The Fluidized Bed-Chemical Vapor Deposition Coating Technology of Micro-Nano Particles: Status and Prospective
Abstract
:1. Introduction
2. The FB-CVD Technology of Micro-Nano Particles: Mechanism and Challenges
2.1. Particle Fluidization Mechanism
2.2. CVD Mechanism
2.3. Challenges: The Aggregation of Micro-Nano Particles
3. Process Intensification of the FB-CVD Coating of Micro-Nano Particles
3.1. The Progress of Particle Design-Assisted Micro-Nano Particle Fluidization
3.2. The Progress of External Force Field-Assisted Micro-Nano Particle Fluidization
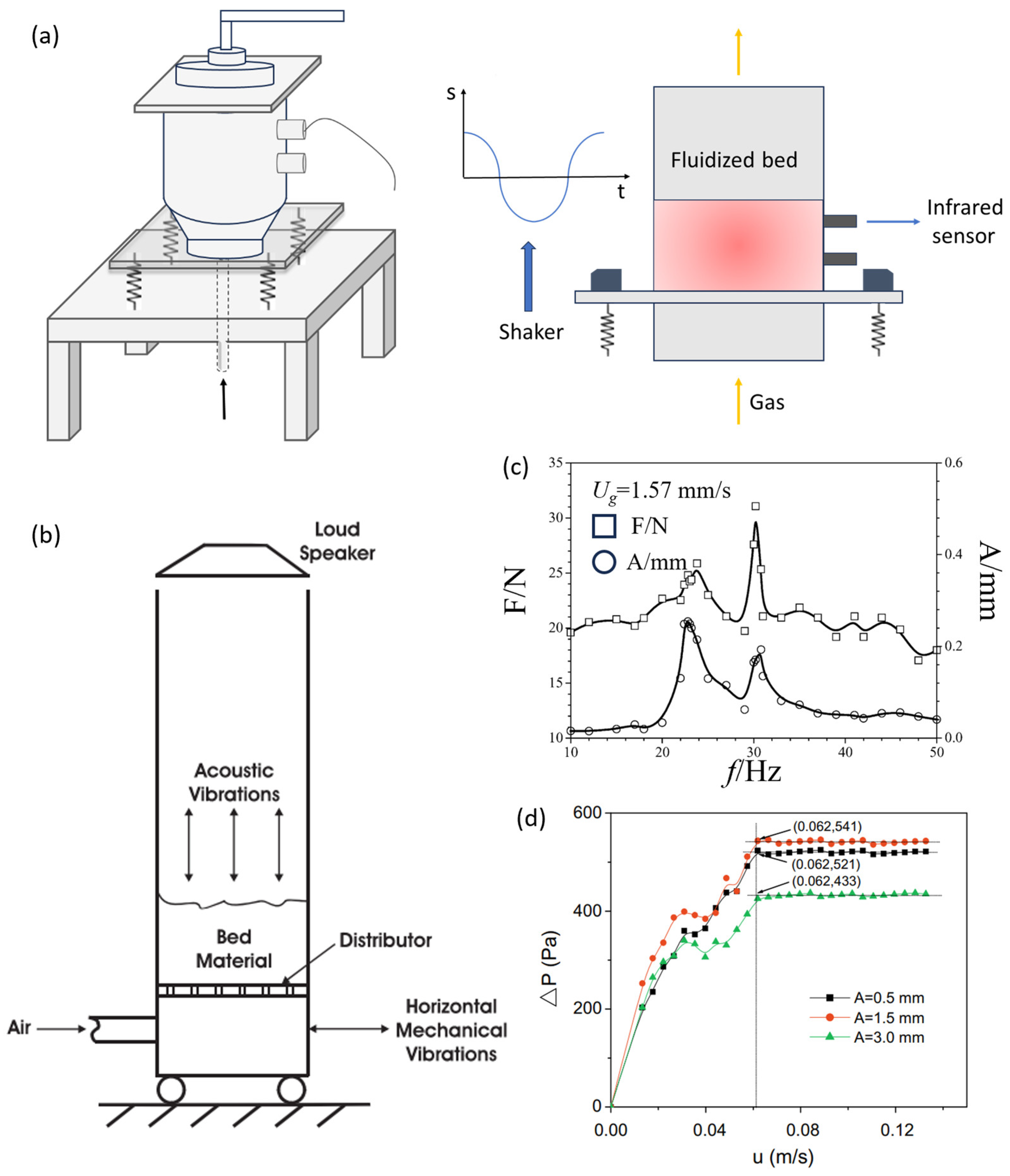
3.2.1. The Progress of Vibration Field-Assisted Micro-Nano Particle Fluidization
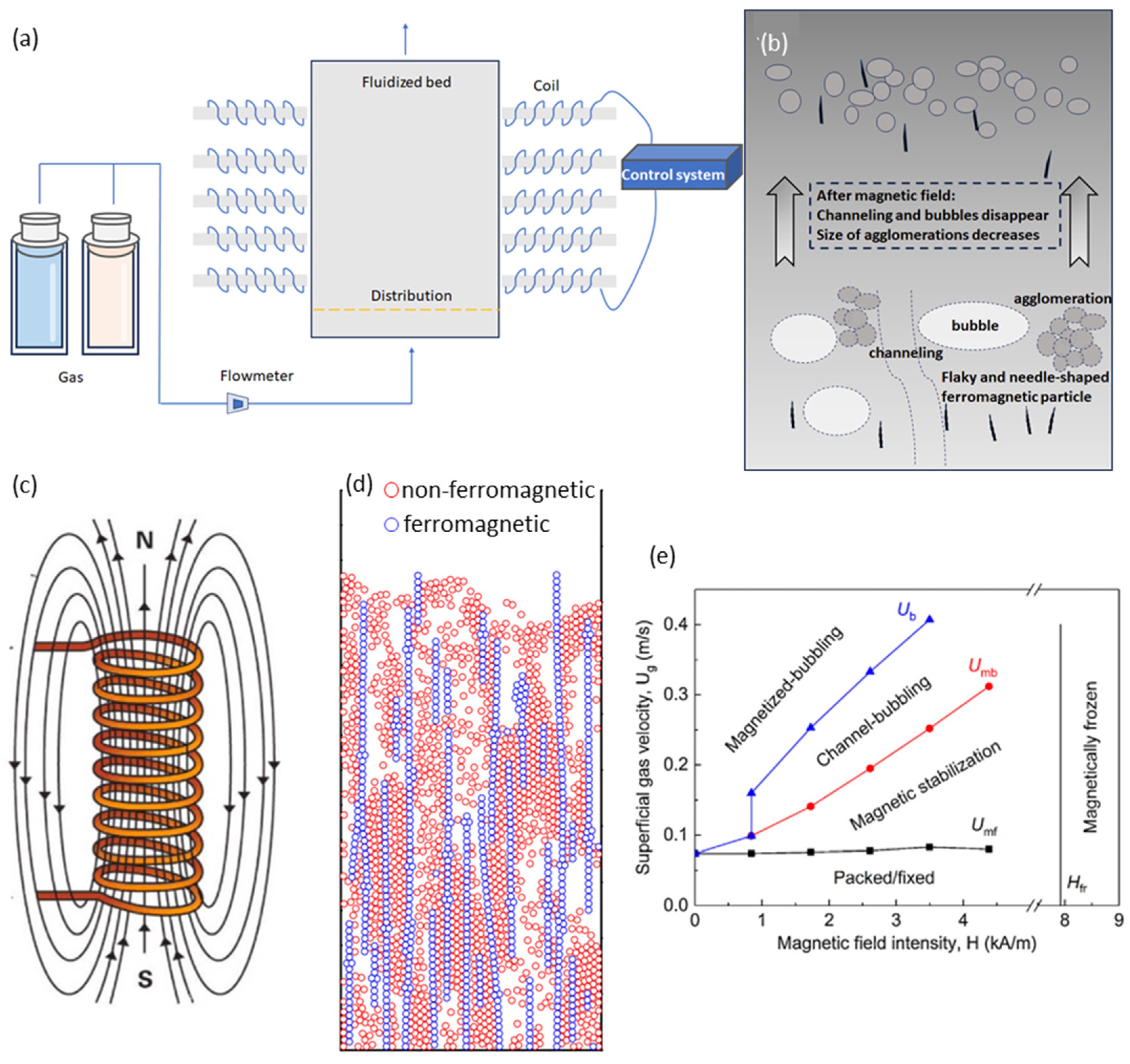
3.2.2. The Progress of Magnetic Field-Assisted Micro-Nano Particle Fluidization
3.2.3. The Progress of Sound Field-Assisted Micro-Nano Particle Fluidization
3.3. The Progress of Micro-Nano Particle CVD Coating
4. The High-Tech Applications of FB-CVD Technology for Micro-Nano Particle Coatings
4.1. Advanced Nuclear Fuel
4.2. Electrode Materials for Battery
4.3. Semiconductor Materials
5. Future Prospectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knowlton, T.M.; Keairns, D.L.; Yang, W.C. History of Fluidization in North America. Powder Technol. 2023, 426, 118609. [Google Scholar] [CrossRef]
- Philippe, R.; Morançais, A.; Corrias, M.; Caussat, B.; Kihn, Y.; Kalck, P.; Plee, D.; Gaillard, P.; Bernard, D.; Serp, P. Catalytic Production of Carbon Nanotubes by Fluidized-Bed CVD. Chem. Vap. Depos. 2007, 13, 447–457. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, W. Numerical Simulations of Particle Growth in a Silicon-CVD Fluidized Bed Reactor via a CFD-PBM Coupled Model. Chem. Eng. Sci. 2014, 111, 112–125. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Zhang, P.; Wang, W.; Duan, J. Technical Challenges and Progress in Fluidized Bed Chemical Vapor Deposition of Polysilicon. Chin. J. Chem. Eng. 2011, 19, 747–753. [Google Scholar] [CrossRef]
- Vahlas, C.; Caussat, B.; Serp, P.; Angelopoulos, G.N. Principles and Applications of CVD Powder Technology. Mater. Sci. Eng. R-Rep. 2006, 53, 1–72. [Google Scholar] [CrossRef]
- Sharma, S.; Kalita, G.; Hirano, R.; Shinde, S.M.; Papon, R.; Ohtani, H.; Tanemura, M. Synthesis of Graphene Crystals from Solid Waste Plastic by Chemical Vapor Deposition. Carbon 2014, 72, 66–73. [Google Scholar] [CrossRef]
- Goto, T. A Review: Structural Oxide Coatings by Laser Chemical Vapor Deposition. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2016, 31, 1–5. [Google Scholar] [CrossRef]
- Chhowalla, M.; Teo, K.B.K.; Ducati, C.; Rupesinghe, N.L.; Amaratunga, G.A.J.; Ferrari, A.C.; Roy, D.; Robertson, J.; Milne, W.I. Growth Process Conditions of Vertically Aligned Carbon Nanotubes Using Plasma Enhanced Chemical Vapor Deposition. J. Appl. Phys. 2001, 90, 5308–5317. [Google Scholar] [CrossRef]
- Tan, S.T.; Chen, B.J.; Sun, X.W.; Fan, W.J.; Kwok, H.S.; Zhang, X.H.; Chua, S.J. Blueshift of Optical Band Gap in ZnO Thin Films Grown by Metal-Organic Chemical-Vapor Deposition. J. Appl. Phys. 2005, 98, 013505. [Google Scholar] [CrossRef]
- Ma, L.; Chen, A.; Lu, J.; Zhang, Z.; He, H.; Li, C. In Situ Synthesis of CNTs/Fe-Ni/TiO2 Nanocomposite by Fluidized Bed Chemical Vapor Deposition and the Synergistic Effect in Photocatalysis. Particuology 2014, 14, 24–32. [Google Scholar] [CrossRef]
- Aslam, S.; Sekkat, A.; Vergnes, H.; Esvan, J.; Pugliara, A.; Samelor, D.; Eshraghi, N.; Vahlas, C.; Auvergniot, J.; Caussat, B. A New Route to Apply Nanometric Alumina Coating on Powders by Fluidized Bed Chemical Vapor Deposition. Chem. Eng. J. Adv. 2023, 16, 100554. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, J.; Yang, X.; Liu, M.; Chang, J.; Shao, Y.; Liu, B. Mass Production of 3D Connective Graphene Networks by Fluidized Bed Chemical Vapor Deposition and Its Application in High Performance Lithium-Sulfur Battery. Nanomaterials 2022, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, P.; Zhao, H.; Li, Z.; Liu, X.; Zhang, K.; Liu, B. SiC Coating on HTR Graphite Spheres Prepared by Fluidized-Bed Chemical Vapor Deposition. Ann. Nucl. Energy 2019, 134, 11–19. [Google Scholar] [CrossRef]
- Arrieta, M.Y.; Keiser, D.D.; Perez-Nunez, D.; McDeavitt, S.M. Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films. Nucl. Technol. 2017, 199, 219–226. [Google Scholar] [CrossRef]
- Sudderth, L.; Perez-Nunez, D.; Keiser, D.; McDeavitt, S. Fabrication of ZrN Barrier Coatings for U-Mo Microspheres Via Fluidized Bed Chemical Vapor Deposition Using a Metalorganic Precursor. Nucl. Technol. 2018, 202, 81–93. [Google Scholar] [CrossRef]
- Ha, J.; Do, Y.; Park, J.; Han, C. Preparation and Photocatalytic Performance of Nano Titania-Coated Beads. In Eco-Materials Processing and Design X; Kim, H., Yang, J.F., Sekino, T., Lee, S.W., Eds.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2009; Volume 620–622, pp. 663–666. [Google Scholar]
- Zhou, J.; Chizhik, A.I.; Chu, S.; Jin, D. Single-Particle Spectroscopy for Functional Nanomaterials. Nature 2020, 579, 41–50. [Google Scholar] [CrossRef]
- Gupta, P.; Jani, K.A.; Yang, D.; Sadoqi, M.; Squillante, E.; Chen, Z. Revisiting the Role of Nanoparticles as Modulators of Drug Resistance and Metabolism in Cancer. Expert Opin. Drug Metab. Toxicol. 2016, 12, 281–289. [Google Scholar] [CrossRef]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. High-Performance Lithium-Ion Anodes Using a Hierarchical Bottom-up Approach (Vol 9, Pg 353, 2010). Nat. Mater. 2010, 9, 461. [Google Scholar] [CrossRef]
- Mondal, A.; Arora, M.; Dubey, B.K.; Mumford, K. Comparative Assessment of the Characteristics and Cr(VI) Removal Activity of the Bimetallic Fe/Cu Nanoparticles Pre- and Post-Coated with Carboxymethyl Cellulose. Chem. Eng. J. 2022, 444, 136343. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Pitchaiya, S.; Muthukumarasamy, N.; Kvamme, K.; Rajesh, G.; Agilan, S.; Pugazhendhi, A.; Velauthapillai, D. Performance of TiO2 Nanoparticles Synthesized by Microwave and Solvothermal Methods as Photoanode in Dye-Sensitized Solar Cells (DSSC). Int. J. Hydrogen Energy 2020, 45, 27036–27046. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Liu, X.; Chen, R. Progress in Enhanced Fluidization Process for Particle Coating via Atomic Layer Deposition. Chem. Eng. Process.-Process Intensif. 2021, 159, 108234. [Google Scholar] [CrossRef]
- Liu, M.; Liu, R.; Liu, B.; Shao, Y. Preparation of the Coated Nuclear Fuel Particle Using the Fluidized Bed-Chemical Vapor Deposition (FB-CVD) Method. Procedia Eng. 2015, 102, 1890–1895. [Google Scholar] [CrossRef]
- Lassegue, P.; Noe, L.; Monthioux, M.; Caussat, B. Iron Deposition on Multi-Walled Carbon Nanotubes by Fluidized Bed MOCVD for Aeronautic Applications. Phys. Status Solidi 2015, 12, 861–868. [Google Scholar] [CrossRef]
- Balaji, S.; Du, J.; White, C.M.; Ydstie, B.E. Multi-Scale Modeling and Control of Fluidized Beds for the Production of Solar Grade Silicon. Powder Technol. 2010, 199, 23–31. [Google Scholar] [CrossRef]
- Liu, R.; Liu, M.; Chang, J.; Shao, Y.; Liu, B. Preparation of Highly Flexible SiC Nanowires by Fluidized Bed Chemical Vapor Deposition. Chem. Vap. Depos. 2015, 21, 196–203. [Google Scholar] [CrossRef]
- Vanni, F.; Caussat, B.; Ablitzer, C.; Iltis, X.; Bothier, M. Silicon Coating on Very Dense Tungsten Particles by Fluidized Bed CVD for Nuclear Application. Phys. Status Solidi-Appl. Mater. Sci. 2015, 212, 1599–1606. [Google Scholar] [CrossRef]
- Dosta, M.; Hoffmann, R.; Schneider, P.; Maus, M. Hybrid Models to Support Development of Fluid Bed Granulation Processes. Powder Technol. 2024, 444, 120005. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, T.; Li, Z.; Ding, Y.; Song, Q.; Zhang, M.; Hu, N.; Yang, H. Experimental Study on Preparation of Inorganic Fibers from Circulating Fluidized Bed Boilers Ash. Materials 2024, 17, 3800. [Google Scholar] [CrossRef]
- Yousuff, M.; Page, N.W. Particle Material, Morphology and Load Effects on Internal Friction in Powders. Powder Technol. 1993, 76, 155–164. [Google Scholar] [CrossRef]
- Zegzulka, J. The Angle of Internal Friction as a Measure of Work Loss in Granular Material Flow. Powder Technol. 2013, 233, 347–353. [Google Scholar] [CrossRef]
- Azad, M.; Moreno, J.; Bilgili, E.; Dave, R. Fast Dissolution of Poorly Water Soluble Drugs from Fluidized Bed Coated Nanocomposites: Impact of Carrier Size. Int. J. Pharm. 2016, 513, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Liu, J.; Yang, Q.; Wu, B.; Xu, X.; Huang, T. Recovery of Sb(III) from Aqueous Solution as Cubic Sb2O3 by Fluidized-Bed Granulation Process. Water 2024, 16, 1690. [Google Scholar] [CrossRef]
- Gänsch, J.; Huskova, N.; Kerst, K.; Temmel, E.; Lorenz, H.; Mangold, M.; Janiga, G.; Seidel-Morgenstern, A. Continuous Enantioselective Crystallization of Chiral Compounds in Coupled Fluidized Beds. Chem. Eng. J. 2021, 422, 129627. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, J.; Luo, H.; Wang, W.; Li, H.; Ye, M.; Liu, Z.; Li, J. Numerical Simulation of Scale-up Effects of Methanol-to-Olefins Fluidized Bed Reactors. Chem. Eng. Sci. 2017, 171, 244–255. [Google Scholar] [CrossRef]
- Verma, A. Advance Coal-Fired Power-Generation Processes Part II. Energy Sources 1980, 5, 259–289. [Google Scholar] [CrossRef]
- Kwauk, M.; Li, J.; Liu, D. Particulate and Aggregative Fluidization—50 Years in Retrospect. Powder Technol. 2000, 111, 3–18. [Google Scholar] [CrossRef]
- Amontree, J.; Yan, X.; DiMarco, C.S.; Levesque, P.L.; Adel, T.; Pack, J.; Holbrook, M.; Cupo, C.; Wang, Z.; Sun, D.; et al. Reproducible Graphene Synthesis by Oxygen-Free Chemical Vapour Deposition. Nature 2024, 630, 636–642. [Google Scholar] [CrossRef]
- Hwang, N.M. Growth Mechanism of CVD Silicon. In Non-Classical Crystallization of Thin Films and Nanostructures in CVD and PVD Processes; Springer Series in Surface Sciences; Springer: Dordrecht, The Netherlands, 2016; Volume 60, pp. 163–180. ISBN 978-94-017-7616-5. [Google Scholar]
- Zhang, Y. Advances in Thermal Modeling of Laser Chemical Vapor Deposition and Infiltration; Evgova, J., Kostadinov, O., Eds.; Mechanical Engineering Theory and Applications; Nova Science Publishers, Inc: Hauppauge, NY, USA, 2010; pp. 283–316. ISBN 978-1-60741-497-1. [Google Scholar]
- Li, W.; Lu, Y. The Study of Friction and Wear Capability of Piston Ring Coatings. In Sae-China Congress 2016: Selected Papers; Springer-Verlag Singapore Pte Ltd.: Singapore, 2017; Volume 418, pp. 241–247. [Google Scholar]
- Lee, S.H.; Lee, G.H.; Lee, H.-S.; Kim, D.; Kang, Y. Characterization of MOCVD-Prepared CIS Solar Cells. Energies 2021, 14, 7721. [Google Scholar] [CrossRef]
- Zhang, L.; Xin, F.; Li, S.; Yang, Y.; Zhu, Q.; Wang, G. Enabling the Low-Cost Preparation of Core-Shell WC-Ni Powder by Developing a Non-Noble Metal-Based Catalytic. J. Am. Ceram. Soc. 2019, 102, 4492–4501. [Google Scholar] [CrossRef]
- Huang, J.; Militzer, C.; Xu, J.; Wijayawardhana, C.A.; Forsberg, U.; Pedersen, H. Growth of Silicon Carbide Multilayers with Varying Preferred Growth Orientation. Surf. Coat. Technol. 2022, 447, 128853. [Google Scholar] [CrossRef]
- Si, W.; Wang, N.; Zong, Y.; Dai, G.; Meng, F.; Yang, Z.; Zhao, L.; Xin, Z.; Jiang, G. A Revisit from CVD Kinetics to CVD Reactor: Investigating Uniform Growth Mechanism of Polysilicon in a Reduction Furnace. Chem. Eng. Sci. 2024, 295, 120162. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.; Xu, C.; Wang, Y.; Cong, H.; Xue, C. Epitaxial Growth of High-Quality Ge Layers on Si with Ge2H6 under UHV-CVD Conditions. Semicond. Sci. Technol. 2024, 39, 015008. [Google Scholar] [CrossRef]
- Musa, N.; Wong, T.W. Design of Polysaccharidic Nano-in-Micro Soft Agglomerates as Primary Oral Drug Delivery Vehicle for Colon-Specific Targeting. Carbohydr. Polym. 2020, 247, 116673. [Google Scholar] [CrossRef]
- Hu, D.; Zhuang, J.; Ding, M. A Review of Studies on the Granular Agglomeration Mechanisms and Anti-Agglomeration Methods. In Progress in Polymer Processing; Zhang, C., Ed.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2012; Volume 501, pp. 515–519. [Google Scholar]
- Mao, D.; Edwards, J.R.; Kuznetsov, A.V.; Srivastava, R. A Model for Fine Particle Agglomeration in Circulating Fluidized Bed Absorbers. Heat Mass Transf. 2002, 38, 379–388. [Google Scholar] [CrossRef]
- Pacek, A.; Nienow, A. Fluidization of Fine and Very Dense Hard-Metal Powders. Powder Technol. 1990, 60, 145–158. [Google Scholar] [CrossRef]
- Hessels, C.J.M.; Lelivelt, D.W.J.; Stevens, N.C.; Tang, Y.; Deen, N.G.; Finotello, G. Minimum Fluidization Velocity and Reduction Behavior of Combusted Iron Powder in a Fluidized Bed. Fuel 2023, 342, 127710. [Google Scholar] [CrossRef]
- Geldart, D. Types of Gas Fluidization. Powder Technol. 1973, 7, 285–292. [Google Scholar] [CrossRef]
- Saleh, K.; Cami, X.B.; Thomas, A.; Guigon, P. An Experimental Study on the Fluidisation Behaviour of Geldart C Glass Powders. Kona Powder Part. J. 2006, 24, 134–145. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, X.; Li, D.; Li, J.; Zhu, Q.; Li, H. SiO2 Microsphere-Assisted Reduction of Ultrafine CuO Powder in a Fluidized Bed. Chem. Eng. Sci. 2023, 269, 118500. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, J. Group C+ Particles: Enhanced Flow and Fluidization of Fine Powders with Nano-Modulation. Chem. Eng. Sci. 2019, 207, 653–662. [Google Scholar] [CrossRef]
- Li, H.; Legros, R.; Brereton, C.; Grace, J.; Chaouki, J. Hydrodynamic Behavior of Aerogel Powders in High-Velocity Fluidized-Beds. Powder Technol. 1990, 60, 121–129. [Google Scholar] [CrossRef]
- Lauga, C.; Chaouki, J.; Klvana, D.; Chavarie, C. Improvement of the Fluidisability of Ni/Sio2 Aerogels by Reducing Interparticle Forces. Powder Technol. 1991, 65, 461–468. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.; Zhou, T.; Liang, X. Agglomeration Mechanism of Nanoparticles by Adding Coarse Fluid Catalytic Cracking Particles. Chem. Eng. Technol. 2016, 39, 1490–1496. [Google Scholar] [CrossRef]
- Duan, H.; Liang, X.; Zhou, T.; Wang, J.; Tang, W. Fluidization of Mixed SiO2 and ZnO Nanoparticles by Adding Coarse Particles. Powder Technol. 2014, 267, 315–321. [Google Scholar] [CrossRef]
- Kono, H.; Huang, C.; Morimoto, E.; Nakayama, T.; Hikosaka, T. Segregation and Agglomeration of Type-C Powders from Homogeneously Aerated Type-a-C Powder Mixtures During Fluidization. Powder Technol. 1987, 53, 163–168. [Google Scholar] [CrossRef]
- Scuzzarella, A.; Fernandez Bertos, M.; Simons, S.J.; Hills, C.D.; Carey, P.J. Expansion of Cohesive Gas Fluidized Binary Solid Systems. Powder Technol. 2006, 163, 18–22. [Google Scholar] [CrossRef]
- Zhou, T.; Li, H.Z. Effects of Adding Different Size Particles on Fluidization of Cohesive Particles. Powder Technol. 1999, 102, 215–220. [Google Scholar] [CrossRef]
- Levy, E.K.; Celeste, B. Combined Effects of Mechanical and Acoustic Vibrations on Fluidization of Cohesive Powders. Powder Technol. 2006, 163, 41–50. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.J.; Yang, Y.; Jin, Y. Resonance Characteristics of a Vibrated Fluidized Bed with a High Bed Hold-Up. Powder Technol. 2002, 127, 196–202. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, T.; Song, L. Agglomerating Vibro-Fluidization Behavior of Nano-Particles. Adv. Powder Technol. 2009, 20, 158–163. [Google Scholar] [CrossRef]
- Mori, S.; Haruta, T.; Yamamoto, A.; Yamada, I.; Mizutani, E. Vibro-Fluidization of Very Fine Particles. Kagaku Kogaku Ronbunshu 1989, 15, 992–997. [Google Scholar] [CrossRef]
- Valverde, J.M.; Castellanos, A.; Quintanilla, M.a.S. Effect of Vibration on the Stability of a Gas-Fluidized Bed of Fine Powder. Phys. Rev. E 2001, 64, 021302. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.H.; Pfeffer, R.; Dave, R.N.; Sundaresan, S. Aerated Vibrofluidization of Silica Nanoparticles. AICHE J. 2004, 50, 1776–1785. [Google Scholar] [CrossRef]
- Mawatari, Y.; Koide, T.; Tatemoto, Y.; Uchida, S.; Noda, K. Effect of Particle Diameter on Fluidization under Vibration. Powder Technol. 2002, 123, 69–74. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, M. Fluidisation Behaviour of Silica Nanoparticles under Horizontal Vibration. J. Exp. Nanosci. 2010, 5, 69–82. [Google Scholar] [CrossRef]
- Barletta, D.; Poletto, M. Aggregation Phenomena in Fluidization of Cohesive Powders Assisted by Mechanical Vibrations. Powder Technol. 2012, 225, 93–100. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Park, Y.; Lee, K. Fluidization Characteristics of Fine Cohesive Particles Assisted by Vertical Vibration in a Fluidized Bed Reactor. Chem. Eng. J. 2020, 380, 122454. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, W.; Cao, Y.; Gai, H.; Zhu, Q. Diverse Gas-Solid Magnetized Fluidized Beds with Different Magnetic Fields. Powder Technol. 2024, 433, 119241. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Li, X.; Gao, J.; Lan, X.; Dong, Q. Simulation of Flow Behavior of Particles in Liquid-Solid Fluidized Bed with Uniform Magnetic Field. Powder Technol. 2013, 237, 314–325. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, H.; Zhu, Q.; Huang, Q. Comparing Flow Regime Transition of Magnetized Fluidized Bed with Geldart-B Particles between Magnetization- FIRST and -LAST Operation Modes. Chem. Eng. J. 2019, 360, 686–700. [Google Scholar] [CrossRef]
- Diao, R.; Zhou, T.; Zhang, F.; Wang, H. Behavior of Non-Magnetic SiO2 Nano-Particles in a Magnetic Fluidized Bed. Guangzhou Chem. 2010, 35, 1–5. [Google Scholar] [CrossRef]
- Zhou, L.; Diao, R.; Zhou, T.; Kage, H.; Mawatari, Y. Characteristics of Non-Magnetic Nanoparticles in Magnetically Fluidized Bed by Adding Coarse Magnets. J. Cent. South Univ. 2011, 18, 1383–1388. [Google Scholar] [CrossRef]
- Yu, Q.; Dave, R.N.; Zhu, C.; Quevedo, J.A.; Pfeffer, R. Enhanced Fluidization of Nanoparticles in an Oscillating Magnetic Field. AICHE J. 2005, 51, 1971–1979. [Google Scholar] [CrossRef]
- Lv, X.; Li, H. Improvement of Fluization Quality of Cohesive by Using Transverse Rotating Magnetic Field. J. Chem. Ind. Eng. (China) 2000, 51, 223–226. [Google Scholar]
- Zhu, Q.S.; Li, H.Z. Study on Magnetic Fluidization of Group C Powders. Powder Technol. 1996, 86, 179–185. [Google Scholar]
- Wang, S.; Li, X.; Lu, H.; Liu, G.; Wang, J.; Xu, P. Simulation of Cohesive Particle Motion in a Sound-Assisted Fluidized Bed. Powder Technol. 2011, 207, 65–77. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, J.; Hao, J. Flow Characteristics in an Acoustic Bubbling Fluidized Bed at High Temperature. Chem. Eng. Process.-Process Intensif. 2011, 50, 331–337. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, G.L.; Yu, Q.; Pfeffer, R.; Dave, R.N.; Nam, C.H. Sound Assisted Fluidization of Nanoparticle Agglomerates. Powder Technol. 2004, 141, 119–123. [Google Scholar] [CrossRef]
- Morse, R. Sonic Energy in Granular Solid Fluidization. Ind. Eng. Chem. 1955, 47, 1170–1175. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P.; Chirone, R. CO2 Adsorption on Fine Activated Carbon in a Sound Assisted Fluidized Bed: Effect of Sound Intensity and Frequency, CO2 Partial Pressure and Fluidization Velocity. Appl. Energy 2014, 113, 1269–1282. [Google Scholar] [CrossRef]
- Levy, E.K.; Shnitzer, I.; Masaki, T.; Salmento, J. Effect of an Acoustic Field on Bubbling in a Gas Fluidized Bed. Powder Technol. 1997, 90, 53–57. [Google Scholar] [CrossRef]
- Herrera, C.A.; Levy, E.K. Bubbling Characteristics of Sound-Assisted Fluidized Beds. Powder Technol. 2001, 119, 229–240. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Wang, M.; Shen, W.; Yang, C. Fluidization Characteristics of SiO2 Nanoparticles in an Acoustic Fluidized. Chem. Eng. Technol. 2006, 29, 78–86. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Q.; Chen, S. Sound-Assisted Fluidization of SiO2 Nanoparticles with Different Surface Properties. Ind. Eng. Chem. Res. 2007, 46, 1345–1349. [Google Scholar] [CrossRef]
- Ammendola, P.; Chirone, R. Aeration and Mixing Behaviours of Nano-Sized Powders under Sound Vibration. Powder Technol. 2010, 201, 49–56. [Google Scholar] [CrossRef]
- Noda, K.; Mawatari, Y.; Uchida, S. Flow Patterns of Fine Particles in a Vibrated Fluidized Bed under Atmospheric or Reduced Pressure. Powder Technol. 1998, 99, 11–14. [Google Scholar] [CrossRef]
- Lehmann, S.E.; Hartge, E.-U.; Jongsma, A.; deLeeuw, I.-M.; Innings, F.; Heinrich, S. Fluidization Characteristics of Cohesive Powders in Vibrated Fluidized Bed Drying at Low Vibration Frequencies. Powder Technol. 2019, 357, 54–63. [Google Scholar] [CrossRef]
- Wang, B.; Tang, T.; Yan, S.; He, Y. Magnetic Segregation Behaviors of a Binary Mixture in Fluidized Beds. Powder Technol. 2022, 397, 117031. [Google Scholar] [CrossRef]
- Espin, M.J.; Quintanilla, M.A.S.; Valverde, J.M. Magnetic Stabilization of Fluidized Beds: Effect of Magnetic Field Orientation. Chem. Eng. J. 2017, 313, 1335–1345. [Google Scholar] [CrossRef]
- Ajbar, A.; Bakhbakhi, Y.; Ali, S.; Asif, M. Fluidization of Nano-Powders: Effect of Sound Vibration and Pre-Mixing with Group A Particles. Powder Technol. 2011, 206, 327–337. [Google Scholar] [CrossRef]
- Lv, B.; Deng, X.; Chen, J.; Fang, C.; Zhu, X. Effects of Sound Fields on Hydrodynamic and Dry Beneficiation of Fine Coal in a Fluidized Bed. Sep. Purif. Technol. 2021, 254, 117575. [Google Scholar] [CrossRef]
- Bizhaem, H.K.; Tabrizi, H.B. Experimental Study on Hydrodynamic Characteristics of Gas-Solid Pulsed Fluidized Bed. Powder Technol. 2013, 237, 14–23. [Google Scholar] [CrossRef]
- Abedi, N.; Esfahany, M.N. Elutriation of Fine Particles in a Pulsed Fluidized Bed. Powder Technol. 2024, 434, 119355. [Google Scholar] [CrossRef]
- Kashyap, M.; Gidaspow, D.; Driscoll, M. Effect of Electric Field on the Hydrodynamics of Fluidized Nanoparticles. Powder Technol. 2008, 183, 441–453. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, H.; Yang, J.; He, Y.; Zhang, T. Application of Electric Field to a Fluidized Bed for Recovering Residual Metals from Fine Particles of the Non-Metallic Fraction of Waste Printed Circuit Boards. J. Clean. Prod. 2018, 187, 1036–1042. [Google Scholar] [CrossRef]
- Nakamura, H.; Watano, S. Fundamental Particle Fluidization Behavior and Handling of Nano-Particles in a Rotating Fluidized Bed. Powder Technol. 2008, 183, 324–332. [Google Scholar] [CrossRef]
- Shi, M.H.; Wang, H.; Hao, Y.L. Experimental Investigation of the Heat and Mass Transfer in a Centrifugal Fluidized Bed Dryer. Chem. Eng. J. 2000, 78, 107–113. [Google Scholar] [CrossRef]
- Schmidt, J.; Werther, J. Simulation and Optimization of a Centrifugal Fluidized Bed Classifier in the Micrometer Range. Chem. Eng. Process. Process Intensif. 2006, 45, 488–499. [Google Scholar] [CrossRef]
- Kang, S.; Wang, C.; Chen, J.; Meng, T.; Jiaqiang, E. Progress on Solvo/Hydrothermal Synthesis and Optimization of the Cathode Materials of Lithium-Ion Battery. J. Energy Storage 2023, 67, 107515. [Google Scholar] [CrossRef]
- Oh, S.H.; Cho, J.S. Dataset on the Effect of the Reaction Temperature during Spray Pyrolysis for the Synthesis of the Hierarchical Yolk-Shell CNT-(NiCo)O/C Microspheres. Data Brief 2019, 25, 104302. [Google Scholar] [CrossRef]
- Kitawaki, S.; Nagai, T.; Sato, N. Chlorination of Uranium Oxides with CCl4 Using a Mechanochemical Method. J. Nucl. Mater. 2013, 439, 212–216. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A Review of Microencapsulation Methods of Phase Change Materials (PCMs) as a Thermal Energy Storage (TES) Medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Saito, Y.; Sekiguchi, Y.; Mizuhata, M.; Deki, S. Continuous Deposition System of SnO2 Thin Film by the Liquid Phase Deposition (LPD) Method. J. Ceram. Soc. Jpn. 2007, 115, 856–860. [Google Scholar] [CrossRef]
- Deng, B.; Liu, Z.; Peng, H. Toward Mass Production of CVD Graphene Films. Adv. Mater. 2019, 31, 1800996. [Google Scholar] [CrossRef]
- Rezaei, S.; Manoucheri, I.; Moradian, R.; Pourabbas, B. One-Step Chemical Vapor Deposition and Modification of Silica Nanoparticles at the Lowest Possible Temperature and Superhydrophobic Surface Fabrication. Chem. Eng. J. 2014, 252, 11–16. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Grenci, G.; Van Gorp, H.; Vanderlinden, W.; De Feyter, S.; Falcaro, P.; De Vos, D.; Vereecken, P.; Ameloot, R. Chemical Vapour Deposition of Zeolitic Imidazolate Framework Thin Films. Nat. Mater. 2016, 15, 304–310. [Google Scholar] [CrossRef]
- Anisur, M.R.; Raman, R.K.S.; Banerjee, P.C.; Al-Saadi, S.; Arya, A.K. Review of the Role of CVD Growth Parameters on Graphene Coating Characteristics and the Resulting Corrosion Resistance. Surf. Coat. Technol. 2024, 487, 130934. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Fu, Y.; Zhang, Y.; Zhu, X. Growth Mechanism and Ablation Behavior of CVD-HfC Coating on the Surface of C/C Composites and CVD-SiC Coating. Corros. Sci. 2021, 192, 109819. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Cao, Y.; Wang, J.; Zhang, Y. CVD Synthesis of Nanometer SiC Coating on Diamond Particles. Ceram. Int. 2021, 47, 16162–16169. [Google Scholar] [CrossRef]
- Morstein, M.; Karches, M.; Bayer, C.; Casanova, D.; von Rohr, P.R. Plasma CVD of Ultrathin TiO2 Films on Powders in a Circulating Fluidized Bed. Chem. Vap. Depos. 2000, 6, 16–20. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Li, X.; Du, Y.; Hou, G.; Xiang, M.; Lv, P.; Zhu, Q. In Situ Fabrication of Dual Coating Structured SiO/1D-C/a-C Composite as High-Performance Lithium Ion Battery Anode by Fluidized Bed Chemical Vapor Deposition. Carbon 2020, 168, 113–124. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Xiao, P.; Zhou, P.; Fang, Z.; Li, Y. Carbon Coating Optimization on Porous Silicon as High-Performance Anode Material via Fluidized Bed Chemical Vapor Deposition. J. Alloys Compd. 2023, 966, 171564. [Google Scholar] [CrossRef]
- Wang, J.; Obrovac, M.N. Lab-Scale Chemical Vapor Deposition onto Powders. AIP Adv. 2022, 12, 075209. [Google Scholar] [CrossRef]
- Choi, S.; Shin, D.; Kim, S.E.; Yun, C.; Tan, Y.Y.; Lee, C.S. Nano-Capsuled Thermal Interface Materials Filler Using Defective Multilayered Graphene-Coated Silver Nanoparticles. Microelectron. Eng. 2023, 281, 112082. [Google Scholar] [CrossRef]
- Yang, Y.; ul Hassan, S.; Zai, M.; Shah, M.; Zafar, S.; Hou, L.; Wang, S. Compositional Design of C-Coated Multi-Elemental Alloy Nanoparticles for Superior Microwave Absorption. J. Alloys Compd. 2024, 988, 174316. [Google Scholar] [CrossRef]
- Shin, D.; Choi, S.; Kim, S.E.; Yun, C.; Tan, Y.Y.; Lee, C.S. Fabrication of Multilayer Graphene-Coated Copper Nanoparticles for Application as a Thermal Interface Material. Appl. Surf. Sci. 2022, 583, 152488. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Chen, X.; Wang, A.; Tian, Z.; Li, J. Graphene-Coated Au Nanoparticle-Enhanced Raman Spectroscopy. J. Raman Spectrosc. 2021, 52, 439–445. [Google Scholar] [CrossRef]
- Tang, C.H.; Tang, Y.P.; Zhu, J.G.; Zou, Y.W.; Li, J.H.; Ni, X.J. Design and Manufacture of the Fuel Element for the 10 MW High Temperature Gas-Cooled Reactor. Nucl. Eng. Des. 2002, 218, 91–102. [Google Scholar] [CrossRef]
- Liu, R.; Liu, M.; Chang, J.; Shao, Y.; Liu, B. An Improved Design of TRISO Particle with Porous SiC Inner Layer by Fluidized Bed-Chemical Vapor Deposition. J. Nucl. Mater. 2015, 467, 917–926. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, X.; Liu, B.; Liu, M.; Shao, Y.; Liu, R. A New Design of Composite Fuel with Zr-Coated TRISO Particles Dispersed in Metal Matrix. Nucl. Eng. Des. 2023, 415, 112702. [Google Scholar] [CrossRef]
- Liu, B.; Liu, C.; Shao, Y.; Zhu, J.; Yang, B.; Tang, C. Deposition of ZrC-Coated Particle for HTR with ZrCl4 Powder. Nucl. Eng. Des. 2012, 251, 349–353. [Google Scholar] [CrossRef]
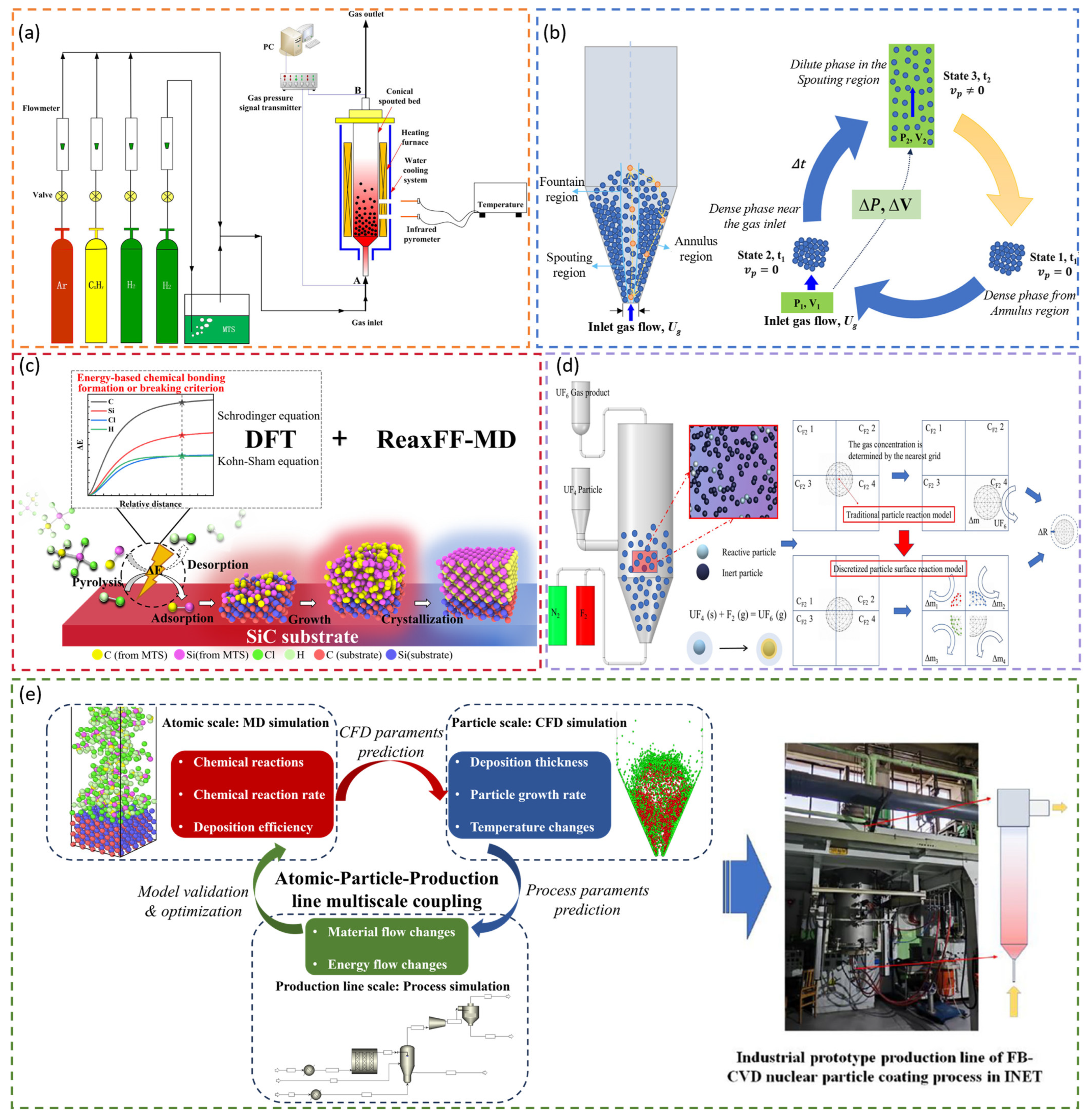
- Yan, Z.; Jiang, L.; Tian, Y.; Liu, R.; Shao, Y.; Liu, B.; Liu, M. Multi-Scale Study of Fluidized Bed-Chemical Vapour Deposition Process in Nuclear Fuel Coated Particle Fabrication for High-Temperature Gas-Cooled Reactor: A Review. Can. J. Chem. Eng. 2024. [Google Scholar] [CrossRef]
- Yan, Z.; Tian, Y.; Liu, R.; Liu, B.; Shao, Y.; Liu, M. Atomistic Insights into Chemical Vapor Deposition Process of Preparing Silicon Carbide Materials Using ReaxFF-MD Simulation. Comput. Mater. Sci. 2024, 241, 113032. [Google Scholar] [CrossRef]
- Liu, R.; Liu, M.; Shao, Y.; Chen, X.; Ma, J.; Liu, B. A Novel Coated-Particle Design and Fluidized-Bed Chemical Vapor Deposition Preparation Method for Fuel-Element Identification in a Nuclear Reactor. Particuology 2017, 31, 35–41. [Google Scholar] [CrossRef]
- Jiang, L.; Qiu, M.; Liu, R.; Liu, B.; Shao, Y.; Liu, M. CFD-DEM Simulation of High Density Particles Fluidization Behaviors in 3D Conical Spouted Beds. Particuology 2024, 88, 266–281. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, Z.; Jiang, L.; Liu, R.; Tang, Y.; Liu, M. Numerical Simulation of Uranium Tetrafluoride Fluorination in a Multistage Spouted Bed Using the Improved CFD-DEM Chemical Reaction Model. Particuology 2023, 75, 119–136. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Hu, C.; Li, S.; Xiang, M.; Lv, P.; Zhu, Q. Efficient Fluidization Intensification Process to Fabricate In-Situ Dispersed (SiO plus G)/CNTs Composites for High-Performance Lithium-Ion Battery Anode Applications. Particuology 2021, 56, 84–90. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, F.; Lin, X.; Zhang, C.; Bai, H.; Yu, C.; He, Z.; Jiang, H.; Wei, F. Mass production of SiOx@C anode material in gas-solid fluidized bed. Energy Storage Sci. Technol. 2022, 11, 1739–1748. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Wang, R.; Liu, M.; Shen, Z.; Chen, J.; Wu, B.; Xiang, M.; Li, J.; Zhang, H.; et al. Modulation of the Interfacial Properties of TiN Coatings to Generate Rigid-Flexible SEI for Better SiO Anodes. Chem. Eng. J. 2023, 461, 142036. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, M.; Chang, J.; Shao, Y.; Liu, B.; Liu, R. Controllable Synthesis of SiC@Graphene Core-Shell Nanoparticles via Fluidized Bed Chemical Vapor Deposition. J. Am. Ceram. Soc. 2020, 103, 5579–5585. [Google Scholar] [CrossRef]
- Hsu, G.; Rohatgi, N.; Houseman, J. Silicon Particle Growth in a Fluidized-Bed Reactor. AICHE J. 1987, 33, 784–791. [Google Scholar] [CrossRef]
- Cadoret, L.; Reuge, N.; Pannala, S.; Syamlal, M.; Rossignol, C.; Dexpert-Ghys, J.; Coufort, C.; Caussat, B. Silicon Chemical Vapor Deposition on Macro and Submicron Powders in a Fluidized Bed. Powder Technol. 2009, 190, 185–191. [Google Scholar] [CrossRef]
- Furusawa, T.; Kojima, T.; Hiroha, H. Chemical Vapor-Deposition and Homogeneous Nucleation in Monosilane Pyrolysis Within Interparticle Spaces—Application of Fines Formation Analysis to Fluidized-Bed Cvd. Chem. Eng. Sci. 1988, 43, 2037–2042. [Google Scholar] [CrossRef]
- Reuge, N.; Cadoret, L.; Caussat, B. Multifluid Eulerian Modelling of a Silicon Fluidized Bed Chemical Vapor Deposition Process: Analysis of Various Kinetic Models. Chem. Eng. J. 2009, 148, 506–516. [Google Scholar] [CrossRef]
- Cadoret, L.; Reuge, N.; Pannala, S.; Syamlal, M.; Coufort, C.; Caussat, B. Silicon CVD on Powders in Fluidized Bed: Experimental and Multifluid Eulerian Modelling Study. Surf. Coat. Technol. 2007, 201, 8919–8923. [Google Scholar] [CrossRef]



| Advantages | Disadvantages | Reference | |
|---|---|---|---|
| LCVD | High deposition accuracy and efficiency, local preparation | High cost, complex operation | [40] |
| PCVD | Low deposition temperature, simple equipment maintenance | Difficult to control reaction process | [41] |
| MOCVD | Large area preparation, high deposition accuracy | High cost and material requirements | [42] |
| FB-CVD | High conversion rate, stable coating quality, and low cost | High deposition temperature, high flowability requirements | [43] |
| Advantages | Disadvantages | Applications | Reference | |
|---|---|---|---|---|
| Vibration field | High vibration force intensity | Great impact on the equipment | The drying of food and pharmaceutical particles | [91,92] |
| Magnetic field | Excellent effect on magnetic particles | Essential magnetic particles and insufficient heat transfer capacity | The adsorption of contaminants from a gas stream | [93,94] |
| Sound field | Directly acting on the powder | Attenuation of acoustic intensity during propagation | Lignite drying, CO2 recovery, and aluminum foam preparation | [95,96] |
| Pulse airflow | Ultra-high strength | Not conducive to stable operation | Elutriation of micro-nano particle | [97,98] |
| Electric field | Enhanced gas–solid flow, heat/mass transfer | Easy stick to the wall | The recovery of residual metals | [99,100] |
| Centrifugal field | Small size and high density of agglomerate | Involves rotating machinery | Drying for food and classification | [101,102,103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Xu, Z.; Duan, G.; Yang, X.; Liu, B.; Shao, Y.; Liu, M.; Liu, R. The Fluidized Bed-Chemical Vapor Deposition Coating Technology of Micro-Nano Particles: Status and Prospective. Coatings 2025, 15, 322. https://doi.org/10.3390/coatings15030322
Li B, Xu Z, Duan G, Yang X, Liu B, Shao Y, Liu M, Liu R. The Fluidized Bed-Chemical Vapor Deposition Coating Technology of Micro-Nano Particles: Status and Prospective. Coatings. 2025; 15(3):322. https://doi.org/10.3390/coatings15030322
Chicago/Turabian StyleLi, Bowen, Zhitong Xu, Gaohan Duan, Xu Yang, Bing Liu, Youlin Shao, Malin Liu, and Rongzheng Liu. 2025. "The Fluidized Bed-Chemical Vapor Deposition Coating Technology of Micro-Nano Particles: Status and Prospective" Coatings 15, no. 3: 322. https://doi.org/10.3390/coatings15030322
APA StyleLi, B., Xu, Z., Duan, G., Yang, X., Liu, B., Shao, Y., Liu, M., & Liu, R. (2025). The Fluidized Bed-Chemical Vapor Deposition Coating Technology of Micro-Nano Particles: Status and Prospective. Coatings, 15(3), 322. https://doi.org/10.3390/coatings15030322







