and the FTIR patterns before and after heat treatment. Anti-corrosion tests demonstrated that superhydrophobic coatings prepared from fluorinated microemulsions containing the thermally decomposable surfactant LDAO were resistant to water, physiological saline, glucose solution, acetic acid solution (pH = 2.42), and diethylamine solution (pH = 12.83) corrosion. Fabrics, glass fiber paper, and wood panel modified with the microemulsions also showed excellent anti-fouling and self-cleaning properties. In addition, the corrosion-resistant superhydrophobic coating presented in this paper significantly improved the impermeability of porous materials. It is expected to be used in construction materials, fibers, and other industries.
Author Contributions
Conceptualization, B.Z. and Z.J.; Methodology, B.Z.; Software, B.Z.; Validation, B.H. and Y.Z.; Investigation, H.Z.; Data curation, B.Z. and Q.R.; Writing—original draft, B.Z.; Supervision, B.Z.; Project administration, Z.J.; Funding acquisition, Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (U22B2076, 52108241, 51878480, 52078369), the National Key Research and Development Projects (2022YFC3803104), the MITT’s 2021 Public Service Platform for Industrial Technology Foundation (2021-H029-1-1), the Program of Shanghai Academic Research Leader (22XD1403300), and the Fundamental Research Funds for the Central Universities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, K.; Ren, S.; Song, J.; Liu, J.; Liu, Z.; Sun, J.; Ling, S. Colorful superhydrophobic concrete coating. Chem. Eng. J. 2021, 403, 126348. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Shi, Z.; Lei, L.; Mao, J.; Qu, L. Study of water repellency and corrosion of STA-PFOA modified mortar. Constr. Build. Mater. 2022, 322, 126363. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Chen, Q.; Jiang, Z. Chemical and mineralogical alterations of concrete subjected to chemical attacks in complex underground tunnel environments during 20–36 years. Cem. Concr. Compos. 2018, 86, 139–159. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, J.; Liao, Y.; Li, H.; Niu, Y. Durability of Cement-Based Materials with Nano SiO2 and VAE Composite Modification. J. Build. Mater. 2023, 26, 687–696. [Google Scholar]
- Wang, Z.; Yao, Z.; He, L.; Wu, H.; Liu, Z. Effect of Nano-SiO2 on Corrosion Resistance and Corrosion Life of Concrete. J. Build. Mater. 2021, 24, 766–773. [Google Scholar]
- Zhao, Y.; Xu, T.; Zhou, J.-H.; Hu, J.-M. Superhydrophobic nanocontainers for passive and active corrosion protection. Chem. Eng. J. 2022, 433, 134039. [Google Scholar] [CrossRef]
- Kobina Sam, E.; Kobina Sam, D.; Lv, X.; Liu, B.; Xiao, X.; Gong, S.; Yu, W.; Chen, J.; Liu, J. Recent development in the fabrication of self-healing superhydrophobic surfaces. Chem. Eng. J. 2019, 373, 531–546. [Google Scholar] [CrossRef]
- Li, A.; Wang, G.; Zhang, Y.; Zhang, J.; He, W.; Ren, S.; Xu, Z.; Wang, J.; Ma, Y. Preparation methods and research progress of superhydrophobic paper. Coordin. Chem. Rev. 2021, 449, 214207. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, Y.; Wu, F.; Qu, G.; Chen, X.; Yang, Y.; Wang, J.; Lu, P. Advances in the research of carbon-, silicon-, and polymer-based superhydrophobic nanomaterials: Synthesis and potential application. Adv. Colloid Interface Sci. 2023, 318, 102932. [Google Scholar] [CrossRef]
- Tian, N.; Chen, K.; Yu, H.; Wei, J.; Zhang, J. Super pressure-resistant superhydrophobic fabrics with real self-cleaning performance. iScience 2022, 25, 104494. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhao, W.; Zhong, X.; Fu, H. Fabrication of Magnetically Inorganic/Organic Superhydrophobic Fabrics and Their Applications. ACS Appl. Mater. Interfaces 2020, 12, 45296–45305. [Google Scholar] [CrossRef]
- Yin, X.; Mu, P.; Wang, Q.; Li, J. Superhydrophobic ZIF-8-Based Dual-Layer Coating for Enhanced Corrosion Protection of Mg Alloy. ACS Appl. Mater. Interfaces 2020, 12, 35453–35463. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X.; Shi, J.; Shen, B.; Huang, J.; Hu, J.; Chen, Z.; Lai, Y. A superhydrophobic TPU/CNTs@SiO2 coating with excellent mechanical durability and chemical stability for sustainable anti-fouling and anti-corrosion. Chem. Eng. J. 2022, 434, 134605. [Google Scholar] [CrossRef]
- Yu, B.; Sun, Z.; Liu, Y.; Zhang, Z.; Wu, Y.; Zhou, F. Improving Anti-Icing and De-Icing Performances via Thermal-Regulation with Macroporous Xerogel. ACS Appl. Mater. Interfaces 2021, 13, 37609–37616. [Google Scholar] [CrossRef]
- Peng, P.; Ke, Q.; Zhou, G.; Tang, T. Fabrication of microcavity-array superhydrophobic surfaces using an improved template method. J. Colloid Interface Sci. 2013, 395, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Q.; Jiao, Y.; Sun, Z.; Yang, X.; Cheng, Z.; Bai, Q.; Zhang, Y.; Wang, K.; Shao, L. Constructing Scalable Superhydrophobic Membranes for Ultrafast Water–Oil Separation. ACS Nano 2021, 15, 3500–3508. [Google Scholar] [CrossRef]
- Peng, S.; Yang, X.; Tian, D.; Deng, W. Chemically Stable and Mechanically Durable Superamphiphobic Aluminum Surface with a Micro/Nanoscale Binary Structure. ACS Appl. Mater. Interfaces 2014, 6, 15188–15197. [Google Scholar] [CrossRef]
- Xu, L.; Tong, F.; Lu, X.; Lu, K.; Lu, Q. Multifunctional polypyrene/silica hybrid coatings with stable excimer fluorescence and robust superhydrophobicity derived from electrodeposited polypyrene films. J. Mater. Chem. C 2015, 3, 2086–2092. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Ben, K.; Chen, Z.; Wang, Y.; Guan, Z. Transparent, durable and thermally stable PDMS-derived superhydrophobic surfaces. Appl. Surf. Sci. 2015, 339, 94–101. [Google Scholar] [CrossRef]
- Kimura, A.; Nagashima, K. A practical strategy for fabrication of transparent, robust and environmentally friendly superhydrophobic surfaces for toys and games. SN Appl. Sci. 2022, 4, 237. [Google Scholar] [CrossRef]
- Deng, W.; Long, M.; Miao, X.; Wen, N.; Deng, W. Eco-friendly preparation of robust superhydrophobic Cu(OH)2 coating for self-cleaning, oil-water separation and oil sorption. Surf. Coat. Technol. 2017, 325, 14–21. [Google Scholar] [CrossRef]
- Wu, X.; Yang, F.; Lu, G.; Zhao, X.; Chen, Z.; Qian, S. A breathable and environmentally friendly superhydrophobic coating for anti-condensation applications. Chem. Eng. J. 2021, 412, 128725. [Google Scholar] [CrossRef]
- Zhao, F.; Guan, J.; Bai, W.; Gu, T.; Liao, S. Transparent, thermal stable and hydrophobic coatings from fumed silica/fluorinated polyacrylate composite latex via in situ miniemulsion polymerization. Prog. Org. Coat. 2019, 131, 357–363. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.; Ma, J. Synthesis of cationic fluorinated polyacrylate copolymer by RAFT emulsifier-free emulsion polymerization and its application as waterborne textile finishing agent. Dyes Pigm. 2017, 139, 102–109. [Google Scholar] [CrossRef]
- Fang, C.; Zhu, K.; Zhu, X.; Lin, Z. Preparation and characterization of self-crosslinking fluorinated polyacrylate latexes and their pressure sensitive adhesive applications. Int. J. Adhes. Adhes. 2019, 95, 102417. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, Y.; Li, J.; Liu, J.; Wang, X.; Yu, F.; Wang, T.; Gao, K.; Zhang, Z. Preparation and characterization of cross-linked waterborne acrylic/PTFE composite coating with good hydrophobicity and anticorrosion properties. Colloids Surf. A 2022, 653, 129872. [Google Scholar] [CrossRef]
- Yin, X.; Sun, C.; Zhang, B.; Song, Y.; Wang, N.; Zhu, L.; Zhu, B. A facile approach to fabricate superhydrophobic coatings on porous surfaces using cross-linkable fluorinated emulsions. Chem. Eng. J. 2017, 330, 202–212. [Google Scholar] [CrossRef]
- Yang, Q.; Cao, J.; Ding, R.; Zhan, K.; Yang, Z.; Zhao, B.; Wang, Z.; Ji, V. The synthesis and mechanism of superhydrophobic coatings with multifunctional properties on aluminum alloys surface: A review. Prog. Org. Coat. 2023, 183, 107875. [Google Scholar] [CrossRef]
- Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Influence of Flow on Longevity of Superhydrophobic Coatings. Langmuir 2012, 28, 9759–9766. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Ma, J. Fluorinated polyacrylate emulsifier-free emulsion mediated by poly(acrylic acid)-b-poly(hexafluorobutyl acrylate) trithiocarbonate via ab initio RAFT emulsion polymerization. Chem. Eng. J. 2013, 223, 8–17. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y. Emulsion copolymerization of fluorinated acrylate in the presence of a polymerizable emulsifier. Colloids Surf. A 2009, 348, 151–156. [Google Scholar] [CrossRef]
- Kawasaki, H.; Maeda, H. FT-IR study on hydrogen bonds between the headgroups of dodecyldimethylamine oxide hemihydrochloride. Langmuir 2001, 17, 2278–2281. [Google Scholar] [CrossRef]
- Karlovska, J.; Devinsky, E.; Balgavy, P. Effect of amphiphilic surfactant LDAO on the solubilization of DOPC vesicles and on the activity of Ca2+-ATPase reconstituted in DOPC vesicles. Gen. Physiol. Biophys. 2007, 26, 290–297. [Google Scholar]
- Kurti, L.; Czako, B. Strategic Applications of Named Reactions in Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2005; pp. 96, 810. [Google Scholar]
- YY 0770.2-2009; Filter Material for Medical Infusion Equipments. Part 2: Air Filter Material. National Technical Committee of Standardization: Beijing, China, 2009.
- Zhu, T.; Kang, W.; Yang, H.; Li, Z.; Zhou, B.; He, Y.; Wang, J.; Aidarova, S.; Sarsenbekuly, B. Advances of microemulsion and its applications for improved oil recovery. Adv. Colloid Interface Sci. 2022, 299, 102527. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, G.; Zhang, F.; Zhang, Y. Preparation of superhydrophobic poly(ethylene terephthalate) fabric by high-temperature sucrose fatty ester inlaying and esterification. Colloids Surf. A 2016, 493, 59–65. [Google Scholar] [CrossRef]
- Prorokova, N.P.; Kumeeva, T.Y.; Kholodkov, I.V. Wear-Resistant Hydrophobic Coatings from Low Molecular Weight Polytetrafluoroethylene Formed on a Polyester Fabric. Coatings 2022, 12, 1334. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, G.; Lu, Y.; Wang, N.; Zhang, Q.; Qu, X. Preparation of core-shell structured particle and its application in toughening PA6/PBT blends. Polym. Adv. Technol. 2017, 28, 699–707. [Google Scholar] [CrossRef]
- Kichigina, G.A.; Kushch, P.P.; Kiryukhin, D.P.; Prorokova, N.P.; Kumeeva, T.Y. Use of Radiation-Synthesized Tetrafluoroethylene Telomers with Silane End Groups for Hydrophobization of Polyester Fabric. High Energy Chem. 2020, 54, 123–129. [Google Scholar] [CrossRef]
- Prorokova, N.P.; Kumeeva, T.Y.; Kiryukhin, D.P.; Kichigina, G.A.; Kushch, P.P. Coatings based on tetrafluoroethylene telomeres synthesized in trimethylchlorosilane for obtaining highly hydrophobic polyester fabrics. Prog. Org. Coat. 2020, 139, 105485. [Google Scholar] [CrossRef]
- Di Mundo, R.; Labianca, C.; Carbone, G.; Notarnicola, M. Recent Advances in Hydrophobic and Icephobic Surface Treatments of Concrete. Coatings 2020, 10, 449. [Google Scholar] [CrossRef]
- Ozkan, E.; Mondal, A.; Douglass, M.; Hopkins, S.P.; Garren, M.; Devine, R.; Pandey, R.; Manuel, J.; Singha, P.; Warnock, J.; et al. Bioinspired ultra-low fouling coatings on medical devices to prevent device-associated infections and thrombosis. J. Colloid Interface Sci. 2022, 608, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.B.; Page, K.; Patir, A.; Nair, S.P.; Allan, E.; Parkin, I.P. The Anti-Biofouling Properties of Superhydrophobic Surfaces are Short-Lived. ACS Nano 2018, 12, 6050–6058. [Google Scholar] [CrossRef] [PubMed]
- Patir, A.; Hwang, G.B.; Lourenco, C.; Nair, S.P.; Carmalt, C.J.; Parkin, I.P. Crystal Violet-Impregnated Slippery Surface to Prevent Bacterial Contamination of Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 5478–5485. [Google Scholar] [CrossRef]
- Xu, F.; Wei, M.; Zhang, X.; Song, Y.; Zhou, W.; Wang, Y. How Pore Hydrophilicity Influences Water Permeability? Research 2019, 2019, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).