Recent Applications and Future Trends of Nanostructured Thin Films-Based Gas Sensors Produced by Magnetron Sputtering
Abstract
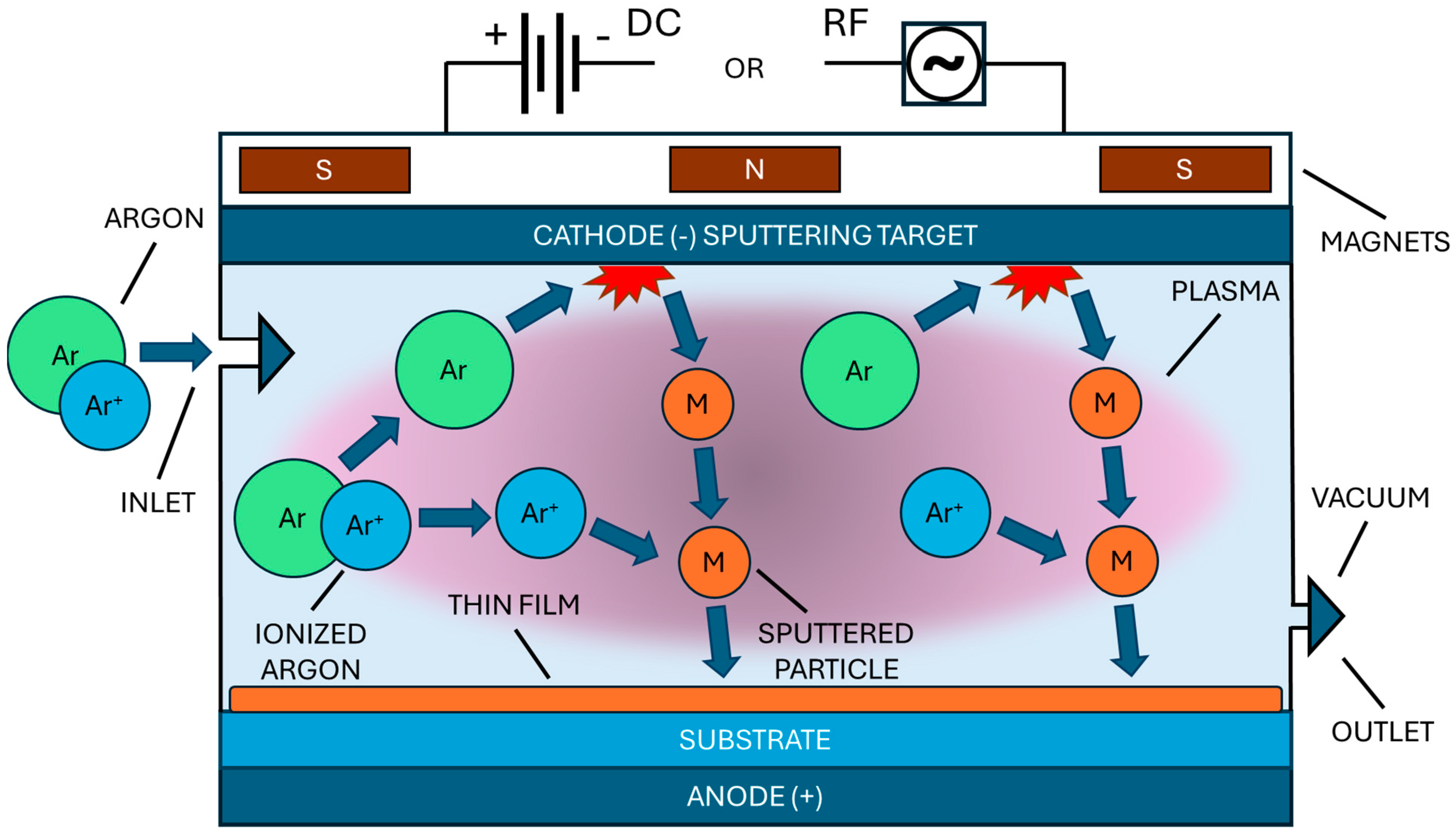
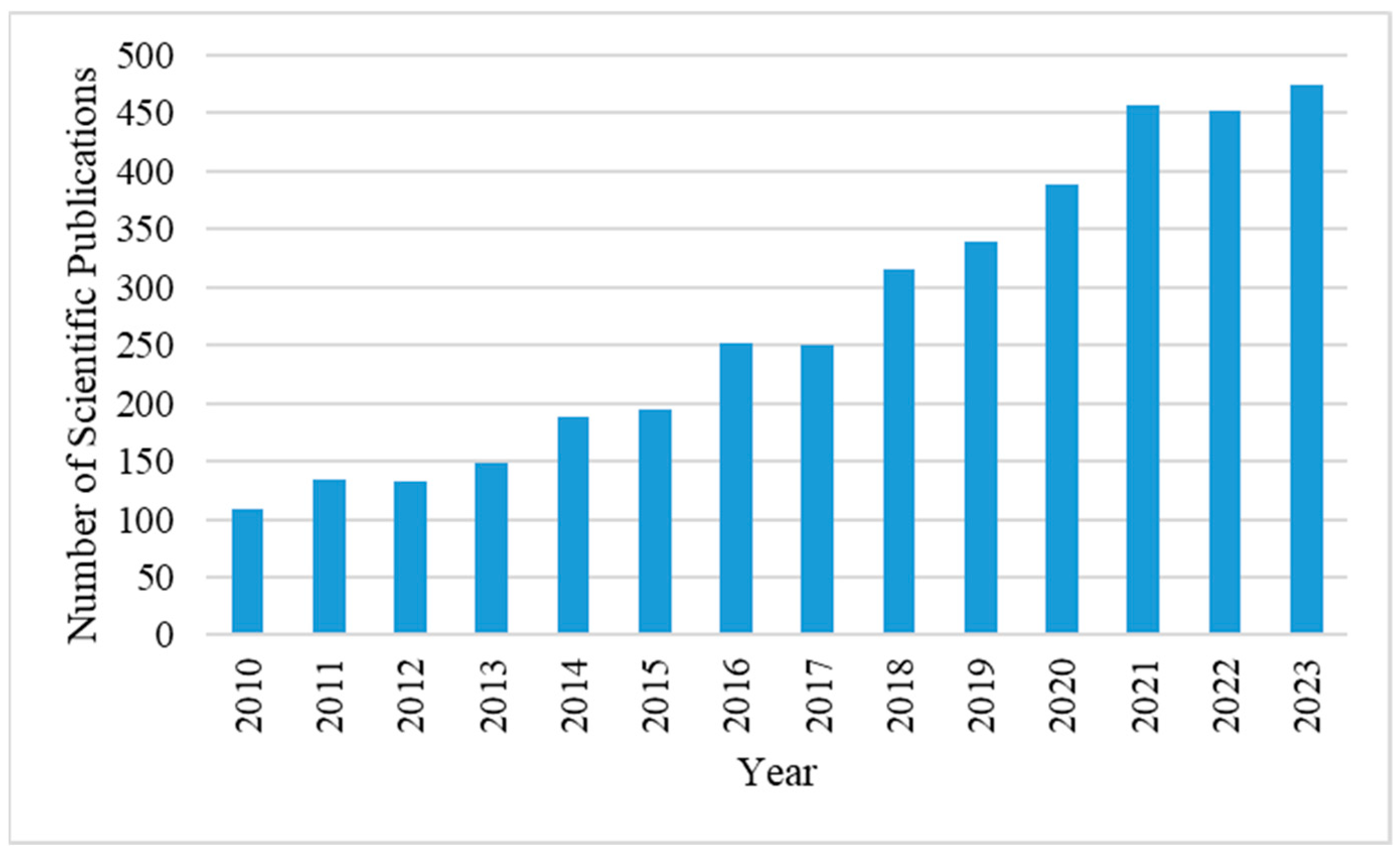
1. Introduction
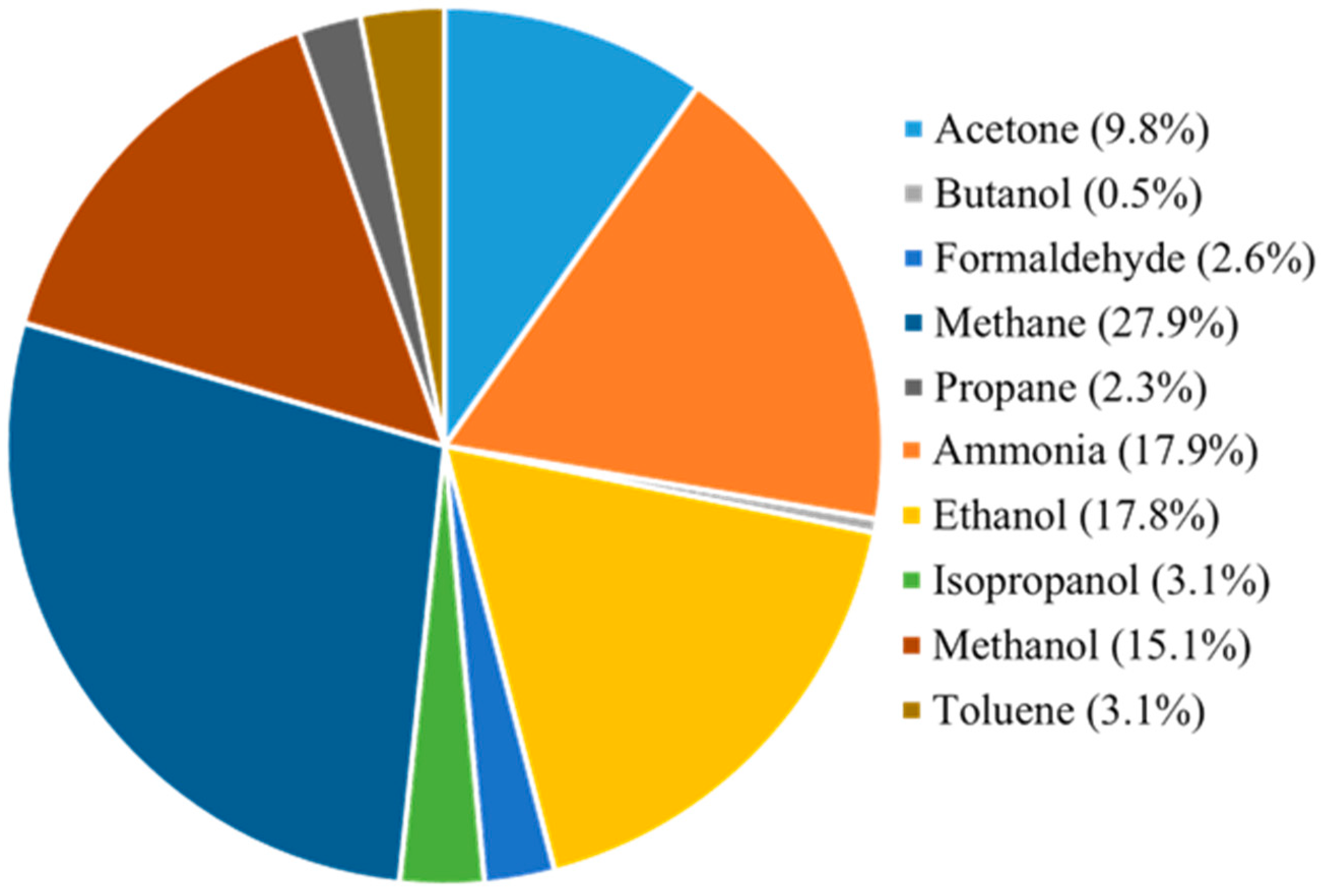
2. Gas Sensors Applications
2.1. Acetone
2.2. Ammonia
2.3. Butanol
2.4. Ethanol
2.5. Formaldehyde
2.6. Isopropanol
2.7. Methane
2.8. Methanol
2.9. Propane
2.10. Toluene
2.11. Future Trends
2.11.1. Acetaldehyde
2.11.2. Acetophenone
2.11.3. Benzene
2.11.4. 2-Butanone
2.11.5. Butyl Acetate
2.11.6. Ethylbenzene
2.11.7. Hexanal
2.11.8. Isoprene
2.11.9. Limonene
2.11.10. Nonanal
2.11.11. Phenol
2.11.12. α-Pinene
2.11.13. Xylene
3. Conclusions
- Sputtering power, which changed between DC and RF, typically ranging from 50 to 1000 W, with higher powers generally leading to higher deposition rates and potentially larger grain sizes.
- Working pressure was usually maintained between 0.1 and 10 Pa, with lower pressures often conducing to denser films and higher pressures promoting more porous structures.
- Target-to-substrate distance, commonly set between 5 and 15 cm, influencing the energy of the sputtered particles and therefore the deposition rate.
- Substrate temperature, which varied from room temperature to 500 °C during deposition, affecting the crystallinity and grain size of the films
- Gas flow rates. Typically, argon flow rates of 10–50 sccm were used, with additional oxygen flow (1–10 sccm) for reactive sputtering of the MOs.
- Deposition time, which ranged from a few minutes to several hours, controlling the film thickness, which typically varied from a 10 nm to a 4 μm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Union Parliament. Directive 2004/42/CE of the European Parliament and of the Council of 21 April 2004 on the limitation of emissions of volatile organic compounds due to the use of organic solvents in certain paints and varnishes and vehicle refinishing products. Off. J. Eur. Un. 2004, L143, 87–96. [Google Scholar]
- Mazzeo, N.A. Air Quality Monitoring, Assessment and Management, 1st ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Moura, P.C.; Vassilenko, V. Gas Chromatography–Ion Mobility Spectrometry as a tool for quick detection of hazardous volatile organic compounds in indoor and ambient air: A university campus case study. Eur. J. Mass Spectrom. 2022, 28, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nie, L.; Li, J.; Wang, Y.; Wang, G.; Wang, J.; Hao, Z. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef]
- Moura, P.C.; Santos, F.; Fujão, C.; Vassilenko, V. Towards the identification of the volatile organic compounds emitted by the coatings used in a car factory painting line. J. Coat. Technol. Res. 2024, 21, 665–682. [Google Scholar] [CrossRef]
- Ulker, O.C.; Ulker, O.; Hiziroglu, S. Volatile Organic Compounds (VOCs) Emitted from Coated Furniture Units. Coatings 2021, 11, 806. [Google Scholar] [CrossRef]
- Montero-Montoya, R.; López-Vargas, R.; Arellano-Aguilar, O. Volatile Organic Compounds in Air: Sources, Distribution, Exposure and Associated Illness in Children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef]
- David, E.; Niculescu, V.C. Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials. In. J. Environ. Res. Public Health 2021, 18, 13147. [Google Scholar] [CrossRef]
- Wah, C.; Yu, F.; Kim, J.T. Building Pathology, Investigation of Sick Building—VOC Emissions. Indoor Built Environ. 2010, 19, 30–39. [Google Scholar]
- Nakaoka, H.; Todaka, E.; Seto, H.; Saito, I.; Hanazato, M.; Watanabe, M.; Mori, C. Correlating the symptoms of sick-building syndrome to indoor VOCs concentration levels and odour. Indoor Built Environ. 2014, 23, 804–813. [Google Scholar] [CrossRef]
- Moura, P.C.; Santos, F.; Fujão, C.; Vassilenko, V. In Situ Indoor Air Volatile Organic Compounds Assessment in a Car Factory Painting Line. Processes 2023, 11, 2259. [Google Scholar] [CrossRef]
- Reis, T.; Moura, P.C.; Gonçalves, D.; Ribeiro, P.A.; Vassilenko, V.; Fino, M.H.; Raposo, M. Ammonia Detection by Electronic Noses for a Safer Work Environment. Sensors 2024, 2024, 3152. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Su, F.; Batterman, S. Volatile Organic Compounds (VOCs) in Conventional and High Performance School Buildings in the U.S. Int. J. Environ. Res. Public Health 2017, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Bessonneau, V.; Mosqueron, L.; Berrubé, A.; Mukensturm, G.; Buffet-Bataillon, S.; Gangneux, J.P.; Thomas, O. VOC Contamination in Hospital, from Stationary Sampling of a Large Panel of Compounds, in View of Healthcare Workers and Patients Exposure Assessment. PLoS ONE 2013, 8, e55535. [Google Scholar] [CrossRef]
- Mishra, N.; Bartsch, J.; Ayoko, G.A.; Salthammer, T.; Morawska, L. Volatile Organic Compounds: Characteristics, distribution and sources in urban schools. Atmos. Environ. 2015, 106, 485–491. [Google Scholar] [CrossRef]
- Rautiainen, P.; Hyttinen, M.; Ruokolainen, J.; Saarinen, P.; Timonen, J.; Pasanen, P. Indoor air-related symptoms and volatile organic compounds in materials and air in the hospital environment. Int. J. Environ. Health Res. 2019, 29, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath Volatile Organic Compounds (VOCs) as Biomarkers for the Diagnosis of Pathological Conditions: A Review. Biomed. J. 2023, 46, 100623. [Google Scholar] [CrossRef]
- Costello, B.L.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Maung, T.Z.; Bishop, J.E.; Holt, E.; Turner, A.M.; Pfang, C. Indoor Air Pollution and the Health of Vulnerable Groups: A Systematic Review Focused on Particulate Matter (PM), Volatile Organic Compounds (VOCs) and Their Effects on Children and People with Pre-Existing Lung Disease. Int. J. Environ. Res. Public Health 2022, 19, 8752. [Google Scholar] [CrossRef]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on Human Health. In Air Pollution and Control, 1st ed.; Sharma, N., Agarwal, A., Eastwood, P., Gupta, T., Eds.; Springer: Singapore, 2017; pp. 119–142. [Google Scholar]
- Garg, A.; Gupta, N.C. A comprehensive study on spatio-temporal distribution, health risk assessment and ozone formation potential of BTEX emissions in ambient air of Delhi, India. Sci. Total Environ. 2019, 659, 1090–1099. [Google Scholar] [CrossRef]
- Mokammel, A.; Rostami, R.; Niazi, S.; Asgari, A.; Fazlzadeh, M. BTEX levels in rural households: Heating system, building characteristic impacts and lifetime excess cancer risk assessment. Environ. Pollut. 2022, 298, 118845. [Google Scholar] [CrossRef]
- Alwis, K.U.; Blount, B.C.; Britt, A.S.; Patel, D.; Ashley, D.L. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal. Chim. Acta 2012, 750, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.; Langenhove, H.; Wittmann, G. Analysis of volatile organic compounds using gas chromatography. Trends Anal. Chem. 2002, 21, 637–646. [Google Scholar] [CrossRef]
- Costa, B.; Martinis, B. Analysis of urinary VOCs using mass spectrometric methods to diagnose cancer: A review. Clin. Mass Spectrom. 2020, 18, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.C.; Vassilenko, V.; Ribeiro, P.A. Ion Mobility Spectrometry Towards Environmental Volatile Organic Compounds Identification and Quantification: A Comparative Overview over Infrared Spectroscopy. Emission Contr. Sc. Technol. 2023, 9, 25–46. [Google Scholar] [CrossRef]
- Moura, P.C.; Vassilenko, V. Contemporary ion mobility spectrometry applications and future trends towards environmental, health and food research: A review. Int. J. Mass Spectrom. 2023, 486, 117012. [Google Scholar] [CrossRef]
- Moura, P.C.; Vassilenko, V. Long-term in situ air quality assessment in closed environments: A gas chromatography–ion mobility spectrometry applicability study. Eur. J. Mass Spectrom. 2023, 29, 231–239. [Google Scholar] [CrossRef]
- Moura, P.C.; Pivetta, T.P.; Vassilenko, V.; Ribeiro, P.A.; Raposo, M. Graphene Oxide Thin Films for Detection and Quantification of Industrially Relevant Alcohols and Acetic Acid. Sensors 2023, 23, 462. [Google Scholar] [CrossRef]
- Günzler, H.; Williams, A. Handbook of Analytical Techniques, 1st ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2001. [Google Scholar]
- Khatib, M.; Haick, H. Sensors for Volatile Organic Compounds. ACS Nano 2022, 16, 7080–7115. [Google Scholar] [CrossRef]
- Moura, P.C.; Ribeiro, P.A.; Raposo, M.; Vassilenko, V. The State of the Art on Graphene-Based Sensors for Human Health Monitoring through Breath Biomarkers. Sensors 2023, 23, 9271. [Google Scholar] [CrossRef]
- Tian, X.; Hu, Z.; Jia, C.; Wang, H.; Wei, X. A review of advanced gas sensor based on sputtering SnO2 thin film—Challenges and opportunities. J. Environ. Chem. Eng. 2023, 11, 111516. [Google Scholar] [CrossRef]
- Gupta, A.; Parida, P.K.; Pal, P. Functional Films for Gas Sensing Applications: A Review. In Sensors for Automotive and Aerospace Applications, 1st ed.; Bhattacharya, S., Agarwal, A., Prakash, O., Singh, S., Eds.; Springer: Singapore, 2018; pp. 7–37. [Google Scholar]
- Gardon, M.; Guilemany, J.M. A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors. J. Mater. Sci. Mater. Electron. 2013, 24, 1410–1421. [Google Scholar] [CrossRef]
- Vidyarthi, V.S.; Hofmann, M.; Savan, A.; Sliozberg, K.; König, D.; Beranek, R.; Schuhmann, W.; Ludwig, A. Enhanced photoelectrochemical properties of WO3 thin films fabricated by reactive magnetron sputtering. Int. J. Hydrogen Energy 2011, 36, 4724–4731. [Google Scholar] [CrossRef]
- Wang, Y.; Rahman, K.H.; Wu, C.C.; Chen, K. A Review on the Pathways of the Improved Structural Characteristics and Photocatalytic Performance of Titanium Dioxide (TiO2) Thin Films Fabricated by the Magnetron-Sputtering Technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Reddy, A.M.; Reddy, A.S.; Lee, K.S.; Reddy, P.S. Growth and characterization of NiO thin films prepared by dc reactive magnetron sputtering. Solid State Sci. 2011, 13, 314–320. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, X.; Chen, H.; Zhang, T.; Debela, T.T. The growth mode of α-Fe2O3 thin films by DC magnetron sputtering. Vacuum 2021, 194, 110625. [Google Scholar] [CrossRef]
- Cho, S. Optical and electrical properties of CuO thin films deposited at several growth temperatures by reactive RF magnetron sputtering. Met. Mater. Int. 2013, 19, 1327–1331. [Google Scholar] [CrossRef]
- Cho, S. Effects of rapid thermal annealing on the properties of In2O3 thin films grown on glass substrate by rf reactive magnetron sputtering. Microelectron. Eng. 2012, 89, 84–88. [Google Scholar] [CrossRef]
- Leng, D.; Wu, L.; Jiang, H.; Zhao, Y.; Zhang, J.; Li, W.; Feng, L. Preparation and Properties of SnO2 Film Deposited by Magnetron Sputtering. Int. J. Photoenergy 2012, 2012, 235971. [Google Scholar] [CrossRef]
- Aissani, L.; Alhussein, A.; Zia, A.W.; Mamba, G.; Rtimi, S. Magnetron Sputtering of Transition Metal Nitride Thin Films for Environmental Remediation. Coatings 2022, 12, 1746. [Google Scholar] [CrossRef]
- Rydosz, A.; Brudnik, A.; Staszek, K. Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range. Materials 2019, 12, 877. [Google Scholar] [CrossRef]
- Omar, S.; Kulkarni, S.N. Effect of simultaneous substitution of Sr and Ca in LaMnO3 thin-film electrode prepared via in situ sol–gel process. J. Mater. Sci. Mater. Electron. 2024, 35, 1542. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Yao, J.; Chen, H.; Yang, G. Pulsed-Laser Deposition of Ge-Doped BiTe Nanofilms and Their Application in Room-Temperature Long-Wave Infrared Photodetection. Adv. Opt. Mater. 2024, 2401937. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.; Jung, M.; Kim, Y.; Kim, S.; Gao, H.; Leer, B.; Jeong, S.; Jeong, H.Y.; Kim, Y. Artifact-free sample preparation of metal thin films using Xe plasma-focused ion beam milling for atomic resolution and in situ biasing analyses. Mater. Charact. 2024, 216, 114260. [Google Scholar] [CrossRef]
- Shin, W.; Nishibori, M.; Itoh, T.; Izu, N.; Matsubara, I. Enhancing the Responsiveness of Thermoelectric Gas Sensors with Boron-Doped and Thermally Annealed SiGe Thin Films via Low-Pressure Chemical Vapor Deposition. Sensors 2024, 24, 3058. [Google Scholar] [CrossRef]
- Siopa, D.; Sério, S.; Jorge, M.E.M.; Viana, A.S.; Gomes, A. ZnO Seed Layers Prepared by DC Reactive Magnetron Sputtering to be Applied as Electrodeposition Substrates. J. Electrochem. Soc. 2016, 163, H697. [Google Scholar] [CrossRef]
- Barrocas, B.; Sério, S.; Rovisco, A.; Nunes, Y.; Sá, A.I.; Pereira, M.I.S.; Jorge, M.E.M. Characterization and electrochemical behaviour of nanostructured calcium samarium manganite electrodes fabricated by RF-Magnetron Sputtering. Electrochim. Acta 2014, 137, 99–107. [Google Scholar] [CrossRef]
- Eleutério, T.; Sério, S.; Vasconcelos, H.C. Growth of Nanostructured TiO2 Thin Films onto Lignocellulosic Fibers through Reactive DC Magnetron Sputtering: A XRD and SEM Study. Coatings 2023, 13, 922. [Google Scholar] [CrossRef]
- Barrocas, B.; Sério, S.; Jorge, M.E.M. Hierarchically Grown CaMn3O6 Nanorods by RF Magnetron Sputtering for Enhanced Visible-Light-Driven Photocatalysis. J. Phys. Chem. 2014, 118, 24127–24135. [Google Scholar] [CrossRef]
- Carreira, D.; Ribeiro, P.A.; Raposo, M.; Sério, S. Engineering of TiO2 or ZnO—Graphene Oxide Nanoheterojunctions for Hybrid Solar Cells Devices. Photonics 2021, 8, 75. [Google Scholar] [CrossRef]
- Magro, C.; Sardinha, M.; Ribeiro, P.A.; Raposo, M.; Sério, S. Magnetron Sputtering Thin Films as Tool to Detect Triclosan in Infant Formula Powder: Electronic Tongue Approach. Coatings 2021, 11, 336. [Google Scholar] [CrossRef]
- Silva, D.; Monteiro, C.S.; Silva, S.O.; Frazão, O.; Pinto, J.V.; Raposo, M.; Ribeiro, P.A.; Sério, S. Sputtering Deposition of TiO2 Thin Film Coatings for Fiber Optic Sensors. Photonics 2022, 9, 342. [Google Scholar] [CrossRef]
- Gupta, J.; Shaik, H.; Kumar, K.N. A review on the prominence of porosity in tungsten oxide thin films for electrochromism. Ionics 2021, 27, 2307–2334. [Google Scholar] [CrossRef]
- Sproul, W.D.; Christie, D.J.; Carter, D.C. Control of reactive sputtering processes. Thin Solid Films 2005, 491, 1–17. [Google Scholar] [CrossRef]
- Berg, S.; Nyberg, T. Fundamental understanding and modeling of reactive sputtering processes. Thin Solid Films 2005, 476, 215–230. [Google Scholar] [CrossRef]
- Anders, A. Tutorial: Reactive high power impulse magnetron sputtering (R-HiPIMS). J. Appl. Phys. 2017, 121, 171101. [Google Scholar] [CrossRef]
- Anders, A. A review comparing cathodic arcs and high-power impulse magnetron sputtering (HiPIMS). Surf. Coat. Technol. 2014, 257, 308–325. [Google Scholar] [CrossRef]
- Magro, C.; Gonçalves, O.C.; Morais, M.; Ribeiro, P.A.; Sério, S.; Vieira, P.; Raposo, M. Volatile Organic Compound Monitoring during Extreme Wildfires: Assessing the Potential of Sensors Based on LbL and Sputtering Films. Sensors 2022, 22, 6677. [Google Scholar] [CrossRef]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath biomarkers in Non-Carcinogenic diseases. Clin. Chim. Acta 2023, 552, 117692. [Google Scholar] [CrossRef]
- Smolinska, A.; Klaassen, E.M.M.; Dallinga, J.W.; Kant, K.D.G.; Jobsis, Q.; Moonen, E.J.C.; Schayck, O.C.P.; Dompeling, E.; Schooten, F.J. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PLoS ONE 2014, 9, e95668. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Happ, J.; Schubbert, J.K.; Staude, H.; Fischer, D.C.; Miekisch, W. Exhaled volatile substances mirror clinical conditions in pediatric chronic kidney disease. PLoS ONE 2017, 12, e0178745. [Google Scholar] [CrossRef]
- Hanouneh, I.A.; Zein, N.N.; Cikach, F.; Dababneh, L.; Grove, D.; Alkhouri, N.; Lopez, R.; Dweik, R.A. The Breathprints in Patients with Liver Disease Identify Novel Breath Biomarkers in Alcoholic Hepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Neerincx, A.H.; Geurts, B.P.; Loon, J.; Tiemes, V.; Jansen, J.J.; Harren, F.; Kluijtmans, L.; Merkus, P.; Cristescu, S.M.; Buydens, L.; et al. Detection of Staphylococcus aureus in cystic fibrosis patients using breath VOC profiles. J. Breath Res. 2016, 10, 046014. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Schmid, A.; Amann, A.; Pratsinis, S.E. Correlations between blood glucose and breath components from portable gas sensors and PTR-TOF-MS. J. Breath Res. 2013, 7, 037110. [Google Scholar] [CrossRef] [PubMed]
- Berna, A.Z.; McCarthy, J.S.; Wang, R.X.; Saliba, K.J.; Bravo, F.G.; Cassells, J.; Padovan, B.; Trowell, S. Analysis of breath specimens for biomarkers of plasmodium falciparum infection. J. Infect. Dis. 2015, 212, 1120–1128. [Google Scholar] [CrossRef]
- Aoki, T.; Nagaoka, T.; Kobayashi, N.; Kurahashi, M.; Tsuji, C.; Takiguchi, H.; Tomomatsu, K.; Oguma, T.; Kobayashi, N.; Magatani, K.; et al. Prospective analyses of volatile organic compounds in obstructive sleep apnea patients. Toxicol. Sci. 2017, 156, 362–374. [Google Scholar] [CrossRef]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2015, 138, 229–236. [Google Scholar] [CrossRef]
- Jung, Y.J.; Seo, H.S.; Kim, J.H.; Song, K.Y.; Park, C.H.; Lee, H.H. Advanced Diagnostic Technology of Volatile Organic Compounds Real Time Analysis from Exhaled Breath of Gastric Cancer Patients Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Front. Oncol. 2021, 11, 560591. [Google Scholar] [CrossRef]
- Handa, H.; Usuba, A.; Maddula, S.; Baumbach, J.I.; Mineshita, M.; Miyazawa, T. Exhaled breath analysis for lung cancer detection using ion mobility spectrometry. PLoS ONE 2014, 9, e114555. [Google Scholar] [CrossRef]
- Shao, S.; Wu, H.; Wang, S.; Hong, Q.; Koehn, R.; Wu, T.; Rao, W.F. Highly crystalline and ordered nanoporous SnO2 thin films with enhanced acetone sensing property at room temperature. J. Mater. Chem. C 2015, 3, 10819–10829. [Google Scholar] [CrossRef]
- Johanson, G. Acetone. In Patty’s Toxicology, 7th ed.; Paustenbach, D.J., Farland, W.H., Klaunig, J., Levy, L., Greim, H., Eds.; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar]
- Gao, W.; Li, Z. ZnO thin films produced by magnetron sputtering. Ceram. Int. 2004, 30, 1155–1159. [Google Scholar] [CrossRef]
- Sachdeva, S.; Agarwal, A.; Agarwal, R. Tungsten Oxide Thin Film Characterizations for Acetone Gas Detection. J. Metrol. Soc. I. 2018, 33, 57–62. [Google Scholar] [CrossRef]
- Sucharitakul, W.; Sukee, A.; Leuasoongnoen, P.; Horprathum, M.; Lertvanithphol, T.; Janphuang, P.; Mitsomwang, P.; Sindhupakorn, B. Fabrication of an acetone gas sensor based on Si-doped WO3 nanorods prepared by reactive magnetron co-sputtering OAD technique. Mater. Res. Express 2021, 8, 125702. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Al-Muhaish, N.A.; Wajih, Y.A.; Alam, M.W.; Yamani, Z.H. Surface composite and morphology tuning of tungsten oxide thin films for acetone gas detection. Chem. Phys. Lett. 2021, 776, 138659. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdullah, M.J.; Aziz, A.A. Performance of Cr-doped ZnO for acetone sensing. Appl. Surf. Sci. 2013, 270, 480–485. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdullah, M.J.; Aziz, A.A.; Ahmad, H.; Low, L.Y. ZnO thin films for VOC sensing applications. Vacuum 2010, 85, 101–1406. [Google Scholar] [CrossRef]
- Kim, S.H.; Shim, G.I.; Choi, S.Y. Fabrication of Nb-doped ZnO nanowall structure by RF magnetron sputter for enhanced gas-sensing properties. J. Alloys Compd. 2017, 698, 77–86. [Google Scholar] [CrossRef]
- Dyndal, K.; Zarzycki, A.; Andrysiewicz, W.; Grochala, D.; Marszalek, K.; Rydosz, A. CuO-Ga2O3 Thin Films as a Gas-Sensitive Material for Acetone Detection. Sensors 2020, 20, 3142. [Google Scholar] [CrossRef]
- Fedoruk, M.J.; Bronstein, R.; Kerger, B.D. Ammonia exposure and hazard assessment for selected household cleaning product uses. J. Expo. Sci. Environ. Epidemiol. 2005, 15, 534–544. [Google Scholar] [CrossRef]
- Sundblad, B.M.; Larsson, B.M.; Acevedo, F.; Ernstgard, L.; Johanson, G.; Larsson, K.; Palmberg, L. Acute respiratory effects of exposure to ammonia on healthy persons. Scand. J. Work Environ. Health 2004, 30, 313–321. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.; Ling, K.; Wurie, S.; O’Connell, N.; Nwokolo, C.U.; Bardhan, K.D.; Skinner, J.; Savage, R.S.; Covington, J.A. Breathomics—exhaled volatile organic compounds analysis to detect hepatic encephalopathy: A pilot study. J. Breath Res. 2016, 10, 016012. [Google Scholar] [CrossRef]
- Vassilenko, V.; Moura, P.C.; Raposo, M. Diagnosis of Carcinogenic Pathologies through Breath Biomarkers: Present and Future Trends. Biomedicines 2023, 11, 3029. [Google Scholar] [CrossRef] [PubMed]
- Boyadzhiev, S.; Georgieva, V.; Rassovska, M. Characterization of reactive sputtered TiO2 thin films for gas sensor applications. J. Phys. Conf. Ser. 2010, 253, 012040. [Google Scholar] [CrossRef]
- Yordanov, R.; Boyadjiev, S.; Georgieva, V. Characterization of RF and DC Magnetron Reactive Sputtered TiO2 Thin Films for Gas Sensors. Digest J. Nanomater. Biostruct. 2014, 9, 467–474. [Google Scholar]
- Vinoth, E.; Gopalakrishnan, N. Ammonia sensing characteristics of Yttrium doped ZnO thin films by RF magnetron sputtering. Mater. Res. Exp. 2018, 5, 066413. [Google Scholar] [CrossRef]
- Fairose, S.; Ernest, S.; Daniel, S. Effect of Oxygen Sputter Pressure on the Structural, Morphological and Optical Properties of ZnO Thin Films for Gas Sensing Application. Sens. Imaging 2018, 19, 1. [Google Scholar] [CrossRef]
- Dhivya, P.; Prasad, A.K.; Sridharan, M. Magnetron sputtered nano structured cadmium oxide films for ammonia sensing. J. Solid State Chem. 2014, 214, 24–29. [Google Scholar] [CrossRef]
- Hien, V.X.; Lee, J.H.; Kim, J.J.; Heo, Y.W. Structure and NH3 sensing properties of SnO thin film deposited by RF magnetron sputtering. Sens. Actuators B Chem. 2014, 194, 134–141. [Google Scholar] [CrossRef]
- Yordanov, R.; Boyadjiev, S.; Georgieva, V.; Vergov, L. Characterization of thin MoO3 films formed by RF and DC-magnetron reactive sputtering for gas sensor applications. J. Phys. Conf. Ser. 2014, 514, 012040. [Google Scholar] [CrossRef]
- Ponmudi, S.; Sivakumar, R.; Sanjeeviraja, C.; Gopalakrishnan, C.; Jeyadheepan, K. Al2O3:Cr2O3:CuO (1:1:1) thin film prepared by radio frequency magnetron sputtering technique: A promising material for high sensitive room temperature ammonia sensor. Mater. Res. Exp. 2019, 6, 066422. [Google Scholar] [CrossRef]
- Dryahina, K.; Sovová, K.; Nemec, A.; Spanel, P. Differentiation of pulmonary bacterial pathogens in cystic fibrosis by volatile metabolites emitted by their in vitro cultures: Pseudomonas aeruginosa Staphylococcus aureus, Stenotrophomonas maltophilia and the Burkholderia cepacia complex. J. Breath Res. 2016, 10, 037102. [Google Scholar] [CrossRef]
- Segal, D.; Bale, A.S.; Phillips, L.J.; Sasso, A.; Schlosser, P.M.; Starkey, C.; Makris, S.L. Issues in assessing the health risks of n-butanol. J. App. Toxicol. 2020, 40, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Dantoft, T.M.; Skovbjerg, S.; Andersson, L.; Claeson, A.S.; Lind, N.; Nordin, S.; Brix, S. Inflammatory Mediator Profiling of n-butanol Exposed Upper Airways in Individuals with Multiple Chemical Sensitivity. PLoS ONE 2015, 10, e0143534. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, T.; Guo, L.; Wang, H.; Wang, X.; Zeng, H.; Feng, Y.; Zhao, W.; Wang, Y.; Liu, X.; et al. Chemiresistive n-butanol gas sensors based on Co3O4@ZnO hollow-sphere-array thin films prepared by template-assisted magnetron sputtering. Sens. Actuators B Chem. 2024, 413, 135862. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, B.; Zhao, Z.; Zhang, Z.; Zhang, S.; Bala, H.; Zhang, Z. Enhanced n-butanol sensing properties of Au modified TiO2 nanorod arrays: A combined experimental and first-principle study. App. Surf. Sci. 2023, 641, 158458. [Google Scholar] [CrossRef]
- Ababii, N.; Hoppe, M.; Shree, S.; Vahl, A.; Ulfa, M.; Pauporté, T.; Viana, B.; Cretu, V.; Magariu, N.; Postica, V.; et al. Effect of noble metal functionalization and film thickness on sensing properties of sprayed TiO2 ultra-thin films. Sens. Actuators A Phys. 2019, 293, 242–258. [Google Scholar] [CrossRef]
- Wongrat, E.; Ta-om, T.; Khamprakaysit, S.; Chanlek, N.; Choopun, S. Effect of Cu or Ni addition to ZnO nanostructures on their n-butanol sensing performance. Thin Solid Films 2023, 774, 139839. [Google Scholar] [CrossRef]
- Martínez, E.E.G.; Matías, I.R.; Melendi-Espina, S.; Hernáez, M.; Zamarreno, C.R. Lossy mode resonance based 1-butanol sensor in the mid-infrared region. Sens. Actuators B Chem. 2023, 388, 133845. [Google Scholar] [CrossRef]
- Zhu, Q.; Meisinger, J.; Emanuele, N.V.; Emanuele, M.A.; LaPaglia, N.; Thiel, D.H. Ethanol Exposure Enhances Apoptosis Within the Testes. Alcohol. Clin. Exp. Res. 2000, 24, 1550–1556. [Google Scholar] [CrossRef]
- Obernier, J.A.; Bouldin, T.W.; Crews, F.T. Binge Ethanol Exposure in Adult Rats Causes Necrotic Cell Death. Alcohol. Clin. Exp. Res. 2002, 26, 547–557. [Google Scholar] [CrossRef]
- Bos, J.D.L.; Meinardi, S.; Blake, D.; Whiteson, K. Bacteria in the airways of patients with cystic fibrosis are genetically capable of producing VOCs in breath. J. Breath Res. 2016, 10, 047103. [Google Scholar] [CrossRef]
- Barash, O.; Zhang, W.; Halpern, J.M.; Hua, Q.L.; Pan, Y.Y.; Kayal, H.; Khoury, K.; Liu, H.; Davies, M.P.A.; Haick, H. Differentiation between genetic mutations of breast cancer by breath volatolomics. Oncotarget 2015, 6, 44864–44876. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Castro, M.; Feller, J.F. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 2013, 1, 4563–4575. [Google Scholar] [CrossRef] [PubMed]
- García, R.A.; Morales, V.; Martín, S.; Vilches, E.; Toledano, A. Volatile Organic Compounds Analysis in Breath Air in Healthy Volunteers and Patients Suffering Epidermoid Laryngeal Carcinomas. Chromatographia 2014, 77, 501–509. [Google Scholar] [CrossRef]
- Chen, J.; Yan, X.; Liu, W.; Xue, Q. The ethanol sensing property of magnetron sputtered ZnO thin films modified by Ag ion implantation. Sens. Actuators B Chem. 2011, 160, 1499–1503. [Google Scholar] [CrossRef]
- Tamvakos, A.; Calestani, D.; Tamvakos, D.; Mosca, R.; Pullini, D.; Pruna, A. Effect of grain-size on the ethanol vapor sensing properties of room-temperature sputtered ZnO thin films. Microchim. Acta 2015, 182, 1991–1999. [Google Scholar] [CrossRef]
- Hassan, M.M.; Khan, W.; Naqvi, A.H.; Mishra, P.; Islam, S.S. Fe dopants enhancing ethanol sensitivity of ZnO thin film deposited by RF magnetron sputtering. J. Mater. Sci. 2014, 49, 6248–6256. [Google Scholar] [CrossRef]
- Khojier, K.; Goudarzi, S.; Firouzi, M. Enhanced, selective, and room temperature detection of ethanol vapor by RF-sputtered TiO2 thin films. App. Phys. A 2023, 129, 835. [Google Scholar] [CrossRef]
- Yan, H.; Tian, X.; Ma, F.; Sun, J. CuO nanoparticles fabricated by direct thermo-oxidation of sputtered Cu film for VOCs quantification. Sens. Actuators B Chem. 2015, 221, 599–605. [Google Scholar] [CrossRef]
- Pandya, H.J.; Chandra, S.; Vyas, A.L. Fabrication and Characterization of Ethanol Sensor Based on RF Sputtered ITO Films. Sens. Transducers 2011, 10, 141–150. [Google Scholar]
- Collins, J.J.; Lineker, G.A. A review and meta-analysis of formaldehyde exposure and leukemia. Regul. Toxicol. Pharmacol. 2004, 40, 81–91. [Google Scholar] [CrossRef]
- Zhang, L.; Steinmaus, C.; Eastmond, D.A.; Xin, X.K.; Smith, M.T. Formaldehyde exposure and leukemia: A new meta-analysis and potential mechanisms. Mutat. Res. Rev. Mutat. Res. 2009, 681, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jahan, S.A.; Lee, J.T. Exposure to Formaldehyde and Its Potential Human Health Hazards. J. Environ. Sci. Health C 2011, 29, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, Y.; Tian, X.; Wang, H.; Wang, J.; Zhang, Q. Magnetron co-sputtering optimized aluminum-doped zinc oxide (AZO) film for high-response formaldehyde sensing. J. Alloys Compd. 2021, 880, 160510. [Google Scholar] [CrossRef]
- Doroftei, C. Formaldehyde sensitive Zn-doped LPFO thin films obtained by rf sputtering. Sens. Actuators B Chem. 2016, 231, 793–799. [Google Scholar] [CrossRef]
- Chen, D.; Chen, R.; Yuan, Y.J. Investigation on Formaldehyde SAW Sensor with ZnO Film Prepared through Radio Frequency Magnetron Sputtering. Russ. J. Phys. Chem. A 2022, 96, S197–S202. [Google Scholar] [CrossRef]
- Castro-Hurtado, I.; Herrán, J.; Mandaya, G.G.; Castano, E. Studies of influence of structural properties and thickness of NiO thin films on formaldehyde detection. Thin Solid Films 2011, 520, 947–952. [Google Scholar] [CrossRef]
- Castro-Hurtado, I.; Malagù, C.; Morandi, S.; Pérez, N.; Mandayo, G.G.; Castaño, E. Properties of NiO sputtered thin films and modeling of their sensing mechanism under formaldehyde atmospheres. Acta Mater. 2013, 61, 1146–1153. [Google Scholar] [CrossRef]
- Prajesh, R.; Goyal, V.; Nahid, M.; Saini, V.; Singh, A.K.; Sharma, A.K.; Bhargava, J.; Agarwal, A. Nickel oxide (NiO) thin film optimization by reactive sputtering for highly sensitive formaldehyde sensing. Sens. Actuators B Chem. 2020, 318, 128166. [Google Scholar] [CrossRef]
- Xu, Y.; Lee, H.; Hu, Y.; Huang, J.; Kim, S.; Yun, M. Detection and Identification of Breast Cancer Volatile Organic Compounds Biomarkers Using Highly-Sensitive Single Nanowire Array on a Chip. J. Biomed. Nanotechnol. 2013, 9, 1164–1172. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; Ramírez-García, S.; Ilizaliturri-Hernández, C.; Gómez-Gómez, A.; Van-Brussel, E.; Díaz-Barriga, F.; Medellín-Garibay, S.; Flores-Ramírez, R. Ultrafast gas chromatography coupled to electronic nose to identify volatile biomarkers in exhaled breath from chronic obstructive pulmonary disease patients: A pilot study. Biomed. Chromatogr. 2019, 33, e4684. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Liu, Y.; Cheng, S.; Duan, Y. Exhaled isopropanol: New potential biomarker in diabetic breathomics and its metabolic correlations with acetone. RSC Adv. 2017, 7, 17480. [Google Scholar] [CrossRef]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography–mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tasar, R.; Wiegand, C.; Elsner, P. How irritant are n-propanol and isopropanol?–A systematic review. Contact Dermat. 2021, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, R.J.; Mason, R.W.; Beasley, D.M.G.; Vale, J.A.; Schep, J. Isopropanol poisoning. Clin. Toxicol. 2014, 52, 470–478. [Google Scholar] [CrossRef]
- Al-Salman, H.S.; Abdullah, M.J. Preparation of ZnO nanostructures by RF-magnetron sputtering on thermally oxidized porous silicon substrate for VOC sensing application. Measurement 2015, 59, 248–257. [Google Scholar] [CrossRef]
- Wang, G.; Wu, P.; Guo, L.; Wang, W.; Liu, W.; Wang, Y.; Chen, T.; Wang, H.; Xu, Y.; Yang, Y. Preparation of Au@ZnO Nanofilms by Combining Magnetron Sputtering and Post-Annealing for Selective Detection of Isopropanol. Chemosensors 2022, 10, 211. [Google Scholar] [CrossRef]
- Gao, W.; Chang, X.; Zhu, X.; Li, J.; Jiang, Y.; Wang, D.; Yang, C.; Sun, S. Al-doped ZnO/WO3 heterostructure films prepared by magnetron sputtering for isopropanol sensors. Rare Met. 2024, 43, 247–256. [Google Scholar] [CrossRef]
- Karthikeyan, P.S.; Dhivya, P.; Raj, P.D.; Sridharan, M. V2O5 thin film for 2-Propanol vapor sensing. Mater. Today Proc. 2016, 3, 1510–1516. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Z.; Wang, J.; Ding, C.; Sun, C.; Liu, P.; Xu, X.; Liu, Y.; Chen, B.; Gu, B. Characterization of the lung microbiome and exploration of potential bacterial biomarkers for lung cancer. Transl. Lung Cancer Res. 2020, 9, 693–704. [Google Scholar] [CrossRef]
- Prasad, S.; Zhao, L.; Gomes, J. Methane and Natural Gas Exposure Limits. Epidemiology 2011, 22, S251. [Google Scholar] [CrossRef]
- Duncan, I.J. Does methane pose significant health and public safety hazards?—A review. Environ. Geosci. 2015, 22, 85–96. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Li, N.; Li, W. Magnetron sputtered Au-decorated vanadium oxides composite thin films for methane-sensing properties at room temperature. J. Alloys Compd. 2016, 671, 283–290. [Google Scholar] [CrossRef]
- Comert, B.; Akin, N.; Donmez, M.; Saglam, S.; Ozcelik, S. Titanium Dioxide Thin Films as Methane Gas Sensors. IEEE Sens. J. 2016, 16, 8890–8896. [Google Scholar] [CrossRef]
- Stankova, M.; Vilanova, X.; Llobet, E.; Calderer, J.; Bittencourt, C.; Pireaux, J.J.; Correig, X. Influence of the annealing and operating temperatures on the gas-sensing properties of rf sputtered WO3 thin-film sensors. Sens. Actuators B 2005, 105, 271–277. [Google Scholar] [CrossRef]
- Dhivya, P.; Prasad, A.K.; Sridharan, M. Effect of sputtering power on the methane sensing properties of nanostructured cadmium oxide films. J. Alloys Compd. 2015, 620, 109–115. [Google Scholar] [CrossRef]
- Arshad, A.Z.; Munajat, Y.; Ibrahim, R.; Hamdan, S.; Mahmood, N. Volatolomics analysis using FTIR spectroscopy for breast cancer identification in vitro. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malasia, 8–10 December 2014. [Google Scholar]
- Ma, H.; Li, X.; Chen, J.; Wang, H.; Cheng, T.; Chen, K.; Xu, S. Analysis of human breath samples of lung cancer patients and healthy controls with solid-phase microextraction (SPME) and flow-modulated comprehensive two-dimensional gas chromatography (GC × GC). Anal. Methods 2014, 6, 6841–6849. [Google Scholar] [CrossRef]
- Nekoukar, Z.; Zakariaei, Z.; Taghizadeh, F.; Musavi, F.; Banimostafavi, E.S.; Sharifpour, A.; Ghuchi, N.E.; Fakhar, M.; Tabaripour, R.; Safanavaei, S. Methanol poisoning as a new world challenge: A review. Ann. Med. Surg. 2021, 66, 102445. [Google Scholar] [CrossRef]
- Moon, C. Estimations of the lethal and exposure doses for representative methanol symptoms in humans. Ann. Occup. Environ. Med. 2017, 29, 44. [Google Scholar] [CrossRef]
- Parmar, M.; Rajanna, K. Copper (II) oxide thin film for methanol and ethanol sensing. Int. J. Smart Sens. Intell. Syst. 2011, 4, 710–725. [Google Scholar] [CrossRef]
- Vinoth, E.; Gowrishankar, S.; Gopalakrishnan, N. RF magnetron sputtered Cd doped ZnO thin films for gas-sensing applications. Mater. Manuf. Process. 2017, 32, 377–382. [Google Scholar] [CrossRef]
- Vinoth, E.; Gowrishankar, S.; Gopalakrishnan, N. Effect of Mg doping in the gas-sensing performance of RF-sputtered ZnO thin films. App. Phys. A 2018, 124, 433. [Google Scholar] [CrossRef]
- Young, S.; Chu, Y. Platinum Nanoparticle-Decorated ZnO Nanorods Improved the Performance of Methanol Gas Sensor. J. Electrochem. Soc. 2020, 167, 147508. [Google Scholar] [CrossRef]
- Ligor, T.; Pater, L.; Buszewski, B. Application of an artificial neural network model for selection of potential lung cancer biomarkers. J. Breath Res. 2015, 9, 027106. [Google Scholar] [CrossRef] [PubMed]
- Kashtan, Y.S.; Nicholson, M.; Finnegan, C.; Ouyang, Z.; Lebel, E.D.; Michanowicz, D.R.; Shonkoff, S.; Jackson, R.B. Gas and Propane Combustion from Stoves Emits Benzene and Increases Indoor Air Pollution. Environ. Sci. Technol. 2023, 57, 9653–9663. [Google Scholar] [CrossRef]
- Thurman, J.T.; Leavitt, R.; Buchanan, J.T.; Shreffler, J.; Huecker, M.R.; Early, T. Carbon Monoxide Levels Produced by Propane/Isobutane Canister Stoves inside a Tent. Wilderness Environ. Med. 2023, 34, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A.; Szkudlarek, A. Gas-Sensing Performance of M-Doped CuO-Based Thin Films Working at Different Temperatures upon Exposure to Propane. Sensors 2015, 15, 20069–20085. [Google Scholar] [CrossRef] [PubMed]
- Sertel, B.C.; Efkere, H.I.; Ozcelik, S. Gas Sensing Properties of Cr Doped TiO2 Films Against Propane. IEEE Sensors J. 2020, 20, 13436–13443. [Google Scholar] [CrossRef]
- Yu, J.; Pan, Y.; Wang, C.; Lai, Z. ZIF-8 membranes with improved reproducibility fabricated from sputter-coated ZnO/alumina supports. Chem. Eng. Sci. 2016, 141, 119–124. [Google Scholar] [CrossRef]
- Regmi, G.; Rohini, M.; Reyes-Figueroa, P.; Maldonado, A.; Olvera, M.L.; Velumani, S. Deposition and characterization of ultrathin intrinsic zinc oxide (i-ZnO) films by radio frequency (RF) sputtering for propane gas sensing application. J. Mater. Sci. Mater. Electron. 2018, 29, 15682–15692. [Google Scholar] [CrossRef]
- Kim, S.; Park, E.; Song, S.; Lee, C.; Kwon, J.; Park, E.Y.; Kim, B. Toluene concentrations in the blood and risk of thyroid cancer among residents living near national industrial complexes in South Korea: A population-based cohort study. Environ. Int. 2021, 146, 106304. [Google Scholar] [CrossRef]
- Win-Shwe, T.T.; Fujimaki, H. Neurotoxicity of toluene. Toxicol. Lett. 2010, 198, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Saidi, T.; Zaim, O.; Moufid, M.; Bari, N.E.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography-mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B Chem. 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Phillips, C.; Parthaláin, N.M.; Syed, Y.; Deganello, D.; Claypole, T.; Lewis, K. Short-term intra-subject variation in exhaled volatile organic compounds (VOCs) in COPD patients and healthy controls and its effect on disease classification. Metabolites 2014, 4, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.M.; Wanjiku, C.; Stanczyk, N.M.; Pulido, H.; Sims, J.W.; Betz, H.; Read, A.F.; Torto, B.; Mescher, M.C. Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans. Proc. Natl. Acad. Sci. USA 2018, 115, 5780–5785. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, C.; Zou, X.; Lu, Y.; Shen, C.; Ding, X.; Wang, H.; Jiang, H.; Chu, Y. Exhaled breath online measurement for cervical cancer patients and healthy subjects by proton transfer reaction mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5603–5612. [Google Scholar] [CrossRef]
- Gregis, G.; Sanchez, J.B.; Bezverkhyy, I.; Weber, G.; Berger, F.; Fierro, V.; Bellat, J.P.; Celzard, A. Detection and quantification of lung cancer biomarkers by a micro-analytical device using a single metal oxide-based gas sensor. Sens. Actuators B Chem. 2018, 255, 391–400. [Google Scholar] [CrossRef]
- Prakasha, B.S.; Shukla, G.; Subramanian, A. Discriminative analysis of volatile organic compounds using machine-learning assisted Au loaded ZnO and TiO2-based thin film sensors. Sens. Actuators A Phys. 2024, 373, 115385. [Google Scholar] [CrossRef]
- Aluri, G.S.; Motayed, A.; Davydov, A.V.; Oleshko, V.P.; Bertness, K.A.; Sanford, N.A.; Rao, M.V. Highly selective GaN-nanowire/TiO2-nanocluster hybrid sensors for detection of benzene and related environment pollutants. Nanotechnology 2011, 22, 295503. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Park, Y.; Kim, J.; Mirzaei, A.; Kim, A.W.; Kim, S.S. Toluene- and benzene-selective gas sensors based on Pt- and Pd-functionalized ZnO nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 294, 78–88. [Google Scholar] [CrossRef]
- Kim, J.H.; Abideen, Z.U.; Zheng, Y.; Kim, S.S. Improvement of Toluene-Sensing Performance of SnO2 Nanofibers by Pt Functionalization. Sensors 2016, 16, 1857. [Google Scholar] [CrossRef]
- Mohajir, A.E.; Yazdi, M.A.; Krystianiak, A.; Heintz, O.; Martin, N.; Berger, F.; Sanchez, J.B. Nanostructuring of SnO2 Thin Films by Associating Glancing Angle Deposition and Sputtering Pressure for Gas Sensing Applications. Chemosensors 2022, 2022, 426. [Google Scholar] [CrossRef]
- Delikhoon, M.; Fazlzadeh, M.; Sorooshian, A.; Baghani, A.N.; Golaki, M.; Ashournejad, Q.; Barkhordari, A. Characteristics and health effects of formaldehyde and acetaldehyde in an urban area in Iran. Environ. Pollut. 2018, 242, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Naddafi, K.; Nabizadeh, R.; Rostami, R.; Ghaffari, H.R.; Fazlzadeh, M. Formaldehyde and acetaldehyde in the indoor air of waterpipe cafés: Measuring exposures and assessing health effects. Build. Environ. 2019, 165, 106392. [Google Scholar] [CrossRef]
- Salaspuro, M. Acetaldehyde and gastric cancer. J. Dig. Dis. 2011, 12, 51–59. [Google Scholar] [CrossRef]
- Maiti, K.S.; Fill, E.; Strittmatter, F.; Volz, Y.; Sroka, R.; Apolonski, A. Towards reliable diagnostics of prostate cancer via breath. Sci. Rep. 2021, 11, 18381. [Google Scholar] [CrossRef]
- Presmanes, L.; Thimont, Y.; Chapelle, A.; Blanc, F.; Talhi, C.; Bonningue, C.; Barnabé, A.; Menini, P.; Tailhades, P. Highly Sensitive Sputtered ZnO:Ga Thin Films Integrated by a Simple Stencil Mask Process on Microsensor Platforms for Sub-ppm Acetaldehyde Detection. Sensors 2017, 17, 1055. [Google Scholar] [CrossRef]
- Cindemir, U.; Lansåker, P.C.; Österlund, L.; Niklasson, G.A.; Granqvist, C.G. Sputter-Deposited Indium–Tin Oxide Thin Films for Acetaldehyde Gas Sensing. Coatings 2016, 6, 19. [Google Scholar] [CrossRef]
- Gunasekaran, E.; Ezhilan, M.; Mani, G.K.; Shankar, P.; Kulandaisamy, A.J.; Balaguru, J.B.; Babu, K.J. Fluorine doped ZnO thin film as acetaldehyde sensor. Semicond. Sci. Technol. 2018, 33, 095005. [Google Scholar] [CrossRef]
- Radha, K.; Selvaraj, B.; Srinivasan, P.; Krishnakumar, A.; Rayappan, J.; Babu, K.J. Room-temperature acetaldehyde-sensing properties of SILAR-deposited ZnO thin films: Role of tungsten doping. J. Mater. Sci. Mater. Electron. 2021, 32, 17700–17715. [Google Scholar] [CrossRef]
- Ibragimova, O.P.; Omarova, A.; Bukenov, B.; Baimatova, N. Solid-Phase Microextraction for the Quantification of Acetophenone Migrated to Air and Water from Toys and School Supplies. J. Anal. Chem. 2023, 78, 1414–1425. [Google Scholar] [CrossRef]
- Zubkov, F.I.; Kouznetsov, V.V. Traveling across Life Sciences with Acetophenone—A Simple Ketone That Has Special Multipurpose Missions. Molecules 2023, 28, 370. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Lockman, K.A.; Homer, N.Z.M.; Bower, E.; Brinkman, P.; Knobel, H.H.; Fallowfield, J.A.; Jaap, A.J.; Hayes, P.C.; Plevris, J.N. Volatomic analysis identifies compounds that can stratify non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100137. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, A.; Meher, S.R.; Alex, Z.C. Metal oxide thin films coated evanescent wave based fiber optic VOC sensor. Sens. Actuators A Phys. 2022, 338, 113459. [Google Scholar] [CrossRef]
- Rana, S.V.; Verma, Y. Biochemical toxicity of benzene. J. Environ. Biol. 2005, 26, 157–168. [Google Scholar]
- Ross, D. The role of metabolism and specific metabolites in benzene-induced toxicity: Evidence and issues. J. Toxicol. Environ. Health A 2010, 61, 357–372. [Google Scholar] [CrossRef]
- Marom, O.; Nakhoul, F.; Tisch, U.; Shiban, A.; Abassi, Z.; Haick, H. Gold Nanoparticle Sensors for Detecting Chronic Kidney Disease and Disease Progression. Nanomedicine 2012, 7, 639–650. [Google Scholar] [CrossRef]
- Dadamio, J.; Velde, S.; Laleman, W.; Hee, P.; Coucke, W.; Nevens, F.; Quirynen, M. Breath biomarkers of liver cirrhosis. J. Chromatogr. B 2012, 905, 17–22. [Google Scholar] [CrossRef]
- Amal, H.; Leja, M.; Funka, K.; Skapars, R.; Sivins, A.; Ancans, G.; Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016, 65, 400–407. [Google Scholar] [CrossRef]
- Luttrell, W.E.; Bellcock, L.R. Methyl ethyl ketone. J. Chem. Health Saf. 2015, 22, 33–36. [Google Scholar] [CrossRef]
- Api, A.M.; Belmonte, F.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; et al. RIFM fragrance ingredient safety assessment, 2-butanone, CAS Registry Number 78-93-3. Food Chem. Toxicol. 2019, 134, 111025. [Google Scholar] [CrossRef]
- Zhang, R.K.; Wang, J.X.; Cao, H. High-Performance Cataluminescence Sensor Based on Nanosized V2O5 for 2-Butanone Detection. Molecules 2020, 25, 3552. [Google Scholar] [CrossRef] [PubMed]
- Acosta, D.; Pérez, A.; Magaña, C.; Hernández, F. V2O5 Thin Films Deposited by RF Magnetron Sputtering: The Influence of Oxygen Content in Physical Properties. J. Mater. Sci. Eng. A 2016, 6, 81–87. [Google Scholar]
- Mastrigt, E.; Reyes-Reyes, A.; Brand, K.; Bhattacharya, N.; Urbach, H.P.; Stubbs, A.P.; Jongste, J.C.; Pijnenburg, M.W. Exhaled breath profiling using broadband quantum cascade laser-based spectroscopy in healthy children and children with asthma and cystic fibrosis. J. Breath Res. 2016, 10, 026003. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.C.; Tyler, T.R.; Klimisch, H.J.; Zimmermann, D.D. Acute Inhalation Toxicity Studies of n-Butyl Acetate. Inhal. Toxicol. 1997, 9, 623–646. [Google Scholar]
- Saillenfait, A.M.; Gallissot, F.; Sabaté, J.P.; Bourges-Abella, N.; Muller, S. Developmental toxic effects of ethylbenzene or toluene alone and in combination with butyl acetate in rats after inhalation exposure. J. App. Toxicol. 2006, 27, 32–42. [Google Scholar] [CrossRef]
- Hotovy, I.; Rehacek, V.; Kemeny, M.; Ondrejka, P.; Kostic, I.; Mikolasek, M.; Spiess, L. Preparation and gas-sensing properties of very thin sputtered NiO films. J. Electr. Eng. 2021, 72, 61–65. [Google Scholar] [CrossRef]
- Cannella, W.J. Xylenes and Ethylbenzene. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Henderson, L.; Brusick, D.; Ratpan, F.; Veenstra, G. A review of the genotoxicity of ethylbenzene. Mutat. Res. Rev. Mutat. Res. 2007, 635, 81–89. [Google Scholar] [CrossRef]
- Gahleitner, F.; Guallar-Hoyas, C.; Beardsmore, C.S.; Pandaya, H.C.; Thomas, C.P. Metabolomics pilot study to identify volatile organic compounds markers of childhood asthma in exhaled breath. Bioanalysis 2013, 5, 2239–2247. [Google Scholar] [CrossRef]
- Saalberg, Y.; Bruhns, H.; Wolff, M. Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation. Sensors 2017, 17, 210. [Google Scholar] [CrossRef]
- Meinardi, S.; Jin, K.; Barletta, B.; Blake, D.R.; Vaziri, N.D. Exhaled breath and fecal volatile organic biomarkers of chronic kidney disease. Biochim. Biophys. Acta 2013, 1830, 2531–2537. [Google Scholar] [CrossRef]
- Janreño-Esteban, J.J.; Muñoz-Lucas, M.A.; Gómez-Martín, O.; Utrilla-Trigo, S.; Gutiérrez-Ortega, C.; Aguilar-Ros, A.; Collado-Yurrita, L.; Callol-Sánchez, L.M. Study of 5 Volatile Organic Compounds in Exhaled Breath in Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2017, 53, 251–256. [Google Scholar] [CrossRef]
- Xu, H.; Wei, Y.; Zhu, L.; Huang, J.; Li, Y.; Liu, F.; Wang, S.; Liu, S. Bifunctional magnetic nanoparticles for analysis of aldehyde metabolites in exhaled breath of lung cancer patients. J. Chromatogr. A 2014, 1324, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Chen, J.; Fei, Q.; Guo, X.; Liu, S.; Wen, L.; Liang, H.; Guo, C.; Nie, L.; Jing, C. The association of aldehydes exposure with diabetes mellitus in US population: NHANES 2013–2014. Chemosphere 2022, 291, 133019. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Wu, N.; Gong, D.; Tang, X.; Yin, T.; Zhang, H.; Li, X. Association of aldehydes exposure with obesity in adults. Ecotoxicol. Environ. Saf. 2020, 201, 110785. [Google Scholar] [CrossRef]
- Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Support Tea Supply Chain. Sensors 2021, 21, 4266. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile biomarkers in the breath of women with breast cancer. J. Breath Res. 2010, 4, 026003. [Google Scholar] [CrossRef]
- Rohr, A.C.; Shore, S.A.; Spengler, J.D. Repeated Exposure to Isoprene Oxidation Products Causes Enhanced Respiratory Tract Effects in Multiple Murine Strains. Inhal. Toxicol. 2003, 15, 1191–1207. [Google Scholar] [CrossRef]
- Lynch, J. Occupational exposure to butadiene, isoprene and chloroprene. Chem.-Biol. Interact. 2001, 135–136, 207–214. [Google Scholar] [CrossRef]
- Lin, P.; Qin, Y.; Qi, X.; Huang, L. Improved isoprene detection performance of Si-doped WO3 films deposited by sputtering and post-annealing. RSC Adv. 2024, 14, 13618–13627. [Google Scholar] [CrossRef]
- Cazzola, M.; Segreti, A.; Capuano, R.; Bergamini, A.; Martinelli, E.; Calzetta, L.; Rogliani, P.; Ciaprini, C.; Ora, J.; Paolesse, R.; et al. Analysis of exhaled breath fingerprints and volatile organic compounds in COPD. COPD Res. Pract. 2015, 1, 7. [Google Scholar] [CrossRef]
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of head-and-neck cancer from exhaled breath. Br. J. Cancer 2011, 104, 1649–1655. [Google Scholar] [CrossRef]
- Anderson, S.E.; Khurshid, S.S.; Meade, B.J.; Lukomska, E.; Wells, J.R. Toxicological analysis of limonene reaction products using an in vitro exposure system. Toxicol. In Vitro 2013, 27, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y.; Bang, D.Y.; Lim, S.K.; Choi, S.M.; Lim, D.S.; Cho, M.C.; Yoon, K.; Kim, H.S.; et al. Safety Evaluation and Risk Assessment Of d-Limonene. J. Toxicol. Environ. Health B 2013, 16, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.N.; Zanella, D.; Stefanuto, P.H.; Bessonov, K.; Smolinska, A.; Dallinga, J.W.; Henket, M.; Paulus, V.; Guissard, F.; Graff, S.; et al. Exhaled Volatile Organic Compounds Are Able to Discriminate between Neutrophilic and Eosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.F.; Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2012, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Song, M.K.; Ryu, J.C. Integrated analysis of microRNA and mRNA expression profiles highlights alterations in modulation of the apoptosis-related pathway under nonanal exposure. Mol. Cell. Toxicol. 2014, 9, 351–364. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, M.; Kim, S. Effect of exposure to aldehyde C9 (nonanal) on the electroencephalographic activity of humans according to time series analysis. J. App. Pharmac. Sci. 2023, 13, 76–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Qiu, Z.; Lv, Y.; Chen, G.; Li, E. Early diagnosis of breast cancer from exhaled breath by gas chromatography-mass spectrometry (GC/MS) analysis: A prospective cohort study. J. Clin. Lab. Anal. 2020, 34, e23526. [Google Scholar] [CrossRef]
- Vietro, N.; Aresta, A.; Rotelli, M.T.; Zambonin, C.; Lippolis, C.; Picciariello, A.; Altomare, D.F. Relationship between cancer tissue derived and exhaled volatile organic compound from colorectal cancer patients. Preliminary results. J. Pharmac. Biomed. Anal. 2020, 180, 113055. [Google Scholar] [CrossRef]
- Hong, Y.; Che, X.; Su, H.; Mai, Z.; Huang, Z.; Huang, W.; Chen, W.; Liu, S.; Gao, W.; Zhou, Z.; et al. Exhaled breath analysis using on-line preconcentration mass spectrometry for gastric cancer diagnosis. J. Mass Spectrom. 2020, 56, e4588. [Google Scholar] [CrossRef]
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Spanel, P.; Smith, D.; Hanna, G.B. Selected Ion Flow Tube Mass Spectrometry Analysis of Exhaled Breath for Volatile Organic Compound Profiling of Esophago-Gastric Cancer. Anal. Chem. 2013, 85, 6121–6128. [Google Scholar] [CrossRef] [PubMed]
- Bruce, W.; Meek, M.E.; Newhook, R. Phenol: Hazard characterization and exposure–response analysis. J. Environ. Sci. Health C 2001, 19, 305–324. [Google Scholar] [CrossRef]
- Berger, K.; Coker, E.; Rauch, S.; Eskenazi, B.; Balmes, J.; Kogut, K.; Holland, N.; Calafat, A.M.; Harley, K. Prenatal phthalate, paraben, and phenol exposure and childhood allergic and respiratory outcomes: Evaluating exposure to chemical mixtures. Sci. Total Environ. 2020, 725, 138418. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Reza, K.K.; Ali, A.; Agrawal, V.V.; Biradar, A.M. Self assembled DC sputtered nanostructured rutile TiO2 platform for bisphenol A detection. Biosens. Bioelectron. 2015, 68, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.A.; Hagberg, M.T.; Löf, A.E.; Wigaeus-Hjelm, E.M.; Zhiping, W. Uptake, distribution and elimination of α-pinene in man after exposure by inhalation. Scand. J. Work Environ. Health 1990, 16, 372–378. [Google Scholar] [CrossRef]
- Satou, T.; Kasuya, H.; Maeda, K.; Koike, K. Daily Inhalation of α-Pinene in Mice: Effects on Behavior and Organ Accumulation. Phytother. Res. 2013, 28, 1284–1287. [Google Scholar] [CrossRef]
- Jisha, P.; Suma, M.S.; Murugendrappa, M.V. Synthesis and characterization of WO3-doped polyaniline to sense biomarker VOCs of Malaria. App. Nanosci. 2020, 11, 29–44. [Google Scholar] [CrossRef]
- Monod, A.; Sive, B.C.; Avino, P.; Chen, T.; Blake, D.R.; Rowland, F.S. Monoaromatic compounds in ambient air of various cities: A focus on correlations between the xylenes and ethylbenzene. Atmos. Environ. 2001, 35, 135–149. [Google Scholar] [CrossRef]
- Robroeks, C.M.; Berkel, J.J.; Jobsis, Q.; Schooten, F.; Dallinga, J.W.; Wouters, E.F.; Dompeling, E. Exhaled volatile organic compounds predict exacerbations of childhood asthma in a 1-year prospective study. Eur. Resp. J. 2013, 42, 98–106. [Google Scholar] [CrossRef]
- Lavra, L.; Catini, A.; Ulivieri, A.; Capuano, R.; Salehi, L.B.; Sciacchitano, S.; Bartolazzi, A.; Nardis, S.; Paolesse, R.; Martinelli, E.; et al. Investigation of VOCs associated with different characteristics of breast cancer cells. Sci. Rep. 2015, 5, 13246. [Google Scholar] [CrossRef]
- Banday, K.M.; Pasikanti, K.K.; Chan, E.; Singla, R.; Rao, K.; Chauhan, V.; Nanda, R. Use of Urine Volatile Organic Compounds to Discriminate Tuberculosis Patients from Healthy Subjects. Anal. Chem. 2011, 83, 5526–5534. [Google Scholar] [CrossRef]
- Lee, C.; Su, Y.; Fu, L.; Jiang, J. A micro gas sensor based on a WO3 thin film for aromatic hydrocarbon detection. In Proceedings of the 2011 Fifth International Conference on Sensing Technology, Palmerston North, New Zealand, 28 November–1 December 2011. [Google Scholar]

| Compound | Formula | CAS | Vapor Pressure | Note |
|---|---|---|---|---|
| Acetone | C3H6O | 67-64-1 | 24.6 KPa | VOC |
| Ammonia | NH3 | 7664-41-7 | 850 KPa | - |
| Butanol | C4H10O | 71-36-3 | 0.6 KPa | VOC |
| Ethanol | C2H6O | 64-17-5 | 5.8 KPa | VOC |
| Formaldehyde | CH20 | 30525-89-4 | 0.5 KPa | VOC |
| Isopropanol | C3H8O | 67-63-0 | 4.2 KPa | VOC |
| Methane | CH4 | 74-82-8 | 14.1 MPa | VOC |
| Methanol | CH4O | 67-56-1 | 12.9 KPa | VOC |
| Propane | C3H8 | 74-98-6 | 289.6 KPa | VOC |
| Toluene | C7H8 | 108-88-3 | 2.9 KPa | VOC |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [76] | WO3 | RF | - | 80/20% | 3 mTorr | 32/8 ccm | 500 °C | 1 h | 100 nm |
| [77] | WO3 | DC | 30 W | 68/32% | 5 × 10−3 Torr | -/- | 400 °C | 4 h | 500 nm |
| [78] | WO3 | DC | 50 W | 80/20% | 3.7 × 10−3 Torr | 70/- sccm | 700 °C | 2 h | 50 nm |
| [79] | ZnO | RF | 150 W | 20/80% | 2.2 × 10−2 mbar | -/- | 500 °C | 6 h | 250 nm |
| [80] | ZnO | RF | 200 W | 20/80% | 2 × 10−2 mbar | -/- | 500 °C | 6 h | 250 nm |
| [81] | ZnO | RF | 150 W | -/- | 2 mTorr | 10/3 sccm | 350 °C | - | - |
| [82] | CuO-Ga2O3 | - | 100 W | -/- | 2 × 10−2 mbar | -/20 sccm | 400 °C | 4 h | 60–70 nm |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
| [76] | 260 °C | 15–20 ppmv | 5 min | 4 min |
| [77] | 350 °C | 100 ppmv | 24 s | 27 s |
| [78] | 300 °C | 0.5–8 ppmv | 45 s | 251 s |
| [79] | 400 °C | 500 ppmv | 70 s | 95 s |
| [80] | 400 °C | 15–1000 ppmv | - | - |
| [81] | 200 °C | 20–100 ppmv | - | - |
| [82] | - | 0.1–1.25 ppmv | 319 s | 901 s |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [87] | TiO2 | DC and RF | - | -/64% | -/- | -/- | - | 2 h | 30–120 nm |
| [88] | TiO2 | DC and RF | 12.5 W | -/- | -/- | -/- | - | 2 h | 30–120 nm |
| [89] | ZnO, Y-ZnO | RF | - | -/- | -/- | -/- | - | 45 min | - |
| [90] | ZnO | DC | 2250 W | -/- | 6 × 10−2/ 3 × 10−4 mbar | -/- | 300 °C | 24 h | - |
| [91] | CdO | DC | 25 W | -/- | 3.8 × 10−3 mbar | -/- | - | - | 294 nm |
| [92] | SnO2 | RF | 50 W | 97/3% | 10 mTorr | -/- | - | - | 100 nm |
| [93] | MoO3 | DC and RF | - | -/- | -/- | -/- | - | - | - |
| [94] | Al2O3: Cr2O3:CuO | RF | 300 W | -/- | 5.6 × 10−3 mbar | 25/- sccm | 300–1000 °C | 1 h | 358–372 nm |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
| [87] | Room Temperature | 1000 ppmv | - | - |
| [88] | Room Temperature | 50–5000 ppmv | - | - |
| [89] | Room Temperature | 50–200 ppmv | 102 s | 101 s |
| [90] | Room Temperature | 1–100 ppmv | 5–50 s | 4–60 s |
| [91] | 150 °C | 50 ppmv | 50–200 s | 50–150 s |
| [92] | 50–200 °C | 50–200 ppmv | - | - |
| [93] | - | 50 ppmv | - | - |
| [94] | Room Temperature | 100 ppmv | 7 s | 7 s |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [98] | Co3O4 @ZnO | - | - | -/- | -/- | -/- | 500 °C | 1 h | 1.2–60 nm |
| [99] | Au-TiO2 | - | - | -/- | -/- | -/- | - | - | - |
| [100] | M-TiO2 M(Au, Ag, Ag-Au, Au-Pt) | DC | 40 W | -/- | 136 Pa | 48/- sccm | 450–600 °C | 2 h | 12–40 nm |
| [101] | Cu or Ni-ZnO | - | - | -/- | -/- | -/- | 400 °C | 4 h | 115 µm |
| [102] | GO-SnO2 | DC | - | -/- | 1.2 × 10−2 mbar | -/- | - | - | - |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
| [98] | 300 °C | 100 ppmv | 1 s | 92 s |
| [99] | 240 °C | 50–100 ppmv | 13 s | 53 s |
| [100] | 200–350 °C | 100 ppmv | - | - |
| [101] | 400–450 °C | 1000 ppmv | 3–4 s | 208–747 s |
| [102] | Room Temp. | 335–1676 ppmv | 27 s | 42 s |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [109] | Ag-ZnO | RF | 300 W | -/- | 1 Pa | 6/20 sccm | - | - | 50 nm |
| [110] | ZnO | RF | 70–150 W | -/- | - | -/- | 400 °C | 1 h | 100–200 nm |
| [111] | ZnO | RF | 100 W | -/- | 3 × 10−2 mbar | -/- | 500 °C | 2 h | - |
| [112] | Fe-TiO2 | RF | 100–300 W | -/- | - | 10/10 sccm | 600 °C | - | 200 nm |
| [113] | CuO | - | - | -/- | -/- | -/- | 400 °C | 3 h | 1 µm |
| [114] | ITO | RF | 200–300 W | -/- | 20 mTorr | -/- | 450 °C | 1 h | 100 nm |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
| [109] | 260 °C | 100 ppmv | - | - |
| [110] | 200–400 °C | 10–50 ppmv | 147 s | 400 s |
| [111] | - | 50–300 ppmv | 20–29 s | 38–60 s |
| [112] | - | 10–50 ppmv | 9–63 s | 12–60 s |
| [113] | 200 °C | 20–500 ppmv | 4–8 s | 4–8 s |
| [114] | 250 °C | 200 ppmv | 180 s | - |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [118] | AZO | - | 50–150 W | -/- | 5.48 mTorr | 30/- sccm | 400 °C | 3 h | 80 nm |
| [119] | LPFO, LPFO-Zn | RF | 75 W | -/- | -/- | -/- | 750 °C | 1.5 h | 330 nm |
| [120] | ZnO | RF | 50–150 W | -/- | -/- | -/- | - | - | 0.5 µm |
| [121] | NiO | RF | 400 W | 50/50% | 5 × 10−3 mbar | -/- | 700 °C | 4 h | 150–300 nm |
| [122] | NiO | RF | 400 W | 50/50% | 5 × 10−3 mbar | -/- | 600–800 °C | 4 h | 150–300 nm |
| [123] | NiO | RF | 200 W | 50–80/20–50% | 10 mTorr | -/- | - | - | 120 nm |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
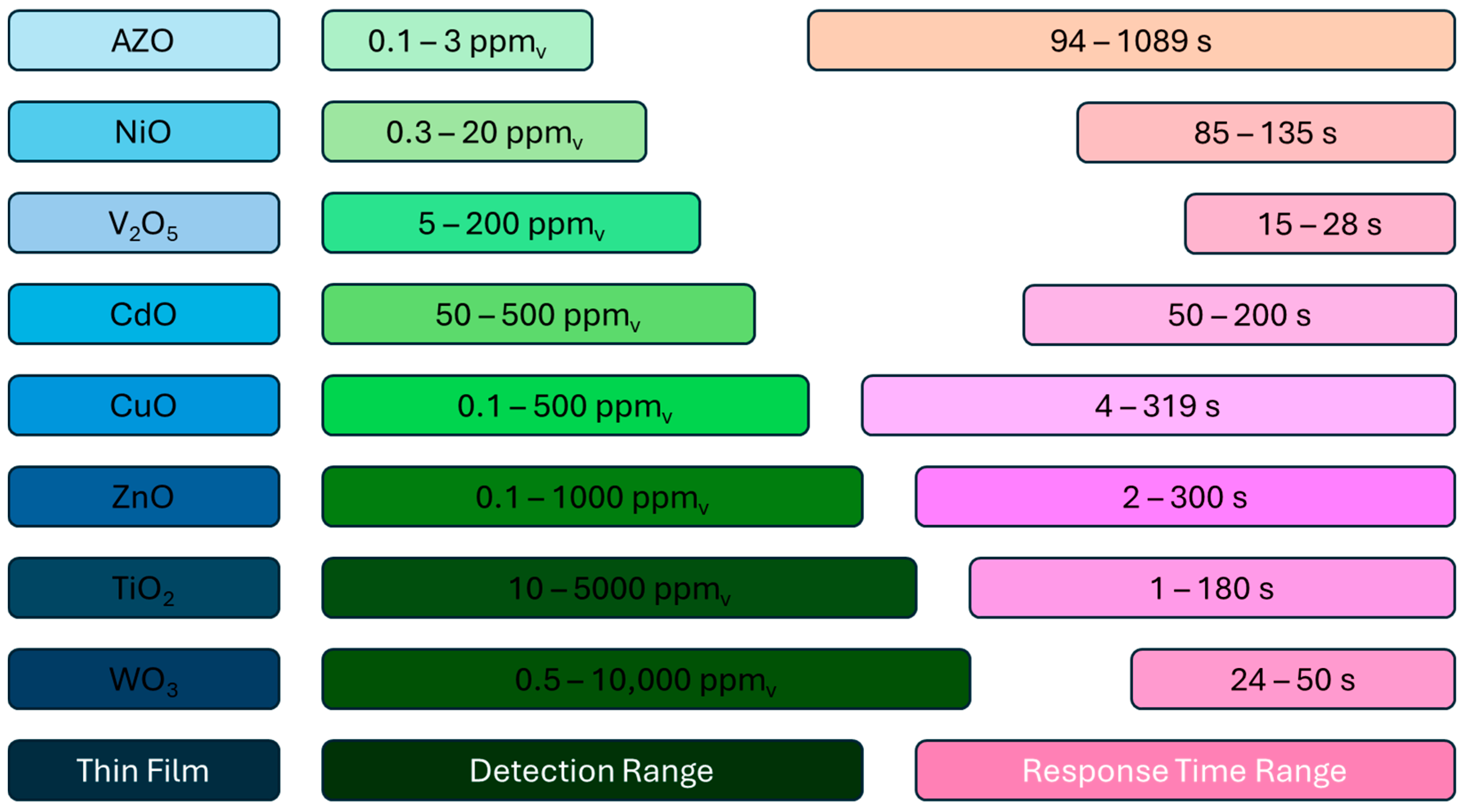
| [118] | 240 °C | 0.1–3 ppmv | 94–1089 s | 206–254 s |
| [119] | 330 °C | 400 ppmv | 12 s | 25 s |
| [120] | Room Temp. | 1–50 ppmv | 60–180 s | 180 s |
| [121] | 300–340 °C | 5–20 ppmv | 420 s | 1800 s |
| [122] | - | 2–20 ppmv | - | - |
| [123] | 200 °C | 0.3–2.5 ppmv | 85–135 s | 85–135 s |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [130] | ZnO | RF | 100 W | -/- | 2 × 10−5 mbar | -/- | 500 °C | 1 h | 260 nm |
| [131] | Au-ZnO | RF | 20–50 W | -/- | 2.5 Pa | 40/- sccm | 600 °C | 2 h | 24 nm |
| [132] | Al-ZnO:WO3 | RF | 100 W | -/- | 2.2 Pa | 25/5 mL/min | 400 °C | 4 h | 600 nm |
| [133] | V2O5 | DC | 70 W | -/- | -/- | 5/1 sccm | - | - | 180–320 nm |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
| [130] | 250 °C | 2–22 ppmv | - | 61–102 s |
| [131] | 300 °C | 1–100 ppmv | 2–6 s | 10–56 s |
| [132] | Room Temp. | 1–500 ppmv | 5 s | 22 s |
| [133] | Room Temp. | 5–200 ppmv | 15–28 s | 13–40 s |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [137] | Au-VOx | DC | 2.2 W | -/- | 2 Pa | -/5 slpm | 470–500 °C | 4 h | 150 nm |
| [138] | TiO2 | RF | 150 W | -/- | 10−3 Torr | -/- | 500–1000 °C | - | 170 nm |
| [139] | WO3 | RF | - | 50/50% | -/- | 1/1 sccm | 350–450 °C | 24 h | - |
| [140] | CdO | DC | 20–50 W | -/- | -/- | 15/5 sccm | 80 °C | 1 h | 240–410 nm |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
| [137] | Room Temp. | 500–2000 ppmv | 1000 s | 500 s |
| [138] | 50 °C | 1000 ppmv | 120 s | 15 s |
| [139] | 300–370 °C | 100–10,000 ppmv | - | - |
| [140] | 100–150 °C | 500 ppmv | - | - |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [44] | CuO, TiO2, SnO2 | DC | 50–1000 W | -/- | 3 × 10−2 mbar | -/0.9–20 sccm | - | - | 200–500 nm |
| [145] | CuO | DC | - | 90/10 | 3 × 10−2 mbar | -/- | 550 °C | 2 h | 85 nm |
| [146] | ZnO, Cd-ZnO | RF | - | -/- | - | -/- | 950 °C | 6 h | 96–103 nm |
| [147] | ZnO, Mg-ZnO | RF | 100 W | -/- | 0.02 mbar | -/- | 950 °C | 8 h | - |
| [148] | ZnO, Pt/ZnO NRs | DC | - | -/- | -/- | -/- | 90 °C | 6 h | 55–58 nm |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
| [44] | Room Temp. | 0–100 ppmv | - | - |
| [145] | 350 °C | 100–2500 ppmv | 235 s | 235 s |
| [146] | - | 50–200 ppmv | 300 s | 360 s |
| [147] | 100 °C | 50–200 ppmv | 283 s | 223 s |
| [148] | 270 °C | 100–1000 ppmv | - | - |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [152] | M-CuO (M = Ag, Au, Cr, Pd, Pt, Sb, Si) | MF | 40 W | 90/10% | 4 × 10−2 mbar | -/- | 400 °C | 4 h | 50 nm |
| [153] | Cr-TiO2 | RF | 150 W | -/- | 30 mTorr | -/- | - | - | 300 nm |
| [154] | ZnO to ZIF-8 | - | 100 W | -/- | 15 mTorr | 10/- sccm | 150 °C | 3 h | 50 nm |
| [155] | ZnO | RF | 100 W | -/- | 2–30 mTorr | -/- | - | - | 300–400 nm |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
| [152] | 250 °C | 0–100 ppmv | 10 s | 24 s |
| [153] | 300 °C | 250–1000 ppmv | 1–3 s | 1–3 s |
| [154] | Room Temp. | - | - | - |
| [155] | 300 °C | 300–500 ppmv | 30 s | 35 s |
| Work | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | |||||
| [163] | Au-ZnTiO3/TiO2 | - | - | -/- | -/- | -/- | 550 °C | 2 h | 75–150 nm |
| [164] | GaN)/TiO2 | RF | 300 W | -/- | -/- | 50/5 sccm | 650–700 °C | - | 150 nm |
| [130] | ZnO | RF | 100–150 W | -/- | 2 × 10−5 mbar | -/- | 500 °C | 2 h | 260 nm |
| [165] | Pt and Pd-ZnO | - | 30 W | -/- | -/- | 300/10 sccm | 550–650 °C | - | 10–30 nm |
| [166] | Pt-SnO2 | - | 30 W | -/- | 2.65 Pa | -/- | 650 °C | 2 h | 3–20 nm |
| [167] | SnO2 | DC | 50 W | -/- | 3 × 10−3– 6 × 10−3 mbar | 6/10 sccm | 350–500 °C | 48 h | 250 nm |
| Work | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|
| [163] | 210 °C | 10–100 ppmv | 4 s | 20 s 90 s |
| [164] | - | 50 ppbv–10,000 ppmv | 60–180 s | 75–150 s |
| [130] | 250 °C | 2–87 ppmv | - | 98 s |
| [165] | - | 0.1–50 ppmv | - | - |
| [166] | 300 °C | 1–10 ppmv | - | - |
| [167] | 300–500 °C | 50–900 ppbv | - | 900 s |
| Compound | Formula | CAS | Vapor Pressure | Note |
|---|---|---|---|---|
| Acetaldehyde | C2H4O | 75-07-0 | 100 KPa | VOC |
| Acetophenone | C8H8O | 98-86-2 | 0.041 KPa | - |
| Benzene | C6H6 | 71-43-2 | 10.0 KPa | VOC |
| 2-Butanone | C4H8O | 78-93-3 | 12.1 KPa | VOC |
| Butyl Acetate | C6H12O2 | 123-86-4 | 1.38 KPa | VOC |
| Ethylbenzene | C8H10 | 100-41-4 | 0.9 KPa | VOC |
| Hexanal | C6H10O | 66-25-1 | 1.4 KPa | VOC |
| Isoprene | C5H8 | 78-79-5 | 61.0 KPa | VOC |
| Limonene | C10H16 | 5989-27-5 | 0.2 KPa | VOC |
| Nonanal | C9H18O | 124-19-6 | 0.04 KPa | VOC |
| Phenol | C6H6O | 108-95-2 | 0.04 KPa | VOC |
| α-Pinene | C10H16 | 80-56-8 | 0.4 KPa | VOC |
| Xylene | C8H10 | 108-38-3 | 0.8 KPa | VOC |
| Work | VOC | Thin Film | DC/RF | Power | Argon/Oxygen | Annealing | Thin Film Thickness | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ratio | Pressure | Flow | Temp. | Time | ||||||
| [172] | Acetaldehyde | ZnO:Ga | RF | 50 W | -/- | 2 Pa | -/- | 400 °C | 4 h | 50–100 nm |
| [173] | Acetaldehyde | In-SnO | DC | 11–55 W | -/- | 0.58 Pa | 25/9 mL/min | 400–500 °C | 6 h | 110–190 nm |
| [179] | Acetophenone | ZnO, AZO and SnO2 | DC | 50 W | -/- | -/- | 6/10 sccm | - | - | 300 nm |
| [167] | Benzene | SnO2 | DC | 50 W | -/- | 3 × 10−3–6 × 10−3 mbar | 6/10 sccm | 350–500 °C | 48 h | 250 nm |
| [192] | Butyl Acetate | NiO | DC | 600 W | 30/70% | -/- | -/- | 500–600 °C | - | 25–50 nm |
| [164] | Ethyl Benzene | GaN/TiO2 | RF | 300 W | -/- | -/- | 50/5 sccm | 700 °C | - | 70 nm |
| [202] | Hexanal | SnO2, CuO | DC | - | -/- | -/- | -/- | - | - | - |
| [206] | Isoprene | Si-WO3 | DC | 700 W | -/- | 20 mTorr | -/- | 500 °C | 1 h | - |
| [202] | Limonene | SnO2, CuO | DC | - | -/- | -/- | -/- | - | - | - |
| [202] | Nonanal | SnO2, CuO | DC | - | -/- | -/- | -/- | - | - | - |
| [221] | Phenol | TiO2 | DC | - | -/- | 5 × 10−6 mTorr | -/- | 95 °C | 3 h | 128 nm |
| [61] | α-Pinene | TiO2, ZnO | DC | 300–1000 W | 50/50% | 0.13 Pa | -/- | - | - | - |
| [229] | Xylene | WO3 | RF | 200 W | 50/50% | 0.01 Torr | -/- | 500 °C | 1.5 h | 4.4 µm |
| Work | VOC | Optimal Sensing Temperature | Detection Range | Response Time | Recovery Time |
|---|---|---|---|---|---|
| [172] | Acetaldehyde | Room Temp.–500 °C | 500 ppbv | - | - |
| [173] | Acetaldehyde | 500 °C | 0.2–25 ppmv | - | - |
| [179] | Acetophenone | Room Temp. | 0–250 ppmv | 17 s | 21 s |
| [167] | Benzene | 300–500 °C | 50–900 ppbv | - | 900 s |
| [192] | Butyl Acetate | 300 °C | 3 ppmv | 124 s | 102 s |
| [164] | Ethyl Benzene | Room Temp. | 50 ppbv–1 ppmv | 60 s | 75 s |
| [202] | Hexanal | 350–400 °C | - | - | - |
| [206] | Isoprene | 325 °C | 5 ppmv | 1 s | 2.5 s |
| [202] | Limonene | 350–400 °C | - | - | - |
| [202] | Nonanal | 350–400 °C | - | - | - |
| [221] | Phenol | Room Temp. | 0.01–1 ppmv | 250 s | - |
| [61] | α-Pinene | Room Temp. | 109–807 ppmv | - | - |
| [229] | Xylene | 300 °C | 0–20 ppmv | - | 30 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, P.C.; Sério, S. Recent Applications and Future Trends of Nanostructured Thin Films-Based Gas Sensors Produced by Magnetron Sputtering. Coatings 2024, 14, 1214. https://doi.org/10.3390/coatings14091214
Moura PC, Sério S. Recent Applications and Future Trends of Nanostructured Thin Films-Based Gas Sensors Produced by Magnetron Sputtering. Coatings. 2024; 14(9):1214. https://doi.org/10.3390/coatings14091214
Chicago/Turabian StyleMoura, Pedro Catalão, and Susana Sério. 2024. "Recent Applications and Future Trends of Nanostructured Thin Films-Based Gas Sensors Produced by Magnetron Sputtering" Coatings 14, no. 9: 1214. https://doi.org/10.3390/coatings14091214
APA StyleMoura, P. C., & Sério, S. (2024). Recent Applications and Future Trends of Nanostructured Thin Films-Based Gas Sensors Produced by Magnetron Sputtering. Coatings, 14(9), 1214. https://doi.org/10.3390/coatings14091214







