Influence of Waste Catalyst Surface Characteristics on High-Temperature Performance and Adhesion Properties of Asphalt Mortar
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WFC-Modified Asphalt Mortar
2.3. MD Simulation and Test Methods
2.4. Gray Relational Degree Theory
- (1)
- Reference sequence and comparison sequences
- (2)
- Non-dimensional processing
- (3)
- First-order difference quotient
- (4)
- Correlation coefficient
- (5)
- Degree of gray correlation
3. Results and Discussion
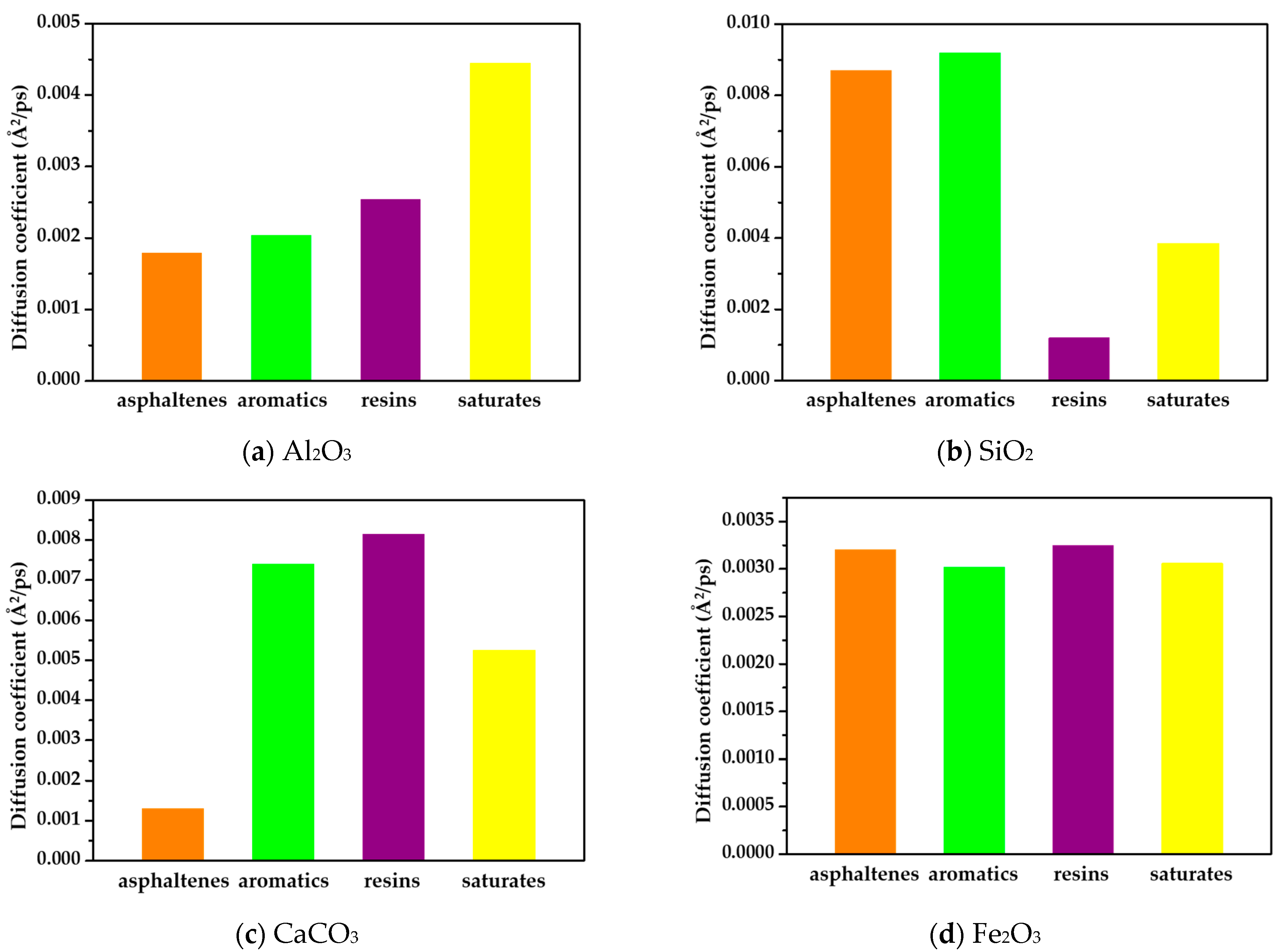
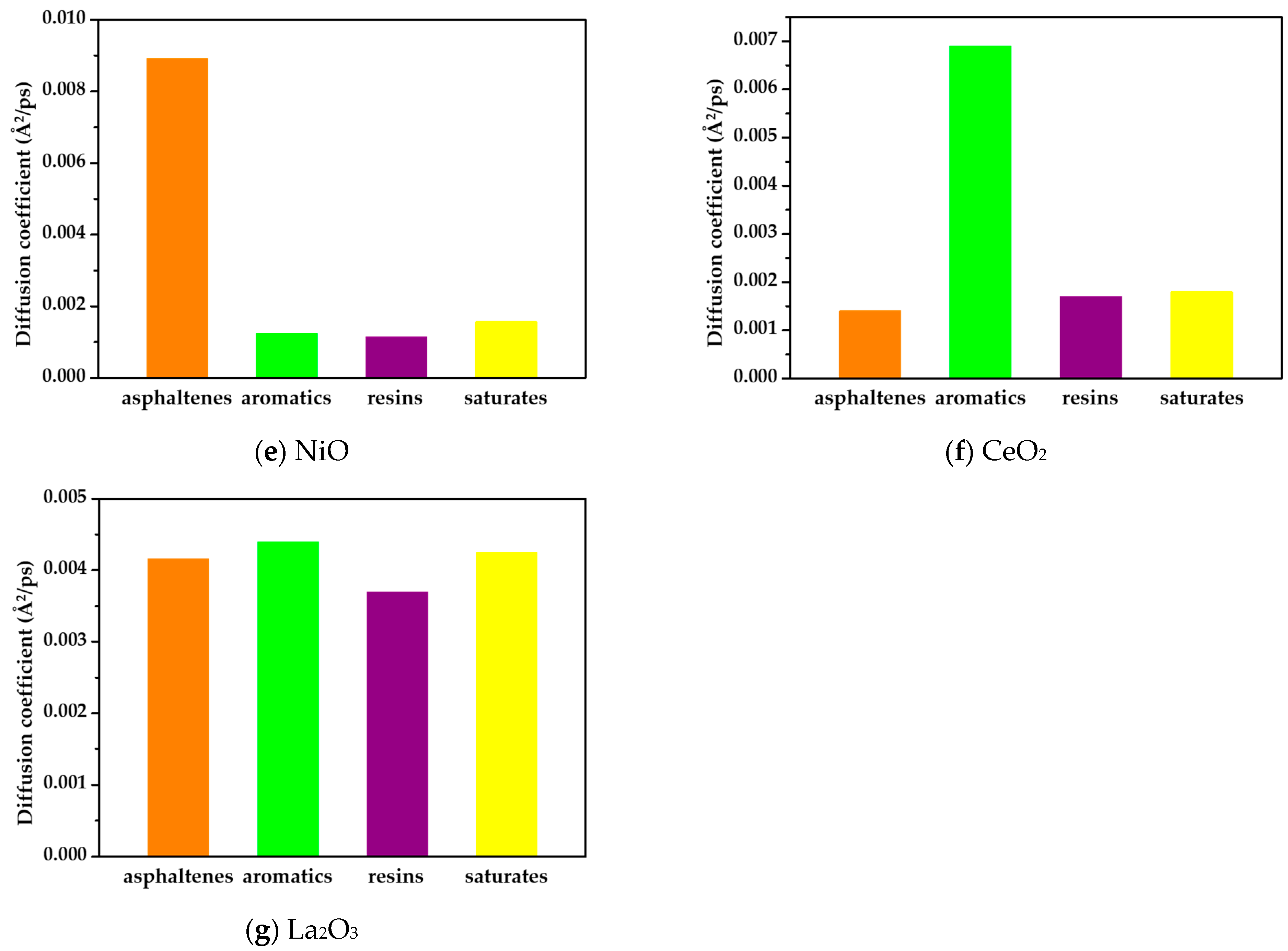
3.1. Diffusion Coefficient
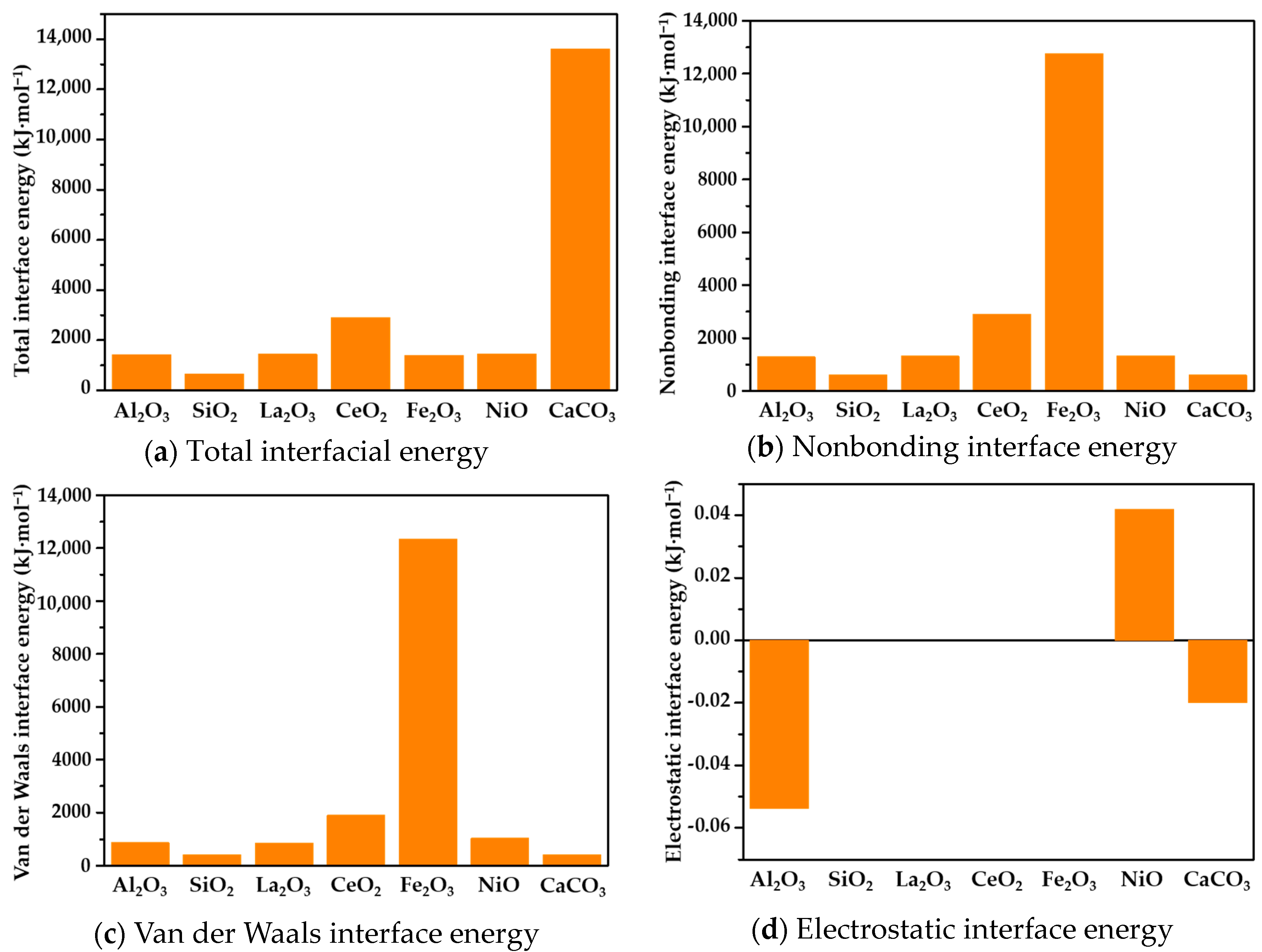
3.2. Interfacial Energy of Asphalt and Components of WFCs
3.3. Adhesion Analysis of Asphalt and Waste Catalyst
3.4. Dynamic Shear Rheometer (DSR)
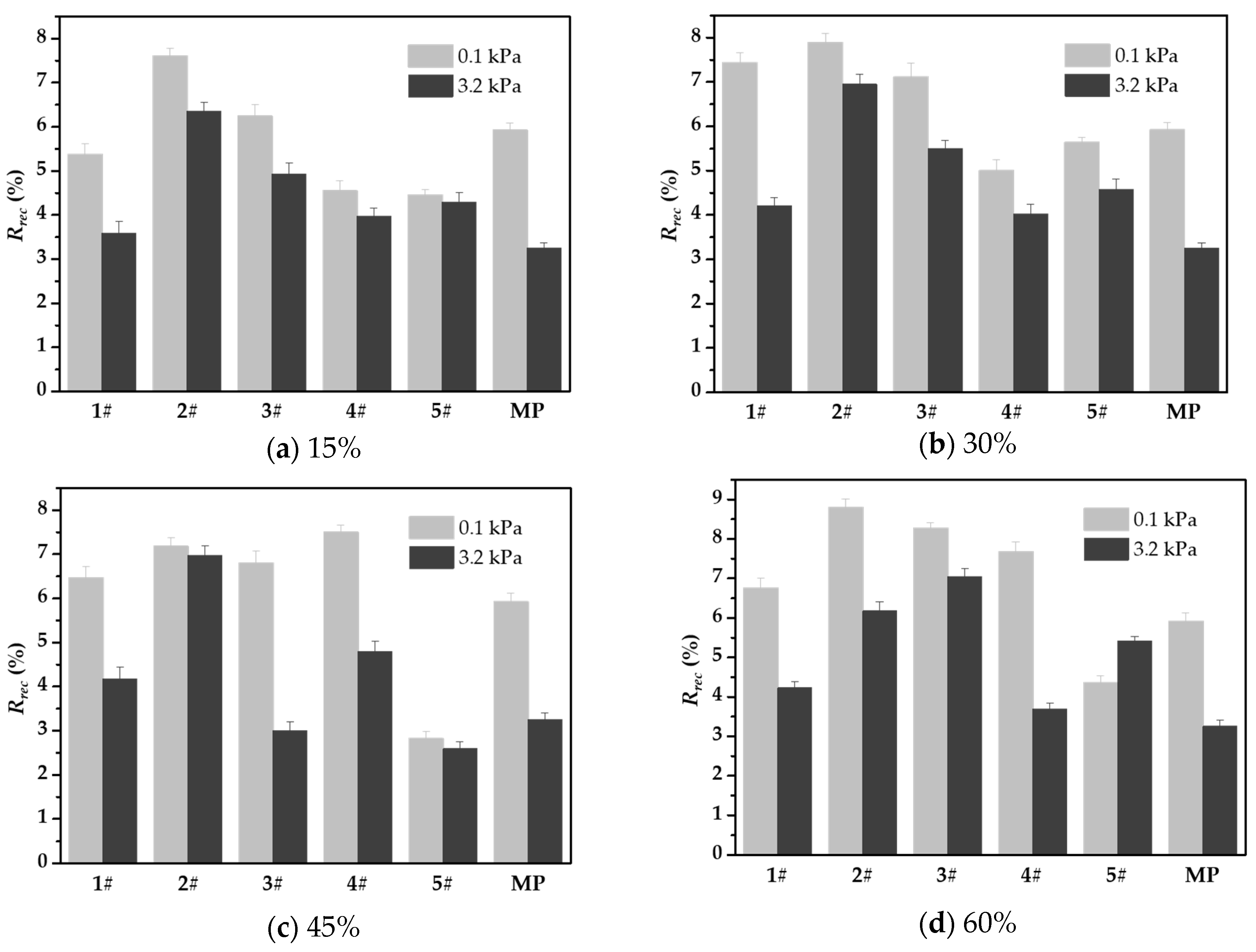
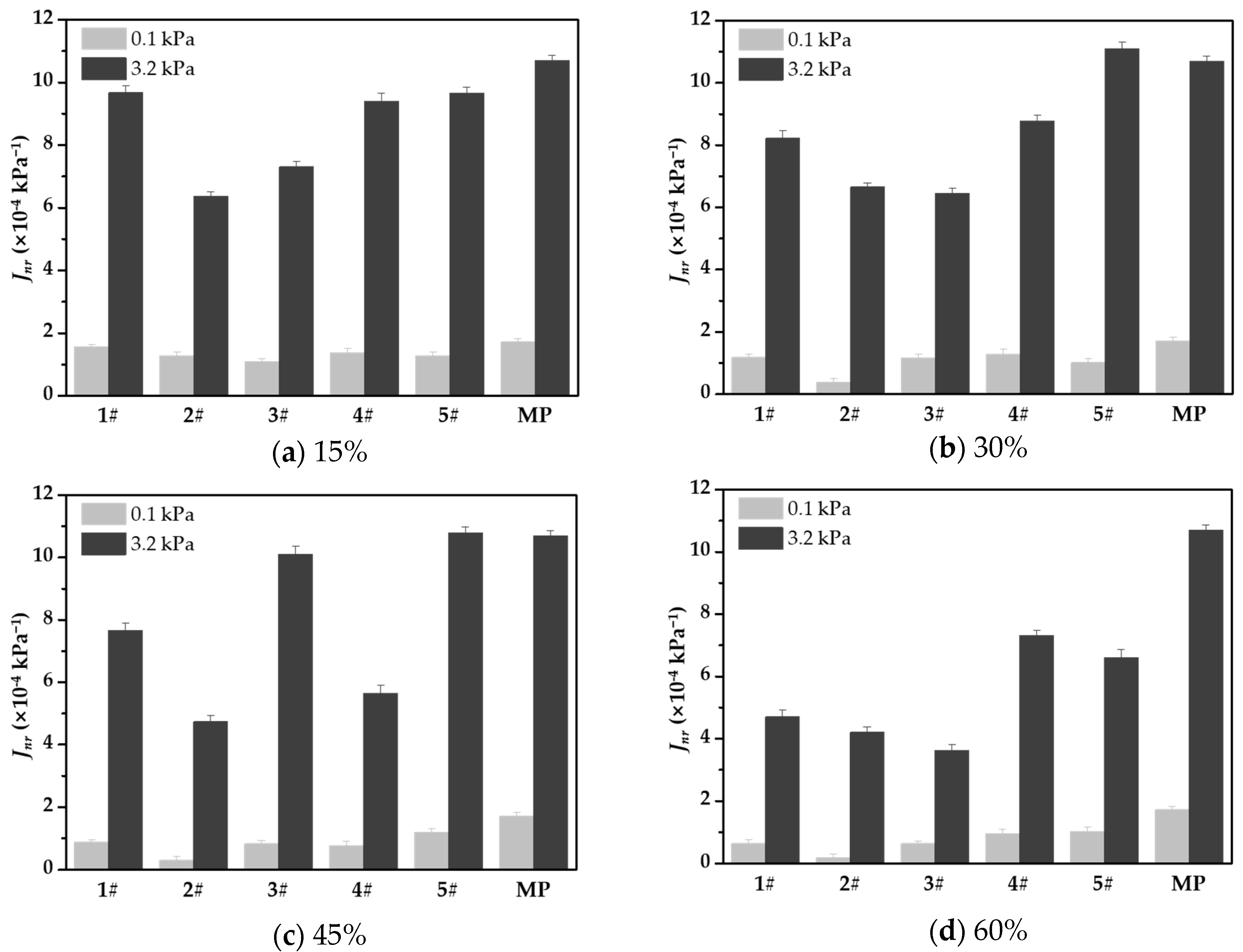
3.5. Resistance to Permanent Deformation of WFC Asphalt Mortar
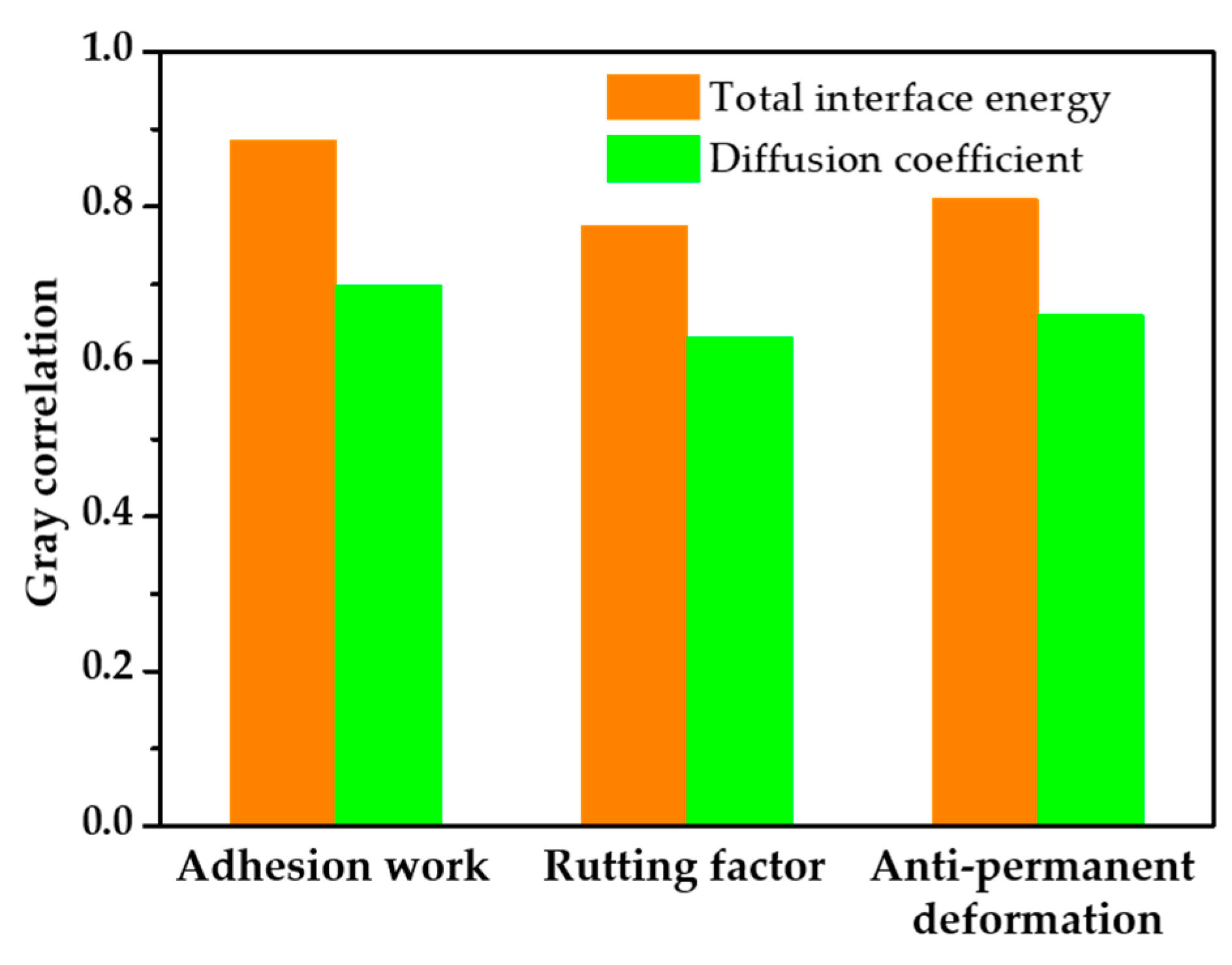
3.6. Gray Correlation Analysis
4. Conclusions
- (1)
- There was a strong correlation between the diffusion behavior of the asphalt components on the oxide surface and the interface between them. On the surfaces of rare-earth metal oxides, the asphalt components exhibited slower diffusion rates, higher interfacial energy, and stronger adhesion. Conversely, on the surfaces of alumina and silicon oxide, the diffusion rates were faster, and the interfacial energy was lower. The presence of rare-earth oxides significantly enhanced the adhesion between the asphalt components and oxide surfaces. This finding is consistent with the results of the adhesion test, which further validates the strong adhesive interactions between the asphalt components and waste catalysts.
- (2)
- With the addition of the waste catalyst instead of mineral powder to asphalt cement, the rutting factor increased with the amount of waste catalyst. When the proportion of waste catalyst replacing the mineral powder reached 30%, the rutting resistance factor increased by 50%, which significantly improved the high-temperature deformation resistance of the waste catalyst-modified asphalt mortar.
- (3)
- Through creep recovery, elastic recovery, and nonrecoverable creep compliance tests, it can be seen that the addition of a waste catalyst effectively improves the average elastic recovery rate of asphalt mortar and significantly reduces the nonrecoverable creep compliance. Under a stress of 3.2 kPa, although nonrecoverable creep compliance increases by orders of magnitude, the nonrecoverable deformation value of the waste catalyst-modified asphalt mortar is always lower than that of the mineral powder asphalt mortar. Among the tested waste catalysts, types 2# and 3# showed the best deformation resistance.
- (4)
- Gray correlation analysis showed that the correlation between the performance indices of the waste catalyst asphalt mortar was strong, with correlation coefficients greater than 0.6. The degree of correlation between the interfacial energy and diffusion coefficient was influenced by adhesion work, resistance to permanent deformation, and the rutting factor. The results showed that the stronger the interfacial interaction between asphalt components and the waste catalyst, the weaker the diffusion behavior and the better the high-temperature performance of the asphalt mortar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FCC | Fluid catalytic cracking |
| MSCR | Multiple stress creep recovery |
| Rrec | Resistance to permanent deformation (elastic recovery parameter) |
| Jnr | Nonrecoverable creep compliance |
References
- Wen, J.; Wang, X.; Yu, F.; Tian, M.; Wang, C.; Huang, G.; Xu, S. Recovery and value-added utilization of critical metals from spent catalysts for new energy industry. J. Clean. Prod. 2023, 419, 138295. [Google Scholar] [CrossRef]
- Pathak, A.; Rana, M.S.; Marafi, M.; Kothari, R.; Gupta, P.; Tyagi, V.V. Waste petroleum fluid catalytic cracking catalysts as a raw material for synthesizing valuable zeolites: A critical overview on potential, applications, and challenges. Sustain. Mater. Technol. 2023, 38, e00733. [Google Scholar] [CrossRef]
- Abdolpour, H.; Niewiadomski, P.; Sadowski, L. Recycling of steel fibres and spent equilibrium catalyst in ultra-high performance concrete: Literature review, research gaps, and future development. Constr. Build. Mater. 2021, 309, 125147. [Google Scholar] [CrossRef]
- Xue, Y.; Wei, X.; Zhao, H.; Wang, T.; Xiao, Y. Interaction of spent FCC catalyst and asphalt binder: Rheological properties, emission of VOCs and immobilization of metals. J. Clean. Prod. 2020, 259, 120830. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Xu, M.; Zhang, X.; Zhang, Y. Preliminary study of using spent fluid catalytic cracking (FCC) catalyst in asphalt binders. In Transportation Research Congress; American Society of Civil Engineers: Reston, VA, USA, 2018; pp. 69–81. [Google Scholar]
- Cui, L.; Xu, J.; Ren, M.; Li, D.; Liu, D.; Cao, F. Modification of FCC slurry oil and deoiled asphalt for making high-grade paving asphalt. Chin. J. Chem. Eng. 2022, 44, 300–309. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Zhang, Z. Road base materials prepared by multi-industrial solid wastes in China: A review. Constr. Build. Mater. 2023, 373, 130860. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Li, C.; Kong, L.; Ling, T.; Wang, H. Study on the influence of FCC waste catalysts on the interaction ability between asphalt and fillers. Int. J. Adhes. Adhes. 2024, 134, 103810. [Google Scholar] [CrossRef]
- Schmitt, R. FCC catalyst finds three safe reuse outlets in Europe. Oil Gas. J. 1991, 89, 101–102. [Google Scholar]
- Furimsky, E. Spent refinery catalysts: Environment, safety and utilization. Catal Today 1996, 30, 223–286. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Y. Grey System Theory and Its Application; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Alshamsi, K.; Baawain, M.; Aljabri, R.; Taha, R.; Kamyani, Z. Utilizing Waste Spent Catalyst in Asphalt Mixtures. Procedia—Soc. Behav. Sci. 2012, 53, 326–334. [Google Scholar] [CrossRef][Green Version]
- Al-Jabri, K.; Baawain, M.; Taha, R.; Al-Kamyani, Z.S.; Al-Shamsi, K.; Ishtieh, A. Potential use of FCC spent catalyst as partial replacement of cement or sand in cement mortars. Constr. Build. Mater. 2013, 39, 77–81. [Google Scholar] [CrossRef]
- Wang, H.; Chen, G.; Kang, H.; Zhang, J.; Rui, L.; Lyu, L.; Pei, J. Asphalt-aggregates interface interaction: Correlating oxide composition and morphology with adhesion. Constr. Build. Mater. 2024, 457, 139317. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, W.; Yuan, D.; Lu, R.; Shan, J.; Xiao, J.; Ogbon, A.W. A review of asphalt-filler interaction: Mechanisms, evaluation methods, and influencing factors. Constr. Build. Mater. 2021, 299, 124279. [Google Scholar] [CrossRef]
- Shi, Z.; Min, Z.; Chen, F.; Huang, W. Adhesion behavior and microscopic mechanism of epoxy asphalt-RAP aggregate interface. Constr. Build. Mater. 2024, 457, 139361. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Fu, C.; Shi, S.; Liu, Q.; Xu, P.; Liu, Q.; Zhou, D.; Cheng, Y.; Jiang, L. Effect and mechanism of waste glass powder silane modification on water stability of asphalt mixture. Constr. Build. Mater. 2023, 366, 130086. [Google Scholar] [CrossRef]
- Hawraa, J.; Amjad, H. Effect of nanomaterials on the durability of hot mix asphalt. Transp. Eng. 2023, 11, 100165. [Google Scholar]
- Lu, Y.; Li, S.; Jiang, Y.; Yang, X.; Li, L. Rheological and aging properties of nano-clay/SBS composite-modified asphalt. Materials 2024, 17, 17. [Google Scholar] [CrossRef]
- Song, S.; Niu, Y.; Kong, L. Correlation analysis of pore structure and frost resistance of carbon nanotube concrete based on gray relational theory. Structural Concrete 2024, 25, 2855–2867. [Google Scholar] [CrossRef]
- Jahroml, S.; Andalibizade, B.; Vossough, S. Engineering properties of nanoclay modified asphalt concrete mixtures. Arab. J. Sci. Eng. 2010, 35, 89–103. [Google Scholar]
- Azarhoosh, A.; Koohmishi, M.; Bazkhaneh, N.K. Sewage sludge ash as filler in asphalt mastic: Low-temperature towards high-temperature performance. Results Eng. 2024, 24, 102948. [Google Scholar] [CrossRef]
- Shi, B.; Zhou, S. Application of fume silica nanoparticles to improve high-temperature rheological performance of terminal blend rubberized asphalt. Case Stud. Constr. Mater. 2024, 20, e03276. [Google Scholar] [CrossRef]
- Bidabehere, C.M.; Sedran, U. Simultaneous diffusion, adsorption, and reaction in fluid catalytic cracking catalysts. Ind. Eng. Chem. Res. 2001, 40, 530–535. [Google Scholar] [CrossRef]
- Su, A.; Yin, H. Grey correlation analysis of asphalt-aggregate adhesion with high and low-temperature performance of asphalt mixtures. Case Stud. Constr. Mater. 2024, 21, e03765. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, N.; Zhu, H.; Xi, Y.; Sheng, W.; Li, Z. Short-term emission behavior and evolution law of organic emissions from asphalt binder materials. Constr. Build. Mater. 2024, 433, 136498. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, Z.; Shao, J.; Tan, J.; Jiang, X. Effect of active Filler on the interfacial adhesion between granite aggregate and asphalt mastic: Investigation of the Mechanism. J. Mater. Civ. Eng. 2025, 37, 18255. [Google Scholar] [CrossRef]
- Shi, W.; Wei, K.; Guo, X.; Ni, T.; Tian, J. Study on the interfacial adhesion performance of polyurethane-modified asphalt based on molecular dynamics simulation. Appl. Surf. Sci. 2025, 685, 162041. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, H.; Duan, H. Effect of catalytic-reactive rejuvenator on structure and properties of aged SBS modified asphalt binders. Constr. Build. Mater. 2020, 246, 118531. [Google Scholar] [CrossRef]
- Lv, S.; Liu, J.; Peng, X.; Liu, H.; Hu, L.; Yuan, J.; Wang, J. Rheological and microscopic characteristics of bio-oil recycled asphalt. J. Clean. Prod. 2021, 295, 126449. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Chen, T.; Tang, Y.; Zhang, D.; Wang, X.; Xie, J.; Wu, S.; Xu, H.; Zhao, P.; et al. Diffusion mechanism of waste crumb rubber composite modified asphalt based on molecular dynamics simulation. J. Clean. Prod. 2024, 482, 144155. [Google Scholar] [CrossRef]
- Zhu, S.; Kong, L.; Fu, Y.; Peng, Y.; Chen, Y.; Wang, H.; Jian, O.; Zhao, P.; Zhang, W. Effect of hydrophilic group substituent position on adhesion at the emulsified asphalt/aggregate interface. Constr. Build. Mater. 2024, 444, 137783. [Google Scholar] [CrossRef]
- Liu, K.; Yang, Q.; Qiu, X.; Xu, W.; Xiao, S.; Gu, Y.; Ye, Y. An investigation toward adhesion characteristics of emulsified asphalt residue–aggregate interface through MD simulation. Constr. Build. Mater. 2024, 438, 137251. [Google Scholar] [CrossRef]
- Xie, J.; Li, S.; He, W.; Ding, Z.; Lu, Z.; Zhao, X. Molecular simulation of graft-activated crumb rubber modified asphalt: A study on high temperature performance and its interface behavior with aggregate. Surf. Interfaces 2025, 58, 105827. [Google Scholar] [CrossRef]
- Deng, J. Control problems of grey systems. Syst. Control Lett. 1982, 1, 288–294. [Google Scholar]
- Song, W.; Zhang, M.; Wu, H. Gray correlation analysis between mechanical performance and pore characteristics of permeable concrete. J. Build. Eng. 2024, 86, 108793. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, R.; Li, J.; Wen, L. Research on preparation and road performance of graphene oxide framework modified asphalt and asphalt mixture based on micro-mechanism and grey correlation analysis. Constr. Build. Mater. 2025, 462, 139980. [Google Scholar] [CrossRef]
- Zhu, S.; Kong, L.; Peng, Y.; Chen, Y.; Zhao, T.; Jian, O.; Zhao, P.; Sheng, X.; Li, Z. Mechanisms of interface electrostatic potential induced asphalt-aggregate adhesion. Constr. Build. Mater. 2024, 438, 137255. [Google Scholar] [CrossRef]
- Peng, C.; Chen, P.; You, Z.; Lv, S.; Zhang, R.; Xu, F.; Zhang, H.; Chen, H. Effect of silane coupling agent on improving the adhesive properties between asphalt binder and aggregates. Constr. Build. Mater. 2018, 169, 591–600. [Google Scholar] [CrossRef]
- Drake, H.F.; Day, G.S.; Xiao, Z.; Zhou, H.; Ryder, M.R. Light-induced switchable adsorption in azobenzene- and stilbene-based porous materials. Trends Chem. 2022, 4, 32–47. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, L.; He, S. High-Temperature rheological properties of asphalt Mortar modified with spent FCC catalysts. Appl. Sci. 2023, 13, 9376. [Google Scholar] [CrossRef]




| WFC | Average Particle Size (μm) | Specific Surface Area (m2/g) | Average Pore Width (nm) | Density (g/cm3) | Hydrophilic Coefficient | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| 1# | 60.1 | 105 | 13.0 | 2.64 | 0.798 | 7.72 |
| 2# | 71.5 | 116 | 11.6 | 2.49 | 0.969 | 7.93 |
| 3# | 69.4 | 112 | 12.3 | 2.54 | 0.895 | 10.47 |
| 4# | 81.0 | 107 | 10.8 | 2.63 | 1.001 | 8.05 |
| 5# | 65.6 | 109 | 13.0 | 2.76 | 0.933 | 7.77 |
| WFC | Al2O3 | SiO2 | La2O3 | CeO2 | Fe2O3 | NiO | CaCO3 | Others |
|---|---|---|---|---|---|---|---|---|
| 1# | 45.99 | 40.78 | 2.54 | 1.11 | 3 | 2.17 | 2.41 | 2 |
| 2# | 44.3 | 42.11 | 2.49 | 1.33 | 2.95 | 2.52 | 2.3 | 2 |
| 3# | 46.01 | 41 | 2.55 | 1.26 | 2.88 | 2.07 | 2.53 | 1.7 |
| 4# | 48.01 | 39.99 | 2.93 | 1.03 | 3.56 | 0.98 | 1 | 2.5 |
| 5# | 39.9 | 46.5 | 1.85 | 2.24 | 3.08 | 2.19 | 2.14 | 2.1 |
| Indicators | Results | Technical Requirement |
|---|---|---|
| Softening point (°C) | 47.8 | >46 |
| Penetration (25 °C, 5 s, 100 g)/0.1 mm | 67.1 | 60~80 |
| Penetration index | 0.51 | −1.5~1 |
| Ductility (5 cm/min, 10 °C)/cm | 31.5 | >20 |
| Ductility (5 cm/min, 15 °C)/cm | >100 | >100 |
| 60 °C dynamic viscosity/(Pa·s) | 226 | >180 |
| Residual needle penetration ratio after TFOT/% | 65.2 | >61 |
| Material ID | Xi (1) | Xi (2) | Xi (3) | Xi (4) | X0 (1) | X0 (2) |
|---|---|---|---|---|---|---|
| Work of Adhesion | Rrec | Jnr | Rutting Factor | Total Interface Energy | Diffusion Coefficient | |
| 1# | 46.28 | 4.05243 | 0.000756725 | 7788.41 | 233.66 | 0.0086 |
| 2# | 44.93 | 6.617825 | 0.000549541 | 10581.09 | 231.76 | 0.0092 |
| 3# | 49.19 | 5.123895 | 0.000687345 | 10336.39 | 229.96 | 0.0085 |
| 4# | 48.18 | 4.124365 | 0.000779037 | 9061.17 | 237.52 | 0.0085 |
| 5# | 53.67 | 4.2187575 | 0.000954275 | 7486.77 | 482.38 | 0.0339 |
| Material ID | Work of Adhesion | Resistance to Permanent Deformation | Rutting Factor | |||
|---|---|---|---|---|---|---|
| Total Interface Energy | Diffusion Coefficient | Total Interface Energy | Diffusion Coefficient | Total Interface Energy | Diffusion Coefficient | |
| 1# | 0.97 | 0.78 | 0.92 | 0.74 | 1.00 | 0.81 |
| 2# | 1.00 | 0.84 | 0.79 | 0.77 | 0.73 | 0.65 |
| 3# | 0.89 | 0.73 | 0.86 | 0.70 | 0.74 | 0.64 |
| 4# | 0.94 | 0.75 | 0.91 | 0.72 | 0.87 | 0.71 |
| 5# | 0.62 | 0.39 | 0.57 | 0.36 | 0.50 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gao, M.; Guo, P.; Chen, Y.; Li, C.; Kong, L. Influence of Waste Catalyst Surface Characteristics on High-Temperature Performance and Adhesion Properties of Asphalt Mortar. Coatings 2025, 15, 187. https://doi.org/10.3390/coatings15020187
Wang Z, Gao M, Guo P, Chen Y, Li C, Kong L. Influence of Waste Catalyst Surface Characteristics on High-Temperature Performance and Adhesion Properties of Asphalt Mortar. Coatings. 2025; 15(2):187. https://doi.org/10.3390/coatings15020187
Chicago/Turabian StyleWang, Zhimei, Mengjie Gao, Peng Guo, Yan Chen, Chuanqiang Li, and Lingyun Kong. 2025. "Influence of Waste Catalyst Surface Characteristics on High-Temperature Performance and Adhesion Properties of Asphalt Mortar" Coatings 15, no. 2: 187. https://doi.org/10.3390/coatings15020187
APA StyleWang, Z., Gao, M., Guo, P., Chen, Y., Li, C., & Kong, L. (2025). Influence of Waste Catalyst Surface Characteristics on High-Temperature Performance and Adhesion Properties of Asphalt Mortar. Coatings, 15(2), 187. https://doi.org/10.3390/coatings15020187






