Crystalline Coating and Its Influence on Chloride Ion Diffusion Resistance of Carbonated Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Concrete Properties
2.2. Concrete Carbonation
2.3. Chloride Ion Concentration
3. Results and Discussion
3.1. Concrete Properties
3.2. Concrete Carbonation
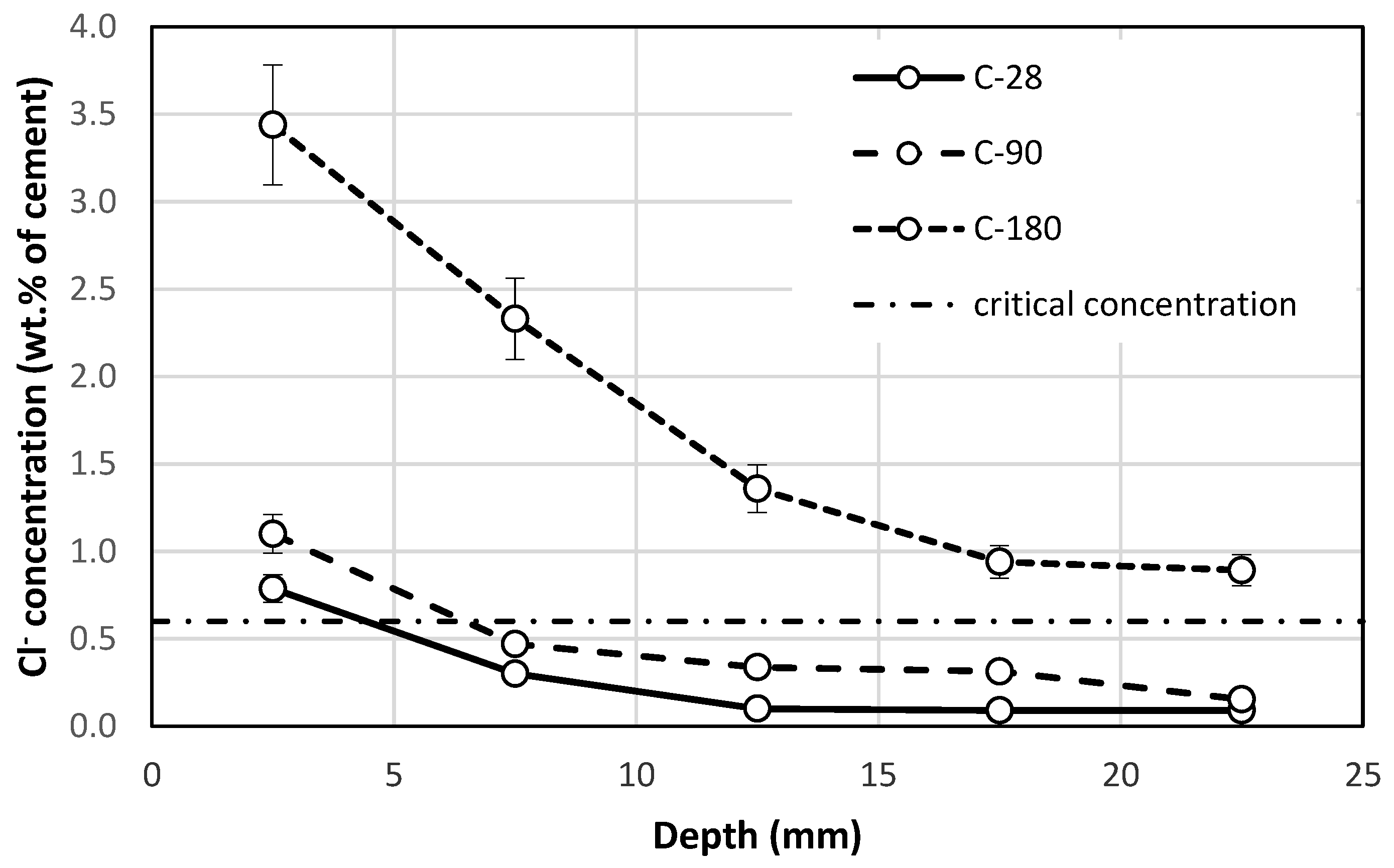
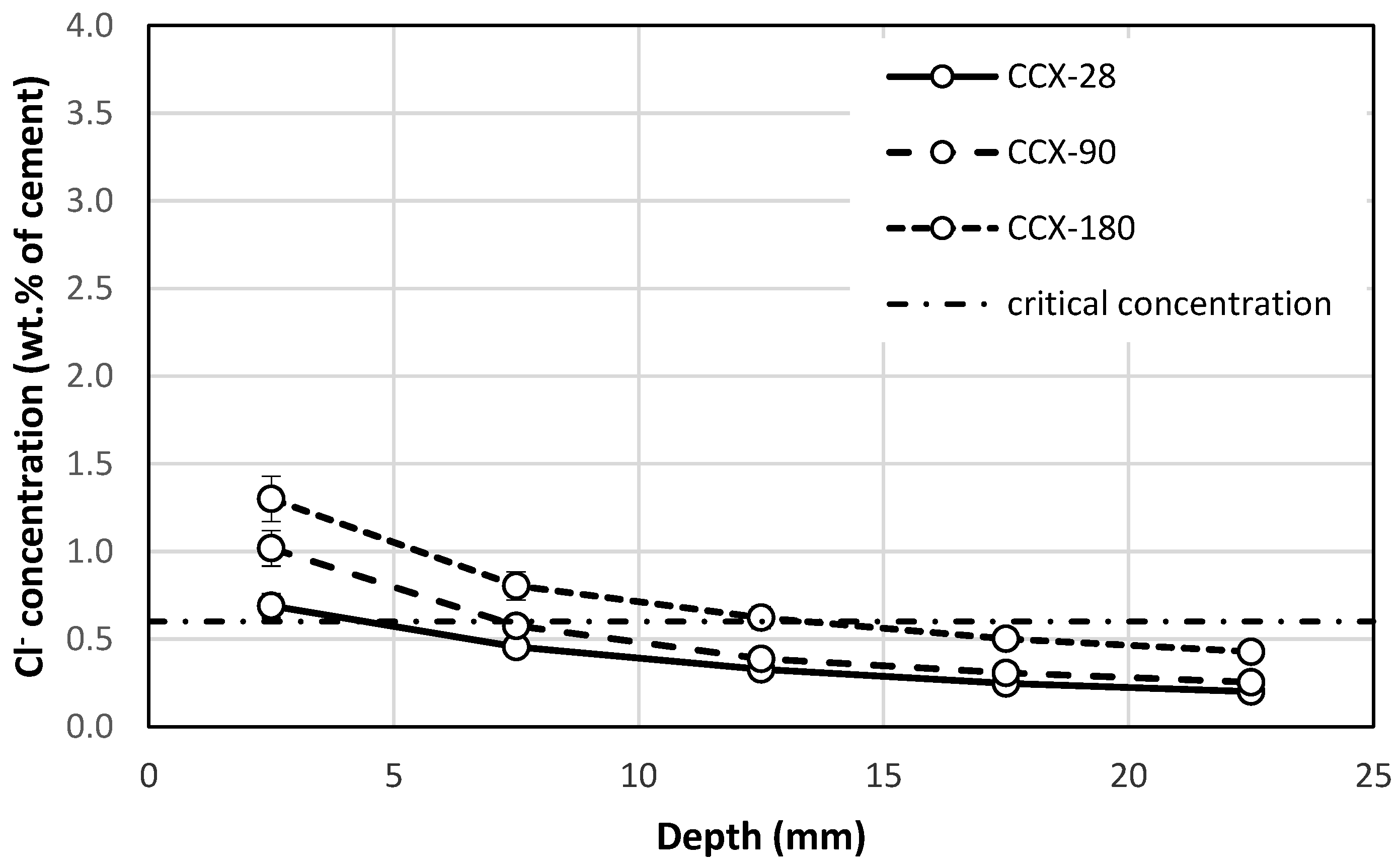
3.3. Chloride Ion Concentration
4. Conclusions
- This experimental study confirmed the previous research presented in the scientific literature and showed that carbonation and chloride ingress are two interrelated processes. Carbonated concrete is more permeable to chloride ion ingress than uncarbonated concrete due to reduced chloride binding. The depth of the critical concentration of chloride ions in the carbonated concrete was up to 116% higher than that in the uncarbonated concrete.
- The results of this study show that crystalline coatings can significantly increase the chloride resistance of carbonated concrete. The crystalline coatings were functional even after 28 days of immersion in the sodium chloride solution when the concentration of chloride ions at depths over 5 mm was under the critical concentration. The same tendency was observed after 90 days of immersion, as the chloride ion concentration was lower than the critical concentration at a depth of 10 mm. The crystalline coating was able to reduce the concentration of chloride ions by 68% under the surface of the concrete and by 65% at depths of 20–25 mm after 180 days of immersion.
- Carbonated concrete treated with a crystalline coating showed chloride ion concentrations below the critical concentration at a depth of 13.45 mm at 180 days of immersion. At the same time of immersion, steel reinforcement in untreated concrete would be fully located in the critical chloride concentration area.
- The results of this study show that crystalline coatings can be used in various applications as an effective solution to protect concrete against chloride ingress, e.g., in marine structures or structures burdened by winter maintenance.
- It can be assumed that the performance of crystalline coatings, in terms of improving resistance to chloride ingress, is at least the same as that of crystalline admixtures. To confirm this comparison of the effectiveness of crystalline coatings and admixtures, additional experimental research should be performed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hájková, K.; Šmilauer, V.; Jendele, L.; Červenka, J. Prediction of reinforcement corrosion due to chloride ingress and its effects on serviceability. Eng. Struct. 2018, 174, 768–777. [Google Scholar] [CrossRef]
- Reiterman, P.; Keppert, M. Effect of various de-icers containing chloride ions on scaling resistance and chloride penetration depth of highway concrete. Roads Bridges Drog. I Mosty 2020, 19, 51–64. [Google Scholar] [CrossRef]
- Kropp, J.; Hilsdorf, H.K.; Grube, H.; Andrade, C.; Nilsson, L.O. Transport mechanisms and definitions. In Performance Criteria for Concrete Durability; Kropp, J., Hilsdorf, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 4–14. [Google Scholar]
- Page, C.L.; Short, N.R.; Terras, A.E. Diffusion of chloride ions in hardened cement pastes. Cem. Concr. Res. 1981, 11, 395–406. [Google Scholar] [CrossRef]
- Erdoǧdu, Ş.; Kondratova, I.L.; Bremner, T.W. Determination of the chloride diffusion coefficient of concrete using open-circuit potential measurements. Cem. Concr. Res. 2004, 34, 603–609. [Google Scholar] [CrossRef]
- Andrade, C.; Whiting, D. A comparison of chloride ion diffusion coefficients derived from concentration gradients and non steady-state accelerated ionic migration. Mater. Struct. 1996, 29, 476–484. [Google Scholar] [CrossRef]
- Lorentz, T.; French, C. Corrosion of reinforcing steel in concrete: Effects of materials, mix composition and cracking. ACI Mater. J. 1995, 92, 181. [Google Scholar]
- Arya, C.; Ofori-Darko, F.K. Influence of crack frequency on reinforcement corrosion in concrete. Cem. Concr. Res. 1996, 26, 345–354. [Google Scholar] [CrossRef]
- Schiessl, P.; Raupach, M. Laboratory studies and calculation on the influence of crack width on chloride induced corrosion of steel in concrete. ACI Mater. J. 1997, 94, 56. [Google Scholar]
- Beeby, A.W. Corrosion of reinforcing steel in concrete and its relation to cracking. Struct. Eng. Part A 1978, 56, 77–81. [Google Scholar]
- Muthena, A.; Andrade, C.; Nilsson, L.-O.; Edvardsen, C. Final Technical Report; DuraCrete: West Valley City, UT, USA, 2000. [Google Scholar]
- Bo, S.; Xiao, R.C.; Ruan, W.D.; Wang, P.B. Corrosion-induced cracking fragility of RC bridge with improved concrete carbonation and steel reinforcement corrosion models. Eng. Struct. 2020, 208, 110313. [Google Scholar]
- Bogas, J.A.; Gomes, M.G.; Ea, L.S. Capillary absorption of structural lightweight aggregate concrete. Mater. Struct. 2014, 48, 2869–2883. [Google Scholar] [CrossRef]
- AL-Ameeri, A.S.; Rafiq, M.I.; Tsioulou, O. Combined impact of carbonation and crack width on the Chloride Penetration and Corrosion Resistance of Concrete Structure. Cem. Concr. Compos. 2021, 115, 103819. [Google Scholar] [CrossRef]
- Zheng, Y.; Russel, M.; Davis, G.; McPolin, D.; Yang, K.; Basheer, P.A.M.; Nanukuttan, S. Influence of carbonation on the bound chloride concentration in different cementitious systems. Constr. Build. Mater. 2021, 302, 124171. [Google Scholar] [CrossRef]
- Teymouri, M.; Atadero, R. Combined effects of carbonation and salt solution on chloride desorption, pH, and composition of fly ash blended pastes. J. Build. Eng. 2024, 97, 110821. [Google Scholar] [CrossRef]
- ACI Committee 212.3R. Report on Chemical Admixtures for Concrete; American Concrete Institute (ACI): Farmington Hills, MI, USA, 2015. [Google Scholar]
- De Belie, N.; Gruyaert, E.; Al-Tabbaa, A.; Antonaci, P.; Baera, C.; Bajare, D.; Darquennes, A.; Davies, R.; Ferrara, L.; Jefferson, T.; et al. A Review of Self-Healing Concrete for Damage Management of Structures. Adv. Mater. Interfaces 2018, 5, 1800074. [Google Scholar] [CrossRef]
- Rahhal, V.; Bonavetti, V.; Delgado, A.; Pedrajas, C.; Talero, R. Scheme of the Portland cement hydration with crystalline mineral admixtures and other aspects. Silic. Ind. 2009, 74, 347–352. [Google Scholar]
- Edvardsen, C. Water penetrability and autogenous healing of separation cracks in concrete. Betonw. Und Fert. Tech. 1996, 62, 77–85. [Google Scholar]
- de Souza Oliveira, A.; Dweck, J.; de Moraes Rego Fairbairn, E.; da Fonesca Martins Gomes, O.; Toledo Filho, R.D. Crystalline admixture effects on crystal formation phenomena during cement pastes’ hydration. J. Therm. Anal. Calorim. 2020, 139, 3361–3375. [Google Scholar] [CrossRef]
- Reterman, P.; Pazderka, J. Crystalline coating and its influence on the water transport in Concrete. Adv. Civ. Eng. 2016, 2016, 2513514. [Google Scholar] [CrossRef]
- Pazderka, J.; Hájková, E. Crystalline admixtures and their effect on selected properties of concrete. Acta Polytech. 2016, 56, 306–311. [Google Scholar] [CrossRef]
- Jahandari, S.; Tao, Z.; Alim, M.; Li, W. Integral waterproof concrete: A comprehensive review. J. Build. Eng. 2023, 78, 107718. [Google Scholar] [CrossRef]
- Wang, G.-M.; Yu, J.-Y. Effect of catalytic crystalline waterproofing coating on performance and microstructure of concrete. J. Wuhan Univ. Technol. 2006, 28, 29–31. [Google Scholar]
- Zhang, Y.; Du, X.; Li, Y.; Yang, F.; Li, Z. Research on cementitious capillary crystalline waterproofing coating for underground concrete works. Adv. Mater. Res. 2012, 450–451, 286–290. [Google Scholar] [CrossRef]
- Gao, L.; Kong, L.; Hui, C. Effect of catalytic crystalline waterproof coatings on steel reinforcement corrosion of concrete. Adv. Mater. Res. 2009, 79–82, 1083–1086. [Google Scholar] [CrossRef]
- Ji, H.-J.; Yang, Y.; Zhou, S.-G.; Yuan, Y. Tentative study of a new cement waterproof paint. J. Xi’an Univ. Archit. Technol. 2008, 40, 80–86. [Google Scholar]
- Munn, R.L.; Kao, G.; Chang, Z.T. Performance and compatibility of permeability reducing and other chemical admixtures in Australian concretes. In Proceedings of the 7th CANMET/ACI International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Berlin, Germany, 20–23 October 2003; pp. 361–379. [Google Scholar]
- Xue, S. Research and development of cement-based permeable crystallization type waterproof materials abroad. China Build. Waterproofing 2001, 6. [Google Scholar]
- Wang, K.; Hu, T.; Xu, S. Influence of permeated crystalline waterproof materials on impermeability of concrete. Adv. Mater. Res. 2012, 446–449, 954–960. [Google Scholar] [CrossRef]
- Tan, Y.; Zhao, B.; Yu, J.; Xiao, H.; Long, X.; Meng, J. Effect of Cementitious Capillary Crystalline Waterproofing Materials on the Mechanical and Impermeability Properties of Engineered Cementitious Composites with Microscopic Analysis. Polymers 2023, 15, 1013. [Google Scholar] [CrossRef] [PubMed]
- Bohus, S.; Drochytka, R. Cement based material with crystal-growth ability under long term aggressive medium impact. Appl. Mech. Mater. 2012, 166–169, 1773–1778. [Google Scholar] [CrossRef]
- Wu, J.; Lu, G.; Lu, L.; Ya, Z.; Yu, J. Influence of Shenkebao permeable crystalline admixture on concrete resistance to chemical corrosion and freeze-thaw cycles. Jiangsu Build. Mater. 2010, 2, 18–21. [Google Scholar]
- Scancella, T.; Robert, J. Use of Xypex admixture to concrete as an inhibitor to reinforcement steel corrosion. Mater. Eng. Conf. 1996, 2, 1276–1280. [Google Scholar]
- Reiterman, P.; Davidová, V.; Pazderka, J.; Kubissa, W. Reduction of Concrete Surface Permeability by Using Crystalline Treatment. Rom. J. Mater. 2020, 50, 69–74. [Google Scholar]
- Azarsa, P.; Gupta, R.; Biparva, A. Assessment of self-healing and durability parameters of concretes incorporating crystalline admixtures and Portland Limestone Cement. Cem. Concr. Compos. 2019, 99, 17–31. [Google Scholar] [CrossRef]
- Antón, C.; Gurdián, H.; de Vera, G.; Climent, M.-Á. Effect of a Crystalline Admixture on the Permeability Properties of Concrete and the Resistance to Corrosion of Embedded Steel. Appl. Sci. 2024, 14, 1731. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Z.-W.; Qin, X.-M. Study on the Corrosion Performance of Reinforced Concrete by Using the Seawater Hot Rain Testing Equipment. Adv. Mater. Res. 2013, 699, 173–178. [Google Scholar] [CrossRef]
- Sisomphon, K.; Copuroglu, O.; Koenders, E.A.B. Self-healing of surface cracks in mortars with expansive additive and crystalline additive. Constr. Build. Mater. 2012, 34, 566. [Google Scholar] [CrossRef]
- EN 12350-2:2019; Testing Fresh Concrete—Part 2: Slump Test. Czech Standardization Agency: Prague, Czech Republic, 2019.
- EN 12390-3:2019; Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens. Czech Standardization Agency: Prague, Czech Republic, 2019.
- EN 12390-8:2019; Testing Hardened Concrete—Part 8: Depth of Penetration of Water Under Pressure. Czech Standardization Agency: Prague, Czech Republic, 2019.
- EN 12390-12:2020; Testing Hardened Concrete—Part 12: Determination of the Carbonation Resistance of Concrete—Accelerated Carbonation Method. Czech Standardization Agency: Prague, Czech Republic, 2020.
- Xypex Concentrate Technical Data Sheet. Available online: https://xypex.cz/downloads/tl_Concentrate.pdf (accessed on 9 January 2025).
- Claisse, P.A. Transport Properties of Concrete: Modelling the Durability of Structures, 2nd ed.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Yuan, C.; Niu, D.; Luo, D. Effect of carbonation on chloride diffusion in fly ash concrete. Comput. Concr. 2012, 5, 312–316. [Google Scholar]
- Qiao, C.; Suraneni, P.; Ying, T.; Choudhary, A.; Weiss, J. Chloride binding of cement pastes with fly ash exposed to CaCl2 solutions at 5 and 23 °C. Cem. Concr. Compos. 2019, 97, 43–53. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Swamy, N. Stability of Friedel’s salt in carbonated concrete structural elements. Cem. Concr. Res. 1996, 26, 729–741. [Google Scholar] [CrossRef]
- Otero, J.; Starinieri, V.; Charola, A.E.; Taglieri, G. Influence of different types of solvent on the effectiveness of nanolime treatments on highly porous mortar substrates. Constr. Build. Mater. 2020, 230, 117112. [Google Scholar] [CrossRef]
- Zhu, X.; Zi, G.; Cao, Z.; Cheng, X. Combined effect of carbonation and chloride ingress in concrete. Constr. Build. Mater. 2016, 110, 369–380. [Google Scholar] [CrossRef]
- Abro, F.U.R.; Buller, A.S.; Lee, K.-M.; Jang, S.Y. Using the Steady-State Chloride Migration Test to Evaluate the Self-Healing Capacity of Cracked Mortars Containing Crystalline, Expansive, and Swelling Admixtures. Materials 2019, 12, 1865. [Google Scholar] [CrossRef] [PubMed]
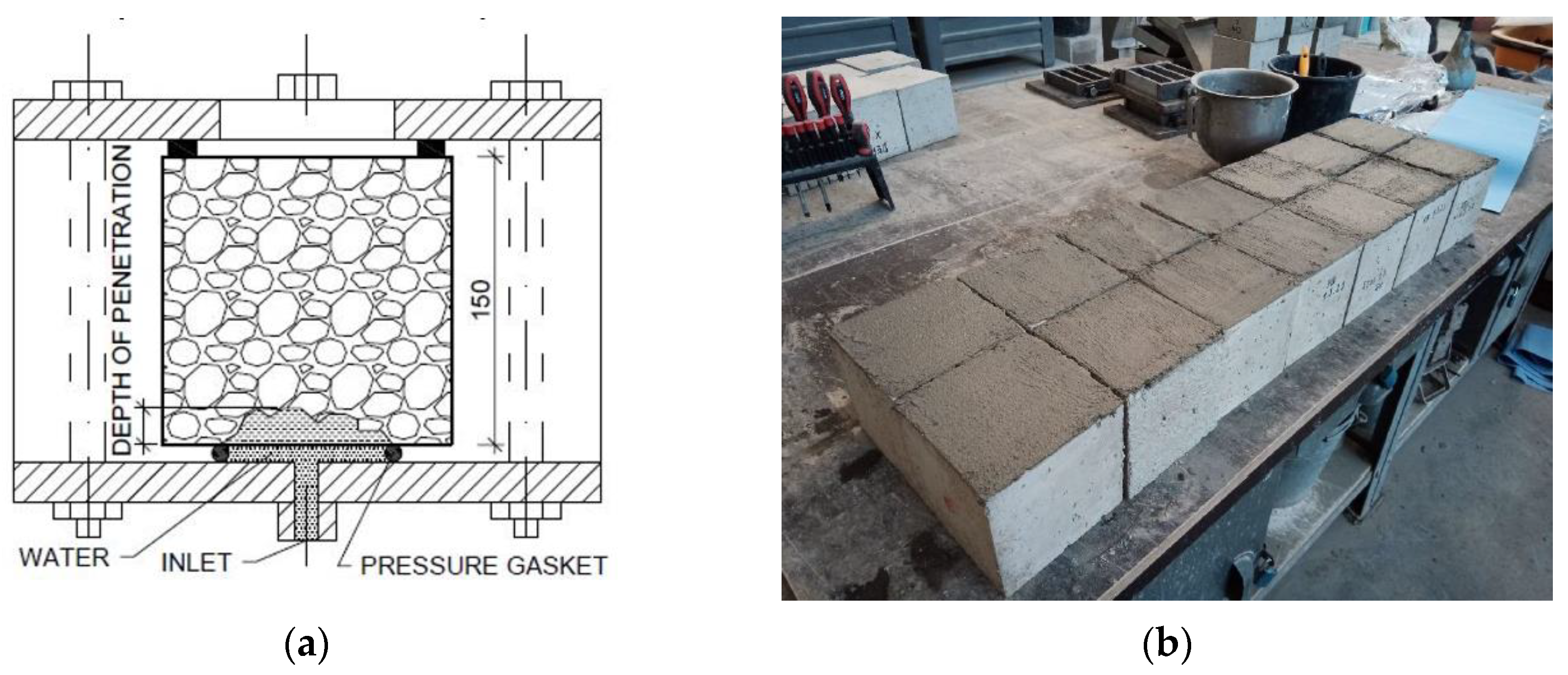


| Material | Specification | Volume (kg/m3) |
|---|---|---|
| Cement | CEM I 42.5 R | 300 |
| Water | w/c 0.51 | 153 |
| Aggregate | 0–4 mm | 855 |
| Aggregate | 4–8 mm | 270 |
| Aggregate | 8–16 mm | 870 |
| Designation | Specification | Depth of Critical Concentration (mm) | ||
|---|---|---|---|---|
| 28 Days | 90 Days | 180 Days | ||
| C | Concrete after 28 days | 4.14 | 6.47 | ˃25 |
| CC | Carbonated concrete | 8.95 | 12.00 | ˃25 |
| CCX | Carbonated concrete with crystalline coating | 4.41 | 7.05 | 13.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottl, M.; Pazderka, J.; Reiterman, P. Crystalline Coating and Its Influence on Chloride Ion Diffusion Resistance of Carbonated Concrete. Coatings 2025, 15, 163. https://doi.org/10.3390/coatings15020163
Mottl M, Pazderka J, Reiterman P. Crystalline Coating and Its Influence on Chloride Ion Diffusion Resistance of Carbonated Concrete. Coatings. 2025; 15(2):163. https://doi.org/10.3390/coatings15020163
Chicago/Turabian StyleMottl, Martin, Jiří Pazderka, and Pavel Reiterman. 2025. "Crystalline Coating and Its Influence on Chloride Ion Diffusion Resistance of Carbonated Concrete" Coatings 15, no. 2: 163. https://doi.org/10.3390/coatings15020163
APA StyleMottl, M., Pazderka, J., & Reiterman, P. (2025). Crystalline Coating and Its Influence on Chloride Ion Diffusion Resistance of Carbonated Concrete. Coatings, 15(2), 163. https://doi.org/10.3390/coatings15020163






