Developments in Dental Implant Surface Modification
Abstract
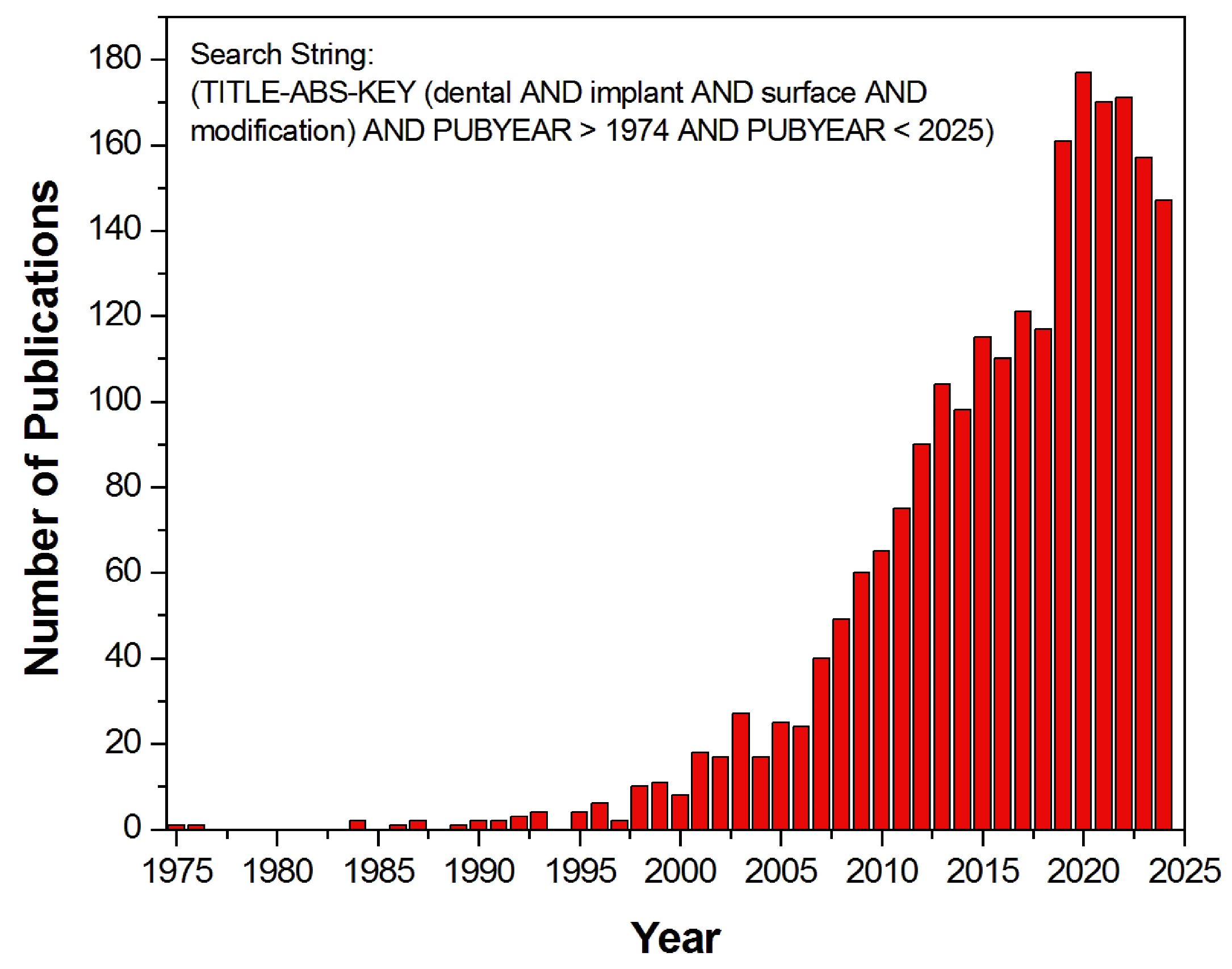
:1. Introduction
2. Mechanism of the Osseointegration Process
3. Types of Dental Implant Surface
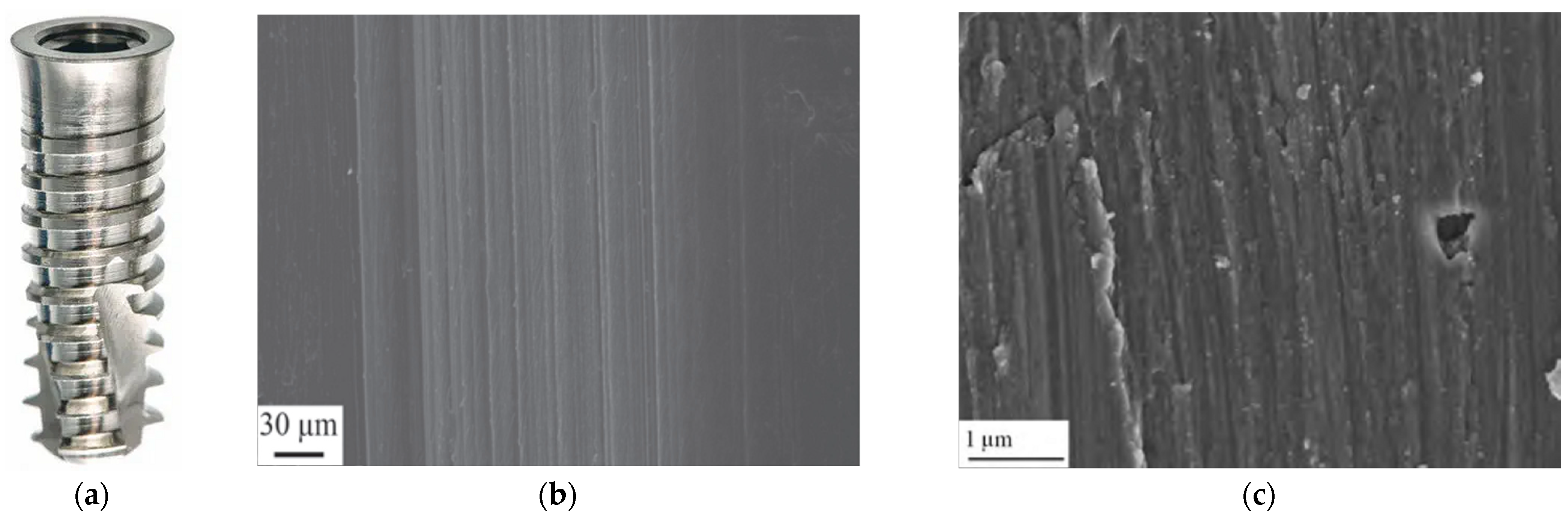
3.1. Machined Surface
3.2. Titanium Plasma Surface
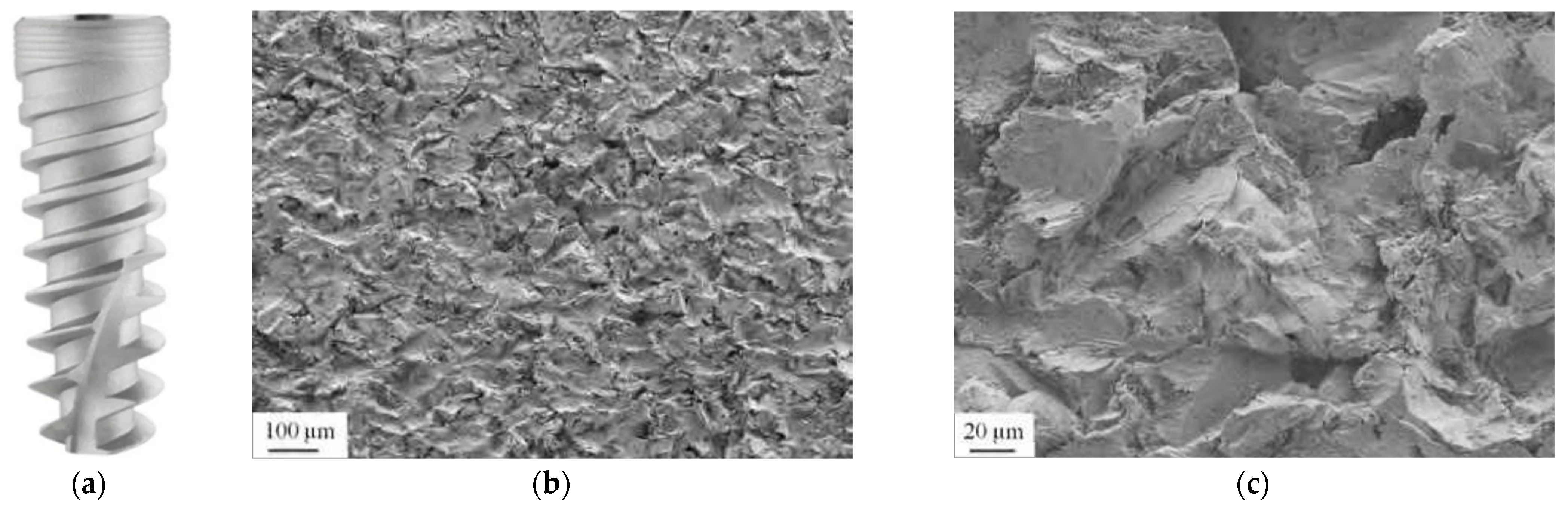
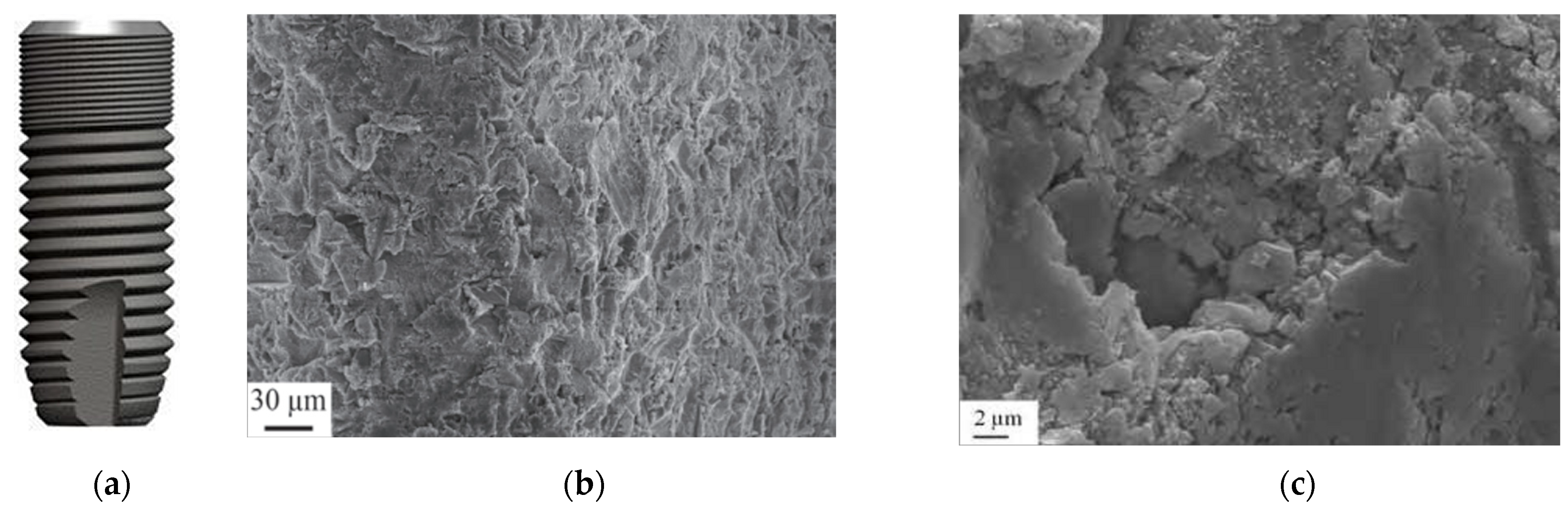
3.3. Sandblasted Surface
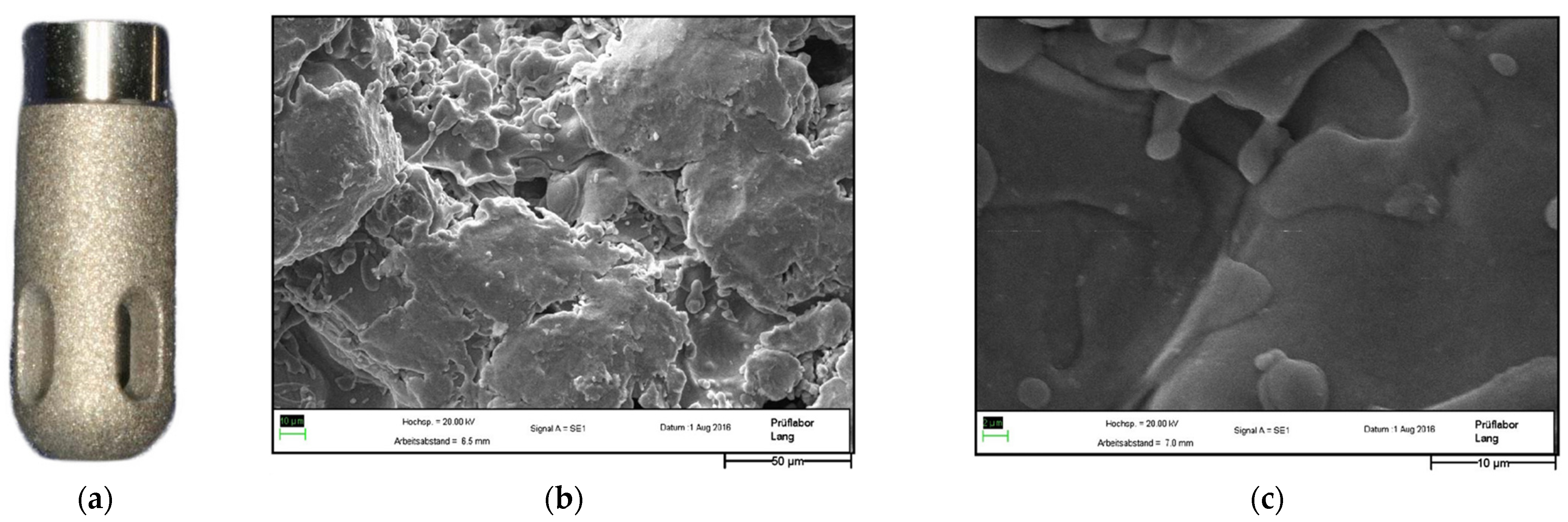
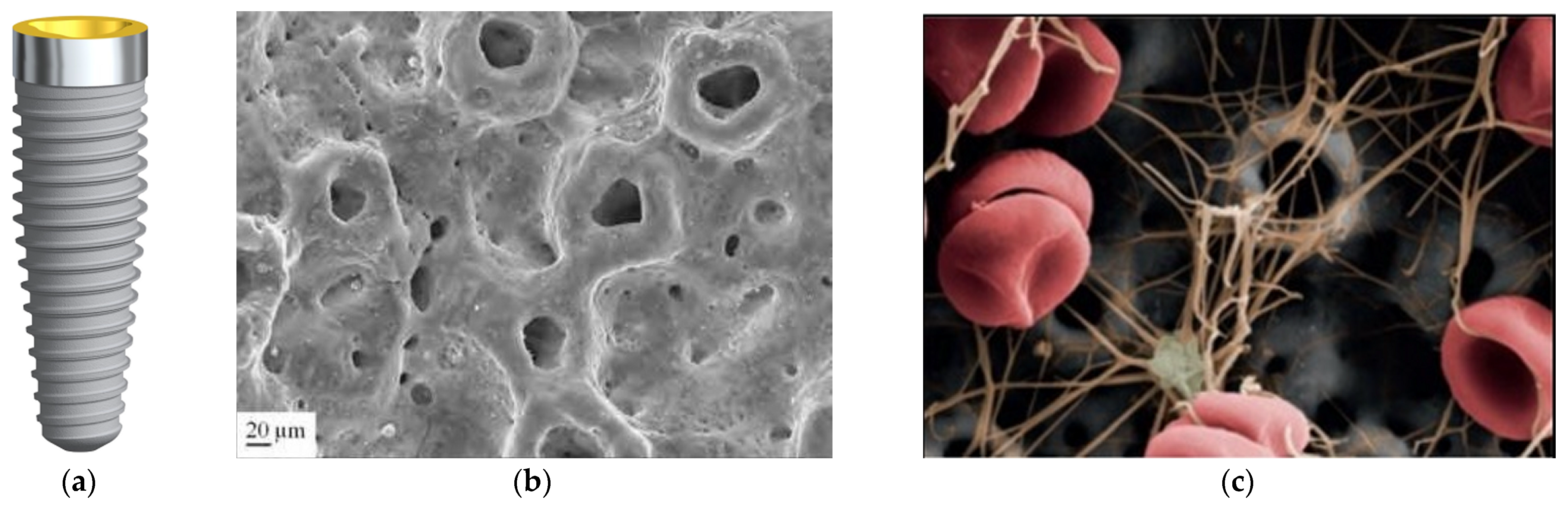
3.4. Hydroxyapatite Surface
3.5. Double-Etched Surface
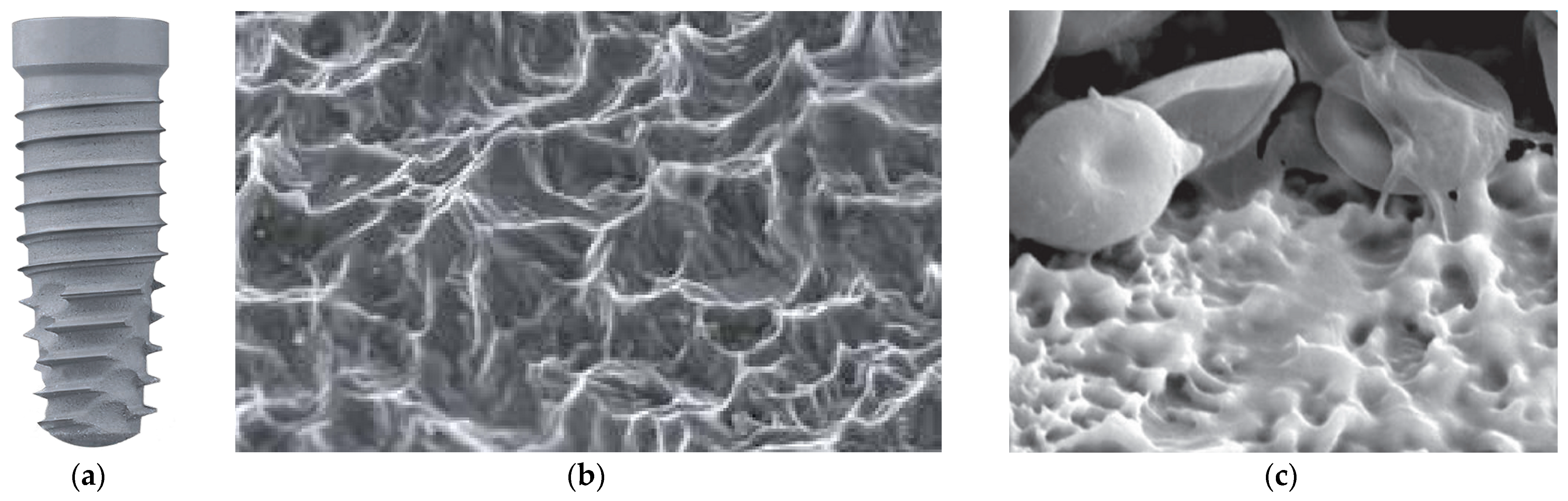
3.6. Sandblasted and Etched Surface
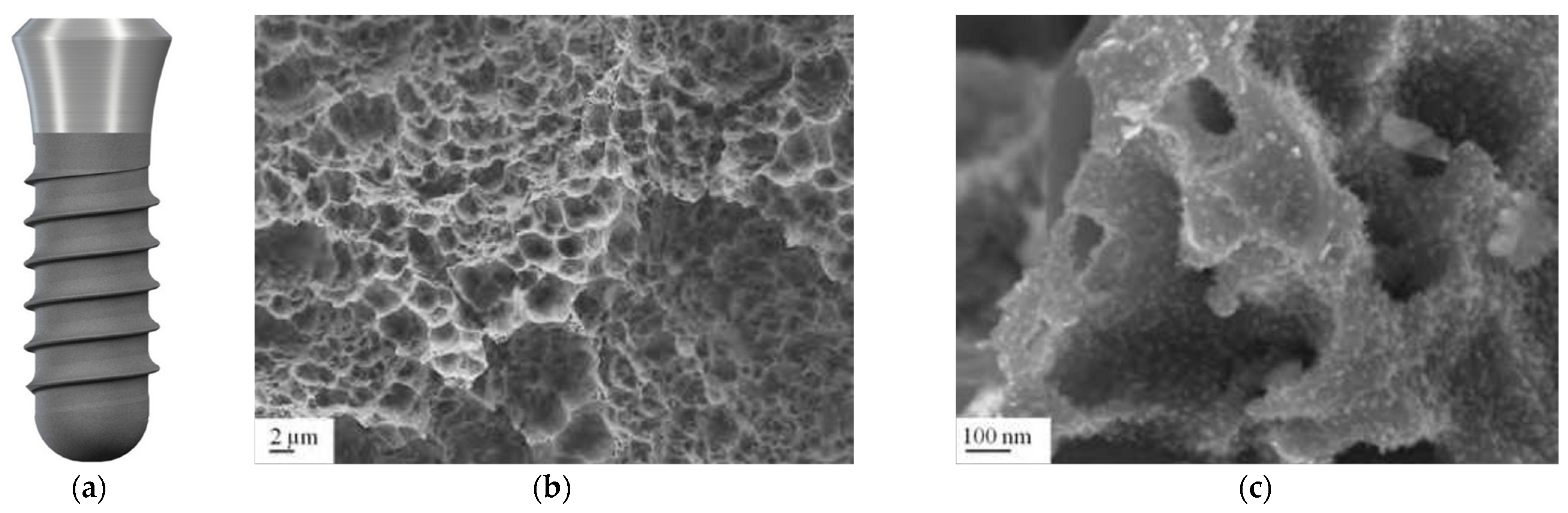
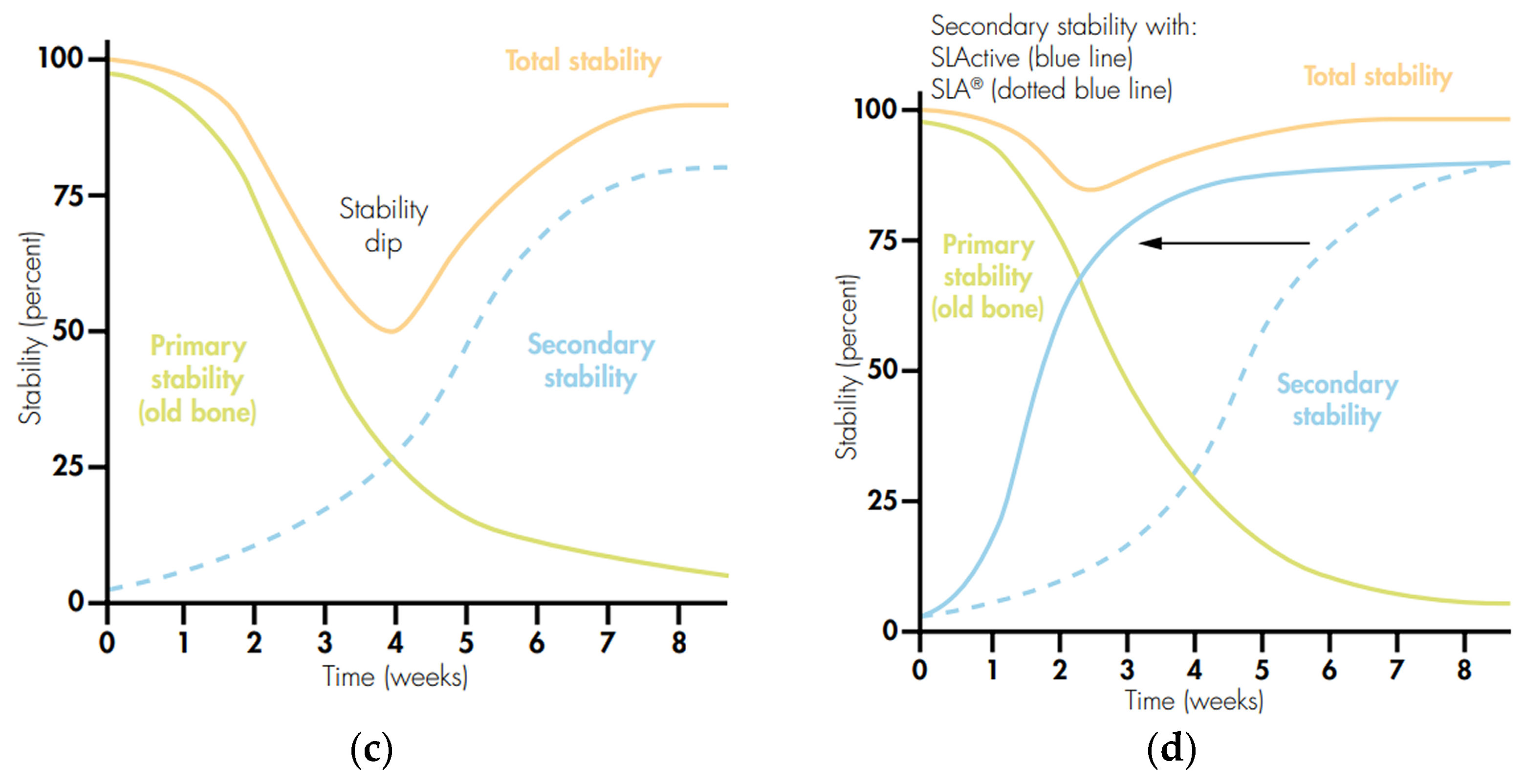
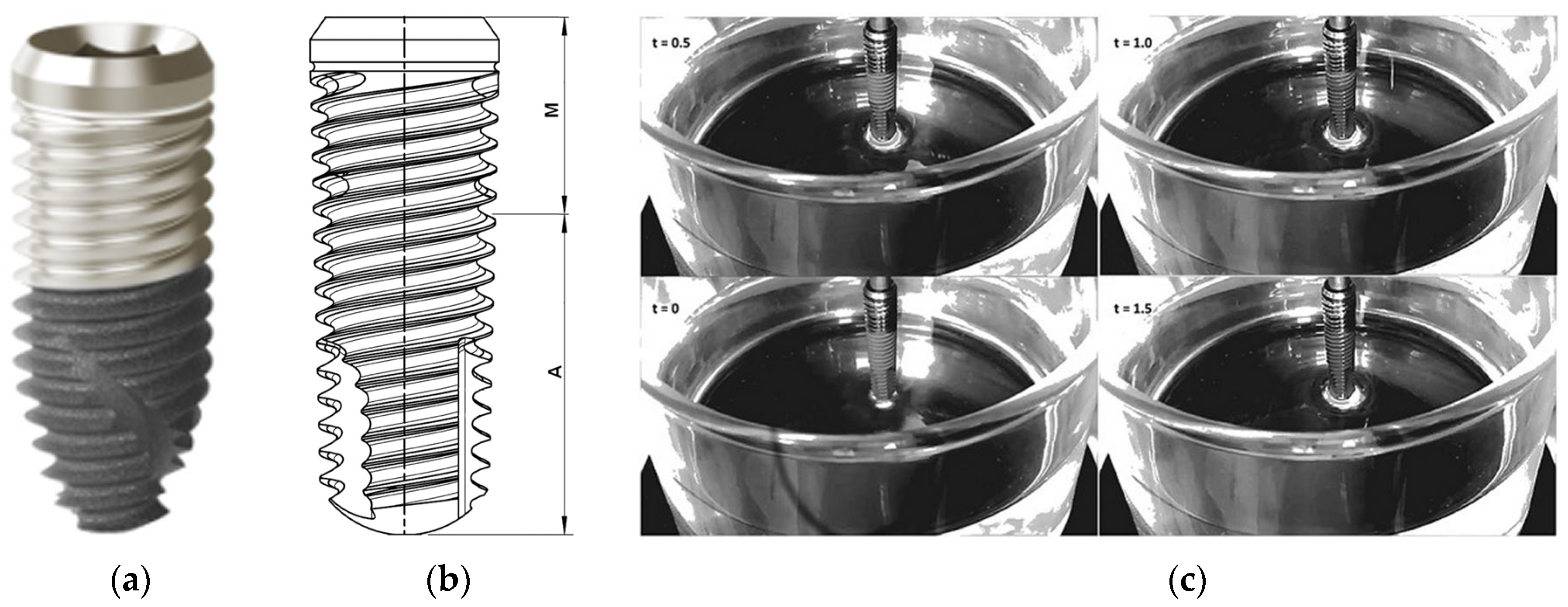
3.7. Hydrophilic Surface
3.8. Oxidized Surface
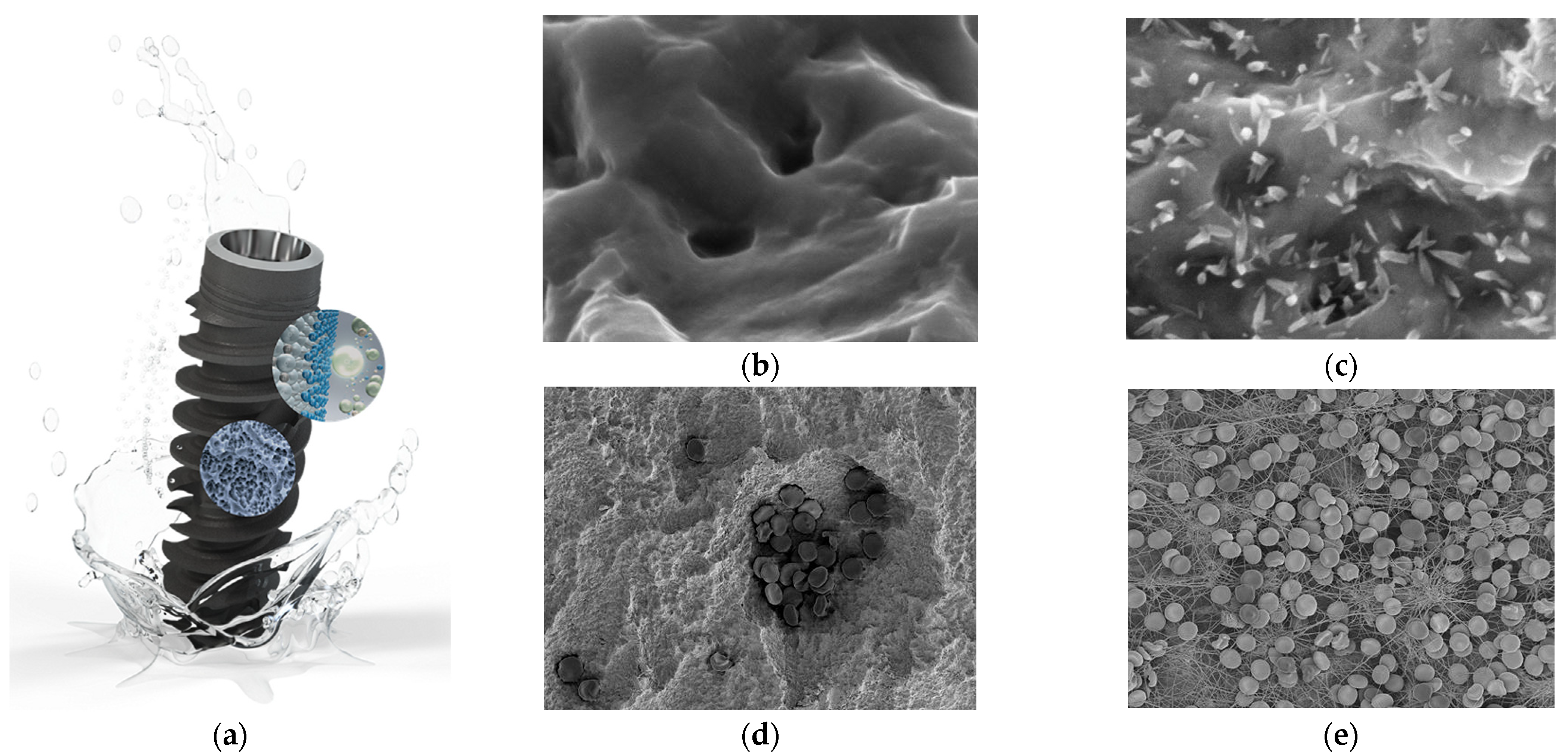
3.9. Biologically Active Surface
3.10. Hybrid Surface
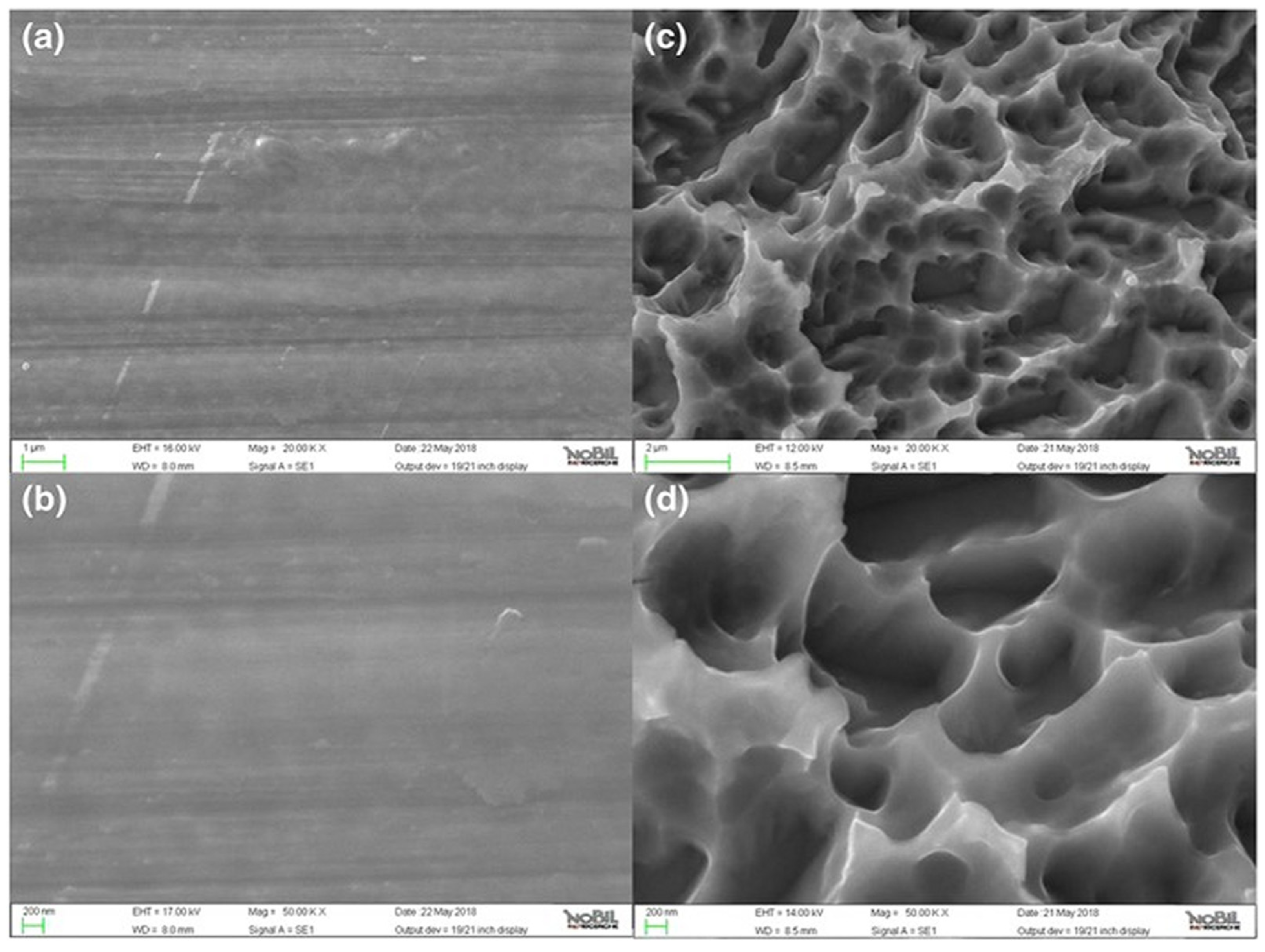
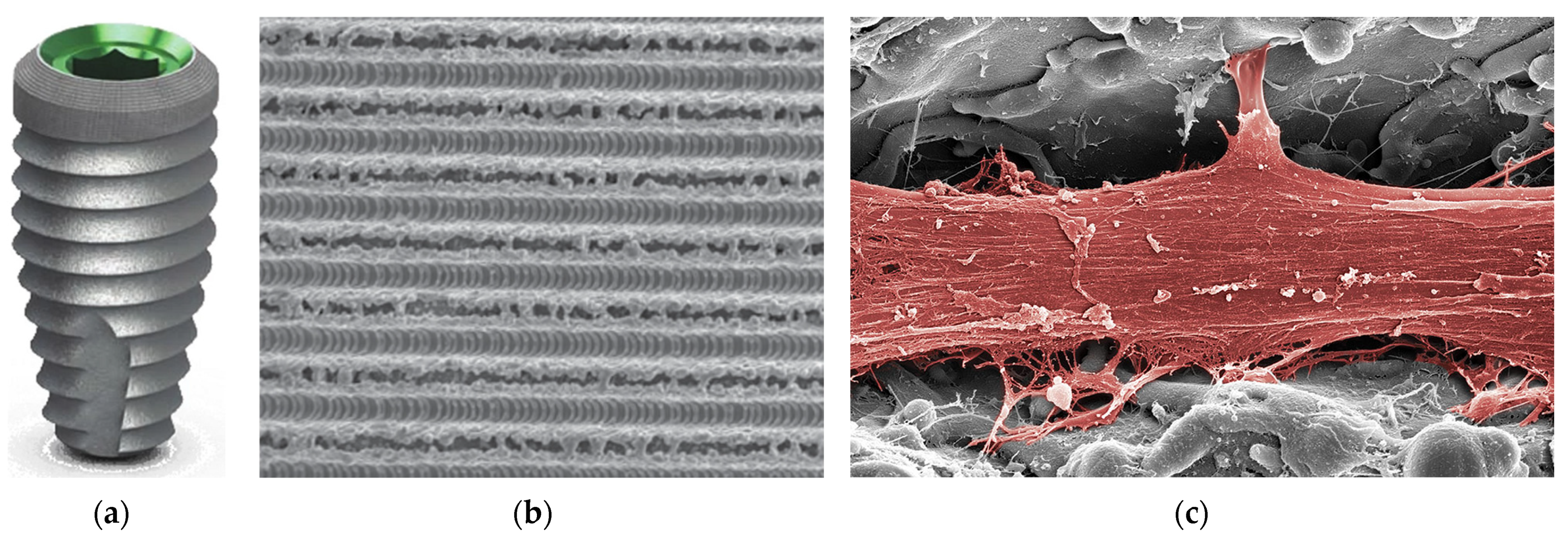
3.11. Laser-Structured Surface
4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Waghmare, G.; Waghmare, K.; Bagde, S.; Deshmukh, M.; Kashyap, D.N.; Shahu, V.T. Materials Evolution in Dental Implantology: A Comprehensive Review. J. Adv. Res. Appl. Mech. 2024, 123, 75–100. [Google Scholar] [CrossRef]
- Lanis, A.; Peña-Cardelles, J.F.; Negreiros, W.M.; Hamilton, A.; Gallucci, G.O. Impact of digital technologies on implant surgery in fully edentulous patients: A scoping review. Clin. Oral Implants Res. 2024, 35, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Shivgotra, R.; Soni, B.; Kaur, M.; Thakur, S. Advancement in Biomaterials in the Form of Implants. In Engineered Biomaterials. Engineering Materials; Malviya, R., Sundram, S., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Nitschke, I.; Krüger, K.; Jockusch, J. Age-related knowledge deficit and attitudes towards oral implants: Survey-based examination of the correlation between patient age and implant therapy awareness. BMC Oral Health 2024, 24, 403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kim, B. The Effects of the Expansion of Dental Care Coverage for the Elderly. Healthcare 2024, 12, 1949. [Google Scholar] [CrossRef]
- Kim, H.-J.; Sung, I.-Y. Analysis of Dental Prosthetic Treatment in Patients with Cancer Aged 65 Years and Older after Expanded Health Insurance Coverage: A Retrospective Clinical Study. Medicina 2024, 60, 1509. [Google Scholar] [CrossRef]
- Gargallo-Albiol, J.; Ortega-Martínez, J.; Salomó-Coll, O.; López-Boado, A.P.; Paternostro-Betancourt, D.; Hernández-Alfaro, F. Mouth opening limitation and influence of age and surgical location for static fully guided dental implant placement: An observational, cross-sectional clinical study. Int. J. Oral Max. Surg. 2024, 53, 526–532. [Google Scholar] [CrossRef]
- Dave, M.; Tattar, R.; Patel, N. Medical considerations in the ageing implant patient. Oral Surgery 2024, 17, 59–66. [Google Scholar] [CrossRef]
- Karlsson, K.; Derks, J.; Wennström, J.L.; Petzold, M.; Berglundh, T. Health economic aspects of implant-supported restorative therapy. Clin Oral Implants Res. 2022, 33, 221–230. [Google Scholar] [CrossRef]
- Brägger, U.; Krenander, P.; Lang, N.P. Economic aspects of single-tooth replacement. Clin Oral Implants Res. 2005, 16, 335–341. [Google Scholar] [CrossRef]
- Massa, L.O.; Fraunhofer, J.A. Economics of Dental Implants. In The ADA Practical Guide to Dental Implants; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 139–143. [Google Scholar] [CrossRef]
- Losenická, J.; Gajdoš, O.; Kamenský, V. Cost-utility analysis of an implant treatment in dentistry. BMC Oral Health 2021, 21, 433. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Li, S.; Cai, Y.J.; Wei, T.; Ye, P. Smoking in relation to early dental implant failure: A systematic review and meta-analysis. J. Dent. 2024, 151, 105396. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Ofec, R.; Levin, L. Long term clinical performance of 10 871 dental implants with up to 22 years of follow-up: A cohort study in 4247 patients. Clin. Implant Dent. Relat. Res. 2021, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Dental Implants Market Growth & Trends. Available online: https://www.grandviewresearch.com/press-release/global-dental-implants-market (accessed on 28 October 2024).
- O’ Dwyer, S.; Riordain, R.N. The patient experience of dental implant surgery: A literature review of pertinent qualitative studies. Ir. J. Med. Sci. 2021, 190, 835–842. [Google Scholar] [CrossRef]
- Chakaipa, S.; Prior, S.J.; Pearson, S.; van Dam, P.J. Improving Patient Experience through Meaningful Engagement: The Oral Health Patient’s Journey. Oral 2023, 3, 499–510. [Google Scholar] [CrossRef]
- Improve competitive advantage, job satisfaction and the patient experience. Br. Dent. J. 2022, 232, 347. [CrossRef]
- Shrivastava, R.; Luxenberg, R.; Sutton, E.; Emami, E. Patients experience and satisfaction with immediate loading of implant-supported overdentures—A qualitative study. J. Dent. 2023, 137, 104644. [Google Scholar] [CrossRef]
- Stróż, A.; Dercz, G.; Chmiela, B.; Stróż, D.; Łosiewicz, B. Electrochemical Formation of Second Generation TiO2 Nanotubes on Ti13Nb13Zr Alloy for Biomedical Applications. Acta Phys. Pol. A 2016, 130, 1079–1080. [Google Scholar] [CrossRef]
- Smołka, A.; Dercz, G.; Rodak, K.; Łosiewicz, B. Evaluation of corrosion resistance of nanotubular oxide layers on the Ti13Zr13Nb alloy in physiological saline solution. Arch. Metall. Mater. 2015, 60, 2681–2686. [Google Scholar] [CrossRef]
- Smołka, A.; Rodak, K.; Dercz, G.; Dudek, K.; Łosiewicz, B. Electrochemical Formation of Self-Organized Nanotubular Oxide Layers on Ti13Zr13Nb Alloy for Biomedical Applications. Acta Phys. Pol. A 2014, 125, 932–935. [Google Scholar] [CrossRef]
- Szklarska, M.; Dercz, G.; Rak, J.; Simka, W.; Łosiewicz, B. The influence of passivation type on corrosion resistance of Ti15Mo alloy in simulated body fluids. Arch. Metall. Mater. 2015, 60, 2687–2693. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Stróż, A.; Kubisztal, J.; Osak, P.; Zubko, M. EIS and LEIS Study on In Vitro Corrosion Resistance of Anodic Oxide Nanotubes on Ti–13Zr–13Nb Alloy in Saline Solution. Coatings 2023, 13, 875. [Google Scholar] [CrossRef]
- Hosseini-Faradonbeh, S.A.; Katoozian, H.R. Biomechanical evaluations of the long-term stability of dental implant using finite element modeling method: A systematic review. J. Adv. Prosthodont. 2022, 14, 182–202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhou, Y.J.; Jiang, Y.B.; Tam, M.S.; Cheang, L.H.; Wang, H.J.; Zha, Z.G.; Zheng, X.F. Effect of Diabetes Mellitus on Implant Osseointegration of Titanium Screws: An Animal Experimental Study. Orthop Surg. 2022, 14, 1217–1228. [Google Scholar] [CrossRef]
- Osak, P.; Maszybrocka, J.; Kubisztal, J.; Ratajczak, P.; Łosiewicz, B. Long-Term Assessment of the In Vitro Corrosion Resistance of Biomimetic ACP Coatings Electrodeposited from an Acetate Bath. J. Funct. Biomater. 2021, 12, 12. [Google Scholar] [CrossRef]
- Shayeb, M.A.; Elfadil, S.; Abutayyem, H.; Shqaidef, A.; Marrapodi, M.M.; Cicciù, M.; Minervini, G. Bioactive surface modifications on dental implants: A systematic review and meta-analysis of osseointegration and longevity. Clin. Oral Investig. 2024, 28, 592. [Google Scholar] [CrossRef]
- Canullo, L.; Menini, M.; Pesce, P.; Iacono, R.; Sculean, A.; Del Fabbro, M. Nano-superhydrophilic and bioactive surface in poor bone environment. Part 1: Transition from primary to secondary stability. A controlled clinical trial. Clin. Oral Investig. 2024, 28, 372. [Google Scholar] [CrossRef]
- Meng, H.W.; Chien, E.Y.; Chien, H.H. Dental implant bioactive surface modifications and their effects on osseointegration: A review. Biomark. Res. 2016, 4, 24. [Google Scholar] [CrossRef]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.M.; Macedo de Sousa, B.; Herrero-Hernández, S.; López-Valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef]
- Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Ž. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings 2019, 9, 612. [Google Scholar] [CrossRef]
- Munisamy, S.; Vaidyanathan, T.K.; Vaidyanathan, J. A bone-like precoating strategy for implants: Collagen immobilization and mineralization on pure titanium implant surface. J. Oral Implantol. 2008, 34, 67–75. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, T.C.S.; Valverde, T.M.; Martins, T.M.d.M.; Oliveira, F.d.P.; Cunha, P.d.S.; Tavares, M.A.B.; Rodrigues, E.M.; Albergaria, J.D.S.; Vieira, G.M.; Gomes, D.A.; et al. Enhanced osteogenic response by collagen type I coating on surface-modified titanium bone implants. Mater. Today Commun. 2024, 39, 108535. [Google Scholar] [CrossRef]
- Belloni, A.; Argentieri, G.; Orilisi, G.; Notarstefano, V.; Giorgini, E.; D’Addazio, G.; Orsini, G.; Caputi, S.; Sinjari, B. New insights on collagen structural organization and spatial distribution around dental implants: A comparison between machined and laser-treated surfaces. J. Transl. Med. 2024, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Erturk, P.A.; Altuntas, S.; Irmak, G.; Buyukserin, F. Bioinspired Collagen/Gelatin Nanopillared Films as a Potential Implant Coating Material. ACS Appl. Bio Mater. 2022, 5, 4913–4921. [Google Scholar] [CrossRef]
- Petrović, Ž.; Šarić, A.; Despotović, I.; Katić, J.; Peter, R.; Petravić, M.; Ivanda, M.; Petković, M. Surface Functionalisation of Dental Implants with a Composite Coating of Alendronate and Hydrolysed Collagen: DFT and EIS Studies. Materials 2022, 15, 5127. [Google Scholar] [CrossRef]
- O’Neill, L.; Twomey, B.; Tan, F.; O’Donoghue, J.; Junt, J.A. Collagen Coating of Titanium Implants Using Non-thermal Plasma. Plasma Med. 2020, 11, 63–79. [Google Scholar] [CrossRef]
- Abdulghafor, M.A.; Mahmood, M.K.; Tassery, H.; Tardivo, D.; Falguiere, A.; Lan, R. Biomimetic Coatings in Implant Dentistry: A Quick Update. J. Funct. Biomater. 2024, 15, 15. [Google Scholar] [CrossRef]
- Lee, S.W.; Hahn, B.D.; Kang, T.Y.; Lee, M.J.; Choi, J.Y.; Kim, M.K.; Kim, S.G. Hydroxyapatite and collagen combination-coated dental implants display better bone formation in the peri-implant area than the same combination plus bone morphogenetic protein-2-coated implants, hydroxyapatite only coated implants, and uncoated implants. J. Oral Maxillofac. Surg. 2014, 72, 53–60. [Google Scholar] [CrossRef]
- Kolarovszki, B.; Ficsor, S.; Frank, D.; Katona, K.; Soos, B.; Turzo, K. Unlocking the potential: Laser surface modifications for titanium dental implants. Lasers Med. Sci. 2024, 39, 162. [Google Scholar] [CrossRef]
- Saran, R.; Ginjupalli, K.; George, S.D.; Chidangil, S.; Unnikrishnan, V.K. LASER as a tool for surface modification of dental biomaterials: A review. Heliyon 2023, 9, e17457. [Google Scholar] [CrossRef]
- Santos, A.F.P.; da Silva, R.C.; Hadad, H.; de Jesus, L.K.; Pereira-Silva, M.; Nímia, H.H.; Oliveira, S.H.P.; Guastaldi, A.C.; Queiroz, T.P.; Poli, P.P.; et al. Early Peri-Implant Bone Healing on Laser-Modified Surfaces with and without Hydroxyapatite Coating: An In Vivo Study. Biology 2024, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- The Use of Lasers for Dental Implant Surgery. Available online: https://www.deserthillsdental.com/dental-implants-and-laser-dentistry/ (accessed on 28 October 2024).
- Papa, S.; Maalouf, M.; Claudel, P.; Sedao, X.; Maio, Y.D.; Hamzeh-Cognasse, H.; Thomas, M.; Guignandon, A.; Dumas, V. Key topographic parameters driving surface adhesion of Porphyromonas gingivalis. Sci. Rep. 2023, 13, 15893. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, T.; Bakr, M.; Walsh, L.J.; George, R. Effects of lasers on titanium dental implant surfaces: A narrative review. Laser Dent. Sci. 2022, 6, 153–167. [Google Scholar] [CrossRef]
- Luczak, W.; Reiner-Rozman, C.; Muck, M.; Heitz, J.; Mitov, G.; Pfaffeneder, F.; See, C.; Hassel, A.W.; Kleber, C. Laser Treatment of Dental Implants toward an Optimized Osseointegration: Evaluation via Tapping-Mode Atomic Force Microscopy and Scanning Electron Microscopy. Phys. Status Solidi Appl. Mater. Sci. 2023, 220, 2200605. [Google Scholar] [CrossRef]
- Alamoudi, A. Nanoengineering and Surface Modifications of Dental Implants. Cureus 2024, 16, e51526. [Google Scholar] [CrossRef]
- Gulati, K. Nano-Engineering Solutions for Dental Implant Applications. Nanomaterials 2022, 12, 272. [Google Scholar] [CrossRef]
- Nagamoto, K.; Nakanishi, K.; Akasaka, T.; Abe, S.; Yoshihara, K.; Nakamura, M.; Hayashi, H.; Takemoto, S.; Tamura, M.; Kitagawa, Y.; et al. Investigation of a new implant surface modification using phosphorylated pullulan. Front. Bioeng. Biotechnol. 2024, 12, 1378039. [Google Scholar] [CrossRef]
- Karthik, K.; Thangaswamy, V. Evaluation of implant success: A review of past and present concepts. J. Pharm. Bioallied. Sci. 2013, 5 (Suppl. S1), S117–S119. [Google Scholar] [CrossRef]
- Han, W.; Fang, S.; Zhong, Q.; Qi, S. Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment. Coatings 2022, 12, 1654. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Cooper, L.F.; Shirazi, S. Osseointegration—The biological reality of successful dental implant therapy: A narrative review. Front. Oral Maxillofac. Med. 2022, 4, 39. [Google Scholar] [CrossRef]
- Lechner, J.; von Baehr, V.; Notter, F.; Schick, F. Osseointegration and osteoimmunology in implantology: Assessment of the immune sustainability of dental implants using advanced sonographic diagnostics: Research and case reports. J. Int. Med. Res. 2024, 52, 3000605231224161. [Google Scholar] [CrossRef] [PubMed]
- Simão, B.S., Jr.; Costa, D.D.; Cangussu, M.C.T.; Sotto-Maior, B.S.; Devita, R.L.; de Carvalho, J.J.; da Silva Brum, I. Observational Study on the Success Rate of Osseointegration: A Prospective Analysis of 15,483 Implants in a Public Health Setting. BioMed 2022, 2, 422–430. [Google Scholar] [CrossRef]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An update. J. Indian Prosthodont. Soc. 2013, 13, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I.; Zarb, G.A.; Albrekson, T. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Quintessence Publishing Company: Batavia, IL, USA, 1985. [Google Scholar]
- Gill, T.; Kühl, S.; Rawlinson, S.; Pippenger, B.; Bellon, B.; Shahdad, S. Primary stability and osseointegration comparing a novel tapered design tissue-level implant with a parallel design tissue-level implant. An experimental in vivo study. Clin. Oral Implants Res. 2024, 35, 1114–1127. [Google Scholar] [CrossRef] [PubMed]
- Lioubavina-Hack, N.; Lang, N.P.; Karring, T. Significance of primary stability for osseointegration of dental implants. Clin. Oral Implants Res. 2006, 17, 244–250. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Xu, L.; Jacobs, R.; Cao, Y.; Sun, X.; Qin, X. Tissue-engineered bone construct promotes early osseointegration of implants with low primary stability in oversized osteotomy. BMC Oral Health 2024, 24, 69. [Google Scholar] [CrossRef]
- Lee, J.; Lim, Y.-J.; Ahn, J.-S.; Kim, B.; Baek, Y.-W.; Lim, B.-S. Correlation of two different devices for the evaluation of primary implant stability depending on dental implant length and bone density: An in vitro study. PLoS ONE 2024, 19, e0290595. [Google Scholar] [CrossRef]
- Barikani, H.; Rashtak, S.; Akbari, S.; Badri, S.; Daneshparvar, N.; Rokn, A. The effect of implant length and diameter on the primary stability in different bone types. J. Dent. 2013, 10, 449–455. [Google Scholar]
- Stoilov, M.; Shafaghi, R.; Stark, H.; Marder, M.; Kraus, D.; Enkling, N. Influence of Implant Macro-Design, -Length, and -Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols-An In Vitro Analysis. J. Funct. Biomater. 2023, 14, 469. [Google Scholar] [CrossRef]
- Gómez-Polo, M.; Ortega, R.; Gómez-Polo, C.; Martín, C.; Celemín, A.; del Río, J. Does Length, Diameter, or Bone Quality Affect Primary and Secondary Stability in Self-Tapping Dental Implants? J. Oral Maxillofac. Surgery 2016, 74, 1344–1353. [Google Scholar] [CrossRef]
- Cucinelli, C.; Pereira, M.S.; Borges, T.; Figueiredo, R.; Leitão-Almeida, B. The Effect of Increasing Thread Depth on the Initial Stability of Dental Implants: An In Vitro Study. Surgeries 2024, 5, 817–825. [Google Scholar] [CrossRef]
- Hiranmayi, V.K. Factors influencing implant stability. J. Dent. Implants 2018, 8, 69–76. [Google Scholar] [CrossRef]
- Huang, S.; Murphy, L.; Xu, W. Genes and functions from breast cancer signatures. BMC Cancer 2018, 18, 473. [Google Scholar] [CrossRef]
- El-Anwar, M.I.; El-Zawahry, M.M.; El-Mofty, M. Load Transfer on Dental Implants and Surrounding Bones. Aust. J. Basic Appl. Sci. 2012, 6, 551–560. [Google Scholar]
- Hansson, S.; Norton, M. The relation between surface roughness and interfacial shear strength for bone-anchored implants. A mathematical model. J. Biomech. 1999, 32, 829–836. [Google Scholar] [CrossRef]
- Bianchi, A.E.; Dolci, G., Jr.; Sberna, M.T.; Sanfilippo, F. Factors affecting bone response around loaded titanium dental implants: A literature review. J. Appl. Biomater. Biomech. 2005, 3, 135–140. [Google Scholar]
- Stanford, C.M. Surface modifications of dental implants. Aust. Dent. J. 2008, 53, S26–S33. [Google Scholar]
- Skalak, R.; Zhao, Y. Interaction of force-fitting and surface roughness of implants. Clin. Implant Dent. Relat. Res. 2000, 2, 219–224. [Google Scholar] [CrossRef]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef]
- Romero, M.; Herrero-Climent, M.; Ríos-Carrasco, B.; Brizuela, A.; Romero, M.M.; Gil, J. Investigation of the Influence of Roughness and Dental Implant Design on Primary Stability via Analysis of Insertion Torque and Implant Stability Quotient: An In Vitro Study. J. Clin. Med. 2023, 12, 4190. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layeolle, P.; Amourinq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.H.; Kazemian, M.; Ghorbanzadeh, S. A brief overview of cellular and molecular mechanisms of osseointegration. Int. J. Contemp. Dent. Med. Rev. 2015, 12, 13. [Google Scholar]
- Yu, M.; Yang, H.; Li, B.; Wang, R.; Han, Y. Molecular mechanisms of interrod spacing-mediated osseointegration via modulating inflammatory response and osteogenic differentiation. Chem. Eng. J. 2023, 454, 140141. [Google Scholar] [CrossRef]
- Nishimura, I. Genetic Networks in Osseointegration. J. Dent. Res. 2013, 92 (Suppl. 12), 109S–118S. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.; Wang, H.; Chen, X.; Shuai, Y.; Wang, H.; Mao, Y.; He, F. Mesenchymal stem cells and dental implant osseointegration during aging: From mechanisms to therapy. Stem. Cell Res. Ther. 2023, 14, 382. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin. Implant Dent. Relat. Res. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Yin, X.; Yang, C.; Wang, Z.; Zhang, Y.; Li, Y.; Weng, J.; Feng, B. Alginate/chitosan modified immunomodulatory titanium implants for promoting osteogenesis in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112087. [Google Scholar] [CrossRef]
- Wu, J.; Chen, M.; Xiao, Y.; Yang, H.; Wang, G.; Zhang, X.; Dai, L.; Yuan, Z. The Bioactive Interface of Titanium Implant with Both Anti-Oxidative Stress and Immunomodulatory Properties for Enhancing Osseointegration under Diabetic Condition. Adv. Healthc. Mater. 2024, 13, e2401974. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Qiu, J.; Zhang, X.; Liu, X.; Qiao, Y.; Liu, X. Synergistic effects of immunoregulation and osteoinduction of ds-block elements on titanium surface. Bioact. Mater. 2020, 6, 191–207. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Y.; Yu, L.; Liu, K.; Fei, Y.; Guo, C.; Zhou, Y.; Hu, J.; Shi, L.; Ji, H. Inhibition of Inflammatory Response and Promotion of Osteogenic Activity of Zinc-Doped Micro-Arc Titanium Oxide Coatings. ACS Omega 2022, 7, 14920–14932. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, W.; Zhou, W.; Zhang, S.; Li, M.; Li, N.; Pan, G.; Zhang, X.; Bai, J.; Zhu, C. Immunomodulatory biomaterials for implant-associated infections: From conventional to advanced therapeutic strategies. Biomater. Res. 2022, 26, 72. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.L.; Yun, S.; Lee, H.; Cao, H.L.; Woo, S.H.; Jeong, Y.H.; Jung, T.G.; Kim, C.M.; Choung, P.H. Osseointegration of 3D-printed titanium implants with surface and structure modifications. Dent. Mater. 2022, 38, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today Proc. 2021, 39, 84–90. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Wennerberg, A.; Lindhe, J. Bone and soft tissue integration to titanium implants with different surface topography: An experimental study in the dog. Int. J. Oral Maxillofac. Implant. 2001, 16, 323–332. [Google Scholar]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2022, 7, e10239. [Google Scholar] [CrossRef]
- Ikeda, E.; Tsuji, T. Growing bioengineered teeth from single cells: Potential for dental regenerative medicine. Expert Opin. Biol. Ther. 2008, 8, 735–744. [Google Scholar] [CrossRef]
- Biguetti, C.C.; Cavalla, F.; Silveira, E.M.; Fonseca, A.C.; Vieira, A.E.; Tabanez, A.P.; Rodrigues, D.C.; Trombone, A.P.F.; Garlet, G.P. Oral implant osseointegration model in C57Bl/6 mice: Microtomographic, histological, histomorphometric and molecular characterization. J. Appl. Oral Sci. 2017, 1, e20170601. [Google Scholar] [CrossRef]
- Perlman, R.L. Mouse models of human disease: An evolutionary perspective. Evol. Med. Public Health 2016, 1, 170–176. [Google Scholar] [CrossRef]
- Setiawati, R.; Rahardjo, P. Bone Development and Growth; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Scarano, A.; Khater, A.G.A.; Gehrke, S.A.; Inchingolo, F.; Tari, S.R. Animal Models for Investigating Osseointegration: An Overview of Implant Research over the Last Three Decades. J. Funct. Biomater. 2024, 15, 83. [Google Scholar] [CrossRef]
- Pazzaglia, U.E. Periosteal and endosteal reaction to reaming and nailing: The possible role of revascularization on the endosteal anchorage of cementless stems. Biomaterials 1996, 17, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Quarterman, J.C.; Phruttiwanichakun, P.; Fredericks, D.C.; Salem, A.K. Zoledronic Acid Implant Coating Results in Local Medullary Bone Growth. Mol. Pharm. 2022, 19, 4654–4664. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, A.J.; Anderson, D.E.; Samii, V.F.; Weisbrode, S.E.; Johnson, J.K.; Noon, M.S.; Tomasko, D.L.; Lannutti, J.L. Effects of orthopedic implants with a polycaprolactone polymer coating containing bone morphogenetic protein-2 on osseointegration in bones of sheep. Am. J. Vet. Res. 2009, 70, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. Bone Healing in the Presence of Orthopaedic Implants. In Handbook of Orthopaedic Trauma Implantology; Banerjee, A., Biberthaler, P., Shanmugasundaram, S., Eds.; Springer: Singapore, 2023; pp. 869–904. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- Gupta, S. Bone Healing in the Presence of Orthopedic Implants. In Handbook of Orthopaedic Trauma Implantology; Banerjee, A., Biberthaler, P., Shanmugasundaram, S., Eds.; Springer: Singapore, 2022; pp. 1–36. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y. Biomaterials Regulating Bone Hematoma for Osteogenesis. Adv. Healthc. Mater. 2020, 9, e2000726. [Google Scholar] [CrossRef]
- Shiu, H.T.; Leung, P.C.; Ko, C.H. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J. Tissue Eng. Regen. Med. 2018, 12, e1911–e1925. [Google Scholar] [CrossRef]
- Milillo, L.; Cinone, F.; Lo Presti, F.; Lauritano, D.; Petruzzi, M. The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil. Materials 2021, 14, 6642. [Google Scholar] [CrossRef]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef]
- Pathak, U.S.; Balasubramanian, A.; Beilan, J.A.; Butaney, M.; Tatem, A.J.; Thirumavalavan, N.; Lipshultz, L.I. Vasoepididymostomy: An insight into current practice patterns. Transl. Androl. Urol. 2019, 8, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Proctor, A.R.; Ren, J.; Benoit, D.S.W.; Choe, R. Temporal blood flow changes measured by diffuse correlation tomography predict murine femoral graft healing. PLoS ONE 2018, 13, e0197031. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.; Stevens, R.; McGrath, K.M. Towards the Development of Artificial Bone Grafts: Combining Synthetic Biomineralisation with 3D Printing. J. Funct. Biomater. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Scridon, A. Platelets and Their Role in Hemostasis and Thrombosis-From Physiology to Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef]
- de Sousa Gomes, P.; Daugela, P.; Poskevicius, L.; Mariano, L.; Fernandes, M.H. Molecular and Cellular Aspects of Socket Healing in the Absence and Presence of Graft Materials and Autologous Platelet Concentrates: A Focused Review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis. Res. Ther. 2007, 9 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Yun, J.H.; Han, S.H.; Choi, S.H.; Lee, M.H.; Lee, S.J.; Song, S.U.; Oh, N. Effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration for osseointegration of dental implants: Preliminary study in canine three-wall intrabony defects. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1021–1030. [Google Scholar] [CrossRef]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell Res. Ther. 2019, 10, 254. [Google Scholar] [CrossRef]
- Fernandes, G.; Yang, S. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 2016, 4, 16036. [Google Scholar] [CrossRef]
- Aniołek, K.; Łosiewicz, B.; Kubisztal, J.; Osak, P.; Stróż, A.; Barylski, A.; Kaptacz, S. Mechanical Properties, Corrosion Resistance and Bioactivity of Oxide Layers Formed by Isothermal Oxidation of Ti-6Al-7Nb Alloy. Coatings 2021, 11, 505. [Google Scholar] [CrossRef]
- Alla, R.K.; Ginjupalli, K.; Upadhya, N.; Mohammed, S.; Sekar, R.; Ravi, R. Surface Roughness of Implants: A Review. Trends Biomater. Artif. Organs 2011, 25, 112–118. [Google Scholar]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Stach, S. Effect of Autoclaving Time on Corrosion Resistance of Sandblasted Ti G4 in Artificial Saliva. Materials 2020, 13, 4154. [Google Scholar] [CrossRef]
- Stróż, A.; Maszybrocka, J.; Goryczka, T.; Dudek, K.; Osak, P.; Łosiewicz, B. Influence of Anodizing Conditions on Biotribological and Micromechanical Properties of Ti–13Zr–13Nb Alloy. Materials 2023, 16, 1237. [Google Scholar] [CrossRef] [PubMed]
- Osak, P.; Maszybrocka, J.; Kubisztal, J.; Łosiewicz, B. Effect of amorphous calcium phosphate coatings on tribological properties of titanium grade 4 in protein-free artificial saliva. Biotribology 2022, 32, 100219. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Bogunia, S.; Ratajczak, P.; Aniołek, K. Effect of Temperature on Electrochemically Assisted Deposition and Bioactivity of CaP Coatings on CpTi Grade 4. Materials 2021, 14, 5081. [Google Scholar] [CrossRef] [PubMed]
- Cylindrical Dental Implant MACHINED WINSIX®. Available online: https://www.medicalexpo.com/prod/biosaf/product-124601-1082647.html (accessed on 28 October 2024).
- Ballo, A.M.; Omar, O.; Xia, W.; Palmquist, A. Dental Implant Surfaces—Physicochemical Properties, Biological Performance, and Trends; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Cochran, D.L. A comparison of endosseous dental implant surfaces. J. Periodontol. 1999, 70, 1523–1539. [Google Scholar] [CrossRef]
- Hong, D.G.K.; Oh, J.H. Recent advances in dental implants. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 33. [Google Scholar] [CrossRef]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Pham, M.H.; Haugen, H.J.; Reseland, J.E. Fluoride Modification of Titanium Surfaces Enhance Complement Activation. Materials 2020, 13, 684. [Google Scholar] [CrossRef]
- Addy, L. An introduction to dental implants. Br. Dent. J. 2024, 236, 753–757. [Google Scholar] [CrossRef]
- Vörös, J.; Wieland, M.; Ruiz-Taylor, L.; Textor, M.; Brunette, D.M. Characterization of Titanium Surfaces. In Titanium in Medicine. Engineering Materials; Brunette, D.M., Tengvall, P., Textor, M., Thomson, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; p. 114. [Google Scholar] [CrossRef]
- Machined Surface Coronal (MSc) Dental Implants. Available online: https://southernimplants.com/solutions/innovative-products/msc-implants/ (accessed on 28 October 2024).
- Brånemark System® Mk III. Available online: https://store.nobelbiocare.com/us/en/media/eifu/IFU1014_EN_US_00.pdf (accessed on 28 October 2024).
- Bredent Medical. Available online: https://www.medicalexpo.com.cn/prod/bredent-medical/product-71642-1088938.html (accessed on 28 October 2024).
- Fousová, M.; Vojtech, D.; Jablonska, E.; Fojt, J.; Lipov, J. Novel Approach in the Use of Plasma Spray: Preparation of Bulk Titanium for Bone Augmentations. Materials 2017, 10, 987. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Hu, R.; Shi, B.; Zhang, L.; Huang, Q.; Yang, Y.; Tang, P.; Lin, C. Advanced surface engineering of titanium materials for biomedical applications: From static modification to dynamic responsive regulation. Bioact. Mater. 2023, 27, 15–57. [Google Scholar] [CrossRef] [PubMed]
- Cizek, J.; Matejicek, J. Medicine Meets Thermal Spray Technology: A Review of Patents. J. Therm. Spray Tech 2018, 27, 1251–1279. [Google Scholar] [CrossRef]
- IMZ Original. Available online: https://www.spotimplant.com/en/dental-implants/imz/imz-original (accessed on 28 October 2024).
- Seidling, R.; Lehmann, L.J.; Lingner, M.; Mauermann, E.; Obertacke, U.; Schwarz, M.L.R. Analysis of the osseointegrative force of a hyperhydrophilic and nanostructured surface refinement for TPS surfaces in a gap healing model with the Göttingen minipig. J. Orthop. Surg. Res. 2016, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.A.; Valiquette, N.; Pillar, R.M. Osseointegration of Sintered Porous-surfaced and Plasma Spray–Coated Implants: An Animal Model Study of Early Postimplantation Healing Response and Mechanical Stability. J. Biomed. Mater. Res. 1999, 47, 127–138. [Google Scholar] [CrossRef]
- Hadzik, J.; Jurczyszyn, K.; Gębarowski, T.; Trytek, A.; Gedrange, T.; Kozakiewicz, M.; Dominiak, M.; Kubasiewicz-Ross, P.; Trzcionka-Szajna, A.; Szajna, E.; et al. An Experimental Anodized and Low-Pressure Oxygen Plasma-Treated Titanium Dental Implant Surface-Preliminary Report. Int. J. Mol. Sci. 2023, 24, 3603. [Google Scholar] [CrossRef]
- Rodriguez y Baena, R.; Rizzo, S.; Manzo, L.; Lupi, S.M. Nanofeatured Titanium Surfaces for Dental Implantology: Biological Effects, Biocompatibility, and Safety. J. Nanomater. 2017, 2017, 6092895. [Google Scholar] [CrossRef]
- Vasilev, O.; Hayles, A.; Campbell, D.; Jaarsma, R.; Johnson, L.; Vasilev, K. Nanoscale antibacterial coatings incorporating silver nanoparticles derived by plasma techniques—A state-of-the-art perspective. Mater. Today Chem. 2024, 41, 102341. [Google Scholar] [CrossRef]
- Ten Good Reasons for IMZ®-TwinPlus—DENTSPLY Friadent. Available online: https://www.yumpu.com/en/document/view/9007132/ten-good-reasons-for-imzr-twinplus-dentsply-friadent (accessed on 28 October 2024).
- Bruggenkate, C.M.; Sutter, F.; Schroeder, A.; Oosterbeek, H.S. Explantation procedure in the F-type and Bonefit ITI implant system. Int. J. Oral Maxillofac. Surg. 1991, 20, 155–158. [Google Scholar] [CrossRef]
- Lifecore Dental in the Restore TPS System. In Brief: Lifecore. Available online: https://insights.citeline.com/MT003637/In-Brief-Lifecore/ (accessed on 28 October 2024).
- Steri-Oss®. Available online: https://www.spotimplant.com/en/dental-implants/steri-oss (accessed on 28 October 2024).
- Fintová, S.; Kuběna, I.; Palán, J.; Mertová, K.; Duchek, M.; Hutař, P.; Pastorek, F.; Kunz, L. Influence of Sandblasting and Acid Etching on Fatigue Properties of Ultra-Fine Grained Ti Grade 4 for Dental Implants. J. Mech. Behav. Biomed. Mater. 2020, 111, 104016. [Google Scholar] [CrossRef]
- Kim, H.-K.; Ahn, B. Effect of Al2O3 Sandblasting Particle Size on the Surface Topography and Residual Compressive Stresses of Three Different Dental Zirconia Grades. Materials 2021, 14, 610. [Google Scholar] [CrossRef]
- Gil, F.; Pérez, R.; Olmos, J.; Herraez-Galindo, C.; Gutierrez-Pérez, J.; Torres-Lagares, D. The Effect of Using Al2O3 and TiO2 in Sandblasting of Titanium Dental Implants. J. Mater. Res. 2022, 37, 2604–2613. [Google Scholar] [CrossRef]
- Guo, C.Y.; Matinlinna, J.P.; Tang, A.T. Effects of surface charges on dental implants: Past, present, and future. Int. J. Biomater. 2012, 2012, 381535. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewska-Kuska, M.; Leda, B.; Gajdus, P.; Hedzelek, W. Evaluation of modified titanium surfaces physical and chemical characteristics. Nucl. Instrum. Methods Phys. Res. B 2017, 411, 94–99. [Google Scholar] [CrossRef]
- Kasemo, B.; Lausmaa, J. Surface science aspects on inorganic biomaterials. Crit. Rev. Biocompat. 1986, 2, 335–380. [Google Scholar]
- Prima Plus 4.1 (RD). Available online: https://osseosource.com/prima-plus-4-1-rd-/p-3167.html (accessed on 28 October 2024).
- Osteoplant Hex. Available online: https://www.spotimplant.com/en/dental-implants/osteoplant/osteoplant-hex (accessed on 28 October 2024).
- Collaert, B.; De Bruyn, H. Immediate functional loading of TiOblast dental implants in full-arch edentulous mandibles: A 3-year prospective study. Clin. Oral Implants Res. 2008, 19, 1254–1260. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Kämmerer, P.W.; Morbach, T.; Ladwein, C.; Wegener, J.; Wagner, W. Ten-Year Retrospective Follow-Up Study of the TiOblast™ Dental Implant. Clin. Implant Dent. Rel. Res. 2012, 14, 127–134. [Google Scholar] [CrossRef]
- Ferguson, R. Renova Internal Hex Implant System: Surgical and restorative versatility. Dent. Implantol. Update 2005, 16, 49–54. [Google Scholar]
- Che Isa, N.N.; Mohd, Y.; Yury, N. Electrochemical Deposition and Characterization of Hydroxyapatite (HAp) on Titanium Substrate. APCBEE Procedia 2012, 3, 46–52. [Google Scholar] [CrossRef]
- Usinskas, P.; Stankeviciute, Z.; Beganskiene, A.; Kareiva, A. Sol-Gel Derived Porous and Hydrophilic Calcium Hydroxyapatite Coating on Modified Titanium Substrate. Surf. Coat. Technol. 2016, 307 Pt A, 935–940. [Google Scholar] [CrossRef]
- Jaafar, A.; Schimpf, C.; Mandel, M.; Hecker, C.; Rafaja, D.; Krüger, L.; Arki, P.; Joseph, Y. Sol–gel derived hydroxyapatite coating on titanium implants: Optimization of sol–gel process and engineering the interface. J. Mater. Res. 2022, 37, 2558–2570. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hędzelek, W.; Dorocka-Bobkowska, B. Hydroxyapatite coating on titanium endosseous implants for improved osseointegration: Physical and chemical considerations. Adv. Clin. Exp. Med. 2018, 27, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Świeczko-Żurek, B.; Bartmański, M. Investigations of Titanium Implants Covered with Hydroxyapatite Layer. Adv. Mater. Sci. 2016, 16, 78–86. [Google Scholar] [CrossRef]
- Kuroda, K.; Okido, M. Hydroxyapatite coating of titanium implants using hydroprocessing and evaluation of their osteoconductivity. Bioinorg. Chem. Appl. 2012, 2012, 730693. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Sandu, A.V.; Nabialek, M.; Vizureanu, P.; Ciobanu, G. Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys. Micromachines 2021, 12, 1447. [Google Scholar] [CrossRef]
- Park, Y.S.; Yi, K.Y.; Lee, I.S.; Han, C.H.; Jung, Y.C. The Effects of Ion Beam– Assisted Deposition of Hydroxyapatite on the Grit-Blasted Surface of Endosseous Implants in Rabbit Tibiae. Int. J. Oral Maxillofac. Implants 2005, 20, 31–38. [Google Scholar]
- 3i T3 Implant. Available online: https://www.dentalproductshopper.com/implants-edentulous-solutions/implants/3i-t3-implant (accessed on 28 October 2024).
- Mautsch, C.; Wolfart, S.; Mautsch, W.; Rittich, A.B. Long-term outcome of the IMZ implant system: A retrospective clinical study with a follow-up between 23 and 34 years. Int. J. Implant Dent. 2022, 8, 54. [Google Scholar] [CrossRef]
- Kallus, T.; Bessing, C.; Homsi, G.; Eklund, I. Five-year evaluation of Lifecore Restore implants: A retrospective comparison with Nobel Biocare MK II implants. Clin. Implant Dent. Relat. Res. 2009, 11, 167–177. [Google Scholar] [CrossRef]
- Nobel Replace External Hex (Steri-Oss). Available online: https://www.spotimplant.com/en/dental-implants/nobel-biocare/nobel-replace-external-hex-steri-oss (accessed on 28 October 2024).
- Petrini, M.; Giuliani, A.; Di Campli, E.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; D’Ercole, S. The Bacterial Anti-Adhesive Activity of Double-Etched Titanium (DAE) as a Dental Implant Surface. Int. J. Mol. Sci. 2020, 21, 8315. [Google Scholar] [CrossRef]
- Xie, Y.; Zuo, J.; Zhou, B.; Ma, L.; Yu, Z.M.; Wei, Q.; Tang, Z.G. Sandblast-free double-etched titanium for dental implants application. Mater. Lett. 2016, 176, 74–77. [Google Scholar] [CrossRef]
- Giner, L.; Mercadé, M.; Torrent, S.; Punset, M.; Pérez, R.A.; Delgado, L.M.; Gil, F.J. Double acid etching treatment of dental implants for enhanced biological properties. J. Appl. Biomater. Funct. Mater. 2018, 16, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Santos Marino, J.; Cortés-Bretón Brinkmann, J.; García-Gil, I.; Martínez-Rodríguez, N.; Fraile, J.F.; Barona Dorado, C.; Martínez-González, J.M. Clinical Evaluation of Dental Implants with a Double Acid-Etched Surface Treatment: A Cohort Observational Study with Up to 10-Year Follow-Up. Materials 2021, 14, 6483. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, J.S.; David, S.R.N.; Zulhilmi, N.R.; Dhaliwal, S.K.S.; Knights, J.; Junior, R.F.d.A. Contamination of titanium dental implants: A narrative review. SN. Appl. Sci. 2020, 2, 1011. [Google Scholar] [CrossRef]
- The Osseotite® Dental Implant System. Available online: https://www.zimvie.com/en/dental/dental-implant-systems/3i-osseotite-implant.html (accessed on 28 October 2024).
- Osseotite® Implant Reference List. Available online: https://www.biomax.it/wp-content/uploads/2020/10/ZB0120_OsseotiteImplantReference_EN.pdf (accessed on 28 October 2024).
- The Osseotite® Implant. Available online: https://www.biomet3i.cz/userFiles/pdf/zb0067_rev_a_osseotite_implant_brochure_final_secured.pdf (accessed on 28 October 2024).
- del Olmo, R.; Czerwiński, M.; Santos-Coquillat, A.; Dubey, V.; Dhoble, S.J.; Michalska-Domańska, M. Nano-scale Surface Modification of Dental Implants: Fabrication. In Surface Modification of Titanium Dental Implants; Gulati, K., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Rungcharassaeng, K.; Kan, J.Y.K. Fabricating a stable record base for completely edentulous patients treated with osseointegrated implants using healing abutments. J. Prosthet. Dent. 1999, 81, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, Y.H.; Herr, Y.; Shin, S.; Chung, J.H. Effect of Erbium-Doped: Yttrium, Aluminium and Garnet Laser Irradiation on the Surface Microstructure and Roughness of Sand-Blasted, Large Grit, Acid-Etched Implants. J. Periodontal Implant. Sci. 2011, 41, 135–142. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Ortiz-Garcia, I.; Jiménez-Guerra, A.; Núñez-Márquez, E.; Moreno-Muñoz, J.; Rondón-Romero, J.L.; Cabanillas-Balsera, D.; Gil, J.; Muñoz-Guzón, F.; Monsalve-Guil, L. Osseointegration of Sandblasted and Acid-Etched Implant Surfaces. A Histological and Histomorphometric Study in the Rabbit. Int. J. Mol. Sci. 2021, 22, 8507. [Google Scholar] [CrossRef]
- Muhammed, H.A.; Mahmoud, E.M.; Fahmy, A.E.; Nasr, D.M. The effect of sandblasting versus acid etching on the surface roughness and biaxial flexural strength of CAD/CAM resin-matrix ceramics (In vitro study). BMC Oral Health 2023, 23, 169. [Google Scholar] [CrossRef]
- Patcas, R.; Zinelis, S.; Eliades, G.; Eliades, T. Surface and interfacial analysis of sandblasted and acid-etched enamel for bonding orthodontic adhesives. Am. J. Orthod. Dentofacial Orthop. 2015, 147 (Suppl. 4), S64–S75. [Google Scholar] [CrossRef]
- Bok, W.M.; Kim, S.Y.; Lee, S.J.; Shin, G.-S.; Park, J.-M.; Lee, M.-H. Surface characteristics and bioactivation of sandblasted and acid-etched (SLA) Ti-10Nb-10Ta alloy for dental implant. Int. J. Precis. Eng. Manuf. 2015, 16, 2185–2192. [Google Scholar] [CrossRef]
- Dental News®. Implant Standard. Available online: https://dentalnews.pl/produkt/implant-standard/ (accessed on 28 October 2024).
- Stafford, G.L. Review Found Little Difference between Sandblasted and Acid-etched (SLA) Dental Implants and Modified Surface (SLActive) Implants. Evid. Based Dent. 2014, 15, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Schupbach, P.; Glauser, R.; Bauer, S. Al2O3 Particles on Titanium Dental Implant Systems following Sandblasting and Acid-Etching Process. Int. J. Biomater. 2019, 1, 6318429. [Google Scholar] [CrossRef] [PubMed]
- Spiral (SPI) By Alpha Bio Tec®. Available online: https://www.spotimplant.com/en/dental-implants/alpha-bio-tec/spi (accessed on 28 October 2024).
- About the DFI Implant. Available online: https://info.alpha-bio.net/dfi-implant (accessed on 28 October 2024).
- Rocci, M.; Rocci, A.; Martignoni, M.; Albrektsson, T.; Barlattani, A.; Gargari, M. Comparing the TiOblast and Osseospeed surfaces. Histomorphometric and histological analysis in humans. Oral Implantol. 2008, 1, 34–42. [Google Scholar]
- Straumann Group. Available online: https://www.straumann.com/group/en/home/about/our-history.html (accessed on 22 December 2024).
- Wennerberg, A.; Galli, S.; Albrektsson, T. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin. Cosmet. Investig. Dent. 2011, 3, 59–67. [Google Scholar] [CrossRef]
- Zinelis, S.; Silikas, N.; Thomas, A.; Syres, K.; Eliades, G. Surface characterization of SLActive dental implants. Eur. J. Esthet. Dent. 2012, 7, 72–92. [Google Scholar]
- SLActive. Available online: http://www.schmidt-dental.pl/wp-content/uploads/2015/11/Straumann_SLActive_Studies.pdf (accessed on 28 October 2024).
- Romero-Ruiz, M.M.; Gil-Mur, F.J.; Ríos-Santos, J.V.; Lázaro-Calvo, P.; Ríos-Carrasco, B.; Herrero-Climent, M. Influence of a Novel Surface of Bioactive Implants on Osseointegration: A Comparative and Histomorfometric Correlation and Implant Stability Study in Minipigs. Int. J. Mol. Sci. 2019, 20, E2307. [Google Scholar] [CrossRef]
- Straumann® SLActive®. Beyond Hydrophilicity—The Science of High Performance. Available online: https://www.straumann.com/en/discover/slactive.html (accessed on 28 October 2024).
- Distinct Nano-Structures Present on the SLActive® Surface27,28. Available online: https://www.straumann.com/en/discover/slactive.html (accessed on 28 October 2024).
- Advanced In-Vitro Research Shows Nano-Structure Support Early Osseointegration23,24. Available online: https://www.straumann.com/en/discover/slactive.html (accessed on 28 October 2024).
- Baier, R.E.; Meyer, A.E. Future directions in surface preparation of dental implants. J. Dent. Educ. 1988, 52, 788–791. [Google Scholar] [CrossRef]
- Şener, I.; Yamaner, G.; Sertgoz, A. Clinical Outcomes of Patients Treated with SLA and SLActive Implants. In Proceedings of the IADR/PER General Session 2010, Barcelona, Spain, 14–17 July 2010. [Google Scholar]
- Birch, J.; Burleigh, T. Oxides Formed on Titanium by Polishing, Etching, Anodizing, or Thermal Oxidizing. Corrosion 2000, 56, 1233–1241. [Google Scholar] [CrossRef]
- Huang, Y.H.; Xiropaidis, A.; Sorensen, R.; Hall, J.; Wikesjö, U. Bone Formation at Titanium Porous Oxide (TiUnite (TM)) Oral Implants in Type IV Bone. Clin. Oral Implants Res. 2005, 16, 105–111. [Google Scholar] [CrossRef]
- Badekas, H.; Panagopoulos, C. Titanium anodization under constant voltage conditions. Surf. Coat. Technol. 1987, 31, 381–388. [Google Scholar] [CrossRef]
- Nowińska, D.; Osak, P.; Maszybrocka, J.; Łosiewicz, B. Anodic Production and Characterization of Biomimetic Oxide Layers on Grade 4 Titanium for Medical Applications. J. Funct. Biomater. 2024, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.; Ouyang, J.; Chen, X.; Xu, Z.; Sun, X. Bioactivity and Osteogenic Cell Response of TiO2 Nanotubes Coupled with Nanoscale Calcium Phosphate via Ultrasonification-Assisted Electrochemical Deposition. Appl. Surf. Sci. 2014, 305, 24–32. [Google Scholar] [CrossRef]
- Kahar, S.; Singh, A.; Patel, V.; Kanetkar, U. Anodizing of Ti and Ti Alloys for Different Applications: A Review. Int. J. Sci. Res. Dev. 2020, 8, 272–276. [Google Scholar]
- Replace Select Tapered TiUnite RP 4.3 × 13 mm. Available online: https://store.nobelbiocare.com/us/en/replace-select-tapered-tiunite-rp-4-3-x-13-mm (accessed on 28 October 2024).
- McCracken, M. Dental implant materials: Commercially pure titanium and titanium alloys. J. Prosthodont. 1999, 8, 40–43. [Google Scholar] [CrossRef]
- Product Catalog 2017/18 Complete Assortment. Available online: https://www.nobelbiocare.com/sites/g/files/wdvifx201/files/81206_ProdCatalog2017-18_GB.pdf (accessed on 28 October 2024).
- Maló, P.; de Araújo Nobre, M.; Gonçalves, Y.; Lopes, A.; Ferro, A. Immediate Function of Anodically Oxidized Surface Implants (TiUnite™) for Fixed Prosthetic Rehabilitation: Retrospective Study with 10 Years of Follow-Up. BioMed Res. Int. 2016, 2016, 2061237. [Google Scholar] [CrossRef]
- Traini, T.; Murmura, G.; Sinjari, B.; Perfetti, G.; Scarano, A.; D’Arcangelo, C.; Caputi, S. The Surface Anodization of Titanium Dental Implants Improves Blood Clot Formation Followed by Osseointegration. Coatings 2018, 8, 252. [Google Scholar] [CrossRef]
- Karl, M.; Albrektsson, T. Clinical performance of dental implants with a moderately rough (TiUnite) surface: A meta-analysis of prospective clinical studies. Int. J. Oral Maxillofac. Implants. 2017, 32, 717–734. [Google Scholar] [CrossRef]
- Jungner, M.; Lundqvist, P.; Lundgren, S. Oxidized titanium implants (Nobel Biocare TiUnite) compared with turned titanium implants (Nobel Biocare mark III) with respect to implant failure in a group of consecutive patients treated with early functional loading and two-stage protocol. Clin. Oral Implants Res. 2005, 16, 308–312. [Google Scholar] [CrossRef]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloys Compd. 2023, 948, 169773. [Google Scholar] [CrossRef]
- Ming, X.; Wu, Y.; Zhang, Z.; Li, Y. Micro-Arc Oxidation in Titanium and Its Alloys: Development and Potential of Implants. Coatings 2023, 13, 2064. [Google Scholar] [CrossRef]
- Wen, X.; Liu, Y.; Xi, F.; Zhang, X.; Kang, Y. Micro-arc oxidation (MAO) and its potential for improving the performance of titanium implants in biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1282590. [Google Scholar] [CrossRef] [PubMed]
- Friedemann, A.E.R.; Thiel, K.; Haßlinger, U.; Ritter, M.; Gesing, T.M.; Plagemann, P. Investigations into the Structure of PEO-Layers for Understanding of Layer Formation. Appl. Surf. Sci. 2018, 443, 467–474. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Biomimetic Implants. Available online: https://implantsystem.avinent.com/wp-content/uploads/sites/4/2019/06/biomimetic-implants-avinent-eng.pdf (accessed on 28 October 2024).
- Łosiewicz, B.; Stróż, A.; Osak, P.; Maszybrocka, J.; Gerle, A.; Dudek, K.; Balin, K.; Łukowiec, D.; Gawlikowski, M.; Bogunia, S. Production, Characterization and Application of Oxide Nanotubes on Ti–6Al–7Nb Alloy as a Potential Drug Carrier. Materials 2021, 14, 6142. [Google Scholar] [CrossRef]
- Osak, P.; Skwarek, S.; Łukowiec, D.; Przeliorz, G.; Łosiewicz, B. Preparation and Characterization of Oxide Nanotubes on Titanium Surface for Use in Controlled Drug Release Systems. Materials 2024, 17, 3753. [Google Scholar] [CrossRef]
- Stróż, A.; Gawlikowski, M.; Balin, K.; Osak, P.; Kubisztal, J.; Zubko, M.; Maszybrocka, J.; Dudek, K.; Łosiewicz, B. Biological Activity and Thrombogenic Properties of Oxide Nanotubes on the Ti-13Nb-13Zr Biomedical Alloy. J. Funct. Biomater. 2023, 14, 375. [Google Scholar] [CrossRef]
- Tran, C.; Walsh, L.J. Novel Models to Manage Biofilms on Microtextured Dental Implant Surfaces; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Lutz, R.; Srour, S.; Nonhoff, J.; Weisel, T.; Damien, C.J.; Schlegel, K.A. Biofunctionalization of Titanium Implants with a Biomimetic Active Peptide (P-15) Promotes Early Osseointegration. Clin. Oral Implants Res. 2010, 21, 726–734. [Google Scholar] [CrossRef]
- Fu, L.; Omi, M.; Sun, M.; Cheng, B.; Mao, G.; Liu, T.; Mendonça, G.; Averick, S.E.; Mishina, Y.; Matyjaszewski, K. Covalent Attachment of P15 Peptide to Ti Alloy Surface Modified with Polymer to Enhance Osseointegration of Implants. ACS Appl. Mater. Interfaces 2019, 11, 38531–38536. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ho, K.-N.; Feng, S.-W.; Huang, H.-M.; Chang, C.-H.; Lin, C.-T.; Teng, N.-C.; Pan, Y.-H.; Chang, W.-J. Fibronectin-Grafted Titanium Dental Implants: An In Vivo Study. BioMed Res. Int. 2016, 2016, 2414809. [Google Scholar] [CrossRef]
- Lo, V.; I-Chun Lai, J.; Sunde, M. Fungal Hydrophobins and Their Self-Assembly into Functional Nanomaterials. Adv. Exp. Med. Biol. 2019, 1174, 161–185. [Google Scholar] [CrossRef]
- Pawar, V.; Bulbake, U.; Khan, W.; Srivastava, R. Chitosan Sponges as a Sustained Release Carrier System for the Prophylaxis of Orthopedic Implant-Associated Infections. Int. J. Biol. Macromol. 2019, 134, 100–112. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Kubisztal, J. The effect of a composite chitosan/copper(II) ion coating on the corrosion resistance of grade 4 titanium in saline: Preliminary results. Prog. Chem. Appl. Chitin Deriv. 2023, 28, 89–102. [Google Scholar] [CrossRef]
- Szklarska, M.; Łosiewicz, B.; Dercz, G.; Maszybrocka, J.; Rams-Baron, M.; Stach, S. Electrophoretic deposition of chitosan coatings on the Ti15Mo biomedical alloy from a citric acid solution. RSC Adv. 2020, 10, 13386–13393. [Google Scholar] [CrossRef] [PubMed]
- OsseoSpeed TX S (Astra Tech). Available online: https://stg.spotimplant.com/en/dental-implants/dentsply-implants/osseospeed-tx-s (accessed on 28 October 2024).
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yu, Y.; Lu, Y.; Quan, K.; Mao, Z.; Zheng, Y.; Qin, L.; Xia, D. UiO-66/AgNPs Coating for Dental Implants in Preventing Bacterial Infections. J. Dent. Res. 2024, 103, 516–525. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Z.; Liu, Z.; Shen, X.; Cai, C.; Li, M.; Luo, Z. Functionally Tailored Metal-Organic Framework Coatings for Mediating Ti Implant Osseointegration. Adv. Sci. 2023, 10, e2303958. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, S.; Xie, W.; Xue, Y.; Qiao, M.; Yang, X.; Zhang, X.; Wan, Q.; Wang, J.; Chen, J.; et al. Surface modification of the Ti surface with nanoscale bio-MOF-1 for improving biocompatibility and osteointegration in vitro and in vivo. Mater. Chem. B 2022, 10, 8535–8548. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Kazemzad, M. Recent advances in surface-mounted metal-organic framework thin film coatings for biomaterials and medical applications: A review. Biomater. Res. 2023, 27, 115. [Google Scholar] [CrossRef]
- Kaur, G. Apatites: A Mark of BioactivityBioactivity. In Bioactive Glasses: Potential Biomaterials for Future Therapy; Kaur, G., Ed.; Series in BioEngineering; Springer International Publishing: Cham, Switzerland, 2017; pp. 145–172. ISBN 978-3-319-45716-1. [Google Scholar]
- Tang, W.; Fischer, N.G.; Kong, X.; Sang, T.; Ye, Z. Hybrid coatings on dental and orthopedic titanium implants: Current advances and challenges. BMEMat 2024, 2, e12105. [Google Scholar] [CrossRef]
- Bravo, E.; Serrano, B.; Ribeiro-Vidal, H.; Virto, L.; Sánchez, I.S.; Herrera, D.; Sanz, M. Biofilm formation on dental implants with a hybrid surface microtopography: An in vitro study in a validated multispecies dynamic biofilm model. Clin. Oral Implant. Res. 2023, 34, 475–485. [Google Scholar] [CrossRef]
- Serrano, B.; Sanz-Sánchez, I.; Serrano, K.; Montero, E.; Sanz, M. One-year outcomes of dental implants with a hybrid surface macro-design placed in patients with history of periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2022, 49, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Liu, T.T.; Wang, Q.Q.; Zhang, J.; Cao, M.S. Surface Modification and Functionalities for Titanium Dental Implants. ACS Biomater. Sci. Eng. 2023, 9, 4442–4461. [Google Scholar] [CrossRef]
- IMAX NHSI. Available online: https://www.spotimplant.com/en/dental-implants/ires/imax-nhsi-internal-hex-c (accessed on 28 October 2024).
- Morra, M.; Cassinelli, C.; Torre, E.; Iviglia, G. Permanent wettability of a novel, nanoengineered, clinically available, hyaluronan-coated dental implant. Clin. Exp. Dent. Res. 2018, 4, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, D.P. Dental implants in periodontal care. Curr. Opin. Periodontol. 1993, 157, 157–162. [Google Scholar]
- Leesungbok, R.; Hong, S.O.; Lee, S.W.; Htay, P.E.E.; Choi, J.J.; Park, J.J. An eight-year retrospective study on the clinical outcomes of laser surface-treated implants. Int. J. Implant. Dent. 2024, 10, 38. [Google Scholar] [CrossRef]
- Lackington, W.A.; Schweizer, P.; Khokhlova, M.; Cancellieri, C.; Guimond, S.; Chopard-Lallier, A.-L.; Hofstetter, J.; Schmutz, P.; Maeder, X.; Rottmar, M. Femtosecond Laser-Texturing the Surface of Ti-Based Implants to Improve Their Osseointegration Capacity. Adv. Mater. Interfaces 2022, 9, 2201164. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Brambilla, E.; Azzola, F.; Ottobelli, M.; Pellegrini, G.; Francetti, L.A. Laser microtextured titanium implant surfaces reduce in vitro and in situ oral biofilm formation. PLoS ONE 2018, 13, e0202262. [Google Scholar] [CrossRef]
- Veiko, V.; Karlagina, Y.; Zernitckaia, E.; Egorova, E.; Radaev, M.; Yaremenko, A.; Chernenko, G.; Romanov, V.; Shchedrina, N.; Ivanova, E.; et al. Laser-Induced µ-Rooms for Osteocytes on Implant Surface: An In Vivo Study. Nanomaterials 2022, 12, 4229. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sakr, H. Implant Surface Topography Following Different Laser Treatments: An In Vitro Study. Cureus 2023, 15, e38731. [Google Scholar] [CrossRef]
- Shapoff, C.A.; Lahey, B.; Wasserlauf, P.; Kim, D. Radiographic Analysis of Crestal Bone Levels on Laser-Lok® Collar Dental Implants. Int. J. Periodontics Restor. Dent. 2010, 30, 129–137. [Google Scholar]
- Laser Etching: Everything You Need to Know. Available online: https://www.laserax.com/blog/laser-etching (accessed on 28 October 2024).
- Tapered Internal Plus (4.5). Available online: https://osseosource.com/tapered-internal-plus-4-5-/p-2495.html (accessed on 18 October 2024).
- Laser-Lok Microchannels. Clinical Overview. Available online: https://www.laser-lok.com (accessed on 18 October 2024).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łosiewicz, B.; Osak, P.; Nowińska, D.; Maszybrocka, J. Developments in Dental Implant Surface Modification. Coatings 2025, 15, 109. https://doi.org/10.3390/coatings15010109
Łosiewicz B, Osak P, Nowińska D, Maszybrocka J. Developments in Dental Implant Surface Modification. Coatings. 2025; 15(1):109. https://doi.org/10.3390/coatings15010109
Chicago/Turabian StyleŁosiewicz, Bożena, Patrycja Osak, Delfina Nowińska, and Joanna Maszybrocka. 2025. "Developments in Dental Implant Surface Modification" Coatings 15, no. 1: 109. https://doi.org/10.3390/coatings15010109
APA StyleŁosiewicz, B., Osak, P., Nowińska, D., & Maszybrocka, J. (2025). Developments in Dental Implant Surface Modification. Coatings, 15(1), 109. https://doi.org/10.3390/coatings15010109





