Abstract
The surface corrosion of magnesium alloys is effectively addressed currently by the creation of a micro-arc oxidation (MAO) ceramic layer. However, oxide film porousness restricts magnesium alloy use. Thus, this work used atomic layer deposition (ALD) to create a TiO2 coating on MAO-coated AZ31B magnesium alloy to plug micropores and increase corrosion resistance and biological characteristics. The samples were analyzed using SEM, EDS, XPS, and XRD to determine their surface appearance, chemical content, and microstructure. Micro-arc oxidation produced a 20 μm oxide coating. The TiO2 film reached 47.41 nm after 400 atomic layer deposition cycles. All corroded samples were tested for corrosion resistance using electrochemical and hydrogen evolution methods and examined for surface morphology. In vitro cell experiments examined biocompatibility. The results indicate that the TiO2 layer sealed the MAO coating’s micro-pores and micro-cracks, enhanced corrosion resistance, and preserved surface morphology following corrosion. The TiO2/MAO composite coating is more biocompatible than the substrate and MAO coating. This research proposes coating AZ31B magnesium alloy for bio-remediation to increase corrosion resistance and biocompatibility.
1. Introduction
As a biodegradable biomedical metal material, magnesium alloy is comparable to human bone, meaning it satisfies the mechanical specifications needed for materials to be implanted into the body [1,2,3,4,5]. The magnesium ions generated by its degradation are easily absorbed by human tissues or excreted through body fluids and do not produce toxicity to harm the human body [6,7]. In addition, magnesium alloy also has good biocompatibility, osteoinductivity, and other biological properties [8,9,10]. On this basis, magnesium alloy has a broad variety of potential applications in the area of orthopedics, including the internal fixing of fractures and the repair of bone deformities. However, magnesium is a very active metal with poor corrosion resistance and a high degradation rate, which can affect cell viability and function [11,12]. Therefore, it is necessary to modify its surface in order to enhance its resistance to corrosion.
Surface modification is the easiest method of lowering the rate of corrosion of implanted materials since it forms a protective coating on the magnesium alloy itself, shielding the material substrate from the corrosive environment. Commonly used surface modification techniques include physical vapor deposition [1], chemical conversion [13], micro-arc oxidation (also known as plasma electrolytic oxidation (PEO)) [14], and the sol–gel method [15]. Among these, the micro-arc oxidation process is a straightforward, fast, and non-toxic technique that is often used for the surface modification of magnesium alloys. After MAO treatment, a layer of oxide ceramic coating with high adhesion and porous roughness will grow on the magnesium alloy’s surface, which can enhance the material’s resistance to corrosion [14]. Tang et al. performed micro-arc oxidation and solution treatment on AZ31 magnesium alloy, and the findings revealed that the MAO coating increased corrosion resistance while also forming apatite [16]. In addition, the rough pore surface stimulates cell proliferation and adhesion, resulting in fast local tissue repair. The results of a study by Ma et al. show that the MAO-treated magnesium samples can effectively improve the corrosion resistance of magnesium samples and promote bone formation [17]. AZ31 magnesium alloy coated with MAO, as proposed by Jian et al., has favorable cytocompatibility and corrosion resistance characteristics [18]. Therefore, this approach offers benefits for the development of novel implant materials and may be used in clinical settings.
However, the high-voltage spark discharges during the experimental process cause micropores and microcracks on the MAO film’s surface, which let hostile ions travel through it and expose the samples to pitting corrosion [19]. Therefore, post-treatment of MAO film is required to improve the film’s density and, hence, corrosion resistance. The main methods can be divided into two categories: conventional sealing (inorganic sealing [20] and organic sealing [21]) and composite film (chemical plating [22] and biofilm coating [23,24]). Among them, the atomic layer deposition (ALD) method is a popular choice for material modification of magnesium alloys because of its many benefits, including strong bonding, broad area homogeneity, and accurate thickness control. Li et al. prepared aluminium-doped zinc oxide (AZO) thin films on AZ31 magnesium alloy coated with a plasma electrolytic oxidation (PEO) layer by atomic layer deposition (ALD) method, which significantly increased the material’s resistance to corrosion [25]. Li et al. discovered that the corrosion resistance of AZ31 magnesium alloy in Hanks’ solution could be effectively improved by the dense, biocompatible tantalum oxide (Ta2O5) nanofilms deposited on MAO-coated AZ31 magnesium alloy by ALD, and the corrosion current density was reduced by three orders of magnitude [26]. Consequently, it is feasible to seal the surface micropores and cracks of MAO coatings via ALD.
Titanium dioxide is frequently utilized in medical implant materials since it is one of the most biocompatible inorganic materials. It is non-toxic to humans and has high mechanical qualities, such as corrosion resistance [27]. On alloys based on magnesium, titanium dioxide coatings have been employed in a number of investigations recently. Kania et al. evaluated the structural and corrosion behavior of TiO2 coatings formed on MgCa4Zn1Gd1 alloys using magnetron sputtering and sol–gel techniques [28]. The results showed that the magnetron-sputtered TiO2 surface had smaller and more uniform grains. Despite this, the samples’ resistance to corrosion was greatly increased by both techniques. In addition, Kania et al. used ALD to deposit uniform TiO2 films on MgCa2Zn1Gd3 alloy [29]. The magnesium base alloys under research saw a decrease in corrosion rate due to the presence of TiO2 coatings, with thicker TiO2 films exhibiting greater corrosion resistance. Nazarov et al. used ALD and in situ oxidation to prepare TiN/TiO2 nanocomposite films [30]. The films adhered well to the substrate made of magnesium alloy. The film’s strong adherence to the magnesium alloy substrate significantly increased the metal’s resistance to corrosion. In conclusion, because of its mechanical qualities and biocompatibility, titanium dioxide is a good option for bone replacement and repair.
In this work, the ALD approach was used to create titanium oxide (TiO2) films on MAO-coated AZ31B magnesium alloy in order to close the micropores and enhance corrosion resistance. Investigations were also conducted on the composite coatings’ biocompatibility and resistance to corrosion.
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Preparation of AZ31B Magnesium Alloy
Utilizing extruded AZ31B magnesium alloy (diameters: t = 2 mm, d = 10 mm, content: 3.000 wt% Al, 1.000 wt% Zn, 0.200 wt% Mn, Fe < 0.005 wt%, bal. Mg) as a substrate, 800 #, 1500 #, and 2000 # SiC sandpaper was employed in a sequential manner. After being sanded, the samples were kept in a dry environment after being ultrasonically cleaned many times in deionized water and acetone to eliminate surface contaminants.
2.1.2. Preparation of MAO
Magnesium alloy and stainless-steel plates were used as anode and cathode for the micro-arc oxidation reaction in an electrolyte containing 10 g/L Na2SiO4, 5 g/L NaOH, and 3 g/L (NaPO3)6, and the whole process was carried out in constant current mode using a 75-kW pulse power source (SAMMAO-100A-800SPM, Shangi Institute For Advanced Materials Co., Ltd., Shanghai, China). Table 1 displays the experimental parameters that were employed.

Table 1.
Parameters of the experiment during microarc oxidation (MAO).
2.1.3. Preparation of TiO2/MAO
The PICOSUN™ R-200 standard atomic layer deposition system (ALD; Espoo, Finland) was used to deposit TiO2 thin films on MAO coatings. The Ti source was Tetrakis (dimethylamino) titanium (IV) or TDMA-Ti, and the O source was H2O. During the deposition process, the temperatures of TDMA-Ti, H2O, and the feed line were 75 °C, 20 °C, and 120 °C, respectively.
Each cycle process included:
- (1)
- TDMA-Ti precursor pulsed for 0.1 s and N2 purged for 5 s.
- (2)
- H2O precursor pulsed for 0.015 s and N2 purged for 5 s.
Both precursors were deposited at a flow rate of 100 sccm for a total of 400 cycles.
Figure 1 depicts the experimental flowchart used in this study to visually represent our design concepts.

Figure 1.
The experimental flowchart.
2.2. Surface Structure and Chemistry Characterization
Surface shape, chemical content, and film thickness of the micro-arc oxidation were examined by scanning electron microscopy (SEM; SU-8010, Hitachi, Tokyo, Japan). Spectrophotometric ellipsometry (ME-L, Wuhan Eoptics Technology Co., Ltd., Wuhan, China) was used to measure the thickness of the titanium dioxide film created via atomic layer deposition. The structure and phase distribution of the samples were investigated by X-ray diffraction (XRD, D8A, Bruker, Berlin, Germany) using Cu-Kα radiation (40 kV, 40 mA, λ = 1.5405 Å, step size 2°/min, test angle: 5°~80°) by Raman spectrometer (Witec alpha300, Ulm, Germany) and by X-ray photoelectron spectroscopy (XPS, Philips PHI-5300 ESCA, Philips, Amsterdam, The Netherlands) using an Al-Kα source, respectively. The water contact angle of the samples was measured using the contact angle equipment (CAMKSV021733, Gothenburg, Sweden). Three distinct surface areas were chosen, and the values were averaged.
2.3. Corrosion Resistance
Potentiodynamic polarization (PDP) measurements were performed in 0.01 M phosphate-buffered saline (PBS: NaCl, KCl, Na2HPO4 and KH2PO4) using an electrochemical workstation (CHI Instruments, Shanghai, China), with a sample exposure area of 0.28 cm2. A calomel electrode served as the reference electrode, a graphite rod (Φ 6 * 65 mm) served as the counter electrode, and a coated sample served as the working electrode in this three-electrode setup. Finally, the potentiodynamic polarization curves were fitted.
Hydrogen evolution tests were carried out by immersion experiments over a period of 7 days using a homemade funnel apparatus. The AZ31B magnesium alloy and modified samples were submerged in 100 mL of Hanks’ solution (composition: Nacl, Kcl, Na2HPO4.12H2O, KH2PO4, NaHCO3, and glucose, pH7.2–7.5) at 37 °C (three samples as a group). The relative height of the liquid level was recorded for each day.
2.4. In Vitro Cytocompatibility Evaluation
Protein electrophoresis using bovine serum albumin was used to assess the samples’ ability to bind proteins. Electrophoretic blots were analyzed using Lane 1D L300 image analysis software.
MC3T3-E1 cells were grown in α-MEM medium with 10% fetal bovine serum and 1% penicillin-streptomycin. Culture conditions were 37 °C, 5% CO2, and 100% humidity. The cells employed in the experiment were from the third to the fifth generation, and the culture medium was replaced every two days throughout the culture phase.
The samples that had been sterilized with UV were placed in α-MEM complete medium for a duration of 24 h at a temperature of 37 °C. The ratio of the sample’s surface area to the extraction medium was 1.25 cm2/mL, following the guidelines of ISO 10993-5:1999 [31]. Extracts were collected and filtered for subsequent cellular experiments.
MC3T3-E1 cells were inoculated into 96-well plates at a concentration of 1 × 104 cells/mL, and each well was filled with 100 μL of cell suspension. The cells were incubated for a duration of 12 h. Once the cells were fully attached to the wall, the original medium was removed and the culture was maintained by replacing the sample extracts every 2 days. The cells were tested for cck-8 at 1, 3, and 5 days, and cell viability was determined by measuring the absorbance at 450 nm in each well using an enzyme-labelled reader (Biotek ELX808, Richmond, VA, USA). Cells were inoculated into 24-well plates at a concentration of 2 × 104 cells/mL. The sample extract was replaced daily, and the cells were cultured until day 3. At this point, the cells were stained using Calcein/PI cell viability/cytotoxicity assay kit (Beyotime, Haimen, China) and examined using inverted fluorescence microscopy.
3. Results
3.1. Surface Characterization
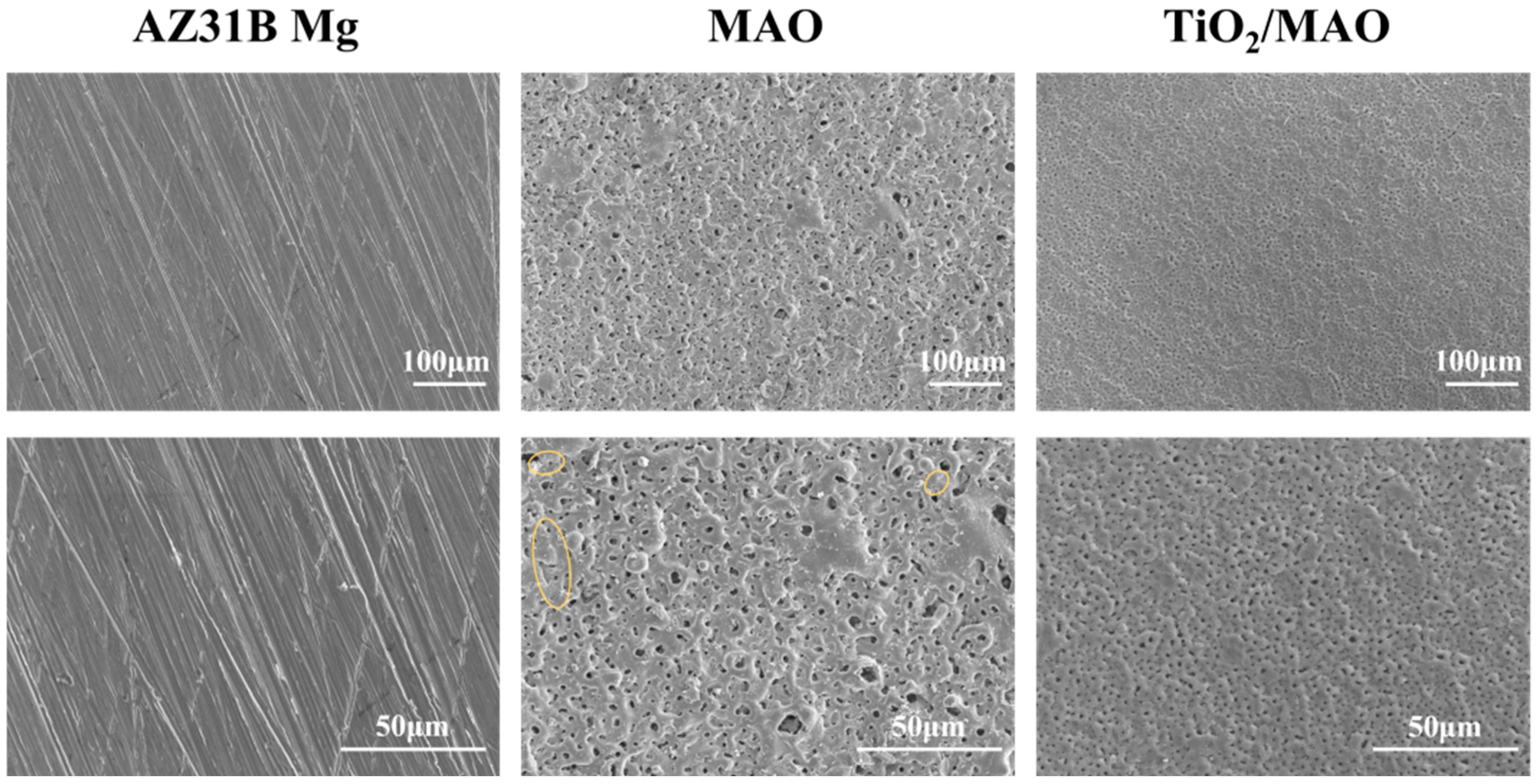
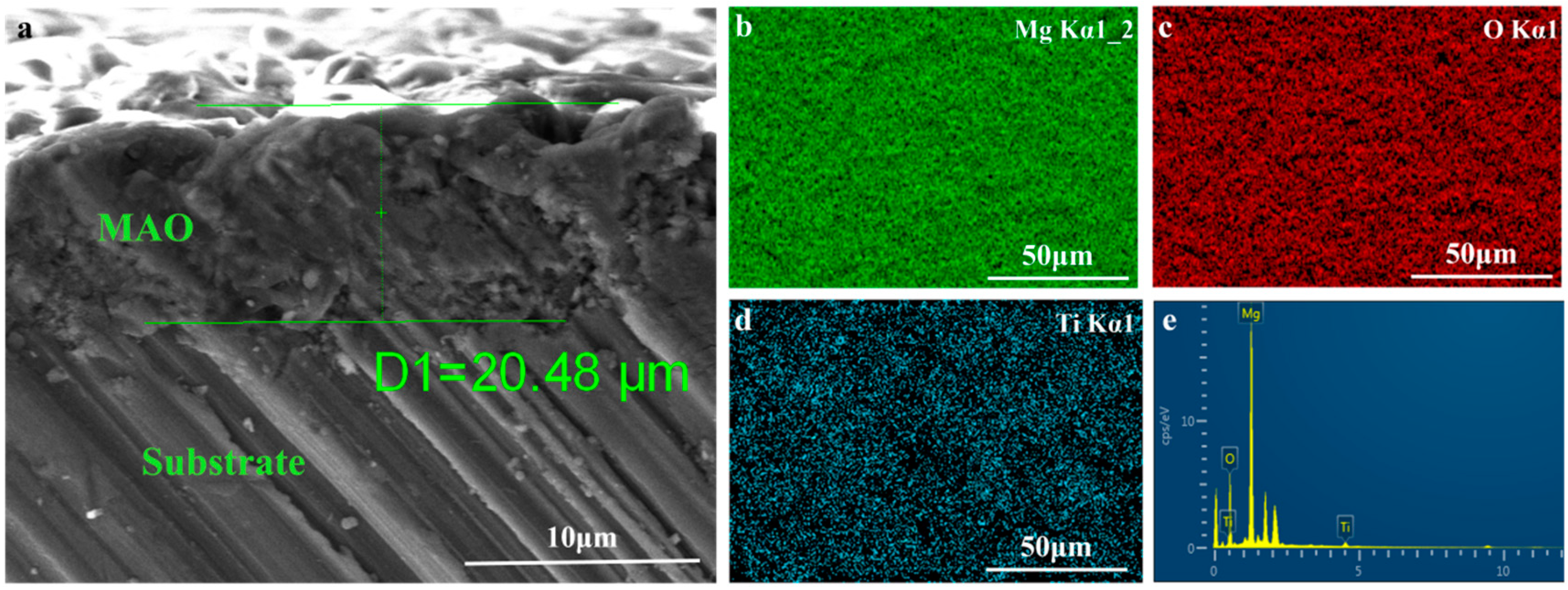
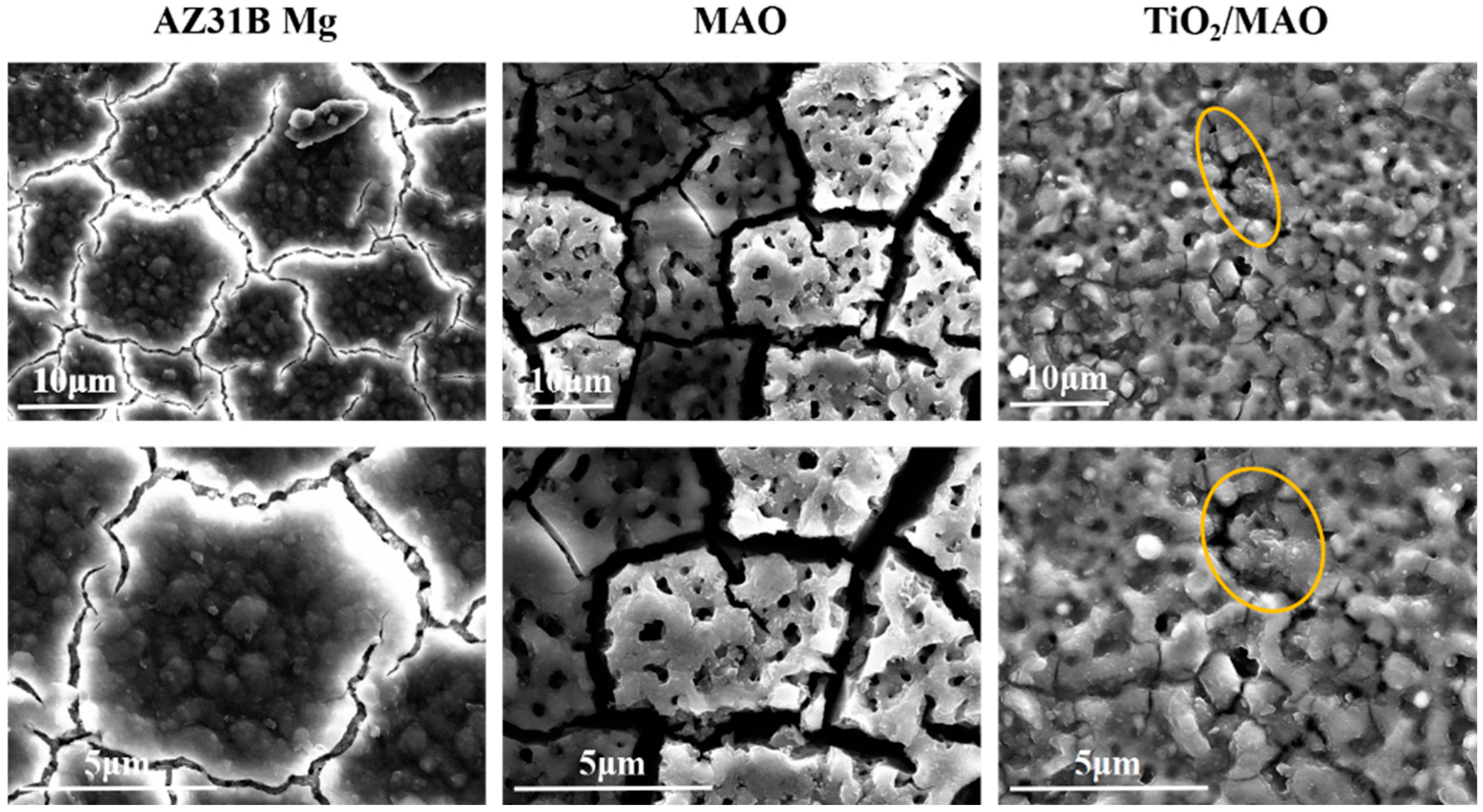
Figure 2 depicts the surface topography of the three sets of samples. As illustrated in Figure 2, the magnesium alloy exhibits numerous scratches on its surface following the polishing treatment. Following the micro-arc oxidation treatment, the surface of the MAO coating exhibited the presence of micro-hole and micro-crack structures (shown by yellow circles). The holes are the result of the discharge channels of the micro-arc oxidation, while the fractures occur owing to the deformation of the magnesium alloy substrate caused by the significant amount of heat released throughout the experiment [32]. The morphology of the TiO2 coating deposited by ALD was significantly altered, with a notable reduction in the number of microcracks on the surface of the MAO coating. Moreover, the size of the surface pores was considerably decreased and partly obscured. This demonstrates that TiO2 has been successfully applied onto the surface of the samples, albeit it does not fully envelop the MAO layer. This might be attributed to the restricted number of ALD deposition cycles leading to the formation of a thin TiO2 layer. Accordingly, we examined the cross-sectional structure of the MAO and found that the thickness of the magnesium oxide (MgO) layer was around 20 μm. The thickness of the ALD-deposited TiO2 film, which is not easily visible in the SEM, was determined to be around 47.41 nm using a spectroscopic ellipsometer. Significant levels of magnesium were found on the sample surface, mostly from the substrate material and the MAO coatings, according to the EDS findings of the TiO2/MAO coatings (Figure 3). On the other hand, the MAO coating and the presence of TiO2 are responsible for elemental oxygen. Furthermore, elemental Ti is less common than Mg, O, but its distribution is uniform overall, supporting the effective deposition of the TiO2 coating on the MAO samples’ surface.
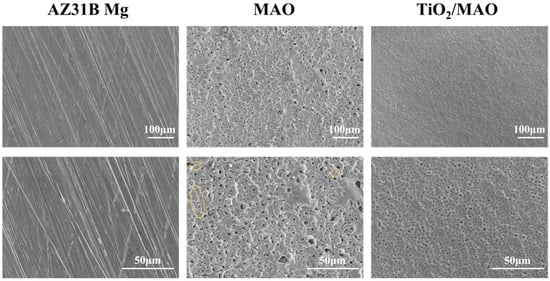
Figure 2.
SEM images of AZ31B Mg, MAO, and TiO2/MAO coatings.
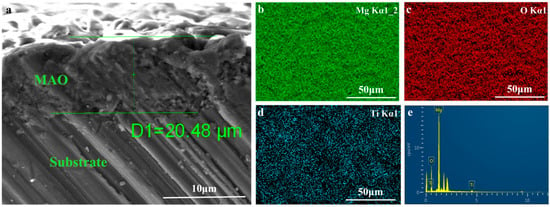
Figure 3.
(a) Cross-sectional morphology of MAO, elemental mapping images of (b) Mg, (c) O, (d) Ti, and (e) EDS surface analysis for TiO2/MAO coating.
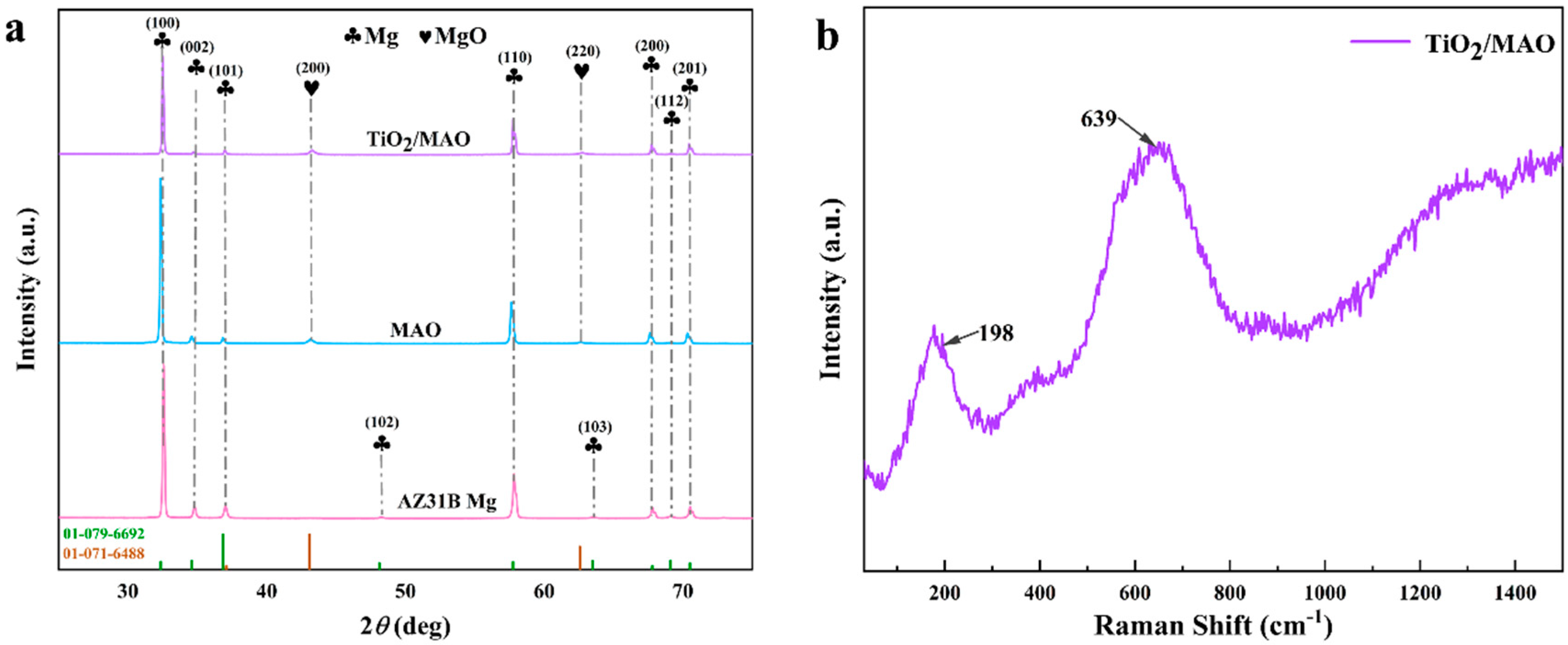
The X-ray diffraction (XRD) spectra were used to characterize the three sets of samples. As illustrated in Figure 4, diffraction peaks of Mg were observed for all samples ((100), (002), (101), (102), (110), (103), (200), (112), and (201)). In comparison to the other diffraction peaks, the (100) direction exhibited a notable increase in crystallinity due to the relatively low growth energy, which led to a large boost in the diffraction intensity of the crystalline surfaces. The MAO and TiO2/MAO coatings exhibited diffraction peaks corresponding to magnesium oxide ((200) and (220)), which suggests that the coatings after the micro-arc oxidation are primarily composed of magnesium oxide. Furthermore, the intensity of the corresponding Mg diffraction peaks (100), (002), and (101) decreased significantly following ALD deposition. Unexpectedly, there were no discernible diffraction peaks seen for TiO2. This absence of peaks may be attributed to the very thin coating, which likely caused TiO2 to reside in an amorphous state. Therefore, the TiO2/MAO sample was subjected to Raman spectroscopy once again. The findings indicated that the Raman signal of the sample was not prominent, and there were no distinct features of anatase TiO2, such as Eg, B1g, A1g + B1g, and Eg at 143, 198, 395, 512, 518, and 639 cm−1 [33]. While peaks at 198 and 639 cm−1 were identified, they were not clearly discernible, as seen in Figure 4b. This may be due to the thinness of the surface TiO2 layer and the weak Raman signal. The indication is that the surface of the TiO2 is in an amorphous state and that there is no occurrence of phase separation in the TiO2 film. This may be verified by the EDS analysis shown in Figure 3 and the subsequent XPS analysis shown in Figure 5.
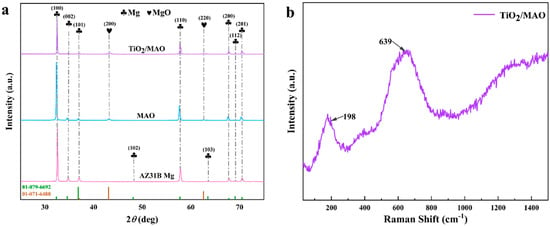
Figure 4.
(a) XRD patterns of AZ31B Mg, MAO, and TiO2/MAO, (b) Raman spectra of TiO2/MAO samples.
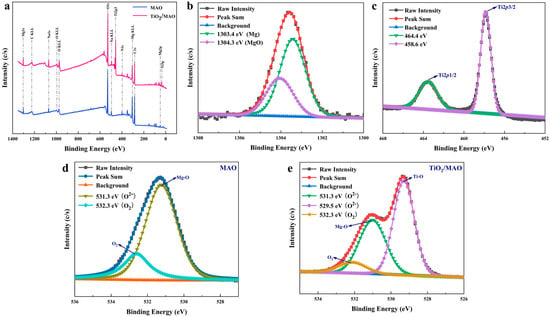
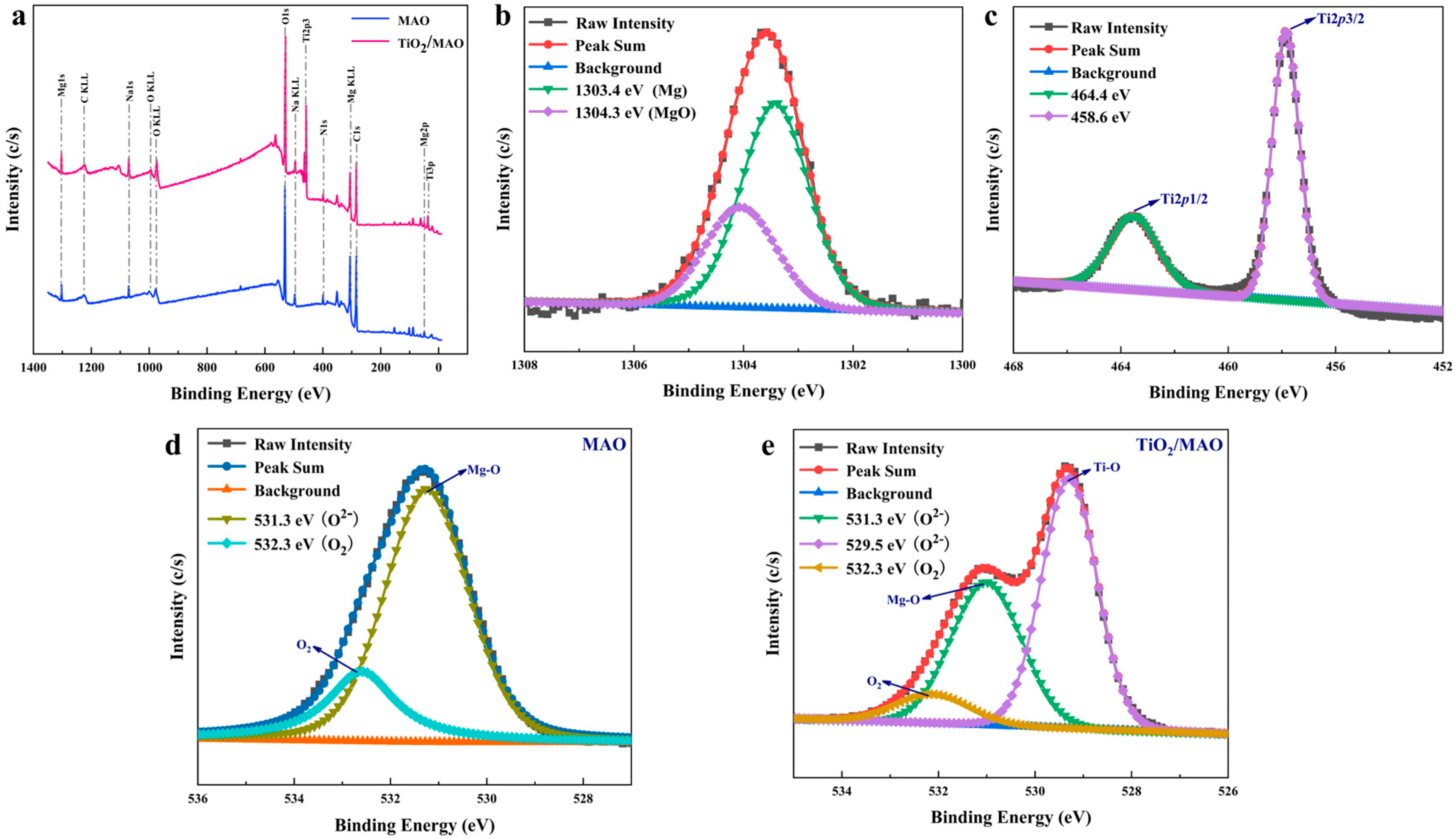
Figure 5.
(a) The XPS spectra of MAO and TiO2/MAO; high-resolution XPS spectra of implanted sample: (b) Mg 1s, (c) Ti 2p, (d) O 1s of MAO, and (e) O 1s of TiO2/MAO.
In addition, the XPS technique was used to investigate the surface chemical composition of both materials. From the full spectra (Figure 5a), it can be seen that both sets of samples contain the elements magnesium, carbon, and oxygen. Figure 5b illustrates that the binding energy of magnesium on the sample surface is 1303.4 eV, corresponding to MgO, and that of magnesium metal is 1304.3 eV [34]. After the ALD deposition of TiO2 on the MAO surface, significant Ti 2p and Ti 3p peaks appeared; peak O 1s also increased significantly (Figure 5a). The Ti 2p spectra, shown in Figure 5c, were comprised of two distinct bands, namely Ti 2p1/2 (464.4 eV) and Ti 2p3/2 (458.6 eV), which were predominantly in the form of TiO2, indicating that TiO2 was successfully deposited on the MAO surface [35]. In addition, the high-resolution O 1s spectra of both MAO and TiO2/MAO samples were also fitted and analyzed (Figure 5d,e). According to the fitting results, the O 1s spectra of the MAO sample is divided into two distinct peaks at 531.3 eV and 532.3 eV [35,36]. The signal seen at 531.3 eV corresponds to O2− ions associated with Mg-O bonds. This finding is consistent with the previous XRD data, suggesting that the surface of the sample consists of MgO after the process of micro-arc oxidation. The peak exhibiting a substantial binding energy of 532.3 eV can be ascribed to the presence of oxygen adsorbed on the surface. On the other hand, the O 1s spectra of TiO2/MAO shows the presence of Ti-O bonds (529.5 eV), Mg-O bonds (531.3 eV), and adsorbed oxygen (532.3 eV) [37,38]. This result also indicates that the TiO2 coating deposited during the experiment does not completely cover the MgO of the substrate, in agreement with the SEM results. Nevertheless, the deposition of TiO2 did indeed have an impact on the Mg peak in Figure 4a, as can be clearly noticed. Both samples exhibit adsorbed oxygen on their surfaces. However, the TiO2/MAO sample has a much lower fraction of adsorbed oxygen compared to the MAO sample. Following micro-arc oxidation, the sample’s microporous surface shape has a higher probability of encountering oxygen upon exposure to air. The following ALD deposition partially fills the surface micropores, resulting in a decreased interaction with air and a corresponding decrease in the quantity of adsorbed oxygen. To summarize, ALD has effectively generated a TiO2 coating, and the altered coating is present in the composition of MgO and TiO2.
3.2. Electrochemical Measurements
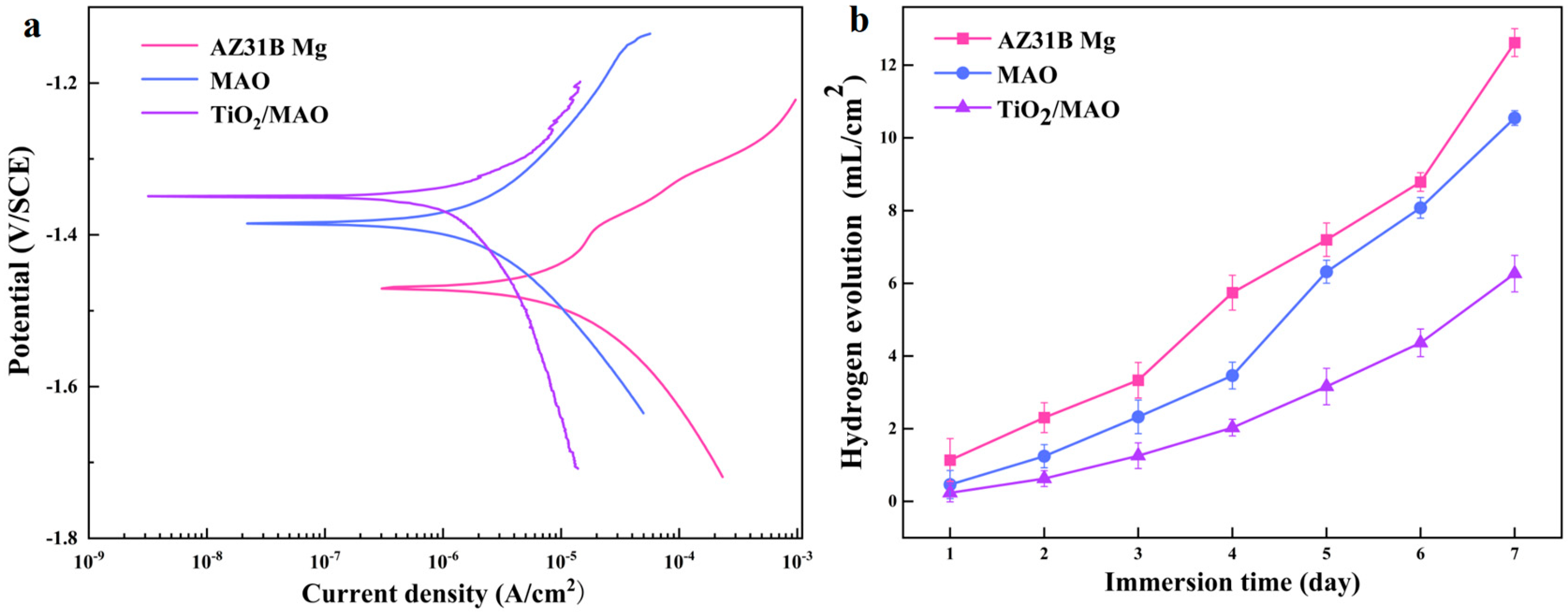
In order to assess the samples’ resistance to corrosion, dynamic potentiodynamic polarization experiments were conducted in a PBS solution. The results of these tests are shown in Figure 6a. The figure clearly demonstrates that the order of corrosion current density is as follows: TiO2/MAO < MAO < AZ31B Mg. Conversely, the corrosion potential follows the exact opposite order: TiO2/MAO > MAO > AZ31B Mg. The corrosion resistance of the samples is strongly correlated with the corrosion current density and corrosion potential. A lower corrosion current density indicates stronger corrosion resistance, whereas a larger corrosion potential suggests a lesser propensity for corrosion [39]. Hence, the TiO2/MAO exhibits superior corrosion resistance and significantly mitigates corrosion tendencies. This may be attributed to the excellent sealing of pores and cracks on the original MAO coating surface by the TiO2 coating, hence decreasing the corrosion of the coating. Table 2 also proves one of the results. Therefore, the TiO2/MAO composite film can provide more effective protection for the magnesium alloy of the substrate and significantly improve the corrosion resistance of the material.
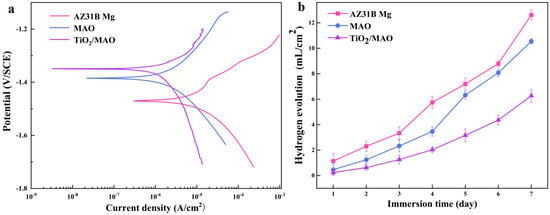
Figure 6.
(a) Potentiodynamic polarization profiles of three samples; (b) HER curves of different samples immersed in Hank’s solution for 7 days.

Table 2.
Important data obtained by the Tafel polarization test.
Following seven days of immersion in Hank’s solution, the hydrogen evolution experimental findings for the three groups of samples are shown in Figure 6b. Among them, the potentiodynamic polarization experiment results are consistent with the significantly lower hydrogen evolution rate of TiO2/MAO samples, suggesting that the TiO2/MAO composite coating provides better substrate protection and can effectively stop the corrosion of AZ31B Mg. During the first three days of immersion, the AZ31B Mg exhibits a low rate of hydrogen evolution. This is attributed to the formation of corrosion products on the surface of the magnesium alloy, which effectively inhibits further corrosion [40]. With an increase in immersion duration, the corrosion products underwent progressive dissolution, leading to an increase in the surface corrosion rate. Consequently, the hydrogen production rate of the AZ31B magnesium alloy saw a substantial rise starting on the fourth day. While the MAO-coated samples showed a lower quantity of hydrogen evolution compared to the AZ31B magnesium alloy during immersion, the hydrogen evolution rate notably increased on the fifth day. This is caused by the deterioration of the permeable MAO coating and the interaction between the corrosive solution and the magnesium substrate. Furthermore, we used SEM to examine the surface structure of the samples after a 7-day immersion period. The results are shown in Figure 7. After 7 days of immersion, the sample’s surface displayed many irregular fissures and a granular appearance due to the strong reactivity of the magnesium alloy. The presence of many cracks in the MAO coating may be attributed to corrosion resulting from the interaction between the solution and the substrate during the immersion process. This interaction leads to the generation of a significant quantity of hydrogen gas, which in turn disrupts and fractures the micro-arc oxidation coating. Conversely, the TiO2/MAO surface exhibited no notable alterations, although there were minor fissures and surface indentations (shown by yellow circles). Hence, the TiO2/MAO composite coating effectively safeguards the substrate and enhances its resistance to corrosion.
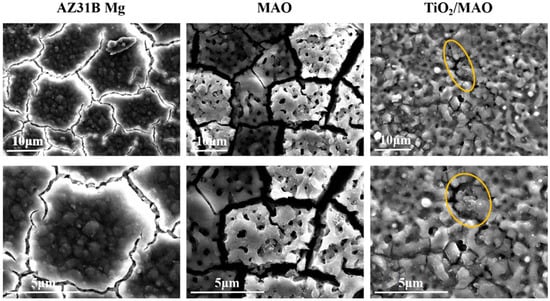
Figure 7.
SEM images of AZ31B Mg, MAO, and TiO2/MAO coatings immersed in Hank’s solution for 7 days.
3.3. In Vitro Cytocompatibility Studies
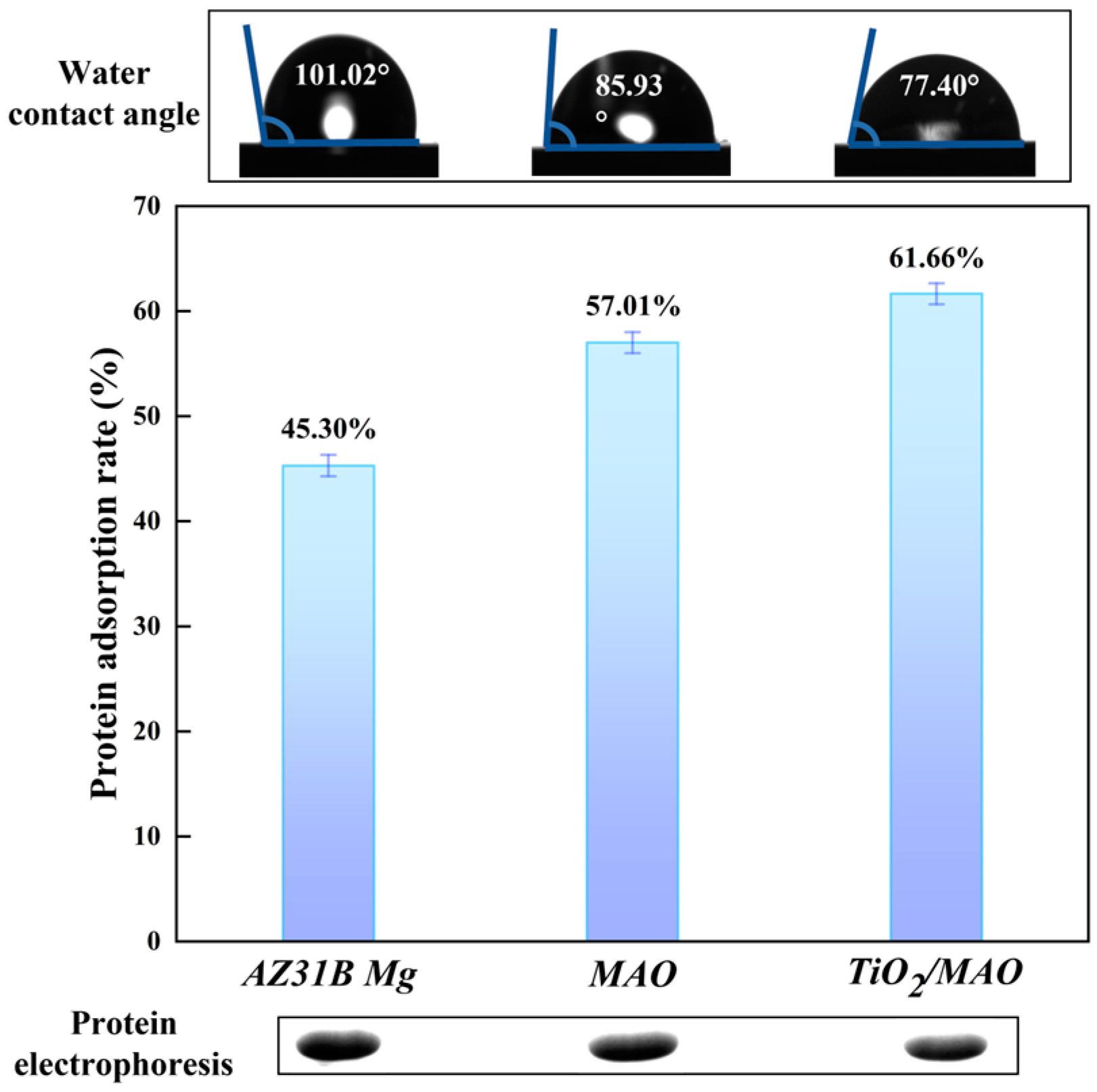
Surface wettability, an essential surface property of biological implant materials, can be assessed using the water contact angle (WCA > 150°, classified as superhydrophobic; 150° > WCA > 90°, considered as hydrophobic; 90° > WCA > 10°, classified as hydrophilic; WCA < 10°, considered as superhydrophilic) [41]. Figure 8 demonstrates a steady reduction in the water contact angle for the three sample groups (101.02° ± 3.17° > 85.93° ± 2.46° > 77.40° ± 1.20°), indicating an increase in hydrophilicity. Furthermore, there is a strong correlation between the surface roughness and the surface wettability [42]. The wettability of the modified samples was enhanced as a result of the progressive augmentation in surface roughness after micro-arc oxidation and ALD deposition. The samples were graded based on their protein adsorption capability in the following order: TiO2/MAO > MAO > AZ31B Mg. In contrast, a decrease in protein residue results in an increase in protein adsorption capability. The TiO2/MAO picture exhibited the lowest protein residue and the highest brightness, confirming its better protein adsorption ability in comparison to the other two groups. Wettability also influences protein adsorption [42]. Surfaces that are superhydrophilic have a higher capacity to absorb water, resulting in a decrease in the adsorption of proteins [43]. Surfaces that are somewhat hydrophilic have the highest ability for protein adsorption [42], which aligns with our experimental findings: the protein adsorption capacity of TiO2/MAO has been enhanced.
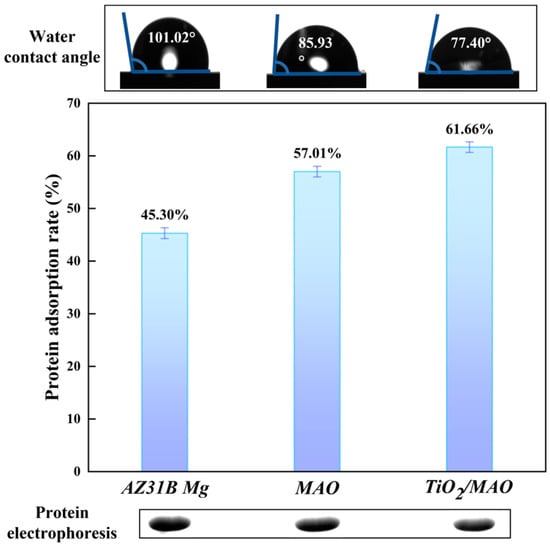
Figure 8.
Protein adsorption of three groups and contact angle images (inset).
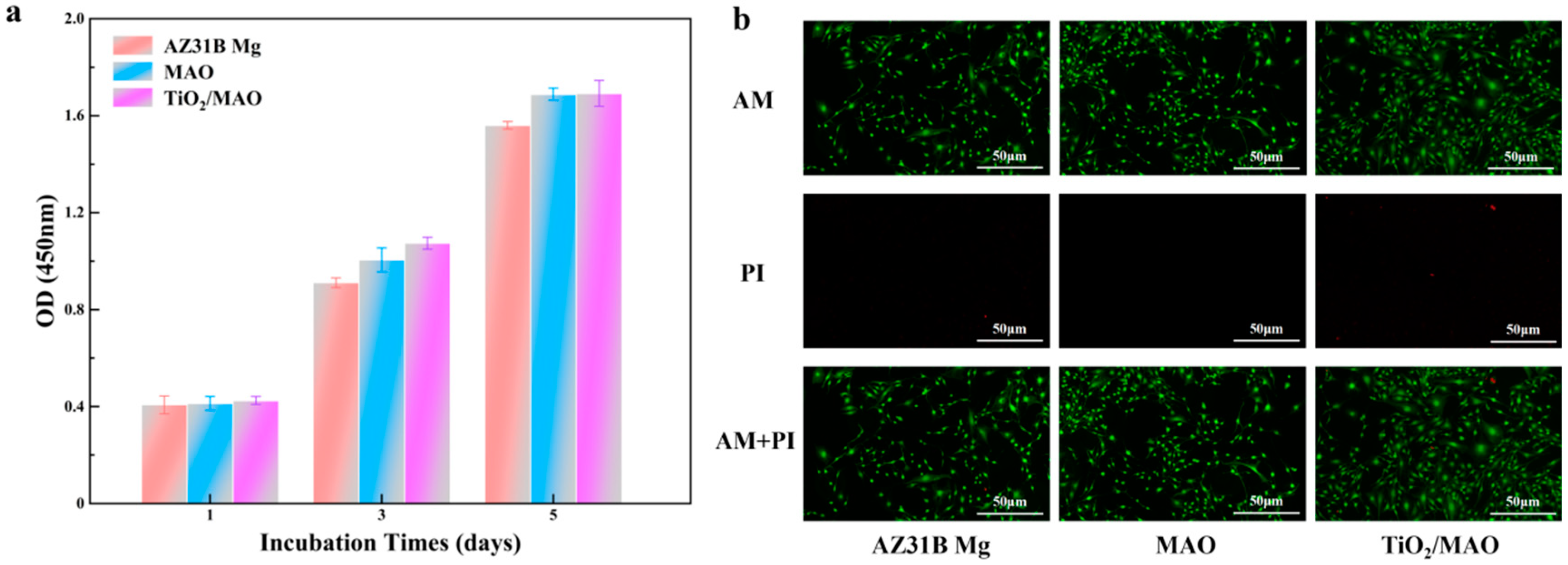
The proliferation results of MC3T3-E1 cells cultured in different sample extracts are shown in Figure 9a. After the first day of culture, the difference in proliferation status among the three groups of samples was not obvious, which might be due to the fact that the cells were sensitive to the growth environment at the early stage of culture and needed some time to adapt to the new culture environment [40]. A difference in cell proliferation between the MAO and TiO2/MAO groups was less pronounced but superior to that of the AZ31B Mg group, suggesting that the MAO coatings and the composite coatings of TiO2/MAO had a beneficial impact on enhancing cell development and proliferation. In order to assess the cytotoxicity of the various solutions, live/dead fluorescence imaging of MC3T3-E1 cells was conducted following a 2-day incubation period in the AZ31B Mg solution. Figure 9b displays the results of live/dead fluorescence imaging of MC3T3-E1 cells following a 2-day incubation in the AZ31B Mg, MAO, and TiO2/MAO leachate samples, which were used to assess their cytotoxicity. The fluorescence images of all the sample groups showed obvious green fluorescence (living cells), while the AZ31B Mg and TiO2/MAO groups had some red fluorescence (dead cells), indicating that the cells cultured in the extracts grew well and very few cells died. The cell growth status of the three sample groups was good; in particular, most of the cells in the TiO2/MAO group were spread out, and the cells were tightly connected to each other. In addition, this outcome also indicates the cellular distribution state. The density of cells in the AZ31B Mg sample extract was marginally lower compared to the MAO and TiO2/MAO sample groups. This finding aligns with the CCK-8 findings. The aforementioned findings suggest that the modified coated samples could promote cell proliferation and did not show cytotoxicity in the extracts of the samples.
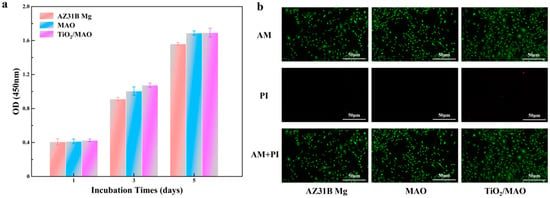
Figure 9.
(a) The OD values of CCK-8 experiments for three groups. (b) Live/dead fluorescence staining results for three samples.
4. Discussion
Atomic layer deposition (ALD) is a precise method for depositing thin films on a substrate. It is based on chemical vapor deposition (CVD) and involves injecting two or more precursor molecules onto the substrate surface. These molecules then react chemically to form a single-atom film, which is deposited layer by layer. During the deposition process, the reactive precursors are sequentially deposited, with each reaction resulting in the deposition of a single layer of atoms. Hence, the quantity of deposition cycles may be regulated to attain meticulous management of film thickness. This work describes the production of titanium dioxide thin films utilizing atomic layer deposition (ALD) technology. The films were created by adsorbing two precursors, TDMA-Ti and H2O, onto the surface of AZ31B magnesium alloy following micro-arc oxidation. This process resulted in the formation of a periodic atomic layer structure. The investigation resulted in the formation of a TiO2 coating with a thickness of about 40 nm via the process of surface cycling, which was repeated 400 times. The samples coated with ALD-deposited TiO2 on MAO exhibited a higher degree of completeness compared to the substrate and single layer MAO coatings. The reason for this is that the thick TiO2 produced by ALD effectively plugs microcracks and holes. Nevertheless, the size of the holes formed during micro-arc oxidation differed, and the TiO2 derived from 400 cycles did not fully envelop the MAO surface. This was mostly due to the excessively thin deposited film layer, which resulted in incomplete sealing of some of the bigger pores.
The TiO2 coating functions as a physical barrier that hinders the entry of corrosive ions, successfully safeguarding the substrate from corrosion during the immersion test. Based on the PDP curve and immersion test, it is evident that the TiO2/MAO coating enhances the corrosion resistance of the magnesium alloy, resulting in a lower corrosion rate. Nevertheless, when the immersion period rises, corrosive ions permeate through the thin TiO2 layer, resulting in pitting corrosion. In this investigation, the TiO2/MAO coating exhibited a lower degree of corrosion resistance compared to general titanium oxide, mostly due to its thin layer. Nevertheless, the presence of a TiO2 film plays a crucial role in controlling the excessive corrosion rate of magnesium alloy. By adjusting the cycle duration, it is feasible to generate a thicker and more evenly distributed TiO2 film, therefore further regulating the corrosion rate.
The biocompatibility of the material is intimately linked to the chemical characteristics and morphology of the implant surface. Upon first contact with the medium, such as mesenchyme or blood, the substance undergoes protein adsorption on its surface, subsequently impacting cell adhesion, proliferation, and migration. To provide the best possible attachment and growth of cells, the material must possess a specific capability for protein adsorption. Surface modification of the samples has been shown to boost the protein adsorption capability in in vitro investigations. This sets the groundwork for the future implantation of the samples in vivo. Furthermore, we specifically examined the impact of corrosion products on cell survival in the implant environment in relation to biocompatibility. In order to achieve this objective, we evaluated the feasibility of MC3T3-E1 cells in the sample extracts by using cck-8 and live/dead cell labeling techniques. The findings demonstrated that the application of ALD coating enhanced the cell survival of the untreated samples and did not exhibit any notable cytotoxic effects. Hence, the changed samples exhibited excellent biocompatibility.
5. Conclusions
- Titanium dioxide (TiO2) thin films were effectively coated upon MAO films utilizing the ALD method. The micropores and fractures that formed during the micro-arc oxidation process were effectively sealed.
- Due to the thin TiO2 coating formed by 400 cycles, the TiO2 film exists mainly in an amorphous form.
- The corrosion resistance of the TiO2/MAO composite coating was shown to be superior by electrochemical and hydrogen evolution studies.
- As shown by in vitro biological tests, the TiO2/MAO composite coating exhibited superior biocompatibility compared to both the alloy matrix and MAO coating.
ALD has been increasingly used for surface modification of biomaterials. However, there has been limited research on the use of ALD coatings, such as TiO2 coatings, on magnesium base alloys following micro-arc oxidation. Based on this, the current research may be considered one of the most efficient bio-coatings. Future studies may use ALD technology and other oxide coatings to enhance the corrosion resistance of magnesium-based alloys. Additionally, future studies will prioritize investigating the biocompatibility of these materials.
Author Contributions
X.H.: conception, methodology, investigation, analysis, writing—original manuscript preparation; Y.W.: investigation, methodology; J.M.: analysis, review and editing; X.M.: analysis, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2022YFC3601904) and the Tianjin Natural Science Foundation Key Program (22JCZDJC00340).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Y.; Zhang, Y.; Wang, Y.-L.; Tian, Y.-Q.; Chen, L.-S. Research progress on surface protective coatings of biomedical degradable magnesium alloys. J. Alloys Compd. 2021, 885, 161001. [Google Scholar] [CrossRef]
- Lu, X.; Cai, H.; Li, Y.R.; Zheng, X.; Yun, J.; Li, W.; Geng, X.; Kwon, J.S.; Jiang, H.B. A Systematic Review and Network Meta-Analysis of Biomedical Mg Alloy and Surface Coatings in Orthopedic Application. Bioinorg. Chem. Appl. 2022, 2022, 4529520. [Google Scholar] [CrossRef]
- Johari, N.A.; Alias, J.; Zanurin, A.; Mohamed, N.S.; Alang, N.A.; Zain, M.Z.M. Recent progress of self-healing coatings for magnesium alloys protection. J. Coat. Technol. Res. 2022, 19, 757–774. [Google Scholar] [CrossRef]
- Peron, M.; Torgersen, J.; Berto, F. Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure. Metals 2017, 7, 252. [Google Scholar] [CrossRef]
- Gawlik, M.M.; Wiese, B.; Desharnais, V.; Ebel, T.; Willumeit-Romer, R. The Effect of Surface Treatments on the Degradation of Biomedical Mg Alloys—A Review Paper. Materials 2018, 11, 2561. [Google Scholar] [CrossRef]
- Hanzi, A.C.; Gerber, I.; Schinhammer, M.; Loffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg-Y-Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef]
- Reinhart, R.A. Magnesium metabolism. A review with special reference to the relationship between intracellular content and serum levels. Arch. Intern. Med. 1988, 148, 2415–2420. [Google Scholar] [CrossRef]
- Kim, B.J.; Piao, Y.; Wufuer, M.; Son, W.C.; Choi, T.H. Biocompatibility and Efficiency of Biodegradable Magnesium-Based Plates and Screws in the Facial Fracture Model of Beagles. J. Oral Maxillofac. Surg. 2018, 76, 1055.e1–1055.e9. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, S.; Roy, M. Recent Developments in Magnesium Metal-Matrix Composites for Biomedical Applications: A Review. ACS Biomater. Sci. Eng. 2020, 6, 4748–4773. [Google Scholar] [CrossRef]
- Li, P.; Zhou, N.; Qiu, H.; Maitz, M.F.; Wang, J.; Huang, N. In vitro and in vivo cytocompatibility evaluation of biodegradable magnesium-based stents: A review. Sci. China Mater. 2018, 61, 501–515. [Google Scholar] [CrossRef]
- Xia, D.; Liu, Y.; Wang, S.; Zeng, R.-C.; Liu, Y.; Zheng, Y.; Zhou, Y. In vitro and in vivo investigation on biodegradable Mg-Li-Ca alloys for bone implant application. Sci. China Mater. 2018, 62, 256–272. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of Recent Developments in the Field of Magnesium Corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Li, L.Y.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Chen, X.B.; Zheng, Y.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Murillo-Gutiérrez, N.V.; Ansart, F.; Bonino, J.P.; Menu, M.J.; Gressier, M. Protection against corrosion of magnesium alloys with both conversion layer and sol–gel coating. Surf. Coat. Technol. 2013, 232, 606–615. [Google Scholar] [CrossRef]
- Tang, H.; Yu, D.; Luo, Y.; Wang, F. Preparation and characterization of HA microflowers coating on AZ31 magnesium alloy by micro-arc oxidation and a solution treatment. Appl. Surf. Sci. 2013, 264, 816–822. [Google Scholar] [CrossRef]
- Ma, W.H.; Liu, Y.J.; Wang, W.; Zhang, Y.Z. Improved biological performance of magnesium by micro-arc oxidation. Braz. J. Med. Biol. Res. 2015, 48, 214–225. [Google Scholar] [CrossRef]
- Jian, S.-Y.; Ho, M.-L.; Shih, B.-C.; Wang, Y.-J.; Weng, L.-W.; Wang, M.-W.; Tseng, C.-C. Evaluation of the Corrosion Resistance and Cytocompatibility of a Bioactive Micro-Arc Oxidation Coating on AZ31 Mg Alloy. Coatings 2019, 9, 396. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Zeng, R.-C.; Guan, S.-K.; Qi, W.-C.; Zhang, F.; Li, S.-Q.; Han, E.-H. Degradation mechanism of micro-arc oxidation coatings on biodegradable Mg-Ca alloys: The influence of porosity. J. Alloys Compd. 2017, 695, 2464–2476. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.; Song, G.-L.; Zheng, D.; Jiang, B.; Atrens, A.; Pan, F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M. Review of electrochemical properties of hybrid coating systems on Mg with plasma electrolytic oxidation process as pretreatment. Surf. Interfaces 2019, 14, 262–295. [Google Scholar] [CrossRef]
- Cui, X.-J.; Ping, J.; Zhang, Y.-J.; Jin, Y.-Z.; Zhang, G.-A. Structure and properties of newly designed MAO/TiN coating on AZ31B Mg alloy. Surf. Coat. Technol. 2017, 328, 319–325. [Google Scholar] [CrossRef]
- Dai, X.-J.; Li, X.-C.; Wang, C.; Yu, S.; Yu, Z.-T.; Yang, X.-R. Effect of MAO/Ta2O5 composite coating on the corrosion behavior of Mg–Sr alloy and its in vitro biocompatibility. J. Mater. Res. Technol. 2022, 20, 4566–4575. [Google Scholar] [CrossRef]
- Dou, J.; Yu, H.; Chen, C.; Lok-Wang Ma, R.; Ming-Fai Yuen, M. Preparation and microstructure of MAO/CS composite coatings on Mg alloy. Mater. Lett. 2020, 271, 127729. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Xiong, Q.; Wu, X.; Zhou, J.; Wu, J.; Wu, X.; Qin, W. Multipurpose surface functionalization on AZ31 magnesium alloys by atomic layer deposition: Tailoring the corrosion resistance and electrical performance. Nanoscale 2017, 9, 8591–8599. [Google Scholar] [CrossRef]
- Li, C.Y.; Yu, C.; Zeng, R.C.; Zhang, B.C.; Cui, L.Y.; Wan, J.; Xia, Y. In vitro corrosion resistance of a Ta2O5 nanofilm on MAO coated magnesium alloy AZ31 by atomic layer deposition. Bioact. Mater. 2020, 5, 34–43. [Google Scholar] [CrossRef]
- Hernández-Montes, V.; Betancur-Henao, C.P.; Santa-Marín, J.F. Titanium dioxide coatings on magnesium alloys for biomaterials: A review. Dyna 2017, 84, 261–270. [Google Scholar] [CrossRef]
- Kania, A.; Pilarczyk, W.; Szindler, M.M. Structure and corrosion behavior of TiO2 thin films deposited onto Mg-based alloy using magnetron sputtering and sol-gel. Thin Solid Film 2020, 701, 137945. [Google Scholar] [CrossRef]
- Kania, A.; Szindler, M.M.; Szindler, M. Structure and Corrosion Behavior of TiO2 Thin Films Deposited by ALD on a Biomedical Magnesium Alloy. Coatings 2021, 11, 70. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Luo, L.; Liu, Y.; Wan, J. Microstructure and corrosion resistance of TiN/TiO2 nano-composite film on AZ31 magnesium alloy. Surf. Coat. Technol. 2021, 406, 126681. [Google Scholar] [CrossRef]
- Li, W.; Tian, A.; Li, T.; Zhao, Y.; Chen, M. Ag/ZIF-8/Mg-Al LDH composite coating on MAO pretreated Mg alloy as a multi-ion-release platform to improve corrosion resistance, osteogenic activity, and photothermal antibacterial properties. Surf. Coat. Technol. 2023, 464, 129555. [Google Scholar] [CrossRef]
- Ahmed, M.; Qi, Y.; Zhang, L.; Yang, Y.; Abas, A.; Liang, J.; Cao, B. Influence of Cu2+ Ions on the Corrosion Resistance of AZ31 Magnesium Alloy with Microarc Oxidation. Materials 2020, 13, 2647. [Google Scholar] [CrossRef] [PubMed]
- Tanskanen, A.; Karttunen, A.J.; Karppinen, M. Substantially enhanced Raman signal for inorganic–organic nanocomposites by ALD-TiO2 capping. RSC Adv. 2016, 6, 41087–41091. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, H.; Liao, K.; Li, X. Improvement on corrosion resistance of micro-arc oxidized AZ91D magnesium alloy by a pore-sealing coating. J. Alloys Compd. 2021, 889, 161460. [Google Scholar] [CrossRef]
- Huang, L.; Su, K.; Zheng, Y.-F.; Yeung, K.W.-K.; Liu, X.-M. Construction of TiO2/silane nanofilm on AZ31 magnesium alloy for controlled degradability and enhanced biocompatibility. Rare Met. 2019, 38, 588–600. [Google Scholar] [CrossRef]
- Lv, L.; Lee, F.Y.; Zhou, J.; Su, F.; Zhao, X.S. XPS study on microporous titanosilicate ETS-10 upon acid treatment. Microporous Mesoporous Mater. 2006, 96, 270–275. [Google Scholar] [CrossRef]
- Mohanta, R.R.; Medicherla, V.R.R.; Mohanta, K.L.; Nayak, N.C.; Majumder, S.; Solanki, V.; Varma, S.; Bapna, K.; Phase, D.M.; Sathe, V. Ion beam induced chemical and morphological changes in TiO2 films deposited on Si(111) surface by pulsed laser deposition. Appl. Surf. Sci. 2015, 325, 185–191. [Google Scholar] [CrossRef]
- Peron, M.; Bin Afif, A.; Dadlani, A.; Berto, F.; Torgersen, J. Comparing physiologically relevant corrosion performances of Mg AZ31 alloy protected by ALD and sputter coated TiO2. Surf. Coat. Technol. 2020, 395, 125922. [Google Scholar] [CrossRef]
- Peron, M.; Cogo, S.; Bjelland, M.; Bin Afif, A.; Dadlani, A.; Greggio, E.; Berto, F.; Torgersen, J. On the evaluation of ALD TiO2, ZrO2 and HfO2 coatings on corrosion and cytotoxicity performances. J. Magnes. Alloys 2021, 9, 1806–1819. [Google Scholar] [CrossRef]
- Peron, M.; Bertolini, R.; Cogo, S. On the corrosion, stress corrosion and cytocompatibility performances of ALD TiO2 and ZrO2 coated magnesium alloys. J. Mech. Behav. Biomed. Mater. 2022, 125, 104945. [Google Scholar] [CrossRef]
- Song, W.; Mano, J.F. Interactions between cells or proteins and surfaces exhibiting extreme wettabilities. Soft Matter 2013, 9, 2985–2999. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2012, 28, 843–863. [Google Scholar] [CrossRef]
- Zheng, J.; Li, L.; Tsao, H.K.; Sheng, Y.J.; Chen, S.; Jiang, S. Strong repulsive forces between protein and oligo (ethylene glycol) self-assembled monolayers: A molecular simulation study. Biophys. J. 2005, 89, 158–166. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).