Influence of Oxygen and Nitrogen Flow Ratios on the Microstructure Evolution in AlCrTaTiZr High-Entropy Oxynitride Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Film Deposition
2.2. Film Characterization
3. Results and Discussion
3.1. Chemical Composition
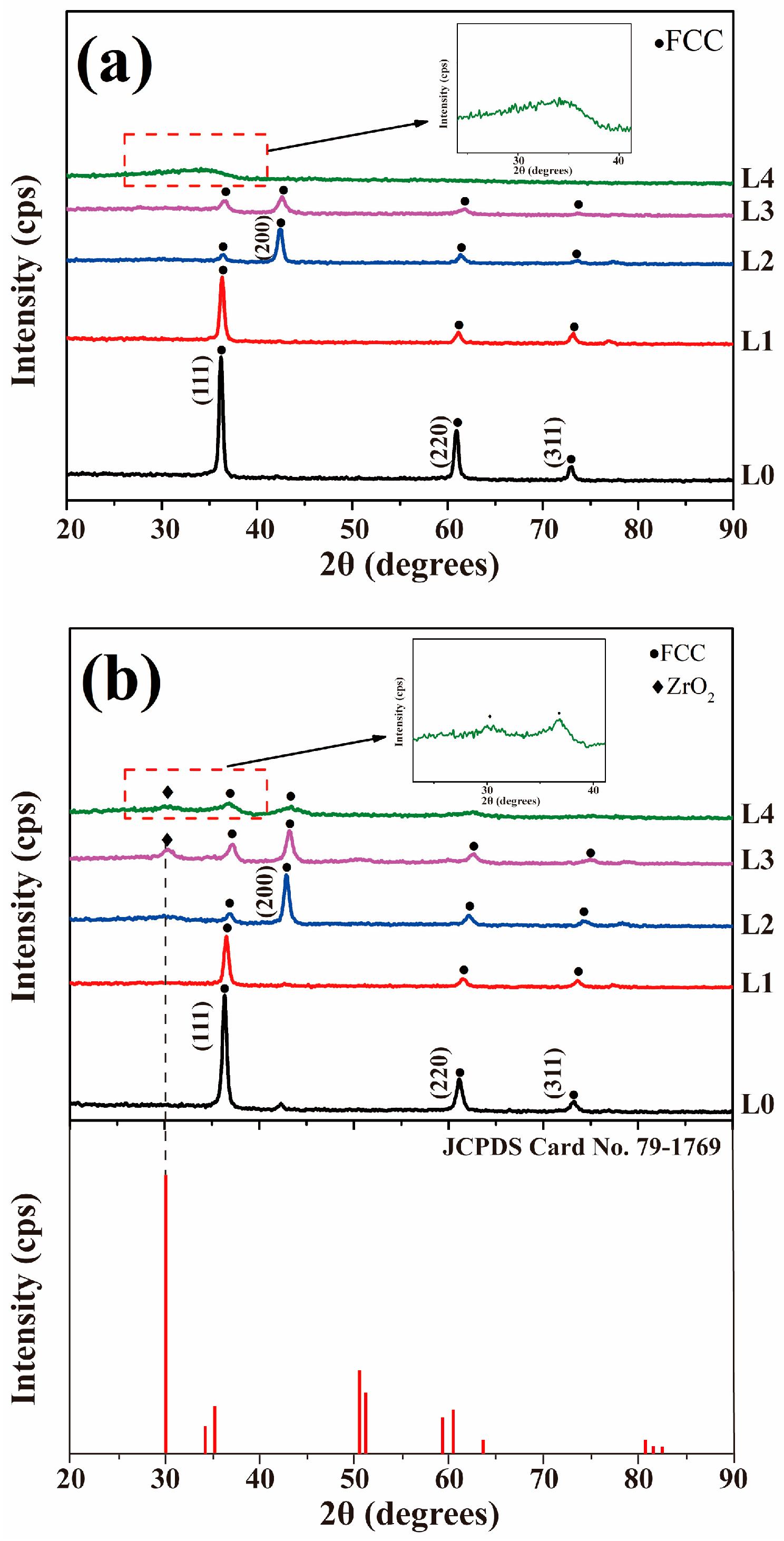
3.2. XRD Analysis
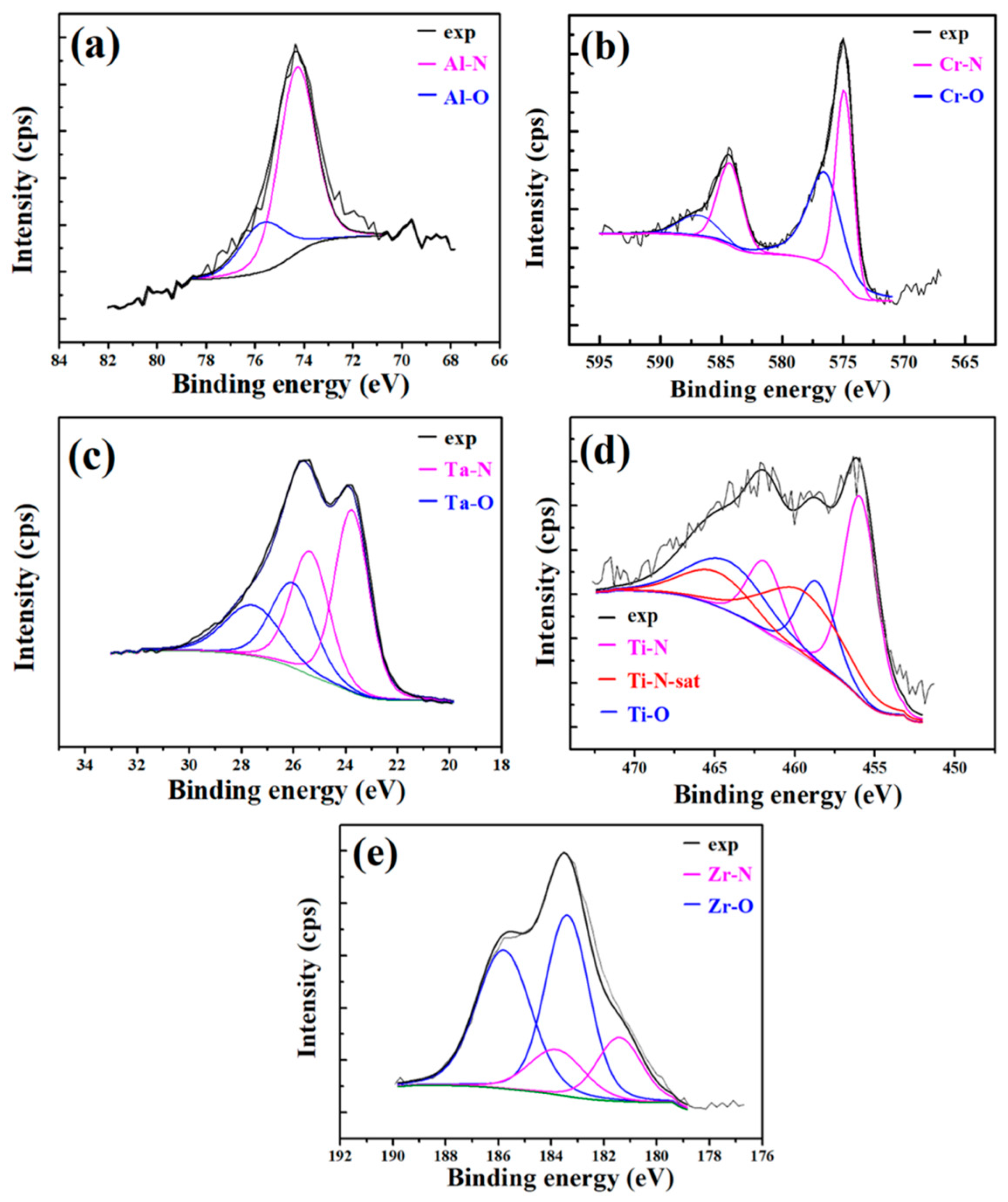
3.3. XPS Analysis
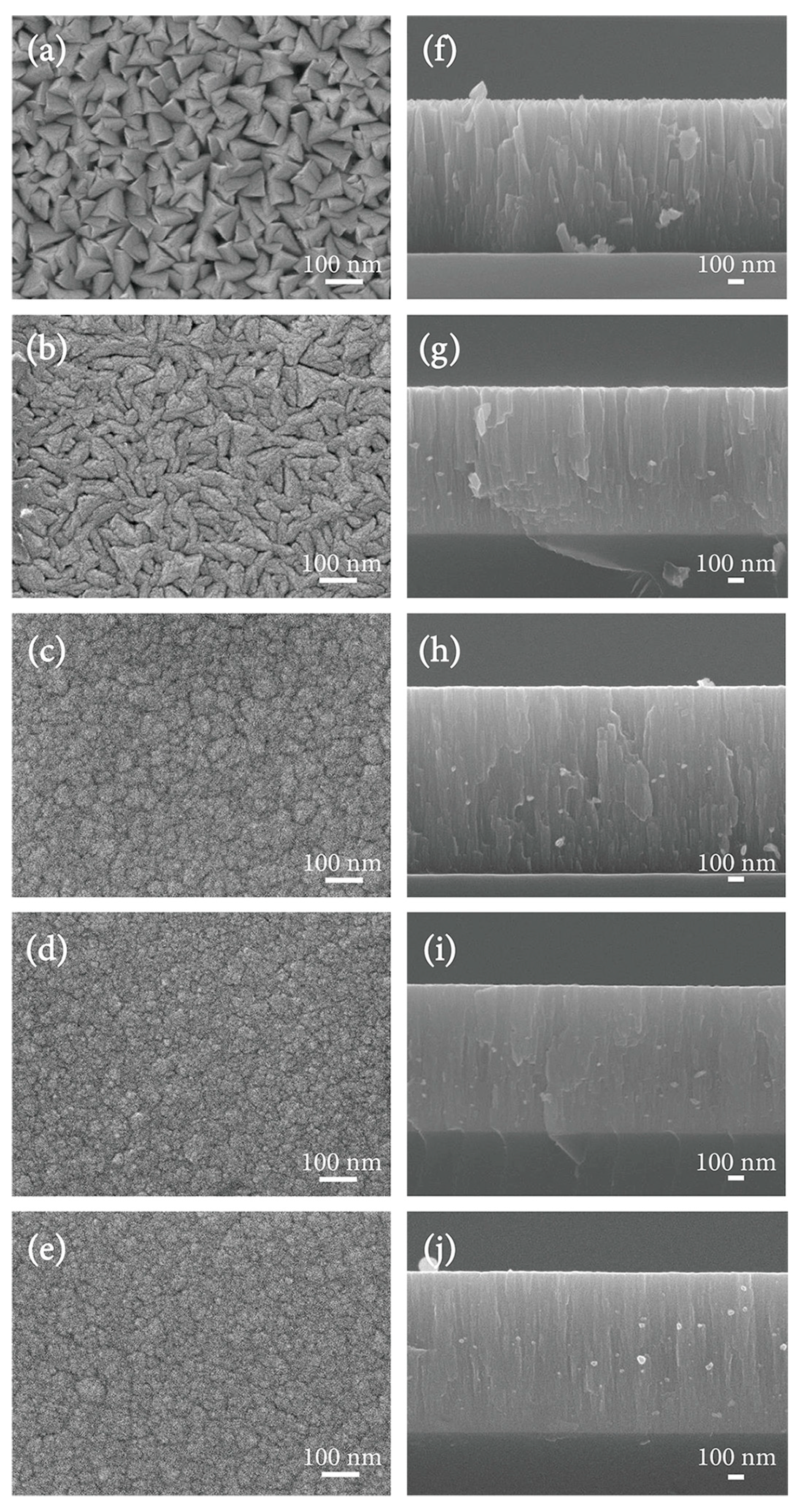
3.4. Surface Morphology
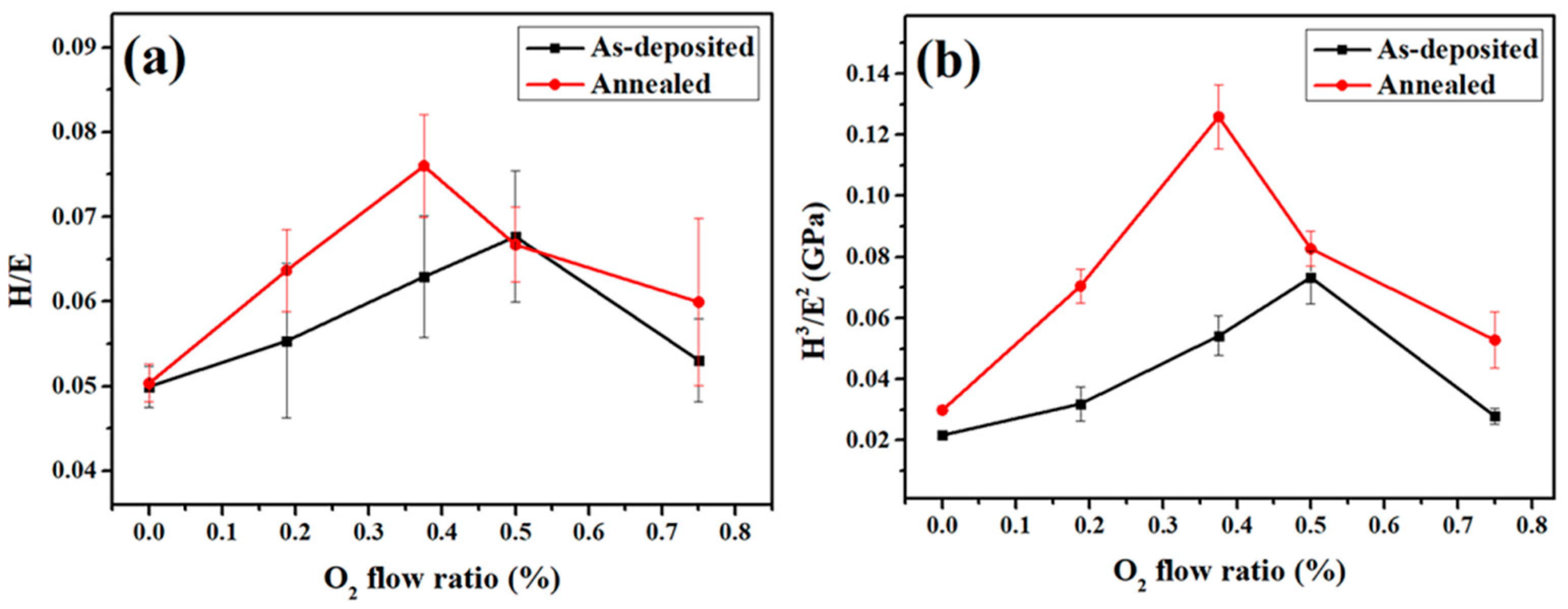
3.5. Mechanical Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- North, B. Six issues for the hard coatings community. Surf. Coat. Technol. 1998, 106, 129–134. [Google Scholar] [CrossRef]
- Münz, W.D. Titanium aluminum nitride films: A new alternative to TiN coatings. J. Vac. Sci. Technol. A 1986, 4, 2717–2725. [Google Scholar] [CrossRef]
- Chim, Y.C.; Ding, X.Z.; Zeng, X.T.; Zhang, S. Oxidation resistance of TiN, CrN, TiAlN and CrAlN coatings deposited by lateral rotating cathode arc. Thin Solid Film. 2009, 517, 4845–4849. [Google Scholar] [CrossRef]
- Keunecke, M.; Stein, C.; Bewilogua, K.; Koelker, W.; Kassel, D.; den Berg, H.V. Modified TiAlN coatings prepared by d.c. pulsed magnetron sputtering. Surf. Coat. Technol. 2010, 205, 1273–1278. [Google Scholar] [CrossRef]
- Tönshoff, K.; Karpuschewski, B.; Mohlfeld, A.; Leyendecker, T.; Erkens, G.; Fuss, H.G.; Wenke, R. Performance of oxygen-rich TiALON coatings in dry cutting applications. Surf. Coat. Technol. 1998, 108–109, 535–542. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high-entropy alloys. Ann. De Chim. Sci. Des Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Tu, M.; Hu, Y.; Wang, H.; Zhang, J.; Li, Z.; Zeng, X.; Wan, Q.; Vasiliy, P.; et al. Structure and mechanical properties of multi-principal-element (AlCrNbSiTi)N hard coating. Surf. Coat. Technol. 2022, 433, 128113. [Google Scholar] [CrossRef]
- Shen, W.J.; Tsai, M.H.; Tsai, K.Y.; Juan, C.C.; Tsai, C.W.; Yeh, J.W.; Chang, Y.S. Superior Oxidation Resistance of (Al0.34Cr0.22Nb0.11Si0.11Ti0.22)50N50 High-Entropy Nitride. J. Electrochem. Soc. 2013, 160, C531. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.T.; Zhao, S.C.; Chen, L.; Li, Y.T.; Jiang, X.; Leng, Y.X. Enhancing wear resistance: In-situ ceramic phase precipitation for strengthening and toughening FeCoNiCrNx high-entropy alloy films. Surf. Coat. Technol. 2024, 478, 130466. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zhao, Y.; Shi, X.; Wang, S.; Zhang, S. Super-hard (MoSiTiVZr)Nx high-entropy nitride coatings. J. Alloys Compd. 2022, 926, 166807. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Yakushchenko, I.V.; Bondar, O.V.; Beresnev, V.M.; Oyoshi, K.; Ivasishin, O.M.; Amekura, H.; Takeda, Y.; Opielak, M.; Kozak, C. Irradiation resistance, microstructure and mechanical properties of nanostructured (TiZrHfVNbTa)N coatings. J. Alloys Compd. 2016, 679, 155–163. [Google Scholar] [CrossRef]
- Lou, B.-S.; Lin, Y.-C.; Lee, J.-W. Mechanical Properties and Corrosion Resistance of AlCrNbSiTiN High Entropy Alloy Nitride Coatings. Coatings 2023, 13, 1724. [Google Scholar] [CrossRef]
- Shams, S.A.A.; Bae, J.W.; Kim, J.N.; Kim, H.S.; Lee, T.; Lee, C.S. Origin of superior low-cycle fatigue resistance of an interstitial metastable high-entropy alloy. J. Mater. Sci. Technol. 2022, 115, 115–128. [Google Scholar] [CrossRef]
- San, S.; Adhikari, P.; Sakidja, R.; Brechtl, J.; Liaw, P.K.; Ching, W.-Y. Porosity modeling in a TiNbTaZrMo high-entropy alloy for biomedical applications. RSC Adv. 2023, 13, 36468–36476. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, R.; Zhou, F.; Amar, A.; Yan, H.; Zhang, Y.; Lu, Y. Remarkable improved strength and ductility in brittle eutectic high-entropy alloy via a novel spheroidization and recrystallization strategy. J. Mater. Sci. Technol. 2024, 187, 177–187. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W. Effects of substrate temperature and post-annealing on microstructure and properties of (AlCrNbSiTiV)N coatings. Thin Solid Film. 2009, 518, 180–184. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Deng, M.-J.; Chang, Z.-C.; Kuo, B.-H.; Chen, E.-C.; Chang, S.-Y.; Shieu, F.-S. Oxidation resistance and characterization of (AlCrMoTaTi)-Six-N coating deposited via magnetron sputtering. J. Alloys Compd. 2015, 647, 179–188. [Google Scholar] [CrossRef]
- Lo, W.-L.; Hsu, S.-Y.; Lin, Y.-C.; Tsai, S.-Y.; Lai, Y.-T.; Duh, J.-G. Improvement of high entropy alloy nitride coatings (AlCrNbSiTiMo)N on mechanical and high temperature tribological properties by tuning substrate bias. Surf. Coat. Technol. 2020, 401, 126247. [Google Scholar] [CrossRef]
- Kirnbauer, A.; Spadt, C.; Koller, C.M.; Kolozsvári, S.; Mayrhofer, P.H. High-entropy oxide thin films based on Al–Cr–Nb–Ta–Ti. Vacuum 2019, 168, 108850. [Google Scholar] [CrossRef]
- Chou, T.C.; Nieh, T.G.; McAdams, S.D.; Pharr, G.M. Microstructures and mechanical properties of thin films of aluminum oxide. Scr. Metall. Mater. 1991, 25, 2203–2208. [Google Scholar] [CrossRef]
- Rui, C.; Haichao, C.; Hang, L.; Wenxue, L.; Yujun, X. Structural, optical and mechanical properties of Ti-doped Ta2O5 films for PV glass covers. Opt. Mater. 2024, 147, 114680. [Google Scholar] [CrossRef]
- Zywitzki, O.; Modes, T.; Sahm, H.; Frach, P.; Goedicke, K.; Glöß, D. Structure and properties of crystalline titanium oxide layers deposited by reactive pulse magnetron sputtering. Surf. Coat. Technol. 2004, 180–181, 538–543. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Weng, C.-H.; Lai, C.-H.; Lin, S.-J. Study on adhesion and wear resistance of multi-element (AlCrTaTiZr)N coatings. Thin Solid Film. 2009, 517, 4989–4993. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lin, S.-J.; Yeh, J.-W.; Chang, S.-Y. Preparation and characterization of AlCrTaTiZr multi-element nitride coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Lai, C.-H.; Cheng, K.-H.; Lin, S.-J.; Yeh, J.-W. Mechanical and tribological properties of multi-element (AlCrTaTiZr)N coatings. Surf. Coat. Technol. 2008, 202, 3732–3738. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Chen, M.-K. High thermal stability of AlCrTaTiZr nitride film as diffusion barrier for copper metallization. Thin Solid Film. 2009, 517, 4961–4965. [Google Scholar] [CrossRef]
- Lin, M.-I.; Tsai, M.-H.; Shen, W.-J.; Yeh, J.-W. Evolution of structure and properties of multi-component (AlCrTaTiZr)Ox films. Thin Solid Film. 2010, 518, 2732–2737. [Google Scholar] [CrossRef]
- Kretschmer, A.; Bohrn, F.; Hutter, H.; Pitthan, E.; Tran, T.T.; Primetzhofer, D.; Mayrhofer, P.H. Analysis of (Al,Cr,Nb,Ta,Ti)-nitride and -oxynitride diffusion barriers in Cu-Si interconnects by 3D-Secondary Ion Mass Spectrometry. Mater. Charact. 2023, 197, 112676. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Pelenovich, V.; Zeng, X.; Zeng, Z.; Hu, L.; Xu, T.; Zhan, X.; Lei, Y.; Chen, Y.; et al. Structure and mechanical properties of multi-principal AlCrNbSiTiON oxynitride coatings. Vacuum 2024, 227, 113366. [Google Scholar] [CrossRef]
- Aghajani, H.; Motlagh, M.S. Effect of temperature on surface characteristics of nitrogen ion implanted biocompatible titanium. J. Mater. Sci. Mater. Med. 2017, 28, 29. [Google Scholar] [CrossRef] [PubMed]
- AzimiRoeen, G.; Kashani-Bozorg, S.F.; Nosko, M.; Lotfian, S. Mechanical and Microstructural Characterization of Hybrid Aluminum Nanocomposites Synthesized from an Al–Fe3O4 System by Friction Stir Processing. Met. Mater. Int. 2020, 26, 1441–1453. [Google Scholar] [CrossRef]
- Takacs, L. Self-sustaining reactions induced by ball milling. Prog. Mater. Sci. 2002, 47, 355–414. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Xia, Y. Synthesis and characterization of super-hard AlCrTiVZr high-entropy alloy nitride films deposited by HiPIMS. Appl. Surf. Sci. 2020, 523, 146529. [Google Scholar] [CrossRef]
- Wang, C.-I.; Chang, T.-J.; Wang, C.-Y.; Yin, Y.-T.; Shyue, J.-J.; Lin, H.-C.; Chen, M.-J. Suppression of GeO x interfacial layer and enhancement of the electrical performance of the high-K gate stack by the atomic-layer-deposited AlN buffer layer on Ge metal-oxide-semiconductor devices. RSC Adv. 2019, 9, 592–598. [Google Scholar] [CrossRef]
- Huang, X.; Xie, Z.; Li, K.; Chen, Q.; Chen, Y.; Gong, F. Thermal Stability of CrWN Glass Molding Coatings after Vacuum Annealing. Coatings 2020, 10, 198. [Google Scholar] [CrossRef]
- Zaman, A.; Meletis, E.I. Microstructure and Mechanical Properties of TaN Thin Films Prepared by Reactive Magnetron Sputtering. Coatings 2017, 7, 209. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Z.; Miao, W. Intense photoluminescence from amorphous tantalum oxide films. Appl. Phys. Lett. 2006, 89, 021915. [Google Scholar] [CrossRef]
- Greczynski, G.; Mráz, S.; Hultman, L.; Schneider, J. Venting temperature determines surface chemistry of magnetron sputtered TiN films. Appl. Phys. Lett. 2016, 108, 041603. [Google Scholar] [CrossRef]
- Zhu, X.; Wen, G.; Liu, H.; Han, S.; Chen, S.; Kong, Q.; Feng, W. One-step hydrothermal synthesis and characterization of Cu-doped TiO2 nanoparticles/nanobucks/nanorods with enhanced photocatalytic performance under simulated solar light. J. Mater. Sci. Mater. Electron. 2019, 30, 13826–13834. [Google Scholar] [CrossRef]
- Cubillos, G.; Bethencourt, M.; Olaya, J.J. Corrosion resistance of zirconium oxynitride coatings deposited via DC unbalanced magnetron sputtering and spray pyrolysis-nitriding. Appl. Surf. Sci. 2015, 327, 288–295. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Birnie, D.P.; Kingery, W.D. Physical Ceramics: Principles for Ceramic Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1996; Available online: https://books.google.com.tw/books?id=cCFmQgAACAAJ (accessed on 14 May 1996).
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Musil, J. Structure–property relationships in single- and dual-phase nanocrystalline hard coatings. Surf. Coat. Technol. 2003, 174–175, 725–731. [Google Scholar] [CrossRef]


| Al | Cr | Ta | Ti | Zr | |
|---|---|---|---|---|---|
| Nominal | 20 | 20 | 20 | 20 | 20 |
| Measured | 20.2 | 20.4 | 18.7 | 18.8 | 21.9 |
| Samples | Oxygen Flow Ratio, RO (%) | Nitrogen Flow Ratio, RN (%) | Oxygen Flow Value (sccm) | Nitrogen Flow Value (sccm) |
|---|---|---|---|---|
| L0 | 0 | 15 | 0 | 6 |
| L1 | 0.1875 | 15 | 0.075 | 6 |
| L2 | 0.375 | 15 | 0.15 | 6 |
| L3 | 0.5 | 15 | 0.2 | 6 |
| L4 | 0.75 | 15 | 0.3 | 6 |
| O2 + N2 + Ar = 40 sccm; RO = O2/40; RN = N2/40 | ||||
| Nitride | ΔH | Oxide | ΔH |
|---|---|---|---|
| AlN | −52.0 | Al2O3 | −1117.1 |
| CrN | −40.0 | Cr2O3 | −756.5 |
| TaN | −118.0 | Ta2O5 | −816.5 |
| TiN | −155.0 | TiO2 | −944.0 |
| ZrN | −185.0 | ZrO2 | −1100.8 |
| Sample | L0 | L1 | L2 | L3 | L4 | |
|---|---|---|---|---|---|---|
| As-deposited | Grain size (nm) | 18.86 | 16.53 | 14.79 | 8.48 | N/A |
| Lattice Constant (Å) | 4.30 | 4.28 | 4.27 | 4.25 | N/A | |
| Annealed | Grain size (nm) | 20.28 | 15.23 | 13.90 | 7.65 | 8.56 |
| Lattice Constant (Å) | 4.28 | 4.26 | 4.22 | 4.19 | 4.20 |
| Elements | Peak | Binding Energy (eV) | Chemical State | Reference |
|---|---|---|---|---|
| Al | 2p3/2 | 74.3 | Al-N (AlN) | [36] |
| 2p3/2 | 75.6 | Al-O (Al2O3) | [36] | |
| Cr | 2p3/2 | 574.8 | Cr-N (CrN) | [37] |
| 2p1/2 | 584.4 | [37] | ||
| 2p3/2 | 576.8 | Cr-O (Cr2O5) | [37] | |
| 2p1/2 | 587.2 | [37] | ||
| Ta | 4f7/2 | 23.8 | Ta-N (TaN) | [38] |
| 4f5/2 | 25.6 | [38] | ||
| 4f7/2 | 27.0 | Ta-O (Ta2O5) | [39] | |
| 4f5/2 | 28.8 | [39] | ||
| Ti | 2p3/2 | 455.9 | Ti-N (TiN) | [40] |
| 2p1/2 | 461.9 | [40] | ||
| 2p3/2 | 458.0 | Ti-O (TiO2) | [41] | |
| 2p1/2 | 464.0 | [41] | ||
| Zr | 3d5/2 | 181.4 | Zr-O-N | [42] |
| 3d3/2 | 184.0 | [42] | ||
| 3d5/2 | 183.0 | Zr-O (ZrO2) | [42] | |
| 3d3/2 | 185.8 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.-C.; Lee, C.-Y.; Lin, M.-I.; Shen, T.-E.; Hung, J.-F.; Yeh, J.-W.; Tsai, C.-W. Influence of Oxygen and Nitrogen Flow Ratios on the Microstructure Evolution in AlCrTaTiZr High-Entropy Oxynitride Films. Coatings 2024, 14, 1199. https://doi.org/10.3390/coatings14091199
Liang Y-C, Lee C-Y, Lin M-I, Shen T-E, Hung J-F, Yeh J-W, Tsai C-W. Influence of Oxygen and Nitrogen Flow Ratios on the Microstructure Evolution in AlCrTaTiZr High-Entropy Oxynitride Films. Coatings. 2024; 14(9):1199. https://doi.org/10.3390/coatings14091199
Chicago/Turabian StyleLiang, Yung-Chu, Ching-Yin Lee, Miao-I Lin, Ting-En Shen, Jung-Fan Hung, Jien-Wei Yeh, and Che-Wei Tsai. 2024. "Influence of Oxygen and Nitrogen Flow Ratios on the Microstructure Evolution in AlCrTaTiZr High-Entropy Oxynitride Films" Coatings 14, no. 9: 1199. https://doi.org/10.3390/coatings14091199







