Abstract
Vegetable-oil-based polyurethane has become a promising sustainable candidate for controlled-release fertilizer based on green chemistry. The purpose of this study was to prepare a series of coatings from selective feedstocks including five vegetable oils with a high saturation degree, mono-unsaturation degree, or poly-unsaturation degree, considering that vegetable oil fatty acids played a key role in the synthesis of polyol and polyurethane. The effect of the type and proportion of fatty acids on the physicochemical properties, microstructure, and macro-properties of vegetable-oil-derived polyols and their resulting coatings was characterized and discussed. The position and number of the hydroxy groups were determined by the type and proportion of fatty acid, and polyol from linseed oil with a high poly-unsaturation degree and three carbon–carbon double bonds had a high hydroxyl value and functionality, whereas polyol from palm oil with a high saturation degree possessed the lowest hydroxyl value and functionality. The resultant coating from linseed-oil-based polyol had a good cross-linking density, and the nitrogen release longevity of coated urea was 56 days at a coating percentage of 3%, and its nitrogen use efficiency was increased by 27.15% compared with conventional urea. Although the palm-oil-based coating had good hydrophobicity, its coated urea was not ideal. Overall, this study has enriched theories of bio-based polyurethane coatings for controlled-release fertilizers; using vegetable oil with a poly-unsaturation degree, it is easy to obtain an excellent coating for controlled-release fertilizer, and this will help provide economic and environmental benefits.
1. Introduction
The application of chemical fertilizers is important to increase crop yield and income of agricultural products in modern agricultural production [1]. Nevertheless, their utilization rates are usually lower than 50%, and excessive and improper application causes serious environmental pollution and resource waste [2,3]. Controlled-release fertilizers (CRFs) can not only improve the uptake of fertilizer nutrients by crops and enhance fertilizer efficiency, but also reduce environmental hazards [4,5,6]. Polymer-coated CRFs have attracted much attention and have been widely used in agricultural production due to their excellent controlled-release performance [7].
Coating material is crucial in nutrient release pattern and release longevity of CRFs [8]. Recently, polyurethane coating materials have been commonly used owing to their adjustable structure and environmentally friendly fabrication process. Regrettably, most of them are derived from petrochemical resources, which are non-renewable and difficult to degrade, resulting in high production cost and a potential pollution problem [9]. For the past decade, green and sustainable development has been the direction of research. Many researchers have focused on the use of renewable resources for the preparation of bio-based coating materials, including vegetable oil [10,11], cellulose [12], lignin [13], and starch [14,15]. Among the broad renewable resources, vegetable oils (VOs) are indisputably the most promising due to their abundant source and low price [16]. The earliest attempt was made to apply castor oil with natural hydroxyl groups in the field of CRFs [17], and the nitrogen release longevity of this type of coated fertilizer was regulated by the molar ratio of hydroxyl and isocyanate groups [18].
The main component of VOs is triglyceride, which consists of three fatty acids attached to glycerol via ester linkage; the fatty acid chains contain 8~24 carbons with C=C bonds ranging from 0 to 5. The type and proportion of fatty acids are the main difference [16]. In order to prepare CRF coatings, VOs are firstly modified by grafting hydroxyl groups in their backbones, considering most of them do not generally contain natural hydroxyl groups. The C=C bond and ester linkage of fatty acids are two main reactive sites, which are amenable to chemical reactions and easily tailored to make vegetable-oil-based polyols (VOPs) [19].
Some studies about vegetable-oil-based polyurethane materials have been reported, and the materials presented different properties [20,21,22]. Different fatty acid monoesters were previously introduced into polyurethane to regulate the hydrophobicity of the membrane, and the length of the alkyl side chain significantly affected permeability: the longer the alkyl side chain, the lower the permeation coefficient [23]. Further, a series of VOPs were prepared by using castor oil, olive oil (OO), linseed oil (LO), and four other oils, their hydroxyl values increased from 190 to 305 mg KOH g−1, and their resulting polyurethane ranged from soft elasticity to hard plasticity [24]. Two different OO-based polyols containing an ether bond and ester bond, respectively, were synthesized, and then bio-based polyurethane foams with different thermal and mechanical properties were fabricated individually or as a mixture [25]. Thus, it is deduced that fatty acids directly determine the property of VOPs and polyurethane. Whether the type and proportion of fatty acids affect coatings for CRFs is still unknown.
Palm oil (PO) with balanced unsaturation was used to enhance the bio-content and biodegradability of coating materials by reducing the amount of curing agent [26]. On this basis, in this study, we go a step further to select five VOs including PO, rapeseed oil (RO), OO, soybean oil (SO), and LO as feedstocks, and these individuals represent the oils with a high saturation degree (PO), mono-unsaturation degree (RO and OO), and poly-unsaturation degree (SO and LO). Their fatty acid compositions are given in Table 1. The effect of the type and proportion of fatty acids on the microstructure, physicochemical, and macro-properties of VOPs and their resulting coatings has been extensively investigated by various detection methods such as 1H nuclear magnetic resonance (1H NMR) and Fourier transform infrared spectroscopy (FTIR). Finally, the effect of fatty acids on coatings was revealed along with their mechanisms.

Table 1.
Information about fatty acids present within vegetable oils.
2. Experimental Section
2.1. Materials
VOs, including PO, RO, OO, SO, and LO, were provided by Jiangxi Xinsen Natural Plant Oil, Co., Ltd. (Ji’an, China). 1,4-butanediol (C4H10O2, 98%, BDO) and methanol (CH3OH, 98%, MA) were obtained from Shanghai Macklin Reagent Co., Ltd. (Shanghai, China). Hydrogen peroxide (H2O2, 30%, HP), hydrochloric acid (HCl, 36%. HA), acetic acid (CH3COOH, 99.5%, AC), and sulfuric acid (H2SO4, 98%, SA) were all procured from Yangzhou Huafu Chemical (Yangzhou, China). Bayer (Leverkusen, Germany) provided polyaryl polymethylene isocyanate (PAPI, Desmodur 44V20L). Large-particle urea (with a diameter of about 2~4 mm and a percentage content of N equal to 46.6%) was procured from Hualu-hengsheng (Dezhou, China).
2.2. Synthesis of VOPs
In the first step, 100 g of VO (PO, RO, OO, SO, or LO) was loaded into a three-necked flask with a reflux condenser, mixer, and an isobaric funnel. Then, 24 g of AC, 70 g of HP, and 0.5 g of SA were loaded into the isobaric funnel and slowly added into the flask under constant stirring at 40 °C. The epoxidation reaction was conducted within an oil bath at 65 °C for 6 h. The solution was poured into a parting funnel once it had cooled to ambient temperature. Following that, the unpurified epoxidized vegetable oil (EVO) was obtained by removing the aqueous layer. In the second step, the EVO and MA with a mass ratio of 1:1 were added into the flask with 20% HA introduced as the catalyst. The hydroxylation reaction was conducted at 60 °C for 2.5 h, after which the oil–water separation was performed. The aqueous phase was decanted following the stratification process, and the oil phase underwent washing using distilled water to obtain a pH value equal to 7. Finally, the VOP was extracted by distillation through rotary evaporation in vacuum conditions at 60 °C for 3 h. The acquired VOPs are denoted as POP, ROP, OOP, SOP, and LOP, respectively.
2.3. Preparation of Coated Urea
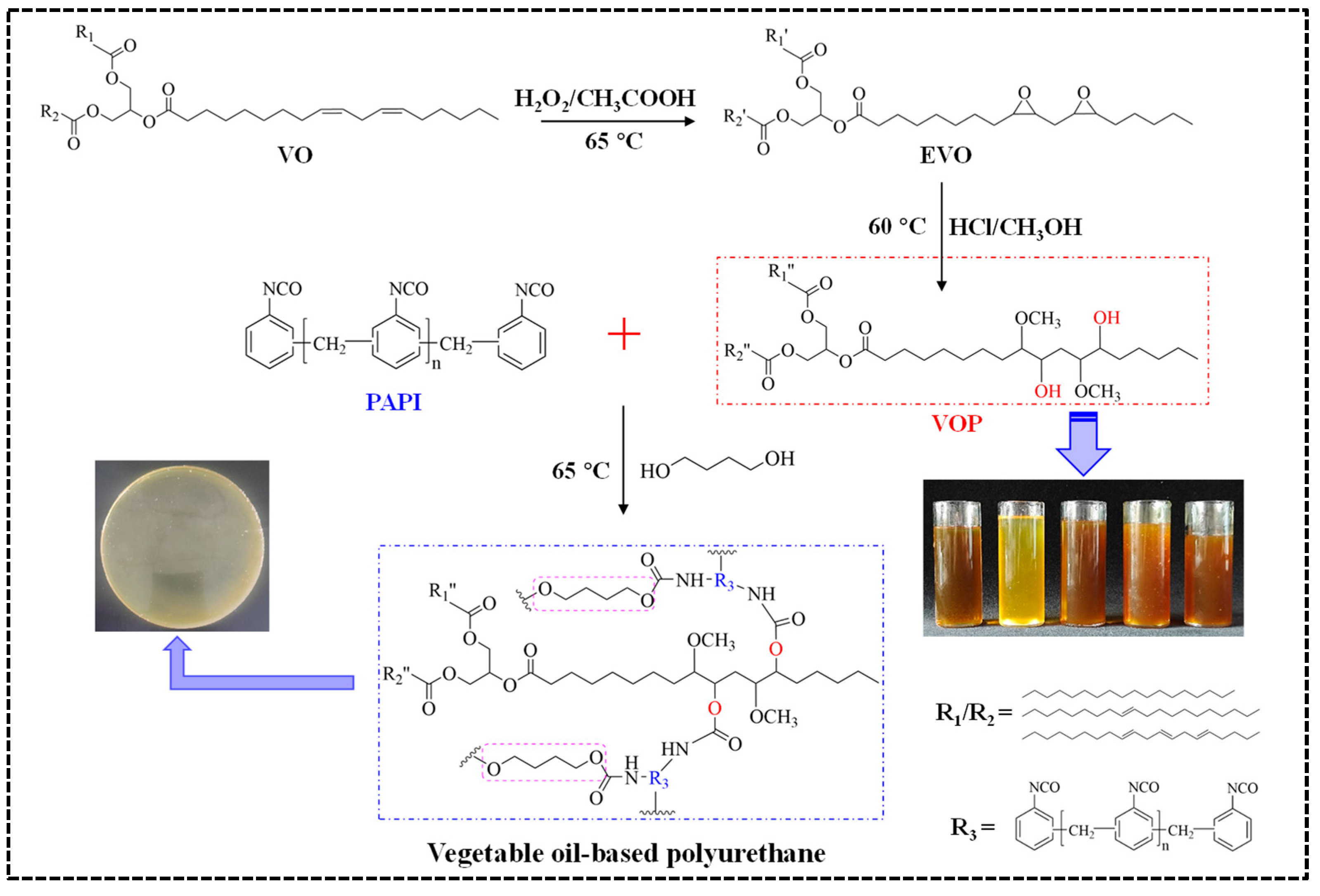
Five VOPs were synthesized by epoxidation/ring-opening method, and then a series of coatings for urea granules were prepared using them. The main reaction diagram and the formula of coatings are displayed in Figure 1 and Table S1 (Supplementary File). First, the preparation of coating liquid was carried out by mixing VOP (POP, ROP, OOP, SOP, or LOP), BDO, and PAPI in a molar ratio equal to OH:NCO = 1:1.5 at 65 °C. Thereafter, 1 kg of urea granules were preheated inside a rotating-drum coating machine at 65 °C for 8 min. Subsequently, 10 g of coating liquid was poured upon the preheated urea granule surface, and the curing reaction was continued for 6 min. Finally, five types of coated urea with approximately 3% coating percentage were obtained by repeating the curing procedure 3 times, and they are denoted as PPCU, RPCU, OPCU, SPCU, and LPCU, respectively. The method for determining the actual coating percentage and the measuring formula are provided in the Supplementary File.
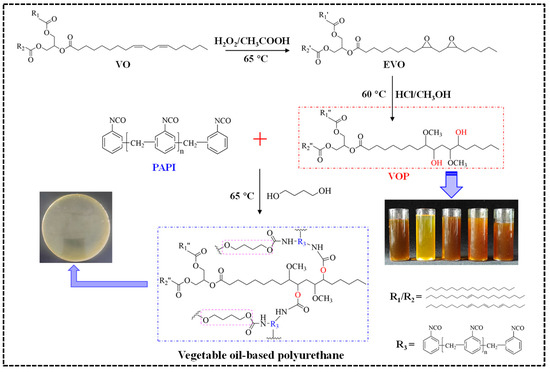
Figure 1.
Reaction diagram of the VOPs and their coatings.
2.4. Preparation of Films
The films were prepared using the film-spreading method based on the formulation (Table S1) in the Supplementary File. In detail, the VOP was continuously mixed with PAPI and BDO for 2 min, poured into a polyfluortetraethylene mold, and then degassed under a vacuum to form a pre-polymer. The pre-polymer was fully reacted for 30 min at 100 °C in an oven to obtain a 2 mm thick film. Five films were prepared and marked as PPU, RPU, OPU, SPU, and LPU, respectively.
2.5. Characterization
The acid and hydroxyl values for the VOPs were measured in accordance with ASTM D4662-08 and ASTM D4274-05 [27,28], respectively. Viscosity (η25) was determined with an LVDV-1 viscometer from Brookfield (Stoughton, MA, USA). The measurement was carried out at 25 °C using a standard spindle with the recommended rotation speed. The molecular weight and polydispersity index (PDI) were obtained by gel permeation chromatography using the DAWN HELEOS II System from Wyatt (Santa Barbara, CA, USA). Tetrahydrofuran was used as an eluent. Functionality (f) was determined employing the formula mentioned in the Supplementary File. The chemical structure of the VOPs was analyzed by FTIR spectra using a Tensor 27 spectrometer (Karlsruhe, Germany) within the range spanning from 4000 to 500 cm−1, and 1H NMR spectra were recorded with an Agilent DD2 spectrometer from Agilent (Santa Clara, CA, USA) with a frequency of 600 MHz in acetone, as a solvent.
For coatings, coated urea was cut up and immersed in water so that the urea was dissolved to obtain the coating. The coating was cleaned in water, dried at 35 °C for 10 h in an oven, and then determined using X-ray photoelectron spectroscopy (XPS) and FTIR. The testing method of FTIR was according to the above method. XPS results were obtained using an AXIS Ultra DLD from Kratos (San Diego, CA, USA) with a 1486.6 eV Al Ka source of radiation. Thermogravimetric analysis (TGA) of the coatings was conducted on an STA449C simultaneous thermal analyzer procured from Netzsch (Selb, Germany). The coatings were subjected to heating (30~700 °C) at a ramp rate equal to 10 °C min−1 in an N2 atmosphere. To analyze the water contact angle (WCA) and icon atomic force microscope (AFM), the films were measured and analyzed. The WCA value was determined by employing an SPCA-X3 goniometer (Harke, Beijing, China). The test was conducted at ambient temperature, and the mean value was evaluated with five parallel tests employing distilled water (5 µL). The surface roughness was examined by AFM using a Bruker Dimension Icon (Billerica, MA, USA) at a scan rate equal to 1.0 Hz. Coated urea was observed using a SUPRA55 scanning electron microscope (SEM) from Zeiss (Jena, Germany), with an accelerating voltage equal to 20 kV.
2.6. Nitrogen Release Behavior
The nitrogen release behavior of coated urea followed the pattern observed in our previous study [26]. Specifically, 10 g of coated urea samples enclosed within a nylon mesh bag were submerged inside a plastic bottle filled with deionized water (250 mL) and then cultivated at 25 °C. The solution from the bottle was extracted at particular intervals of time (1, 3, 7, 14, 21, 28, 35 d, etc.) and exchanged with fresh deionized water (250 mL) until the cumulative nitrogen released surpassed 80%. The content of nitrogen was quantified through UV spectroscopy, with absorbance measured at λ = 430 nm. The test was replicated three times.
2.7. Pot Experiment
To verify the practical viability of coated urea, the pot experiment was performed on Chinese cabbage. According to the growth period, LPCU was selected as a test fertilizer, and four treatments were set up. The test was carried out inside the phytotron of Beijing Academy of Agriculture and Forestry Sciences. A mass of 3 kg of soil was added into the pot, and the physicochemical indexes of the soil are shown in Table S2 in the Supplementary File. Four treatments were set as follows: (1) 0 nitrogen control (CK), (2) 0.9 g of urea/pot (T1), (3) 0.94 g of LPCU/pot (T2), and (4) 0.27 g of urea and 0.67 g of LPCU/pot (T3). Twenty seeds were planted in each treatment and repeated four times, with six seedlings retained after seed germination. Following 50 days, the plant dry weight, fresh weight, and height were determined, and the nitrogen utilization efficiency was evaluated.
3. Results and Discussion
3.1. Physicochemical Properties of VOPs
The physicochemical indexes of the prepared VOPs were tested, and the findings are shown in Table 2. The hydroxyl value is a key index to ensure the overall quality of polyurethane materials [29]. Usually, the VOPs with a hydroxyl value ranging from 100 to 300 mg KOH g−1 are suitable for the preparation of coated fertilizers [26]. As shown, the hydroxyl value of the obtained VOPs varied from 128 to 165 mg KOH g−1. The existence of fatty acids with three C=C bonds is beneficial to the formation of high hydroxyl value. This is because more C=C bonds in the fatty acid increased reaction reactivity. It is worth noting that the hydroxyl value of SOP was a little bit lower than that of ROP and OOP despite its high number of C=C bonds. This is because the higher number of C=C bonds in fatty acid chains of SO led to the increased ring-opening side reactions, although their total contents of C=C bonds were near [30]. The acid value generally has a negative effect on the quality of polyurethane and needs to be kept within a certain range of 0~10 mg KOH g−1 [31,32]. Here, the acid value of the VOPs was in the range of 5.18~7.11 mg KOH g−1. The weight-average molecular weight of POP was 1193 g mol−1, which was the lowest among the VOPs, which depended on the molecular weight of PO, and the PDIs of the VOPs were between 1.07 and 1.33, suggesting the obtained polyols had a relatively narrow distribution. Their viscosity range was 679~2855 g mol−1, which would not affect the coating process as expected. Functionality is another important factor to ensure the quality of polyurethane materials, and it determines their cross-linking density. The functionalities of the VOPs were calculated based on molecular weights and hydroxyl values and ranged from 2.55 to 3.76 [33]. These above-mentioned results indicate that the existence of fatty acids with three C=C bonds and a high unsaturation degree is beneficial to the synthesis of polyol with a high hydroxyl value and functionality.

Table 2.
Physicochemical properties of VOPs.
3.2. Chemical Structure of VOPs
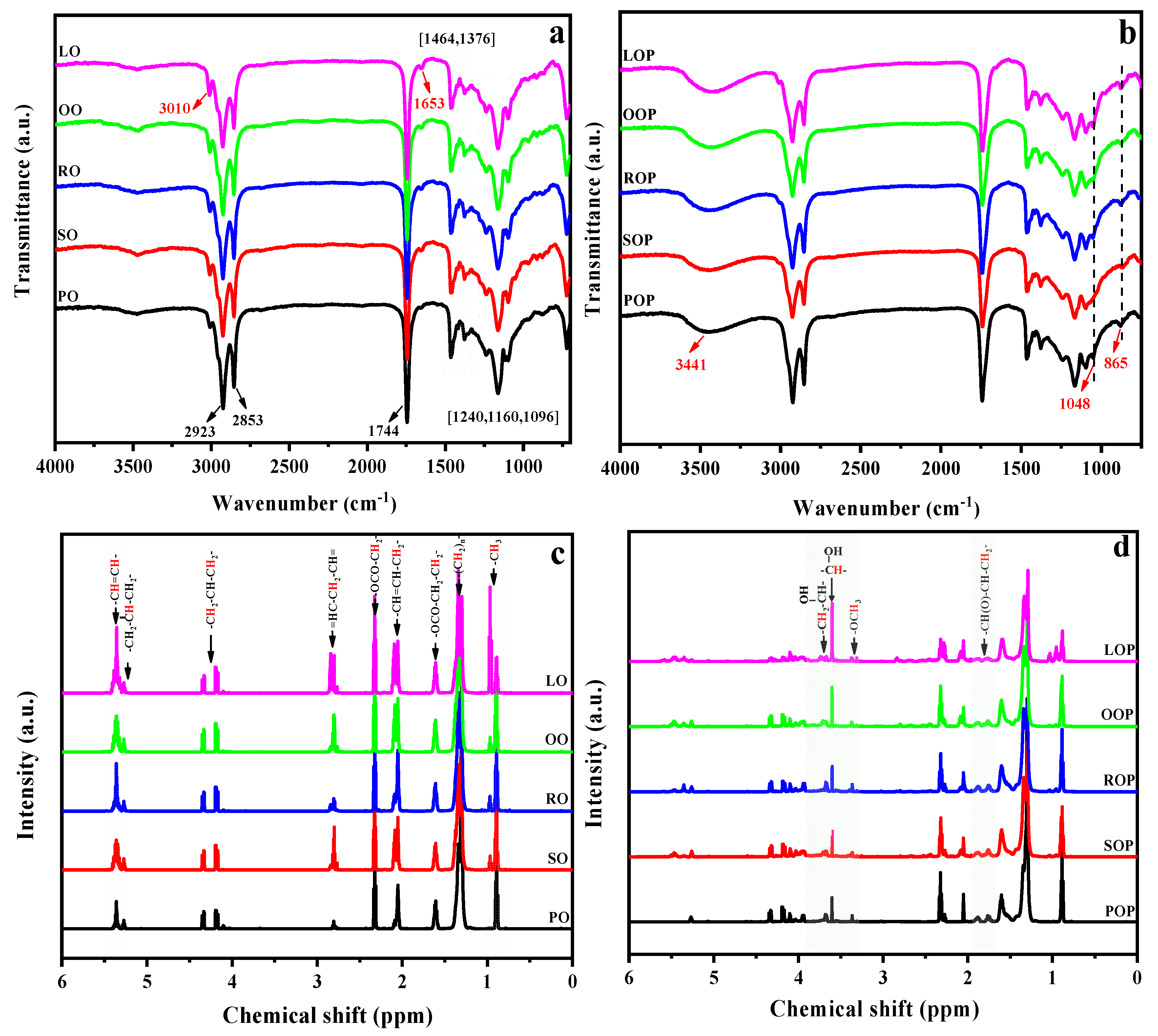
The chemical structures of the VOs and their polyols were explored through FTIR and 1H NMR. The FTIR spectra of VOs are provided in Figure 2a. The distinctive signals were similar for all samples; the characteristic peaks at 3010 cm−1 and 1653 cm−1 belong to the stretching vibrations of the C=C bond. The strong peak is observed at 1744 cm−1, corresponding to the C=O stretching vibration of ester group, and the signals observed at 1240, 1161, and 1094 cm−1 were associated with the C–O stretching vibrations of the glycerol unit. The results prove the main component of various VOs is triglycerides [31]. After VOs were transformed into VOPs (Figure 2b), the distinctive signals at 3010 and 1653 cm−1 disappeared or weakened, and a novel wide absorption peak appeared at 3441 cm−1, associated with the O–H stretching vibration, which indicated the effective grafting of hydroxyl groups via the epoxidation/ring-opening reaction. A new band appeared at 865 cm−1 corresponding to the residual epoxy group. In addition, another new weak signal was observed at 1048 cm−1, associated with the C–O stretching vibration from ether group, implying the obtained VOPs were all polyetherester polyols.
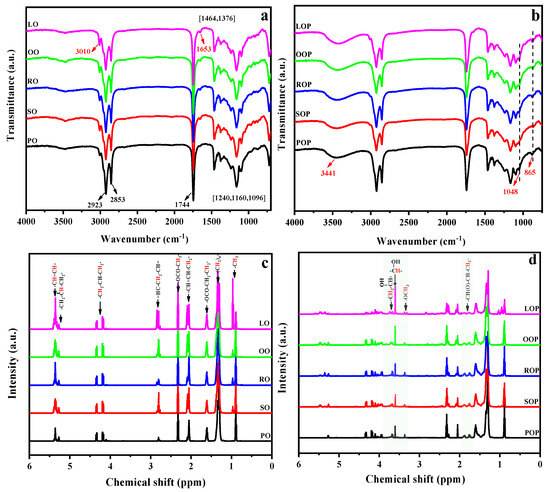
Figure 2.
ATR-FTIR (a,b) and 1H NMR (c,d) spectra of VOs and their polyols, respectively.
Furthermore, 1H NMR spectra of VOs showed characteristic chemical shifts for fatty acids (Figure 2c). In detail, peaks due to the olefinic protons were observed at 5.42–5.30 ppm. The methylene and methane protons of the glycerol unit were presented at about 5.27 and 4.38–4.12 ppm. The protons of methylene attached to olefin groups were presented at about 2.87–2.76 and 2.13–2.01 ppm, the protons of methylene attached to ester groups were presented at 2.36–2.28 and 1.67–1.56 ppm, and the methylene protons in fatty acid chains were presented at about 1.43–1.23 ppm, respectively. In addition, the methyl protons of ending groups were presented at about 0.93–0.84 ppm. It should be noted that peaks due to the methyl protons of linolenic acid appeared at 1.03–0.93 ppm, which are the strongest in LOP and the lowest in POP. After epoxidation and hydroxylation, the peaks attributed to olefin groups exhibited a decrease in intensity or even vanished entirely, while novel peaks emerged, as displayed in Figure 2d. The weak peaks corresponding to the protons of methoxy and methylene attached to the hydroxyl groups were observed at 3.36 and 3.60 ppm, respectively. The methane protons attached to hydroxyl groups appeared at about 3.70 ppm, and the peak intensities were significantly different owing to the type and proportion of fatty acids [29,34]. The original carbon chain structure of the VOs was not destroyed after undergoing the epoxidation/ring-opening reaction. The main carbon chain structure of VOPs was similar, and the position and number of their hydroxyl groups were determined by the type and proportion of fatty acids.
3.3. Chemical Structure of Coatings
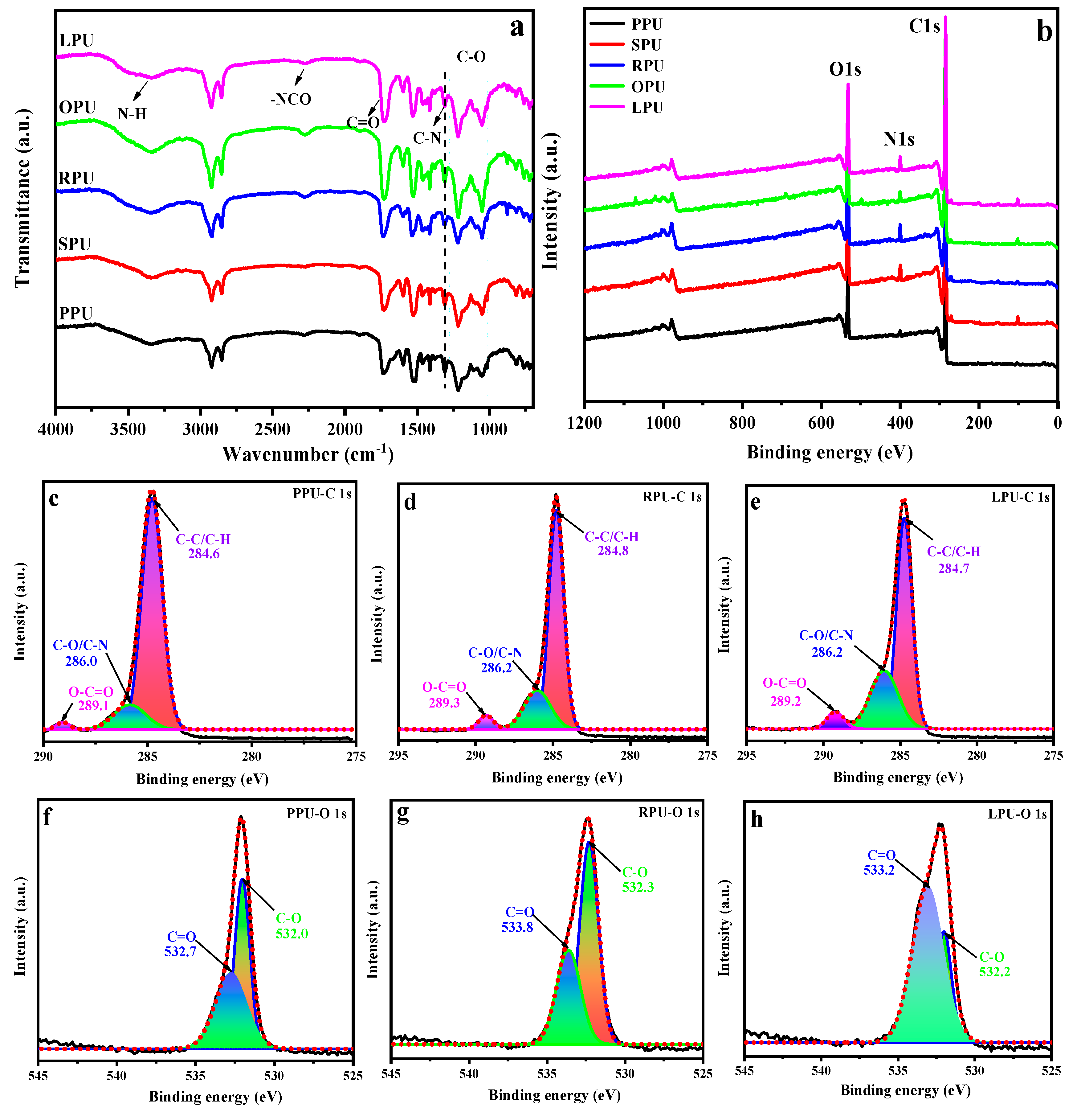
FTIR was also employed for evaluating the chemical structure of the coatings (Figure 3a). Compared with Figure 2, the characteristic signals ascribed to –CH2, –CH3, C–O, and C=O of VOPs were still observed in the spectra of all coatings. Three novel distinctive bands at 1600, 3349, and 1314 cm−1 appeared, which were associated with the C=C, N–H, and C–N stretching vibrations, respectively. This suggested all VOPs can interact with isocyanate to produce polyurethane. Notably, the highly intense band found at 1743 cm−1 here was a superposition of the stretching vibrations of C=O from the ester and amide groups, and the peaks in the range of 1000~1270 cm−1 were associated with the C–O stretching vibrations from the ether and ester groups. The intensity of these peaks has obvious differences owing to the different degrees of reaction between VOPs and isocyanate, which is closely related to the type and proportion of fatty acids in the VOs. Furthermore, the low absorption signal appearing at 2278 cm−1 was assigned to the –NCO of the unreacted curing agent [21,35].
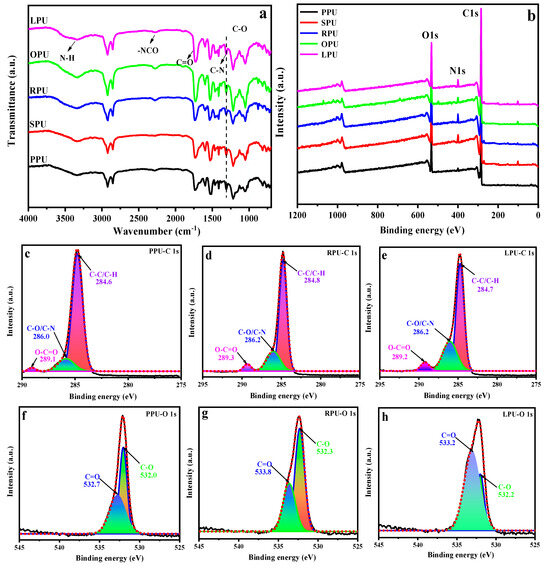
Figure 3.
ATR-FTIR (a) and XPS (b) spectra of coatings; XPS C1s (c–e) and O1s (f–h) fitting curves of PPU, RPU, and LPU, respectively.
XPS was used to determine the elemental composition and chemical bonding state on the coating surface (as shown in Figure 3b). The peaks at O1s (530 eV), N1s (397 eV), and C1s (282 eV) are shown in all coatings, indicating that these obtained VOPs with different hydroxyl values reacted with isocyanate to form polyurethane. On the other hand, the N1s peak on the PPU surface was the weakest. This is because the POP with low hydroxyl values and functionality resulted in a low content of urethane groups in its resultant PPU. To clearly analyze the effect of fatty acids on the chemical structure of coatings, PPU, RPU, and LPU with a high saturation degree, mono-unsaturation degree, and poly-unsaturation degree, respectively, were selected as typical coatings. Subsequently, the C1s and O1s peak fittings were conducted on the surface of these coating materials (Figure 3c–h). It is shown that the surface binding states were C–C/C–H, C=O, C–O, and –COO, which were the characteristic chemical bonds of polyurethane. As seen from the results, the peaks at 289.1, 289.3, and 289.2 eV of PPU, RPU, and LPU correspond to the carbon of –COO from urethane groups (Figure 3c–e), and the peaks at 532.7, 533.8, and 533.2 eV correspond to the oxygen of C=O form urethane and ester groups (Figure 3f–h). It is worth noting that the peak area of –COO and C=O increased in the order of PPU, RPU, and LPU, implying that the existence of fatty acids with both three C=C bonds and a high unsaturation degree played an important role in increasing the reaction degree between the VOP and isocyanate and improving the cross-linking density of the coating material.
3.4. Morphology of Coatings
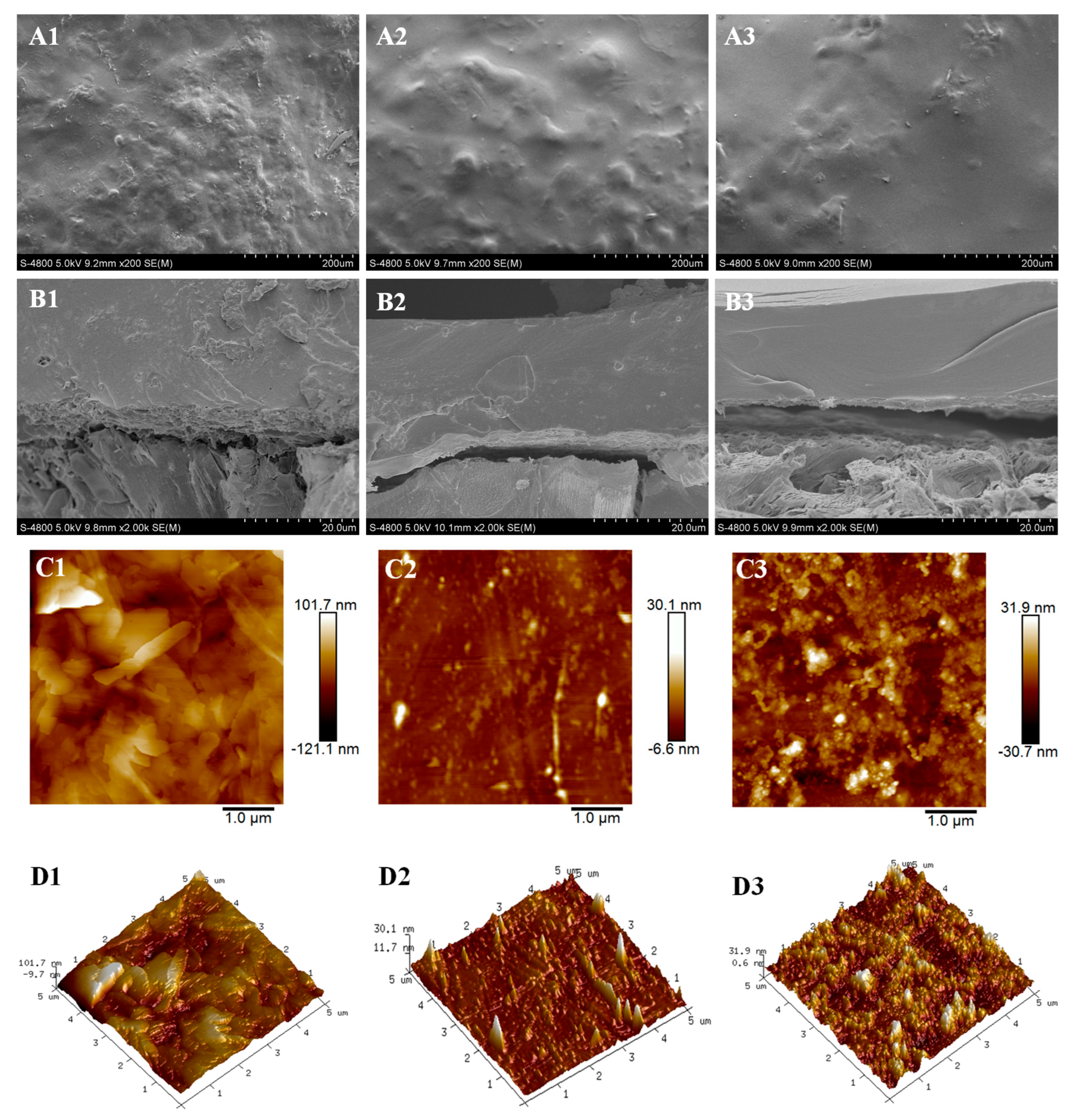
The morphologies of coatings, PPU, RPU, and LPU were characterized by SEM and AFM. The surface of PPU was rough with lots of bulges and wrinkles (Figure 4A1), and some microholes were observed in the cross section of PPU (Figure 4B1). The reason for this phenomenon was the presence of a large number of saturated fatty acids (palmitic acid) in POP, and these non-film-forming substances reduced the reaction degree, leading to the formation of irregular bulges and microholes during the coating process [36]. Fewer irregular bulges and microholes appeared on the surface section of RPU and LPU conversely (Figure 4A2,A3). By comparison, the cross section image of LPU (Figure 4B3) was smoother and more compact, indicating that the VOP with high hydroxyl value and functionality can improve the compactness and uniformity of the coating and then prolong the nitrogen release longevity. As seen from AFM images (Figure 4C1–D3), the average roughness (Ra) of PPU was 101 nm, suggesting a coating surface with a relatively high roughness range, while Ra values of RPU and LPU were about 30 nm, evidently slow. These results again demonstrate that the rough structure of the film was caused by plentiful non-film-forming saturated fatty acids from VOPs.
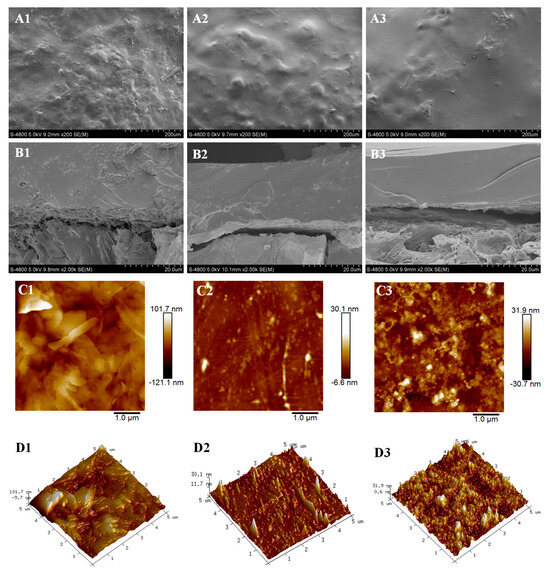
Figure 4.
SEM and AFM images of PPU, RPU, and LPU coatings and films. SEM surface (A1–A3) and cross sections (B1–B3); AFM (C1,D1), (C2,D2), and (C3,D3), respectively.
3.5. Macro-Properties of Coatings
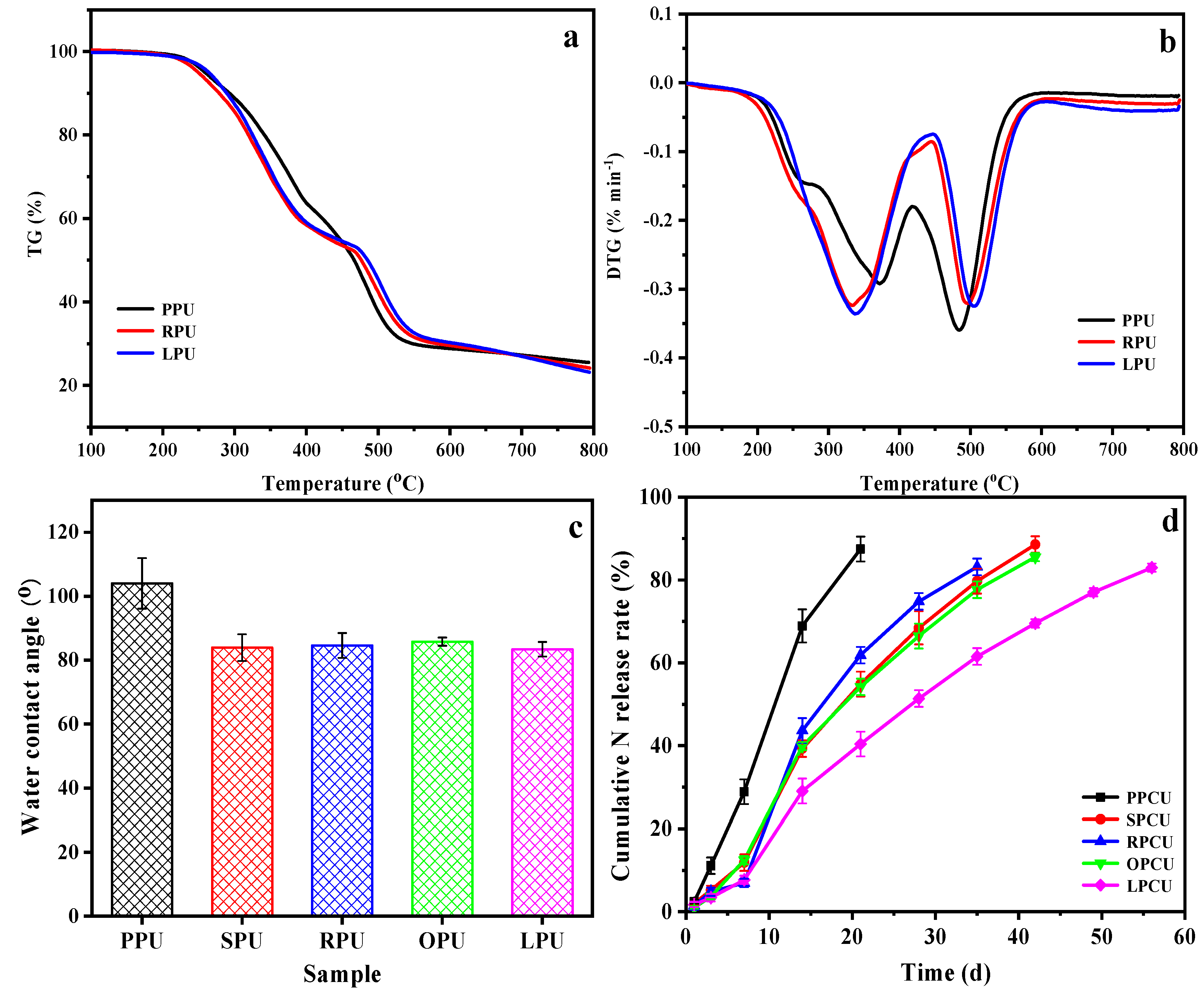
In order to compare the macro-properties of the coatings, TGA, WCA, and nitrogen release behavior were measured. The thermal stability of three typical coatings was detected using TG/DTG, and the findings are presented in Figure 5a,b and Table S3 (in the Supplementary File). The main difference for the three coatings was that there was an obvious weight loss of PPU and RPU at 170~250 °C, implying the existence of easily decomposable small molecules. The 5% mass losses of PPU, RPU, and LPU occurred at 257 °C, 262 °C, and 264 °C, respectively. The thermal degradation at 250~450 °C was attributed to the decomposition of urethane bonds into isocyanates and polyols, leading to the formation of amines, carbon dioxide, and other small molecules. Notably, at this stage, the weight loss of PPU was lower than that of other samples. This is because the content of urethane bonds in PPU was less than in other coatings due to the low hydroxyl value and functionality of POP. Finally, the thermal degradation at 450~550 °C was attributed to the dissociation of isocyanate and polyol molecules [37]. At this stage, the weight loss of three samples was in the order of PPU > RPU > LPU; the maximum thermal weight loss rates of PPU, RPU, and LPU occurred at 484 °C, 502 °C, and 500 °C, respectively; the residual weights were all around 24%, indicating that the thermal stability of LPU exceeded that of other samples, implying its excellent cross-linking density.
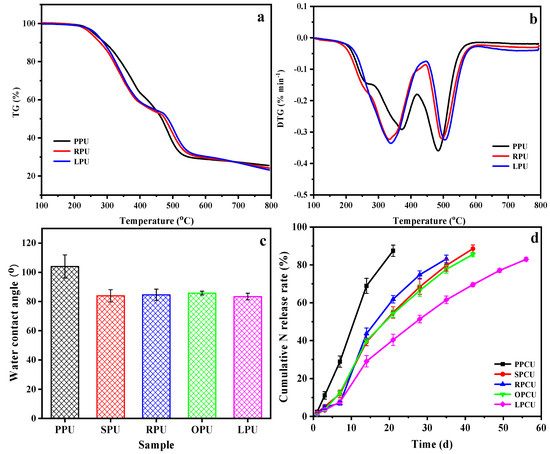
Figure 5.
TG (a)/DTG (b) and WCAs (c) of coatings, and nitrogen release behaviors (d) of their coated urea.
WCAs of all films are provided in Figure 5c. The WCA of PPU (104°) was significantly larger than that of SPU (84°), RPU (85°), OPU (86°), and LPU (83°) by 21%~25%, indicating that only the PPU exhibited a hydrophobic behavior. This is because the plentiful saturated fatty acids in POP existed in the resulting film as a side chain or free substances, resulting in the formation of irregular protrusions and increasing the roughness of the surface, which was consistent with the SEM and AFM analyses. Figure 5d shows their nitrogen controlled-release property. The cumulative nitrogen release rate of PPCU reached as high as 80% at 18 d, while LPCU was the best among all coated urea samples, and the cumulative nitrogen release rate at 28 d was 51.3%, demonstrating that LPCU will supply more sufficient nutrients in the whole growth stage of crops as expected. Interestingly, the nitrogen release behaviors of SPCU, RPCU, and OPCU were basically consistent, and their nitrogen release longevities were more than 30 d. The findings demonstrate that the type and proportion of fatty acids play a significant role, and the cross-linking density is more important than the hydrophobicity of coating to improve the nitrogen controlled-release property of coated urea.
3.6. Application Effect of Coated Urea
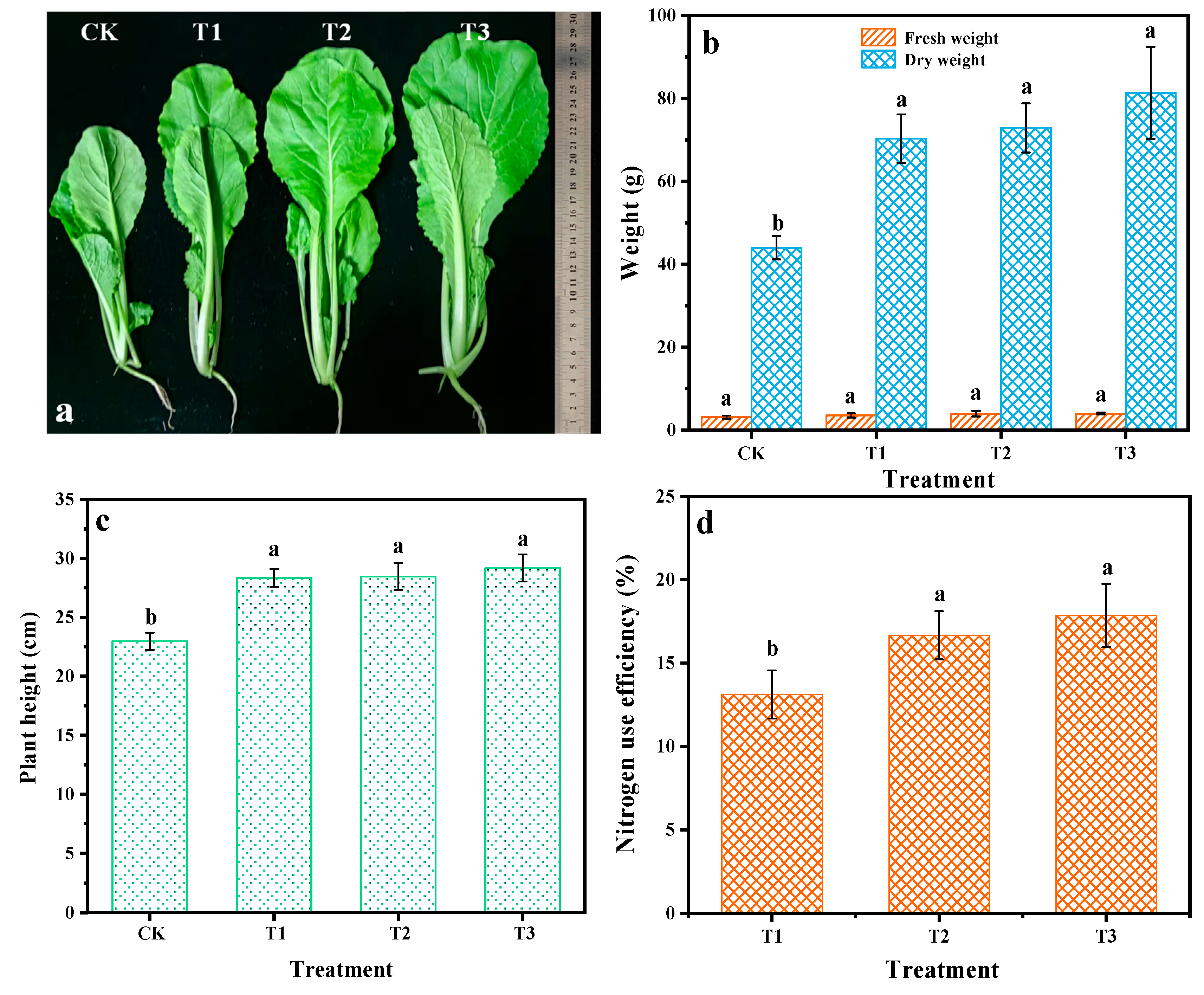
Considering the controlled-release property of LPCU, its fertilizer efficiency was further evaluated. Figure 6 shows the result of the pot experiment. As seen in Figure 6b,c, the fresh weight and plant height of Chinese cabbage with three fertilization treatments were considerably higher than CK, but the difference between them was not evident. Compared with T1 treatment, the T2 and T3 treatments increased the nitrogen use efficiency by 27.15 and 36.16%, respectively (Figure 6d). It can be concluded that a small amount of conventional urea provided enough nutrients for crop growth in the early growing period; thus, the coated fertilizer can release the amount of nutrients to meet the needs of crop growth in the middle and last growing period, while most of the conventional urea nutrients were lost because of the rapid transformation. The coating obtained by VOPs with suitable physicochemical properties showed evident nitrogen controlled-release properties and then improved nitrogen use efficiency.
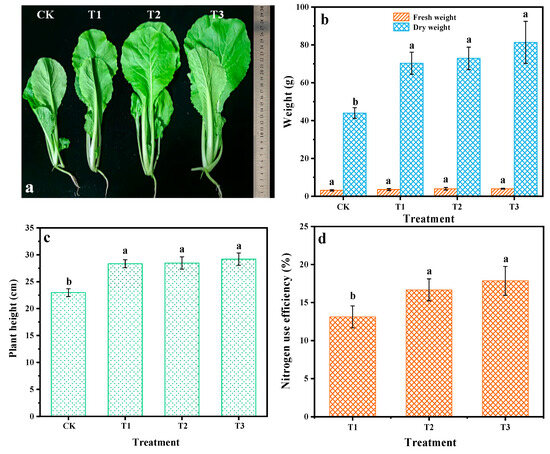
Figure 6.
The results of the pot experiment. Picture of Chinese cabbage after growth for 45 days (a); effect of different treatments on fresh weight and dry weight (b), plant height (c), and nitrogen use efficiency (d) of Chinese cabbage. Various lowercase letters (a and b) demonstrate notable differences between the treatments (p < 0.05).
4. Conclusions
The type and proportion of fatty acids had a significant influence on the physicochemical property, microstructure, and macro-properties of VOPs and their resulting coatings. The existence of fatty acids with both three C=C bonds and a high unsaturation degree helped to produce polyols with multiple hydroxyl groups and high functionality, and their coatings possessed good cross-linking density. The controlled-release property of the resultant coated urea and nitrogen use efficiency were improved. Nitrogen release longevity of linseed-oil-based coated urea was 56 d at the coating percentage of 3%, and its nitrogen use efficiency was increased by 27.15% compared with conventional urea. Thus, this research provided a theoretical framework and technical backing for research and development on polyurethane-coated fertilizer with excellent controlled-release properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings14091183/s1. Table S1: The formulation of vegetable oil-based polyurethane coatings; Table S2: Physicochemical indexes of soil; Table S3: TGA of vegetable oil-based coatings; Table S4: Pot experiment results.
Author Contributions
Conceptualization, L.L. (Lixia Li); methodology, M.P.; software, Z.L.; validation, Z.L.; formal analysis, M.P.; investigation, H.L.; resources, L.L. (Lina Liang); data curation, Z.L.; writing—original draft preparation, M.P. and Z.L.; writing—review and editing, L.L. (Lixia Li); visualization, H.L.; supervision, L.L. (Lina Liang); project administration, M.P.; funding acquisition, L.L. (Lixia Li). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [National Key R&D Program of China, Youth Research Foundation and team facilitation program by Beijing Academy of Agriculture and Forestry Sciences] grant number [2022YFD170060103, QNJJ202209, and ZHS202301].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository. The data presented in this study are available on request from the author.
Conflicts of Interest
The authors do not have any conflicting financial interests or personal relationships to report that might have influenced the work reported in this study.
References
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The future of food security and food safety. Microorganisms 2022, 10, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.C.; Li, F.F.; Chen, Y.W.; Chang, Y.Y.; Lian, X.F.; Li, Y.S.; Ye, L.; Yin, T.; Lu, X.P. Effects of fertilization approaches on plant development and fertilizer use of citrus. Plants 2022, 11, 2547–2558. [Google Scholar] [CrossRef] [PubMed]
- Jadon, P.; Selladurai, R.; Yadav, S.S.; Coumar, M.V.; Dotaniya, M.L.; Singh, A.K.; Bhadouriya, J.; Kundu, S. Volatilization and leaching losses of nitrogen from different coated urea fertilizers. J. Soil Sci. Plant Nut. 2018, 18, 1036–1047. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, Y.Z.; Jin, J.; Sun, J.Y.; Tian, X.H. Polyvinyl alcohol composite hydrogels/epoxidized natural rubber composites (CMCS/PVA/CS-ENR) with core-shell structure as biomass coating material for slow-release nitrogen fertilizer. Prog. Org. Coat. 2023, 183, 107744. [Google Scholar] [CrossRef]
- Shen, Y.M.; Wang, H.; Li, W.K.; Liu, Z.J.; Liu, Y.H.; Wei, H.L.; Li, J.J. Synthesis and characterization of double-network hydrogels based on sodium alginate and halloysite for slow release fertilizers. Int. J. Biol. Macromol. 2020, 164, 671–677. [Google Scholar] [CrossRef]
- Li, L.X.; Wang, M.; Wu, X.D.; Yi, W.P.; Xiao, Q. Bio-based polyurethane nanocomposite thin coatings from two comparable POSS with eight same vertex groups for controlled release urea. Sci. Rep. 2021, 11, 9917. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Contr. Release 2014, 181, 11–21. [Google Scholar]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled release fertilizers: A review on coating materials and mechanism of release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Fertahi, S.; Ilsouk, M.; Zeroual, Y.; Oukarroum, A.; Barakat, A. Recent trends inorganic coating based on biopolymers and biomass for controlled and slow release fertilizers. J. Contr. Release 2021, 330, 341–361. [Google Scholar] [CrossRef]
- Wang, S.P.; Li, X.; Ren, K.; Huang, R.; Lei, G.C.; Shen, L.J.; Zhan, Y.Y.; Jiang, L.L. Surface modification of pyrophyllite for optimizing properties of castor oil-based polyurethane composite and its application in controlled-release fertilizer. Arab. J. Chem. 2023, 16, 104400. [Google Scholar] [CrossRef]
- Liang, D.S.; Wang, Y.; Shi, H.B.; Luo, Z.L.; Quirino, R.L.; Lu, Q.M.; Zhang, C.Q. Controllable release fertilizer with low coating content enabled by superhydrophobic castor oil-based polyurethane nanocomposites prepared through a one-step synthetic strategy. Ind. Crop. Prod. 2022, 189, 115803. [Google Scholar] [CrossRef]
- Pang, M.H.; Dong, S.Q.; Zhao, J.G.; Li, H.Y.; Liu, D.S.; Li, L.X. Preparation of high bio-content polyurethane coatings from co-liquefaction of cellulosic biomass and starch for controlled release fertilizers. Coatings 2023, 13, 148. [Google Scholar] [CrossRef]
- Dong, H.P.; Tang, S.F.; Zhang, L.; Tong, Z.H.; Wu, Z.P.; Zhan, P.; Shao, L.S.; Qing, Y.; Liu, J. Wood-derived bio-coating materials incorporating hydrophobic lignin and hierarchically porous biochar for high-efficiency coating slow-release fertilizers. Int. J. Biol. Macromol. 2023, 242, 124769. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.Y.; Li, Z.L.; Lu, P.F.; Wang, Y.; Jia, C.; Wang, H.L.; Liu, Z.G.; Zhang, M. Starch and castor oil mutually modified, cross-linked polyurethane for improving the controlled release of urea. Carbohyd. Polym. 2021, 251, 117060. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Geng, K.Q.; Liu, D.S.; Song, H.H.; Li, H.Y. Relationship between starch liquefaction behavior and properties of polymer coated urea from liquefied starch. Prog. Org. Coat. 2020, 147, 105759. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable oil based polyurethane coatings-a sustainable approach: A review. Prog. Org. Coat. 2021, 156, 106267. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Ribeiro, C.; Polito, W.L. Controlled release of nitrogen-source fertilizers by natural-oil-based poly (urethane) coatings: The kinetic aspects of urea release. Inc. J. Appl. Polym. Sci. 2016, 13, 43790. [Google Scholar] [CrossRef]
- Liang, D.S.; Zhang, Q.; Zhang, W.B.; Liu, L.X.; Liang, H.Y.; Quirino, R.L.; Chen, J.; Liu, M.H.; Lu, Q.M.; Zhang, C.Q. Tunable thermo-physical performance of castor oil-based polyurethanes with tailored release of coated fertilizers. J. Clean. Prod. 2019, 210, 1207–1215. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Polyurethanes from seed oil-based polyols: A review of synthesis, mechanical and thermal properties. Ind. Crop. Prod. 2019, 142, 111841. [Google Scholar] [CrossRef]
- Feng, G.D.; Ma, Y.; Zhang, M.; Jia, P.Y.; Hu, L.H.; Liu, C.G.; Zhou, Y.H. Polyurethane-coated urea using fully vegetable oil-based polyols: Design, nutrient release and degradation. Prog. Org. Coat. 2019, 133, 267–275. [Google Scholar] [CrossRef]
- Acik, G.; Kamaci, M.; Altinkok, C.; Karabulut, H.F.; Tasdelen, M.A. Synthesis and properties of soybean oil-based biodegradable polyurethane films. Prog. Org. Coat. 2018, 123, 261–266. [Google Scholar] [CrossRef]
- Garrison, T.F.; Kessler, M.R.; Larock, R.C. Effects of unsaturation and different ring-opening methods on the properties of vegetable oil-based polyurethane coatings. Polymer 2014, 55, 1004–1011. [Google Scholar] [CrossRef]
- Watanabe, A.; Takebayashi, Y.; Ohtsubo, T.; Furukawa, M. Permeation of urea through various polyurethane membranes. Pest. Manag. Sci. 2009, 65, 1233–1240. [Google Scholar] [CrossRef]
- Liang, H.Y.; Feng, Y.C.; Lu, J.Y.; Liu, L.X.; Yang, Z.H.; Luo, Y.; Zhang, Y.; Zhang, C.Q. Bio-based cationic waterborne polyurethanes dispersions prepared from different vegetable oils. Ind. Crop. Prod. 2018, 122, 448–455. [Google Scholar] [CrossRef]
- Coman, A.E.; Peyrton, J.; Hubca, G.; Sarbu, A.R.; Gabor, A.; Nicolae, C.A.; Iordache, T.V.; Averous, L. Synthesis and characterization of renewable polyurethane foams using different biobased polyols from olive oil. Eur. Polym. J. 2021, 149, 110363. [Google Scholar] [CrossRef]
- Pang, M.H.; Dong, S.Q.; Zou, G.Y.; Zhao, J.G.; Li, H.Y.; Li, L.X. Study on reduction potential of curing agent in sustainable bio-based controlled release coatings. Polym. Test. 2023, 127, 108193. [Google Scholar] [CrossRef]
- Pillai, P.K.S.; Li, S.J.; Bouzidi, L.; Narine, S.S. Metathesized palm oil & novel polyol derivatives: Structure, chemical composition and physical properties. Ind. Crop. Prod. 2016, 84, 205–223. [Google Scholar]
- Zhang, J.M.; Hori, N.; Takemura, A. Optimization of agricultural wastes liquefaction process and preparing bio-based polyurethane foams by the obtained polyols. Ind. Crop. Prod. 2019, 138, 111455. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M. Synthesis and application of new bio-polyols based on mustard oil for the production of selected polyurethane materials. Ind. Crop. Prod. 2020, 155, 112831. [Google Scholar] [CrossRef]
- Kong, X.H.; Liu, G.G.; Qi, H.; Curtis, J.M. Preparation and characterization of high-solid polyurethane coating systems based on vegetable oil derived polyols. Prog. Org. Coat. 2019, 76, 1151–1160. [Google Scholar] [CrossRef]
- Jayavani, S.; Sunanda, S.; Varghese, T.O.; Nayak, S.K. Synthesis and characterizations of sustainable polyester polyols from non-edible vegetable oils: Thermal and structural evaluation. J. Clean. Prod. 2017, 162, 795–805. [Google Scholar] [CrossRef]
- Yuan, S.N.; Cheng, L.; Tan, Z.X. Characteristics and preparation of oil-coated fertilizers: A review. J. Control. Release 2022, 345, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Prociak, A.; Szczepkowski, L.; Ryszkowska, J.; Kurańska, M.; Auguścik, M.; Malewska, E.; Gloc, M.; Michałowski, S. Influence of chemical structure of petrochemical polyol on properties of bio-polyurethane foams. J. Polym. Environ. 2019, 27, 2360–2368. [Google Scholar] [CrossRef]
- Alagi, P.; Choi, Y.J.; Hong, S.C. Preparation of vegetable oil-based polyols with controlled hydroxyl functionalities for thermoplastic polyurethane. Eur. Polym. J. 2016, 78, 46–60. [Google Scholar] [CrossRef]
- Liao, Y.; Cao, B.; Liu, L.; Wu, X.D.; Guo, S.; Mi, C.H.; Li, K.W.; Wang, M. Structure and properties of bio-based polyurethane coatings for controlled-release fertilizer. J. Appl. Polym. Sci. 2021, 138, 5017. [Google Scholar] [CrossRef]
- Liu, P.; Lu, K.; Li, J.L.; Wu, X.W.; Qian, L.; Wang, M.J.; Gao, S.X. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Jiao, L.L.; Xiao, H.H.; Wang, Q.S.; Sun, J.H. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stabil. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).