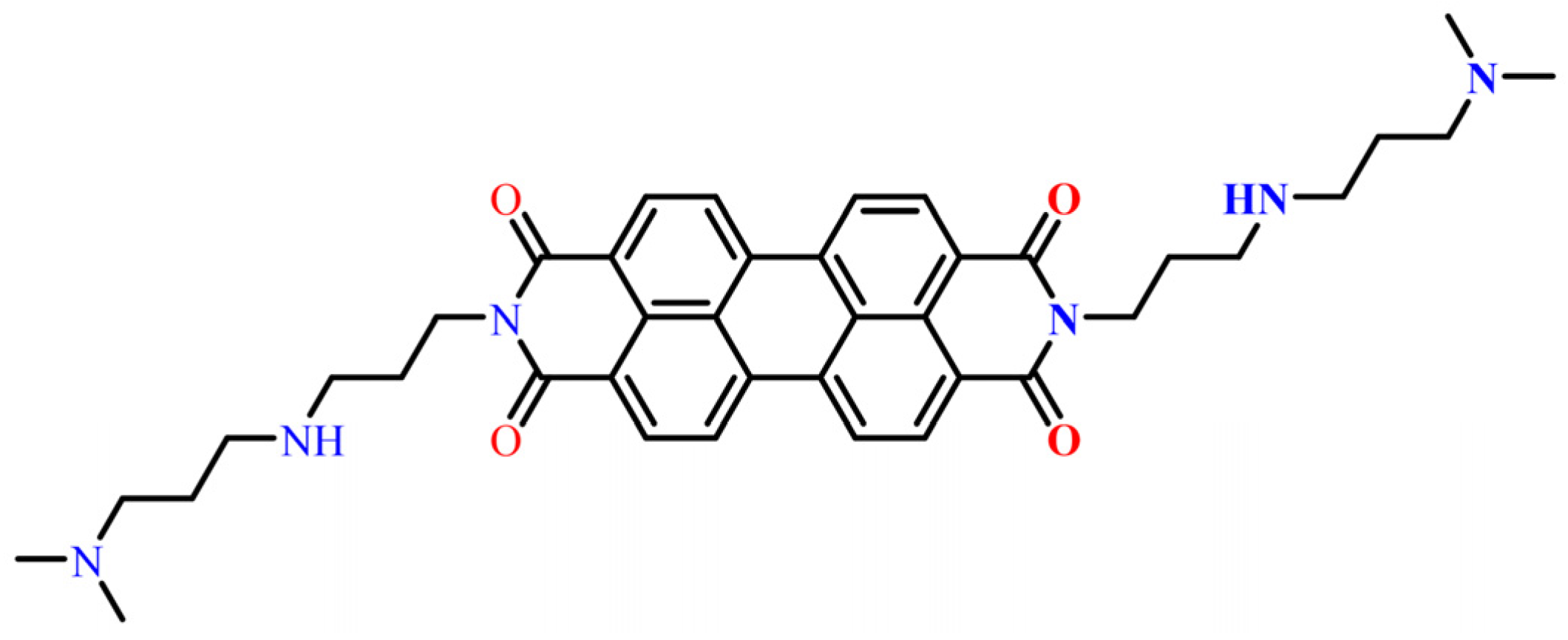
PDINN as an Efficient and Environmentally Friendly Corrosion Inhibitor for Mild Steel in HCl: A Comprehensive Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Solution
2.2. Electrochemical Tests
2.3. Surface Characterization
2.4. Quantum Chemical Calculations
2.5. MD Simulations
3. Results and Discussions
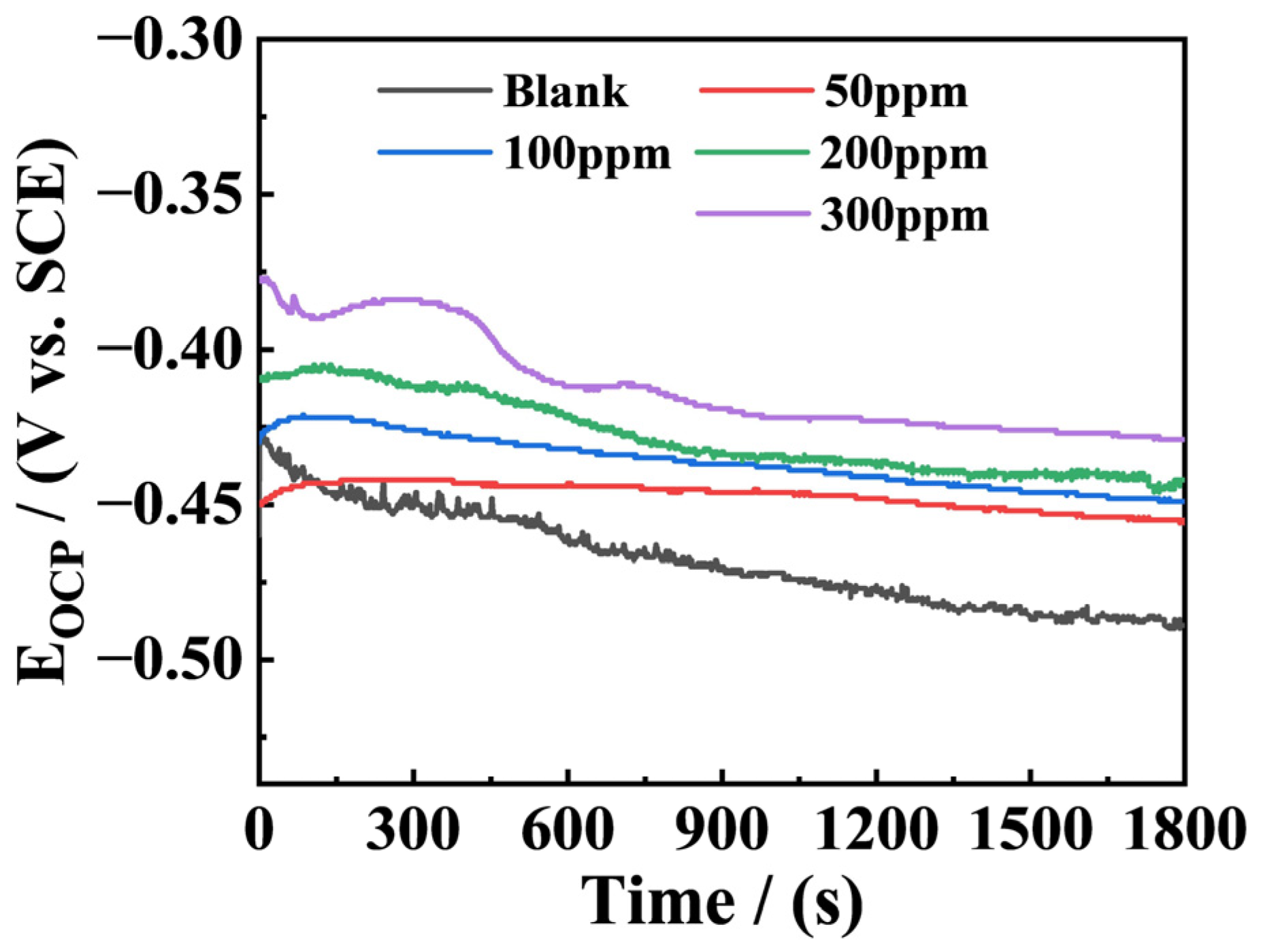
3.1. OCP Test
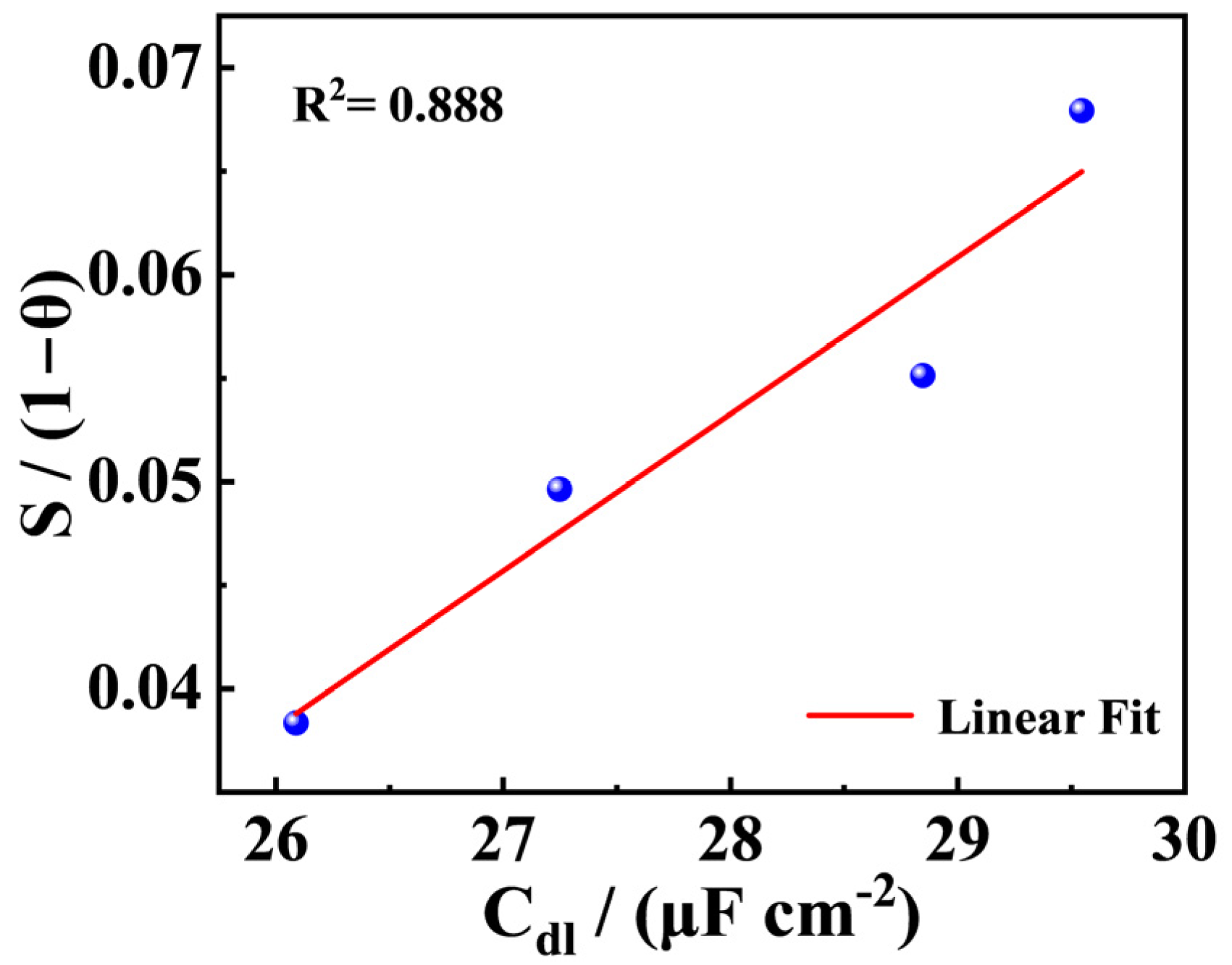
3.2. EIS Measurement
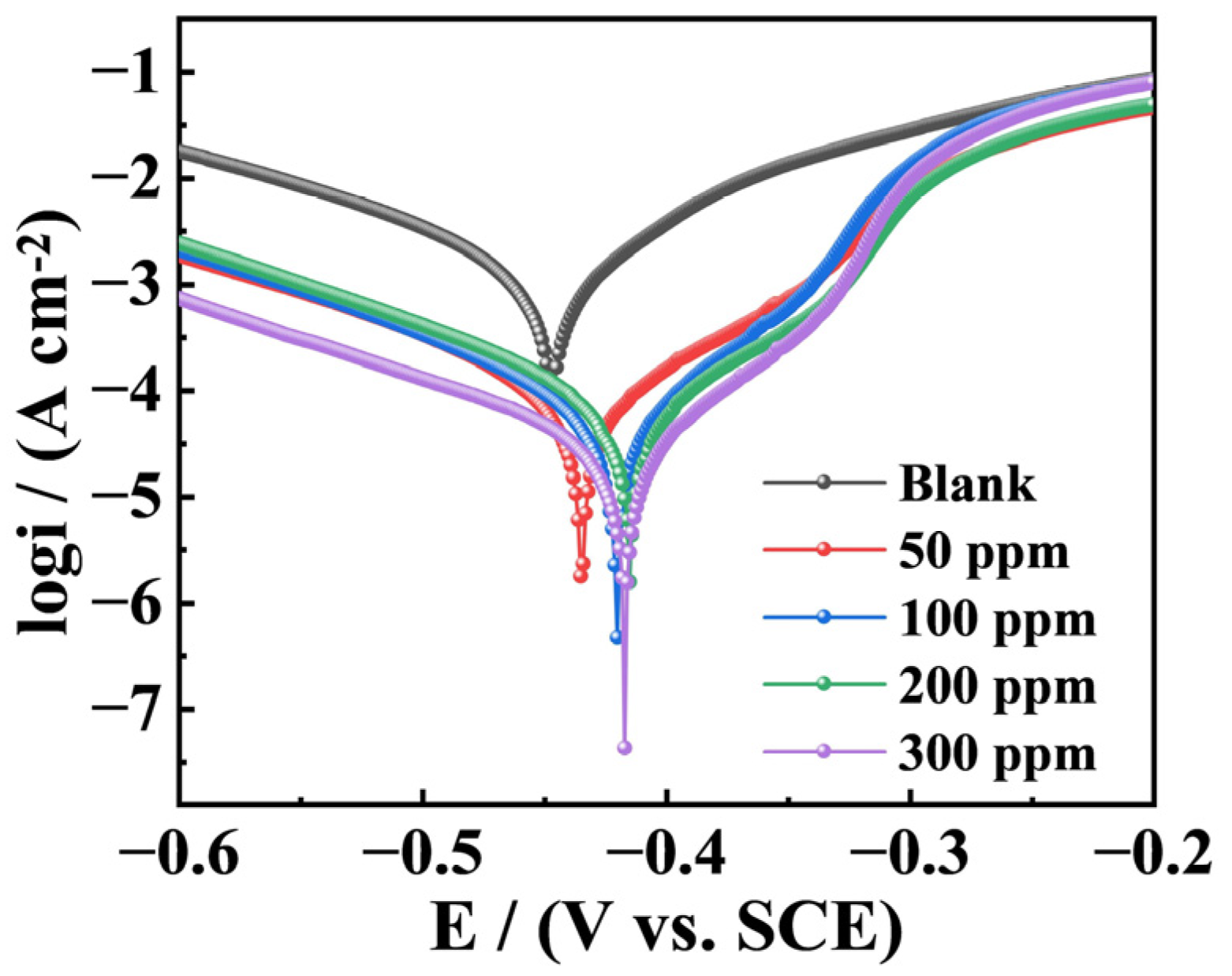
3.3. PDP Measurements
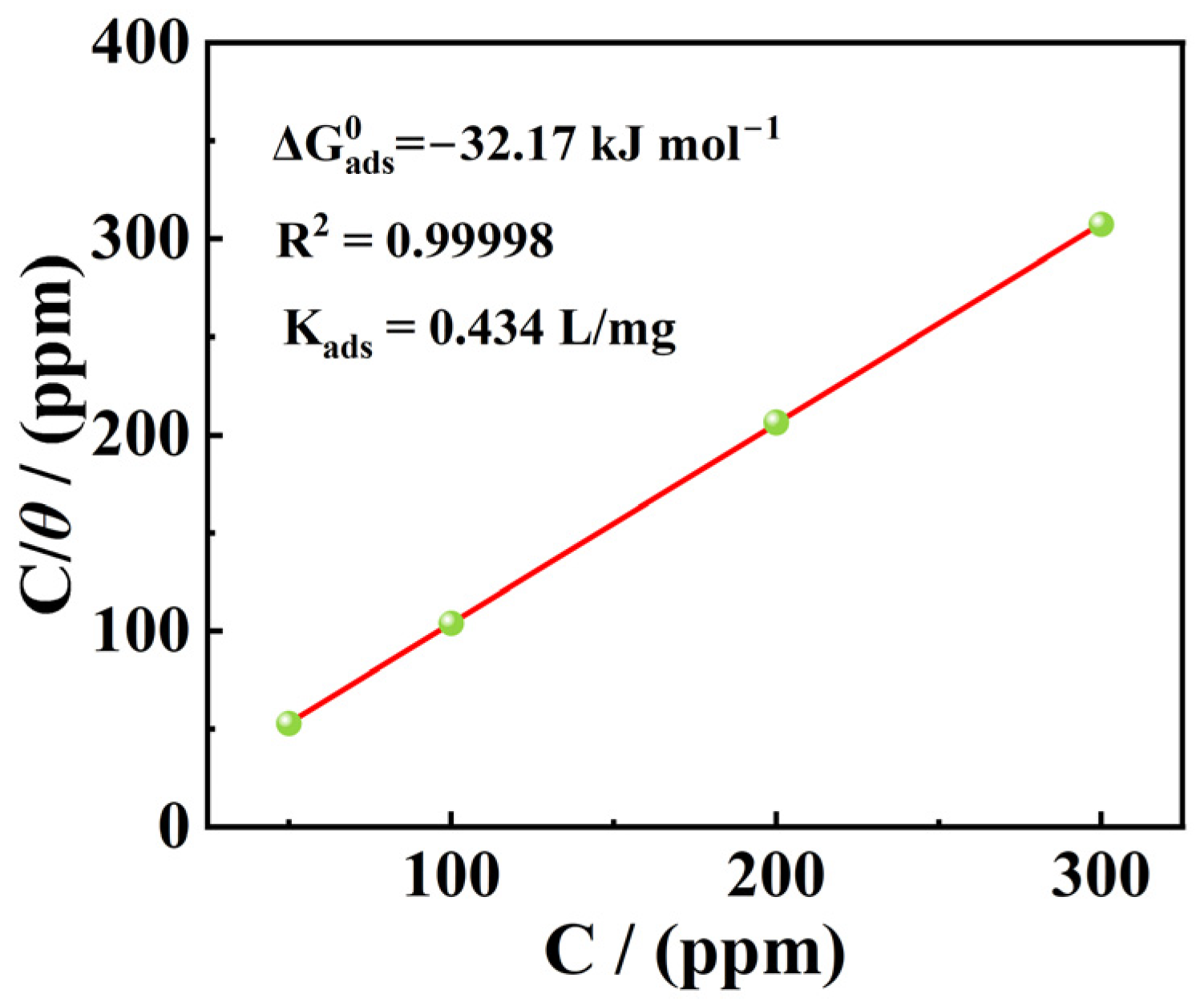
3.4. Thermodynamic Parameters and Adsorption Isotherm
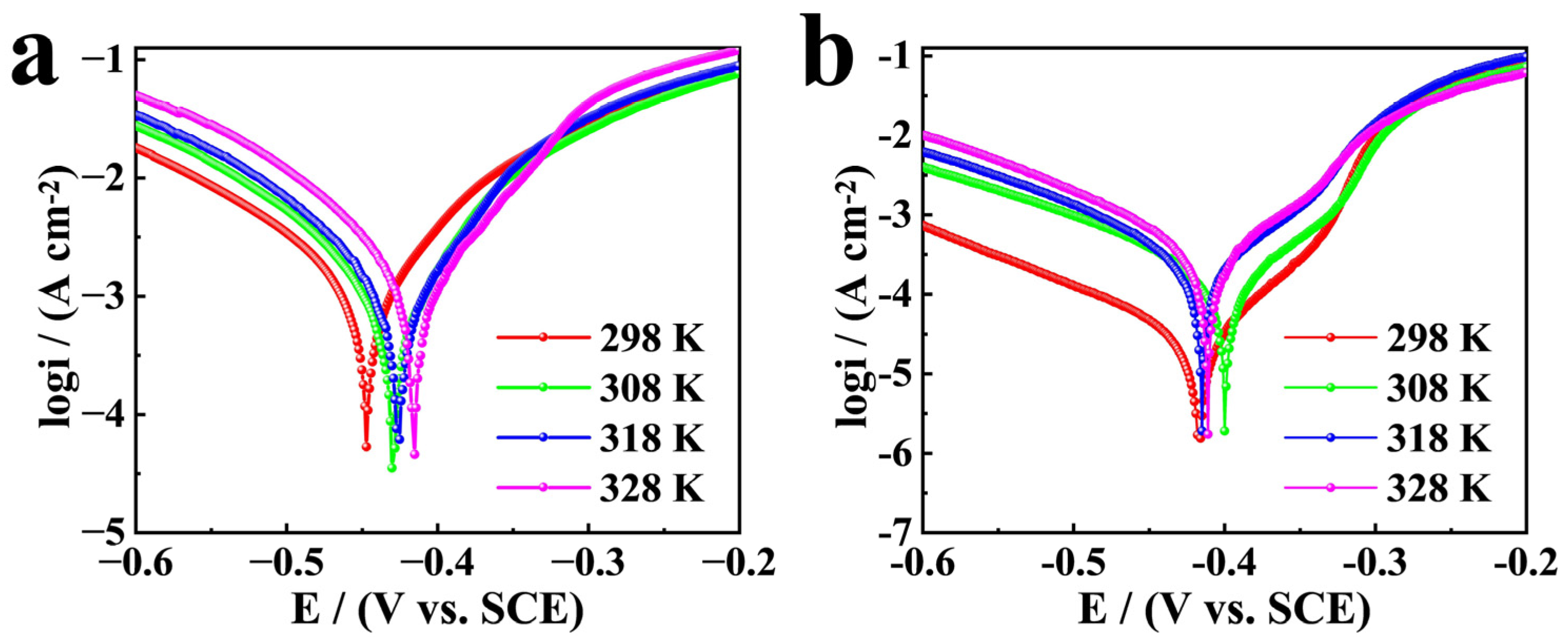
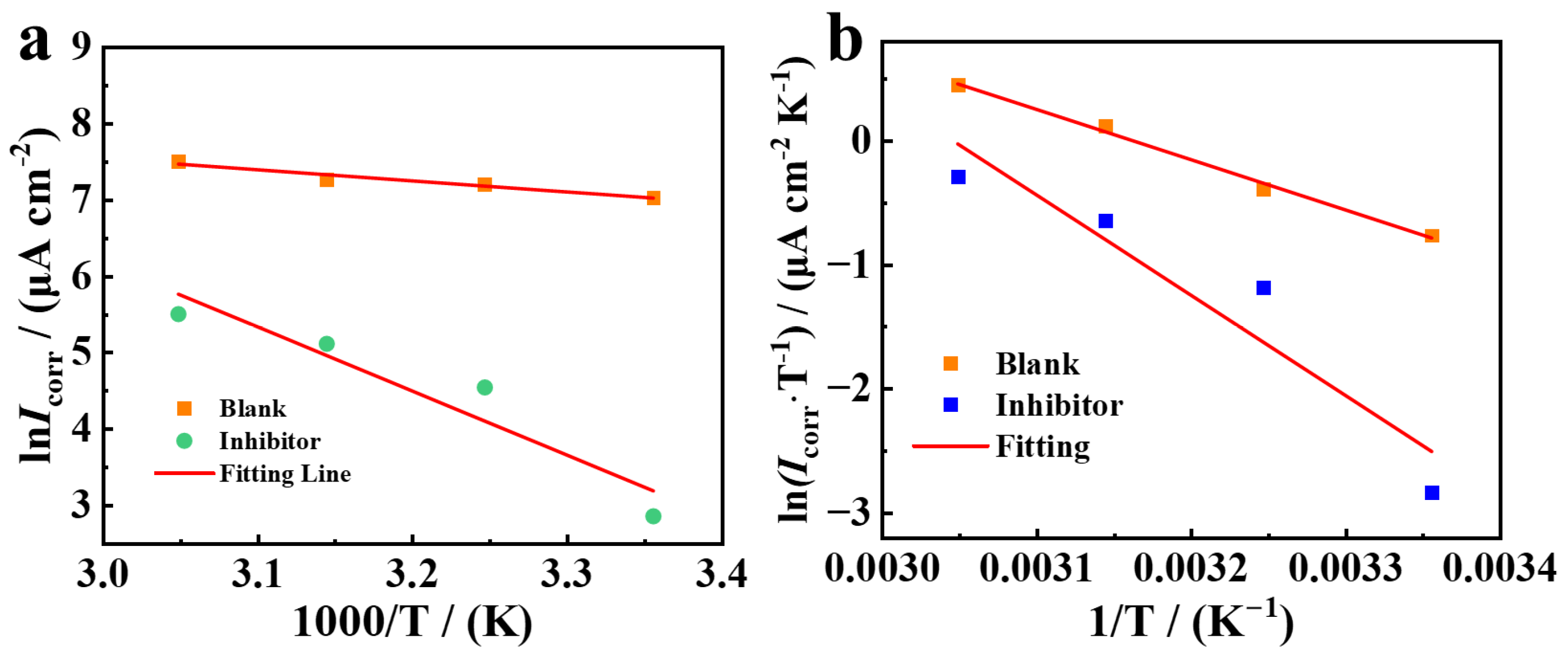
3.5. Influence of Temperature
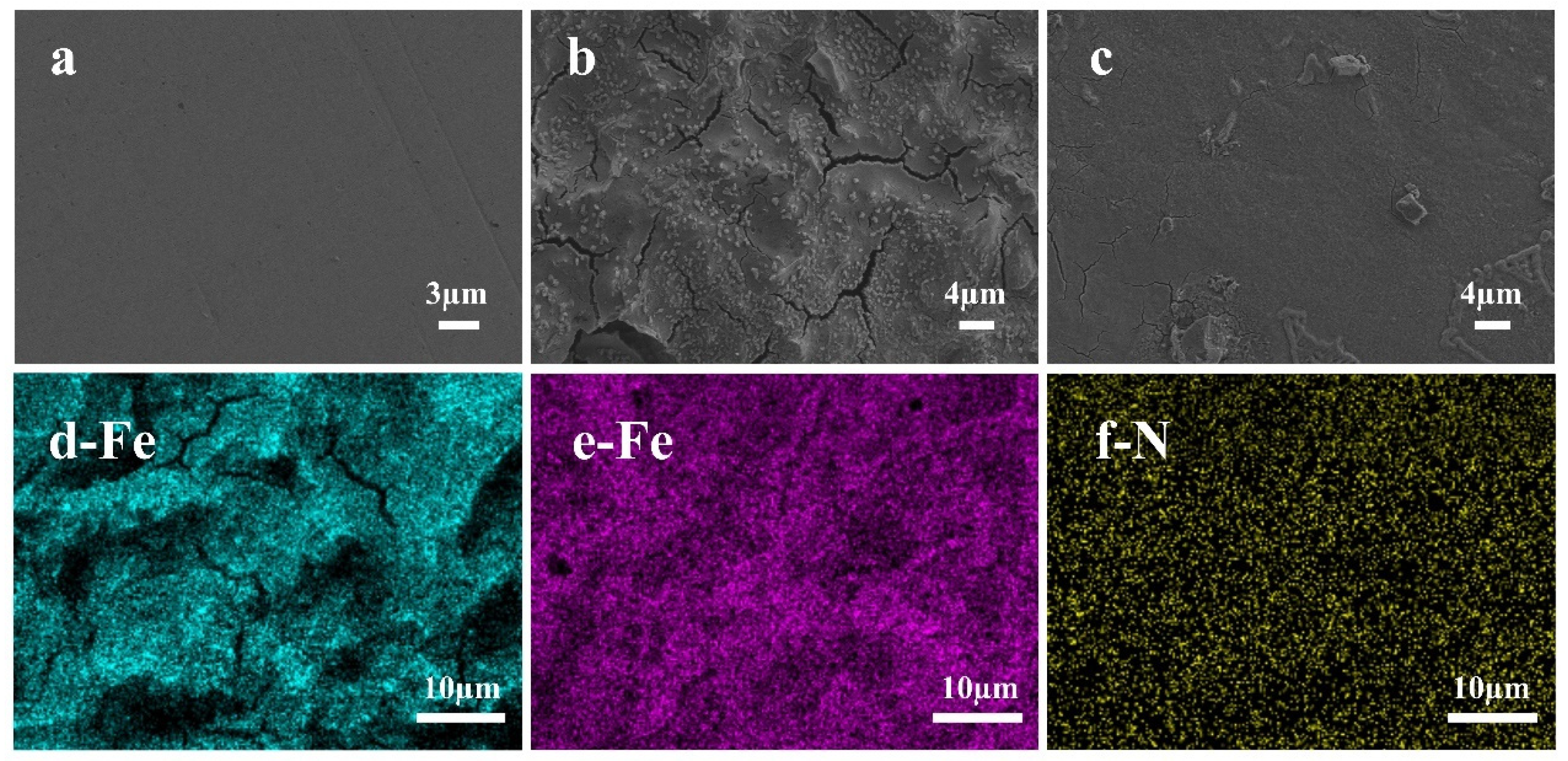
3.6. Surface Morphological Studies
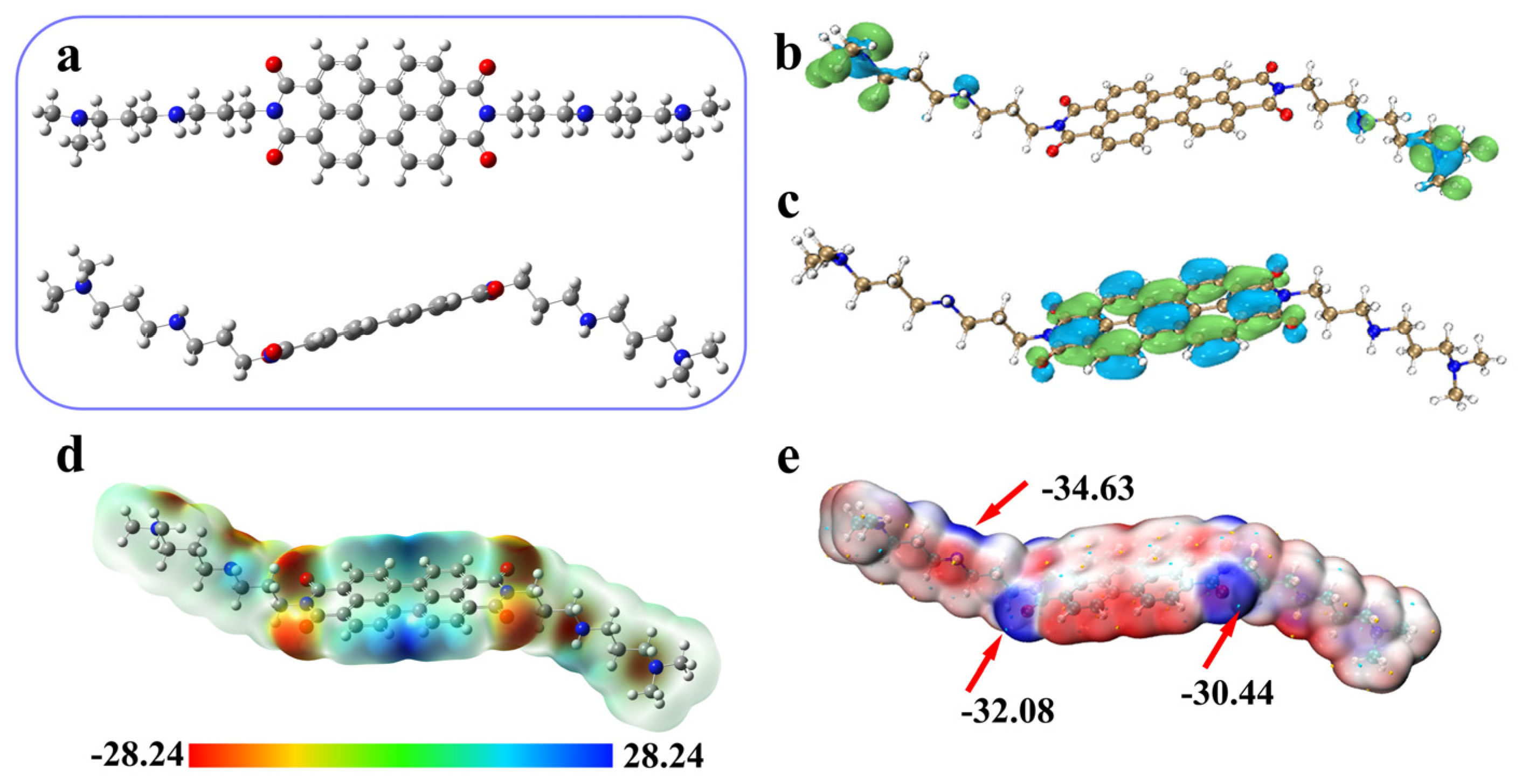
3.7. Computational Studies
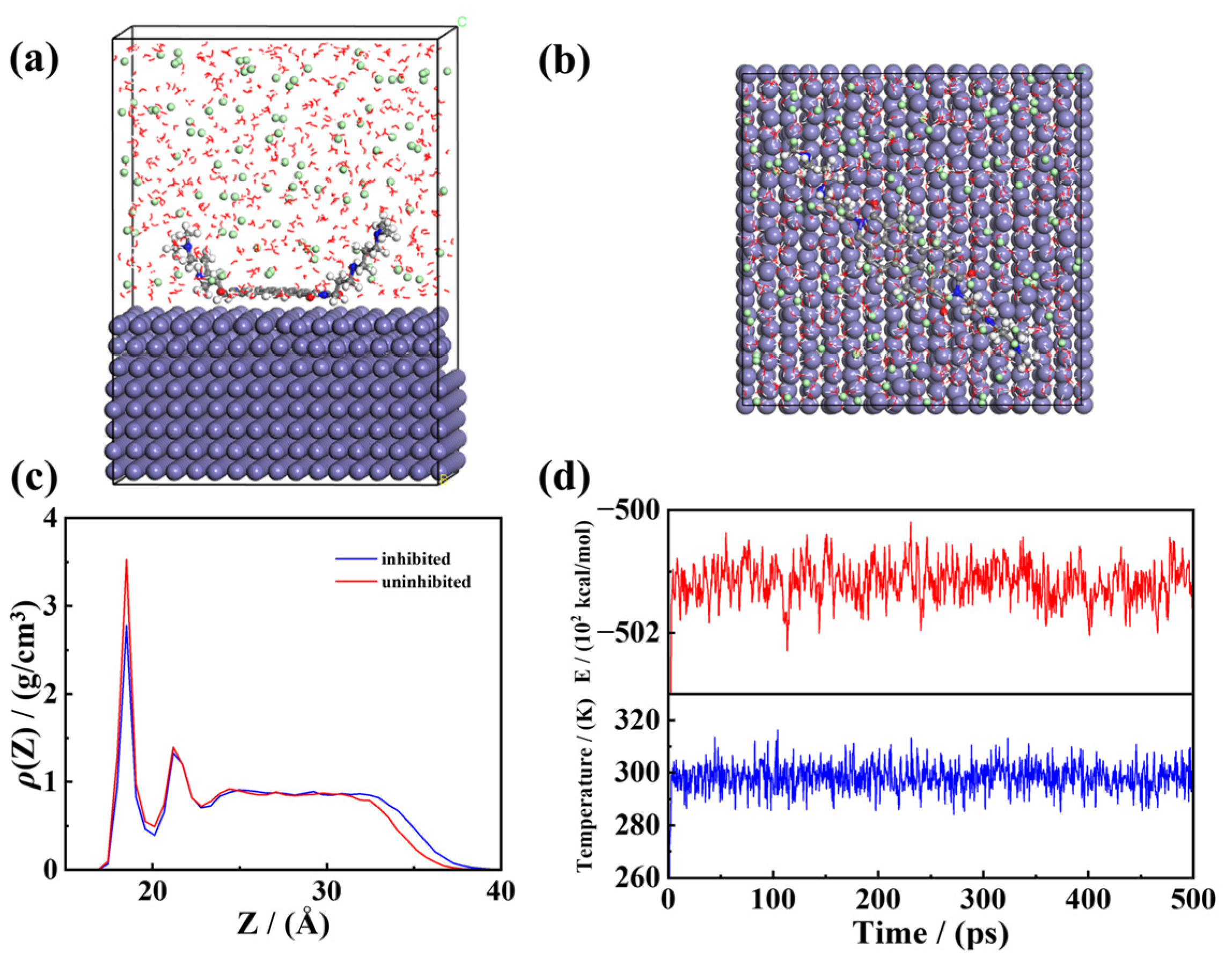
3.8. Molecular Dynamic Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Ganjoo, R.; Saha, S.K.; Kang, N.; Thakur, A.; Assad, H.; Sharma, V.; Kumar, A. Experimental and Theoretical Analysis of Baclofen as a Potential Corrosion Inhibitor for Mild Steel Surface in HCl Medium. J. Adhes. Sci. Technol. 2022, 36, 2067–2092. [Google Scholar] [CrossRef]
- Ganjoo, R.; Sharma, S.; Thakur, A.; Assad, H.; Kumar Sharma, P.; Dagdag, O.; Berisha, A.; Seydou, M.; Ebenso, E.E.; Kumar, A. Experimental and Theoretical Study of Sodium Cocoyl Glycinate as Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Medium. J. Mol. Liq. 2022, 364, 119988. [Google Scholar] [CrossRef]
- Bashir, S.; Lgaz, H.; Chung, I.-M.; Kumar, A. Effective Green Corrosion Inhibition of Aluminium Using Analgin in Acidic Medium: An Experimental and Theoretical Study. Chem. Eng. Commun. 2021, 208, 1121–1130. [Google Scholar] [CrossRef]
- Khan, G.; Basirun, W.J.; Kazi, S.N.; Ahmed, P.; Magaji, L.; Ahmed, S.M.; Khan, G.M.; Rehman, M.A.; Badry, A.B.B.M. Electrochemical Investigation on the Corrosion Inhibition of Mild Steel by Quinazoline Schiff Base Compounds in Hydrochloric Acid Solution. J. Colloid Interface Sci. 2017, 502, 134–145. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Advincula, R.C. Polymeric Corrosion Inhibitors for the Oil and Gas Industry: Design Principles and Mechanism. React. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Ueda, M. 2006 FN Speller Award Lecture: Development of Corrosion-Resistance Alloys for the Oil and Gas Industry—Based on Spontaneous Passivity Mechanism. Corrosion 2006, 62, 856–867. [Google Scholar] [CrossRef]
- Bashir, S.; Thakur, A.; Lgaz, H.; Chung, I.-M.; Kumar, A. Corrosion Inhibition Performance of Acarbose on Mild Steel Corrosion in Acidic Medium: An Experimental and Computational Study. Arab. J. Sci. Eng. 2020, 45, 4773–4783. [Google Scholar] [CrossRef]
- Hegazy, M.; Rashwan, S.; Kamel, M.; El Kotb, M. Synthesis, Surface Properties and Inhibition Behavior of Novel Cationic Gemini Surfactant for Corrosion of Carbon Steel Tubes in Acidic Solution. J. Mol. Liq. 2015, 211, 126–134. [Google Scholar] [CrossRef]
- Pareek, S.; Jain, D.; Hussain, S.; Biswas, A.; Shrivastava, R.; Parida, S.K.; Kisan, H.K.; Lgaz, H.; Chung, I.-M.; Behera, D. A New Insight into Corrosion Inhibition Mechanism of Copper in Aerated 3.5 wt.% NaCl Solution by Eco-Friendly Imidazopyrimidine Dye: Experimental and Theoretical Approach. Chem. Eng. J. 2019, 358, 725–742. [Google Scholar] [CrossRef]
- Solmaz, R. Investigation of the Inhibition Effect of 5-((E)-4-Phenylbuta-1, 3-Dienylideneamino)-1, 3, 4-Thiadiazole-2-Thiol Schiff Base on Mild Steel Corrosion in Hydrochloric Acid. Corros. Sci. 2010, 52, 3321–3330. [Google Scholar] [CrossRef]
- Xu, B.; Ji, Y.; Zhang, X.; Jin, X.; Yang, W.; Chen, Y. Experimental and Theoretical Studies on the Corrosion Inhibition Performance of 4-Amino-N, N-Di-(2-Pyridylmethyl)-Aniline on Mild Steel in Hydrochloric Acid. RSC Adv. 2015, 5, 56049–56059. [Google Scholar] [CrossRef]
- Chebabe, D.; Abbout, S.; Damej, M.; Oubair, A.; Lakbaibi, Z.; Dermaj, A.; Benassaoui, H.; Doubi, M.; Hajjaji, N. Electrochemical and Theoretical Study of Corrosion Inhibition on Carbon Steel in 1M HCl Medium by 1, 10-Bis (4-Amino-3-Methyl-1, 2, 4-Triazole-5-Thioyl) Decane. J. Fail. Anal. Prev. 2020, 20, 1673–1683. [Google Scholar] [CrossRef]
- Damej, M.; Benassaoui, H.; Chebabe, D.; Benmessaoud, M.; Erramli, H.; Dermaj, A.; Hajjaji, N.; Srhiri, A. Inhibition Effect of 1, 2, 4-Triazole-5-Thione Derivative on the Corrosion of Brass in 3% NaCl Solution. J. Mater. Environ. Sci. 2016, 7, 738–745. [Google Scholar]
- El-Hajjaji, F.; Merimi, I.; Ouasif, L.; Ghoul, M.E.; Achour, R.; Hammouti, B.; Belghiti, M.; Chauhan, D.; Quraishi, M. 1-Octyl-2-(Octylthio)-1H-Benzimidazole as a New and Effective Corrosion Inhibitor for Carbon Steel in 1 M HCl. Port. Electrochim. Acta 2019, 37, 131–145. [Google Scholar] [CrossRef]
- Ma, Q.; Qi, S.; He, X.; Tang, Y.; Lu, G. 1,2,3-Triazole Derivatives as Corrosion Inhibitors for Mild Steel in Acidic Medium: Experimental and Computational Chemistry Studies. Corros. Sci. 2017, 129, 91–101. [Google Scholar] [CrossRef]
- Revie, R.W. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kushwaha, A.K.; Sahoo, M.R.; Ray, M.; Das, D.; Nayak, S.; Maity, A.; Sarkar, K.; Bhagat, A.N.; Pal, A.R.; Rout, T.K.; et al. Functional Pyromellitic Diimide as a Corrosion Inhibitor for Galvanized Steel: An Atomic-Scale Engineering. ACS Omega 2022, 7, 27116–27125. [Google Scholar] [CrossRef]
- Kokalj, A.; Lozinšek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Stavber, S.; Crespo, D.; Renner, F.U.; et al. Simplistic Correlations between Molecular Electronic Properties and Inhibition Efficiencies: Do They Really Exist? Corros. Sci. 2021, 179, 108856. [Google Scholar] [CrossRef]
- Anantharaj, R.; Banerjee, T. Fast Solvent Screening for the Simultaneous Hydrodesulfurization and Hydrodenitrification of Diesel Oil Using Ionic Liquids. J. Chem. Eng. Data 2011, 56, 2770–2785. [Google Scholar] [CrossRef]
- Yang, X.; Fu, S.; Wang, Q.; Sun, Q.; Zhang, J.; Peng, Y.; Liang, Z.; Li, J. Protective Behaviour of Naphthylamine Derivatives for Steel Reinforcement in the Simulated Concrete Pore Solutions: Detailed Experimental and Computational Explorations. J. Mol. Struct. 2022, 1270, 133898. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, S.; Yang, X.; Sun, Q.; Zhang, J.; Peng, Y.; Zhang, R.; Liang, Z.; Li, J. An Imide-Based Organic Polymer as an Inhibitor for HRB400 Steel in Simulated Concrete Pore Solution: Experimental and Theoretical Calculations. J. Mol. Struct. 2022, 1265, 133426. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, L.; Wang, L.; Cao, Y.; Wen, Y.; Lu, Z.; Shang, W. Study of Functional Perylene Diimides for Corrosion Protection on Aluminum Alloy Surfaces. J. Taiwan Inst. Chem. Eng. 2023, 147, 104933. [Google Scholar] [CrossRef]
- Kohl, M.; Alafid, F.; Bouška, M.; Krejčová, A.; Raycha, Y.; Kalendová, A.; Hrdina, R.; Burgert, L. New Corrosion Inhibitors Based on Perylene Units in Epoxy Ester Resin Coatings. Coatings 2022, 12, 923. [Google Scholar] [CrossRef]
- Yao, J.; Qiu, B.; Zhang, Z.-G.; Xue, L.; Wang, R.; Zhang, C.; Chen, S.; Zhou, Q.; Sun, C.; Yang, C.; et al. Cathode Engineering with Perylene-Diimide Interlayer Enabling over 17% Efficiency Single-Junction Organic Solar Cells. Nat. Commun. 2020, 11, 2726. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zeng, X.Q.; Jiang, Z.N.; Duan, J.M.; Li, Y.R.; Peng, S.Y.; Dong, C.F.; Zhang, G.A. Developing a Novel Amino Acid Derivative as High-Efficient Green Corrosion Inhibitor for Mild Steel in Acidic Solution: Experiments and First-Principles Calculations. Ind. Crops Prod. 2024, 210, 118032. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, G.; Niu, F.; Shi, Y.; Du, Z.; Lu, G.; Liu, Z. Understanding the Inhibition Performance of Novel Dibenzimidazole Derivatives on Fe (110) Surface: DFT and MD Simulation Insights. J. Mater. Res. Technol. 2022, 17, 211–222. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, J.; Wang, M.; Liu, Q.; Wang, J.; Chong, Y.; Jia, G. Synergistic Corrosion Inhibition Effects of Quaternary Ammonium Salt Cationic Surfactants and Thiourea on Q235 Steel in Sulfuric Acid: Experimental and Theoretical Research. Corros. Sci. 2022, 199, 110199. [Google Scholar] [CrossRef]
- Salarvand, Z.; Amirnasr, M.; Talebian, M.; Raeissi, K.; Meghdadi, S. Enhanced Corrosion Resistance of Mild Steel in 1M HCl Solution by Trace Amount of 2-Phenyl-Benzothiazole Derivatives: Experimental, Quantum Chemical Calculations and Molecular Dynamics (MD) Simulation Studies. Corros. Sci. 2017, 114, 133–145. [Google Scholar] [CrossRef]
- Alamry, K.A.; Aslam, R.; Khan, A.; Hussein, M.A.; Tashkandi, N.Y. Evaluation of Corrosion Inhibition Performance of Thiazolidine-2,4-Diones and Its Amino Derivative: Gravimetric, Electrochemical, Spectroscopic, and Surface Morphological Studies. Process Saf. Environ. Prot. 2022, 159, 178–197. [Google Scholar] [CrossRef]
- Fu, S.; Yang, X.; Peng, Y.; Wang, Q.; Sun, Q.; Zhang, J.; Wang, X.; Liang, Z.; Li, J. Corrosion Behaviors of Tetrasodium Iminodisuccinate (IDS) as an Environmentally Friendly Inhibitor: Experimental and Theoretical Studies. Coatings 2023, 13, 613. [Google Scholar] [CrossRef]
- Li, M.; Fu, S.; Peng, Y.; Sang, T.; Cui, C.; Ma, H.; Dai, J.; Liang, Z.; Li, J. An Extensive Analysis of Isoindigotin Derivatives as Effective Corrosion Inhibitors for Mild Steel in Acidic Corrosive Environments: An Electrochemical and Theoretical Investigation. Prog. Org. Coat. 2025, 200, 108960. [Google Scholar] [CrossRef]
- Peimani, A.; Nasr-Esfahani, M. Application of Anise Extract for Corrosion Inhibition of Carbon Steel in CO 2 Saturated 3.0% NaCl Solution. Prot. Met. Phys. Chem. Surf. 2018, 54, 122–134. [Google Scholar] [CrossRef]
- El-Hashemy, M.A.; Sallam, A. The Inhibitive Action of Calendula Officinalis Flower Heads Extract for Mild Steel Corrosion in 1 M HCl Solution. J. Mater. Res. Technol. 2020, 9, 13509–13523. [Google Scholar] [CrossRef]
- Farag, A.A.; Ismail, A.S.; Migahed, M. Environmental-Friendly Shrimp Waste Protein Corrosion Inhibitor for Carbon Steel in 1 M HCl Solution. Egypt. J. Pet. 2018, 27, 1187–1194. [Google Scholar] [CrossRef]
- El Bribri, A.; Tabyaoui, M.; Tabyaoui, B.; El Attari, H.; Bentiss, F. The Use of Euphorbia Falcata Extract as Eco-Friendly Corrosion Inhibitor of Carbon Steel in Hydrochloric Acid Solution. Mater. Chem. Phys. 2013, 141, 240–247. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Alvarez, L.X.; dos Santos, N.E.; Maldonado Barrios, A.C.; Ponzio, E.A. Green Synthesis of 1-Benzyl-4-Phenyl-1H-1,2,3-Triazole, Its Application as Corrosion Inhibitor for Mild Steel in Acidic Medium and New Approach of Classical Electrochemical Analyses. Corros. Sci. 2019, 149, 185–194. [Google Scholar] [CrossRef]
- Boumezzourh, A.; Ouknin, M.; Chibane, E.; Costa, J.; Bouyanzer, A.; Hammouti, B.; Majidi, L. Inhibition of Tinplate Corrosion in 0.5 M H2C2O4 Medium by Mentha Pulegium Essential Oil. Int. J. Corros. Scale Inhib. 2020, 9, 152–170. [Google Scholar]
- Aljourani, J.; Raeissi, K.; Golozar, M. Benzimidazole and Its Derivatives as Corrosion Inhibitors for Mild Steel in 1M HCl Solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, S.; Peng, Y.; Li, P.; Ma, H.; Fang, Z.; Ma, T.; Zhang, R.; Liang, Z.; Li, J. Isoindigo Derivatives as Some Potential Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solution: Synthesis, Experimental and Theoretical Studies. J. Mol. Struct. 2024, 1312, 138480. [Google Scholar] [CrossRef]
- Fu, S.; Peng, Y.; Sun, Q.; Wang, Q.; Li, P.; Ma, H.; Fang, Z.; Ma, T.; Du, S.; Liang, Z.; et al. A Comprehensive Study of Highly Soluble Cationic Polyfluorene as a Polymer Anticorrosive Coating: Anticorrosion Mechanisms and Performance Evaluation. Prog. Org. Coat. 2024, 191, 108455. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Abai, E.J. Poly(Methacrylic Acid)/Silver Nanoparticles Composites: In-Situ Preparation, Characterization and Anticorrosion Property for Mild Steel in H2SO4 Solution. J. Mol. Liq. 2015, 212, 340–351. [Google Scholar] [CrossRef]
- El Aoufir, Y.; Lgaz, H.; Bourazmi, H.; Kerroum, Y.; Ramli, Y.; Guenbour, A.; Salghi, R.; El-Hajjaji, F.; Hammouti, B.; Oudda, H. Quinoxaline Derivatives as Corrosion Inhibitors of Carbon Steel in Hydrochloridric Acid Media: Electrochemical, DFT and Monte Carlo Simulations Studies. J. Mater. Environ. Sci. 2016, 7, 4330–4347. [Google Scholar]
- Damej, M.; Kaya, S.; Ibrahimi, B.E.; Lee, H.; Molhi, A.; Serdaroğlu, G.; Benmessaoud, M.; Ali, I.; Hajjaji, S.E.; Lgaz, H. The Corrosion Inhibition and Adsorption Behavior of Mercaptobenzimidazole and Bis-Mercaptobenzimidazole on Carbon Steel in 1.0 M HCl: Experimental and Computational Insights. Surf. Interfaces 2021, 24, 101095. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, Y.; Fu, S.; Yang, X.; Sun, Q.; Wang, X.; Zhang, J.; Liang, Z.; Li, J. Experimental and Theoretical Investigations of 1,1′-Dibenzyl-[4,4′-Bipyridine]−1,1′-Diium Chloride as Effective Corrosion Inhibitor for Q235 Steel in 1 M HCl. Mater. Today Commun. 2023, 35, 106169. [Google Scholar] [CrossRef]
- Ould Abdelwedoud, B.; Damej, M.; Tassaoui, K.; Berisha, A.; Tachallait, H.; Bougrin, K.; Mehmeti, V.; Benmessaoud, M. Inhibition Effect of N-Propargyl Saccharin as Corrosion Inhibitor of C38 Steel in 1 M HCl, Experimental and Theoretical Study. J. Mol. Liq. 2022, 354, 118784. [Google Scholar] [CrossRef]
- Han, P.; He, Y.; Chen, C.; Yu, H.; Liu, F.; Yang, H.; Ma, Y.; Zheng, Y. Study on Synergistic Mechanism of Inhibitor Mixture Based on Electron Transfer Behavior. Sci. Rep. 2016, 6, 33252. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.; Li, W.; Guo, L.; Xu, S.; Feng, L.; Madkour, L.H. Experimental and Theoretical Investigations of Some Pyrazolo-Pyrimidine Derivatives as Corrosion Inhibitors on Copper in Sulfuric Acid Solution. Appl. Surf. Sci. 2018, 459, 612–620. [Google Scholar] [CrossRef]
- Hsissou, R. Review on Epoxy Polymers and Its Composites as a Potential Anticorrosive Coatings for Carbon Steel in 3.5% NaCl Solution: Computational Approaches. J. Mol. Liq. 2021, 336, 116307. [Google Scholar] [CrossRef]
- Guo, L.; Safi, Z.S.; Kaya, S.; Shi, W.; Tüzün, B.; Altunay, N.; Kaya, C. Anticorrosive Effects of Some Thiophene Derivatives against the Corrosion of Iron: A Computational Study. Front. Chem. 2018, 6, 155. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Masroor, S.; Kim, S.-H.; Kwon, C.; Kim, S.Y.; Yang, Y.-J.; Chung, I.-M. Assessing Corrosion Inhibition Characteristics of Hydrazone Derivatives on Mild Steel in HCl: Insights from Electronic-Scale DFT and Atomic-Scale Molecular Dynamics. J. Mol. Liq. 2020, 308, 112998. [Google Scholar] [CrossRef]
- Boutouil, A.; Laamari, M.R.; Elazhary, I.; Bahsis, L.; Anane, H.; Stiriba, S.-E. Towards a Deeper Understanding of the Inhibition Mechanism of a New 1,2,3-Triazole Derivative for Mild Steel Corrosion in the Hydrochloric Acid Solution Using Coupled Experimental and Theoretical Methods. Mater. Chem. Phys. 2020, 241, 122420. [Google Scholar] [CrossRef]
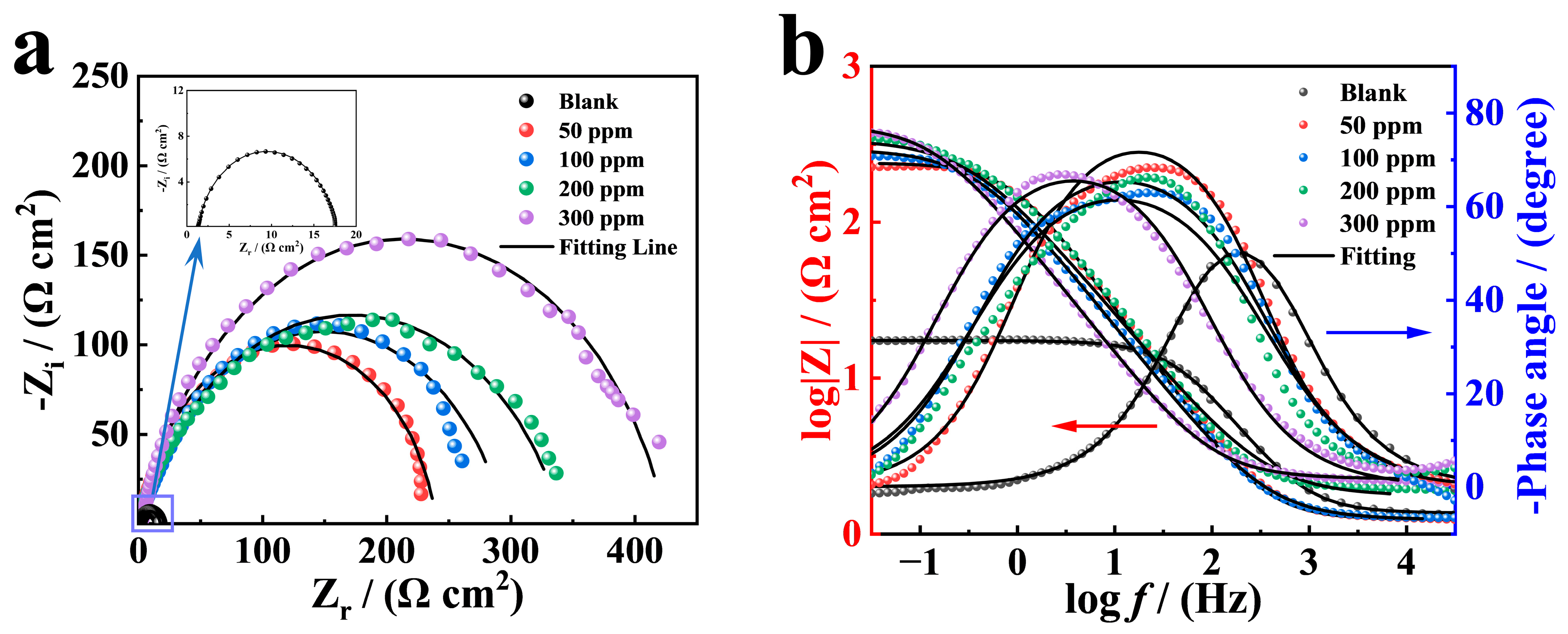






| Inhibitor | Conc. (ppm) | Rs (Ω cm2) | Rct (Ω cm2) | Y0 (×10−5 sn Ω−1 cm−2) | n | Cdl (×10−5 F cm−2) | IEEIS% |
|---|---|---|---|---|---|---|---|
| Blank | 0 | 1.35 | 16.23 | 44.60 | 0.88 | 43.62 | — |
| PDINN | 50 | 1.23 | 239.20 | 35.76 | 0.88 | 29.55 | 93.21 |
| 100 | 1.23 | 294.70 | 34.35 | 0.80 | 28.85 | 94.49 | |
| 200 | 1.77 | 326.90 | 31.58 | 0.79 | 27.25 | 95.04 | |
| 300 | 1.87 | 423.50 | 25.71 | 0.82 | 26.09 | 96.17 |
| Inhibitor | Conc. (ppm) | Ecorr (V) | βa (mv/Dec) | –βc (mv/Dec) | Icorr (µA/cm2) | IETP (%) |
|---|---|---|---|---|---|---|
| Blank | 0 | −0.446 | 115.26 | 157.01 | 1134.02 | — |
| PDINN | 50 | −0.411 | 56.84 | 123.70 | 65.46 | 94.23 |
| 100 | −0.393 | 36.19 | 233.34 | 38.06 | 96.64 | |
| 200 | −0.387 | 35.36 | 77.38 | 20.12 | 98.23 | |
| 300 | −0.385 | 31.32 | 132.55 | 17.53 | 98.45 |
| Inhibitor | Ea/(kJ·mol−1) | K/(μA cm−2) | R2 | ∆Ha/(kJ·mol−1) | ∆Sa/(kJ·mol−1) |
|---|---|---|---|---|---|
| Blank | 12.06 | 0.15 × 106 | 0.927 | 33.7 | −91.1 |
| PDINN | 69.69 | 40 × 1012 | 0.855 | 67.09 | 6.82 |
| Particles | Eads (kJ/mol) |
|---|---|
| H2O | −6.505 |
| H3O+ | −7.251 |
| Cl− | −6.920 |
| PDINN | −301.710 |
| Diffusion Models | H3O+ | H2O | Cl− |
|---|---|---|---|
| H2O box | 2.03 × 10−9 | 3.45 × 10−9 | 2.03 × 10−9 |
| PDINN box | 4.40 × 10−10 | 1.31 × 10−9 | 6.96 × 10−10 |
| ETotal (kJ/mol) | ESurface+Solution (kJ/mol) | ESolution+Inhibitir (kJ/mol) | ESolution (kJ/mol) | EAdsorption (kJ/mol) |
|---|---|---|---|---|
| 1.308 × 106 | 1.301 × 106 | 0.118 × 106 | 0.111 × 106 | −247.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, J.; Fu, S.; Song, J.; Ma, L.; Liu, X.; Liang, Z.; Li, J.; Dai, J. PDINN as an Efficient and Environmentally Friendly Corrosion Inhibitor for Mild Steel in HCl: A Comprehensive Investigation. Coatings 2025, 15, 352. https://doi.org/10.3390/coatings15030352
Kuang J, Fu S, Song J, Ma L, Liu X, Liang Z, Li J, Dai J. PDINN as an Efficient and Environmentally Friendly Corrosion Inhibitor for Mild Steel in HCl: A Comprehensive Investigation. Coatings. 2025; 15(3):352. https://doi.org/10.3390/coatings15030352
Chicago/Turabian StyleKuang, Jiakai, Shaopeng Fu, Jiaqi Song, Lanlan Ma, Xueqi Liu, Zezhou Liang, Jianfeng Li, and Jinpeng Dai. 2025. "PDINN as an Efficient and Environmentally Friendly Corrosion Inhibitor for Mild Steel in HCl: A Comprehensive Investigation" Coatings 15, no. 3: 352. https://doi.org/10.3390/coatings15030352
APA StyleKuang, J., Fu, S., Song, J., Ma, L., Liu, X., Liang, Z., Li, J., & Dai, J. (2025). PDINN as an Efficient and Environmentally Friendly Corrosion Inhibitor for Mild Steel in HCl: A Comprehensive Investigation. Coatings, 15(3), 352. https://doi.org/10.3390/coatings15030352





