Comprehensive Evaluation of 45S5 Bioactive Glass Doped with Samarium: From Synthesis and Physical Properties to Biocompatibility and Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioactive Glass Preparation
2.2. Thermogravimetric Measurements
2.3. SEM Microscopy
2.4. X-Ray Diffraction Analysis
2.5. Density Determination and BET Analysis
2.6. Bioactivity Evaluation In Vitro
2.7. Determination of pH Variation, Ionic Strength, and Mass Loss
2.8. Biocompatibility Test
2.8.1. Cell Culture Model
2.8.2. Biocompatibility Assays
2.8.3. Live/Dead Assays
2.8.4. MTT Assay
2.8.5. LDH Assay
2.8.6. Statistical Analysis
2.9. Antimicrobial and Anti-Biofilm Properties and the Influence of 45S5 Bioactive Glass on the Soluble Virulence Factors’ Modulation
2.9.1. Qualitative Screening of 45S5 Bioactive Glass Against Reference Microbial Strains
2.9.2. Quantitative Evaluation of the Antimicrobial Activity of the 45S5 Bioactive Glass
2.9.3. Assessment of the Influence of 45S5 Bioactive Glass on Microbial Adherence
2.9.4. The Influence of 45S5 Bioactive Glass on the Soluble Virulence Factors Modulation
2.9.5. Statistical Data Analysis
3. Results and Discussion
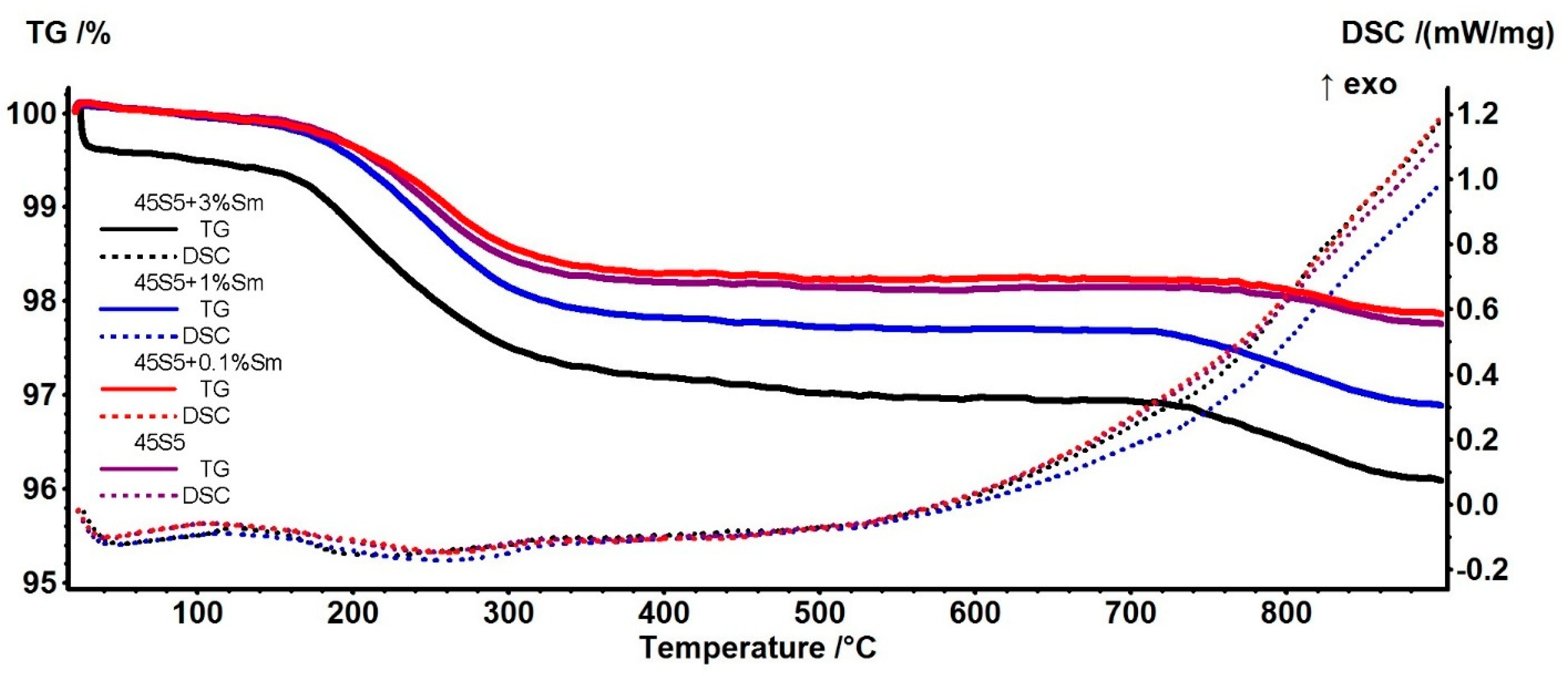
3.1. Thermogravimetry (TG) and Differential Scanning Calorimetry (DSC) Analysis
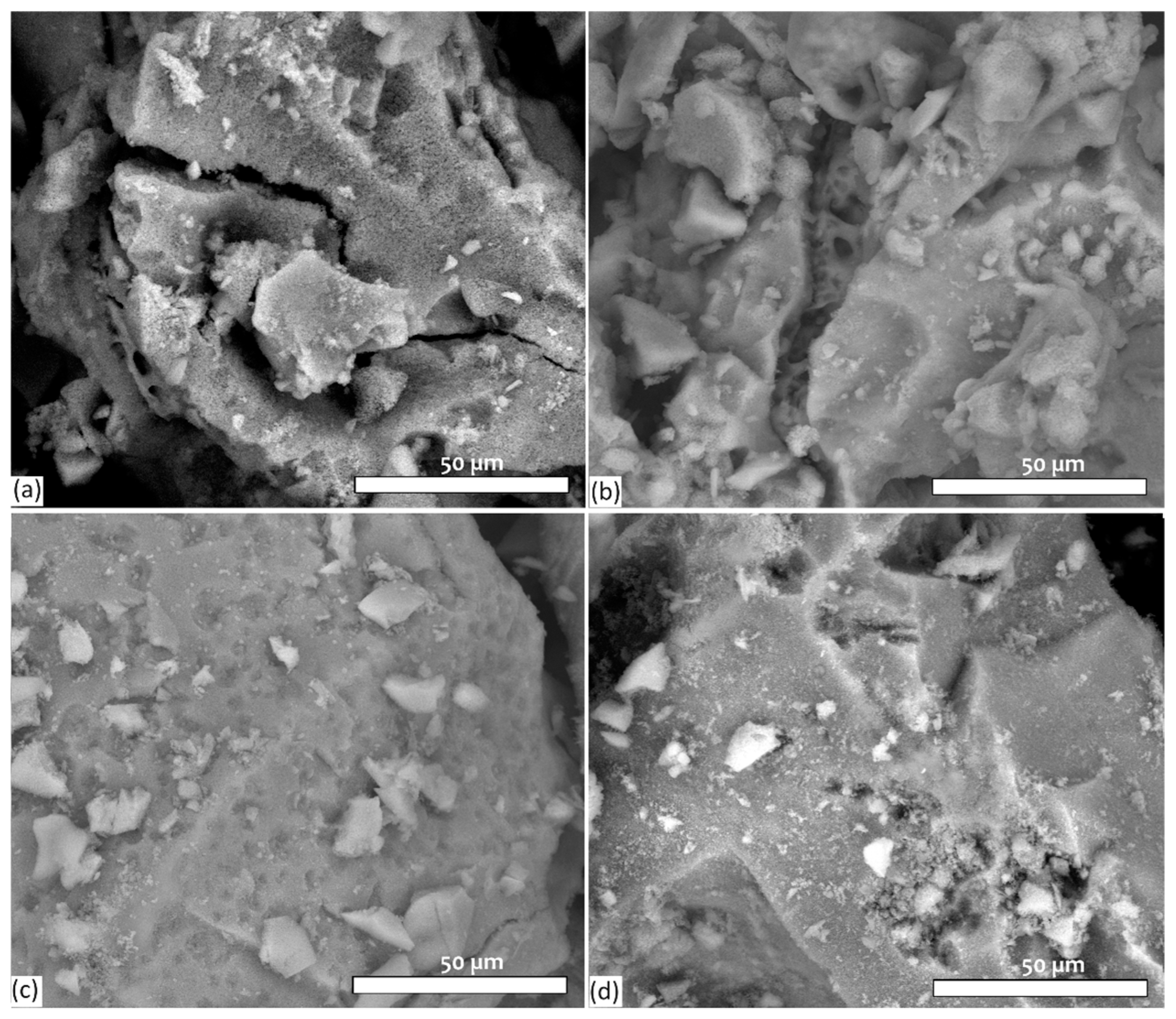
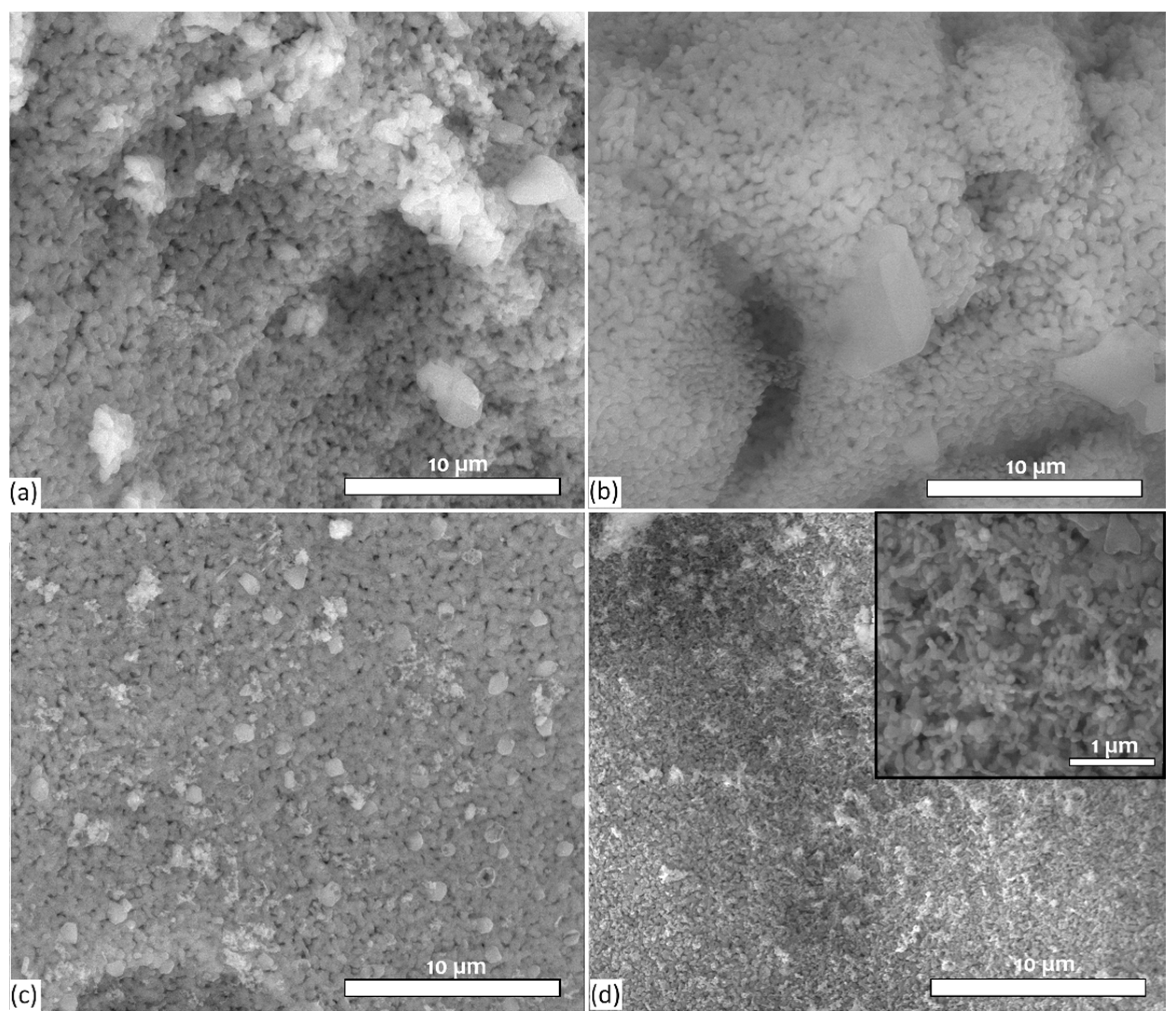
3.2. Evaluation of Surface Morphology of Bioactive Glass Samples by SEM
3.3. FTIR Spectroscopy
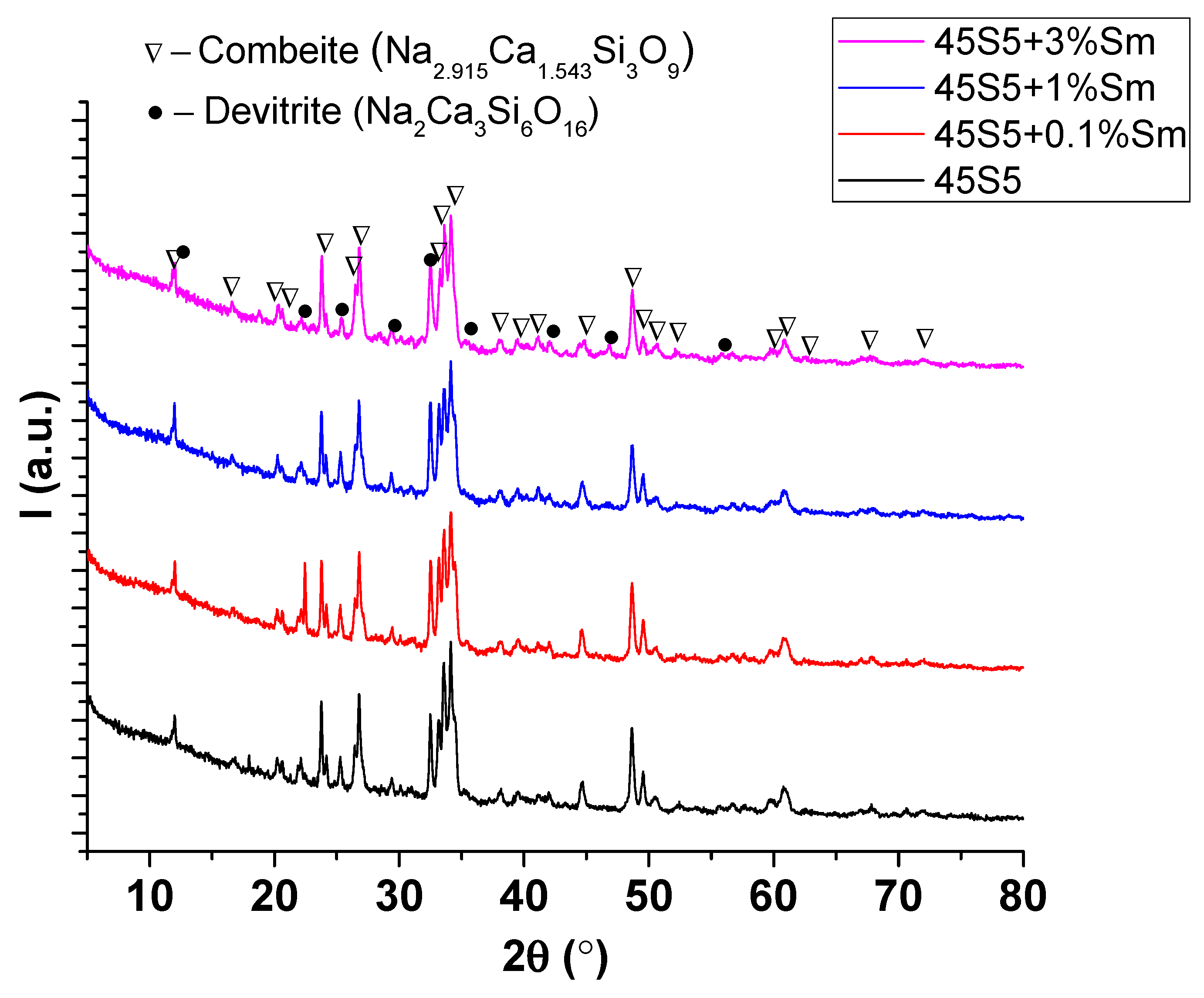
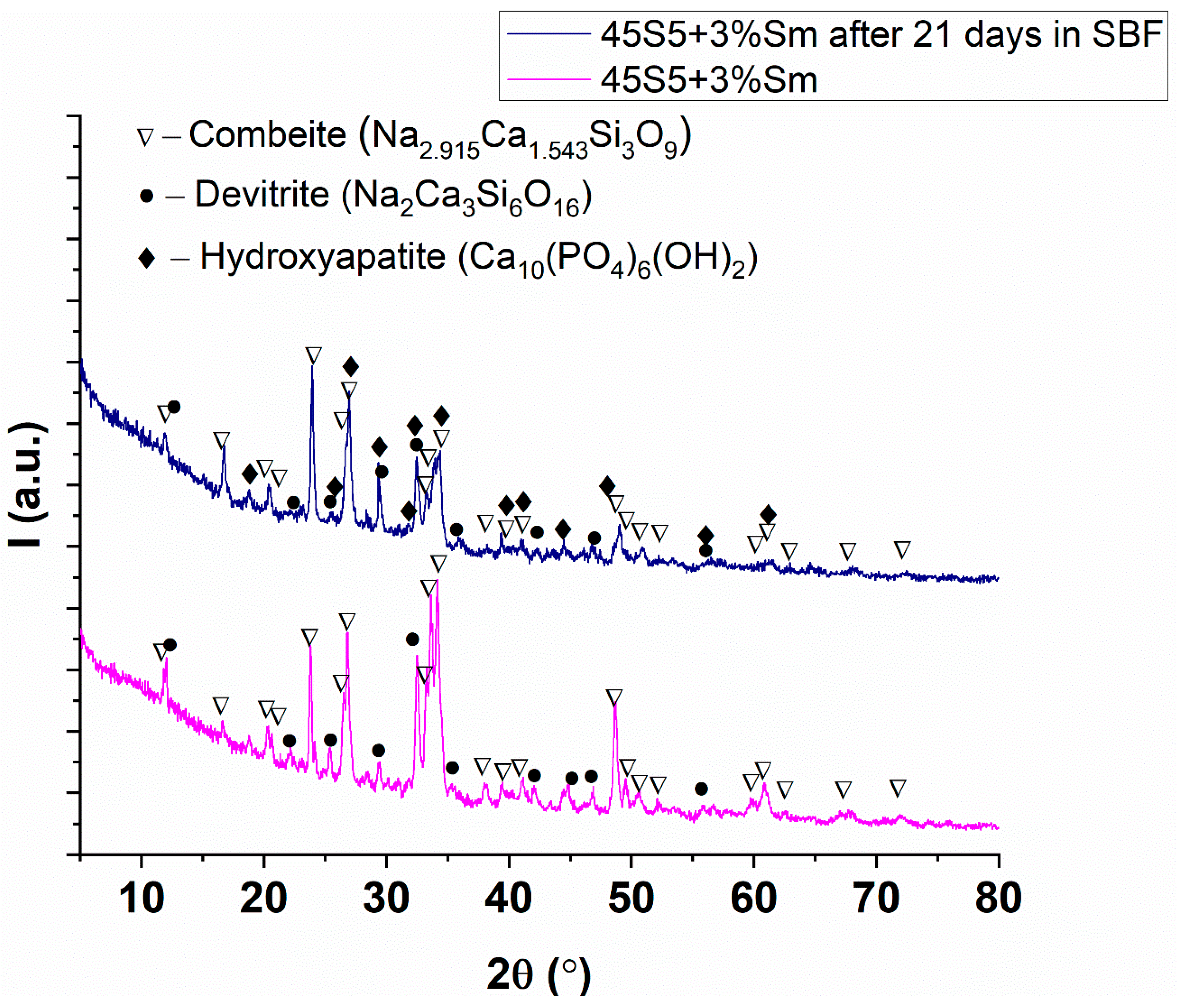
3.4. X-Ray Diffraction Analysis
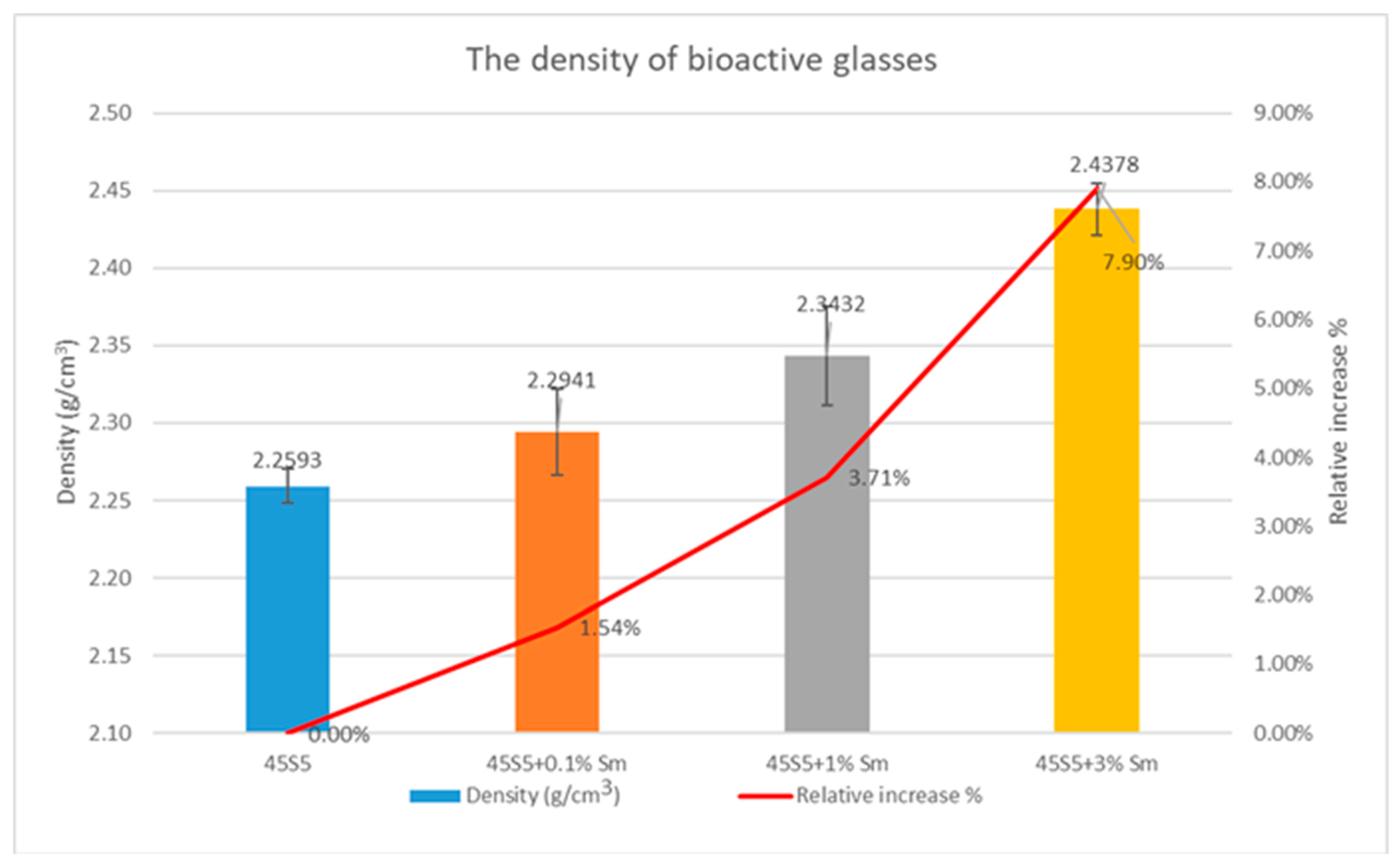
3.5. Density Determination and Textural Characteristics of the Bioactive Glasses
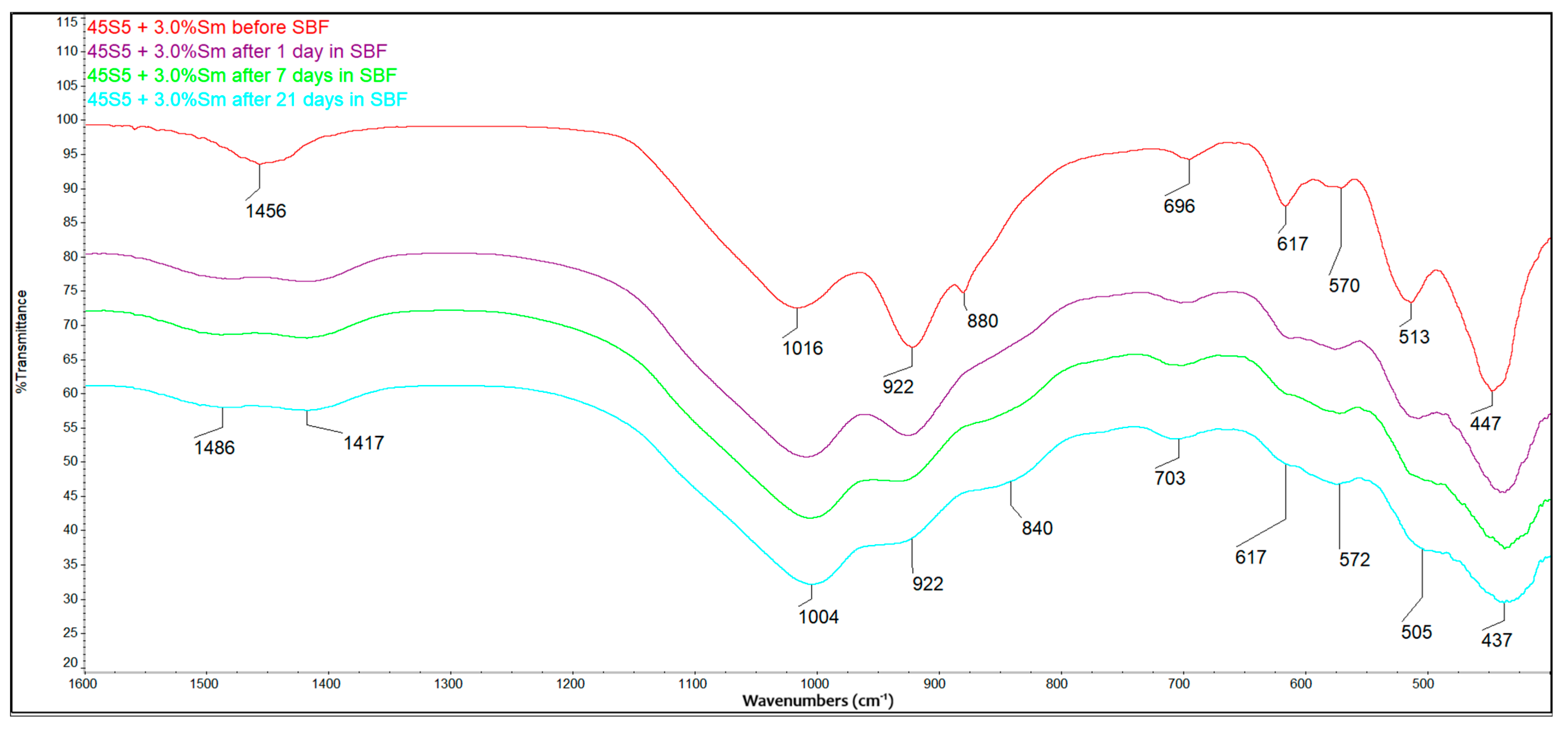
3.6. Bioactivity Evaluation Using FTIR
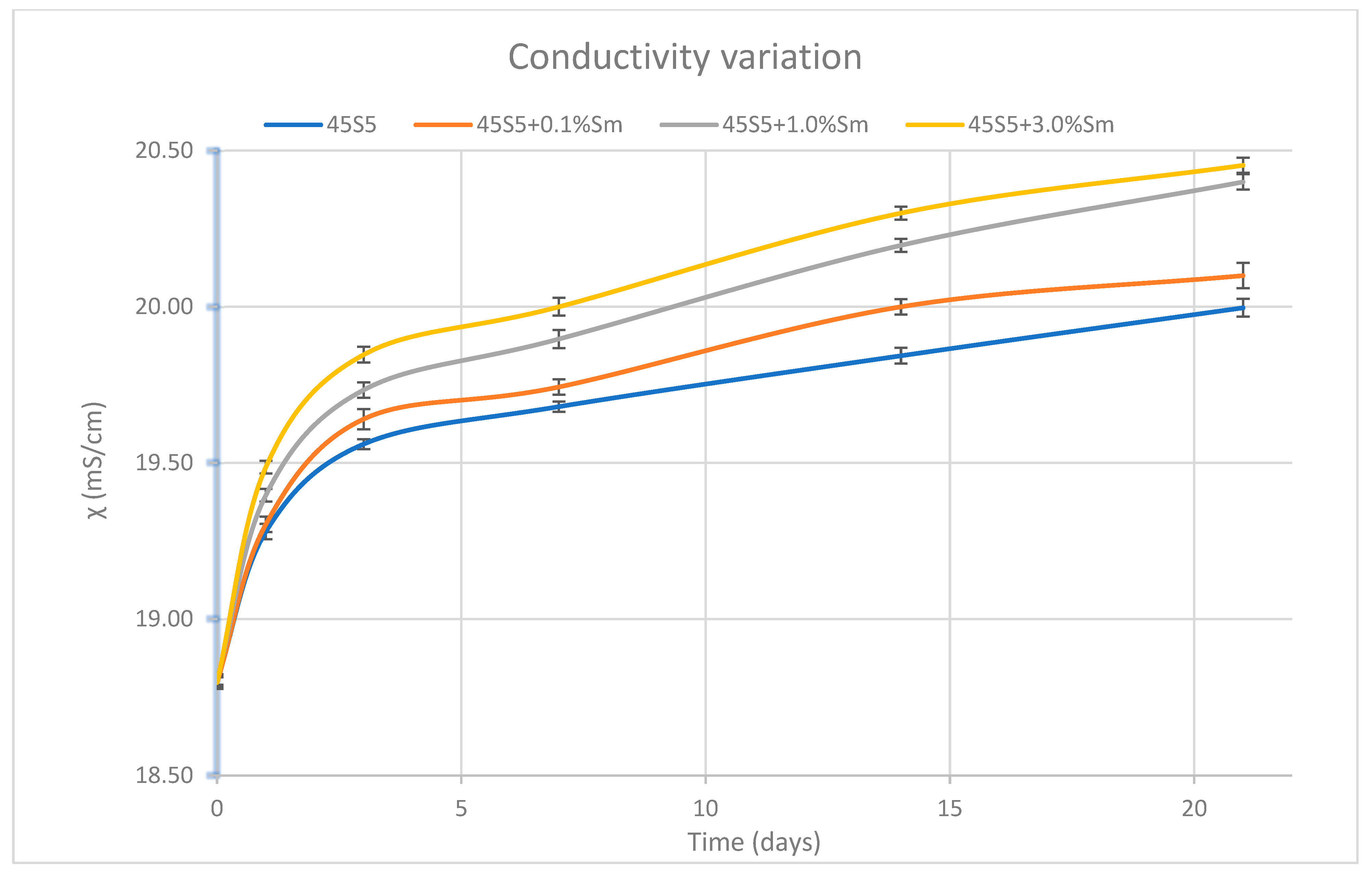
3.7. Determination of the pH Variation and the Ionic Strength of SBF Solution
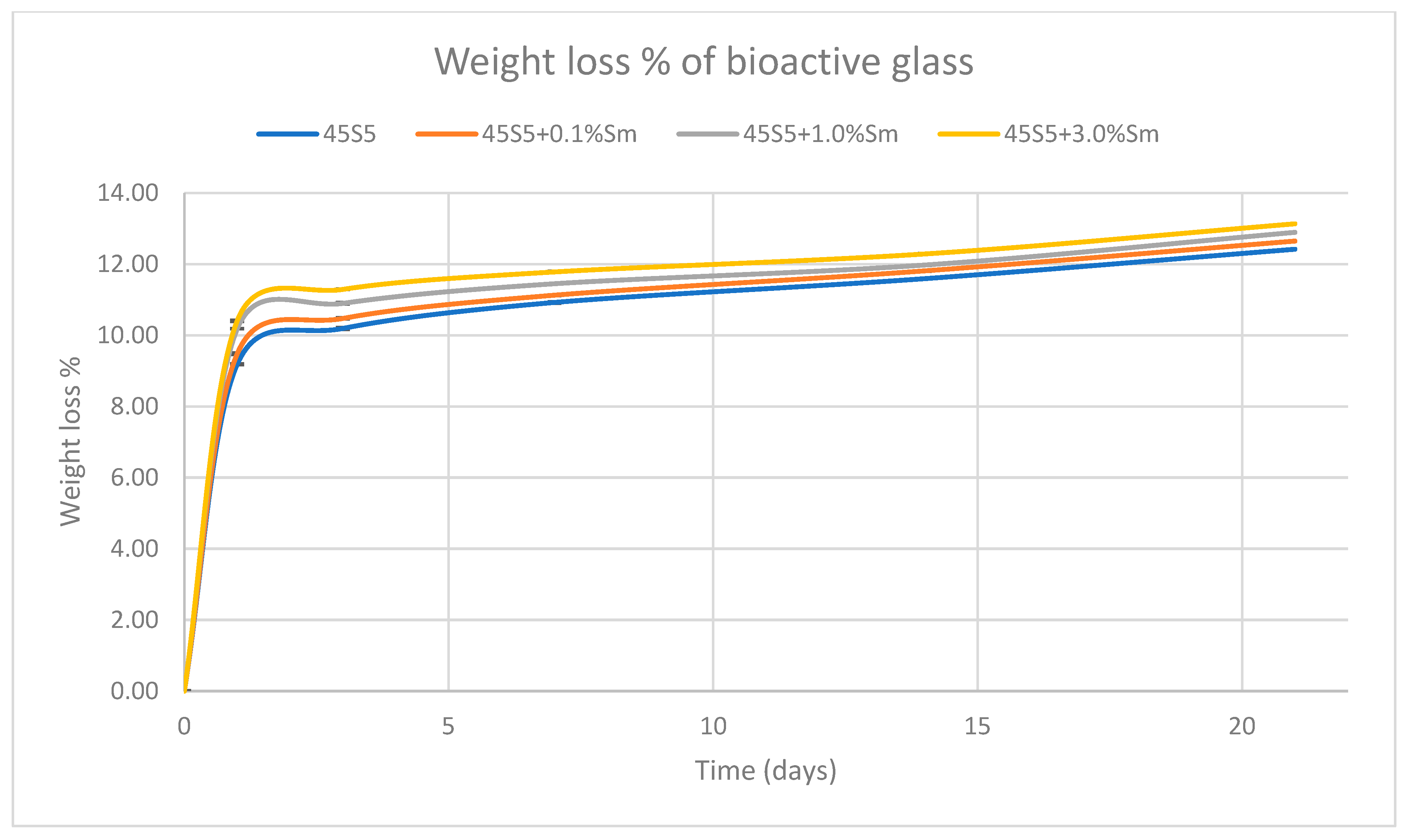
3.8. Mass Loss Determination
3.9. Biocompatibility Test Results
3.10. Antimicrobial and Anti-Adherence Activity and the Influence of Bioactive Glass 45S5 Doped with Samarium Against Virulence Factors’ Production
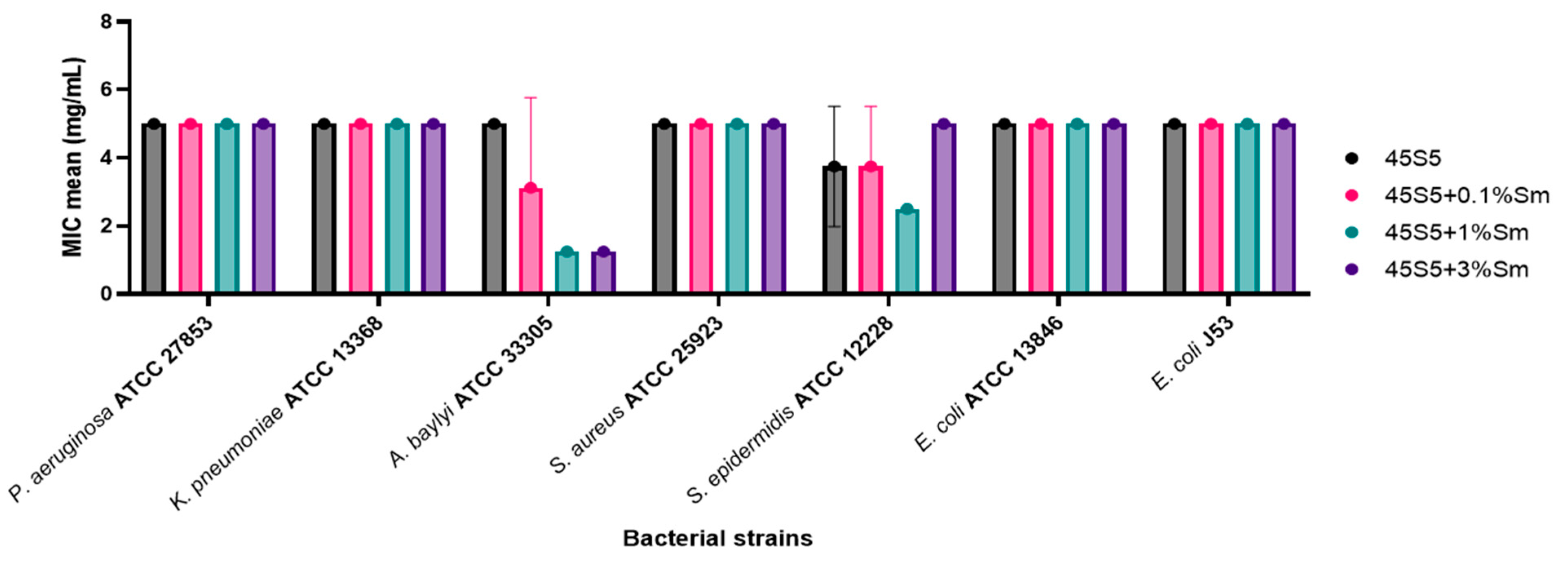
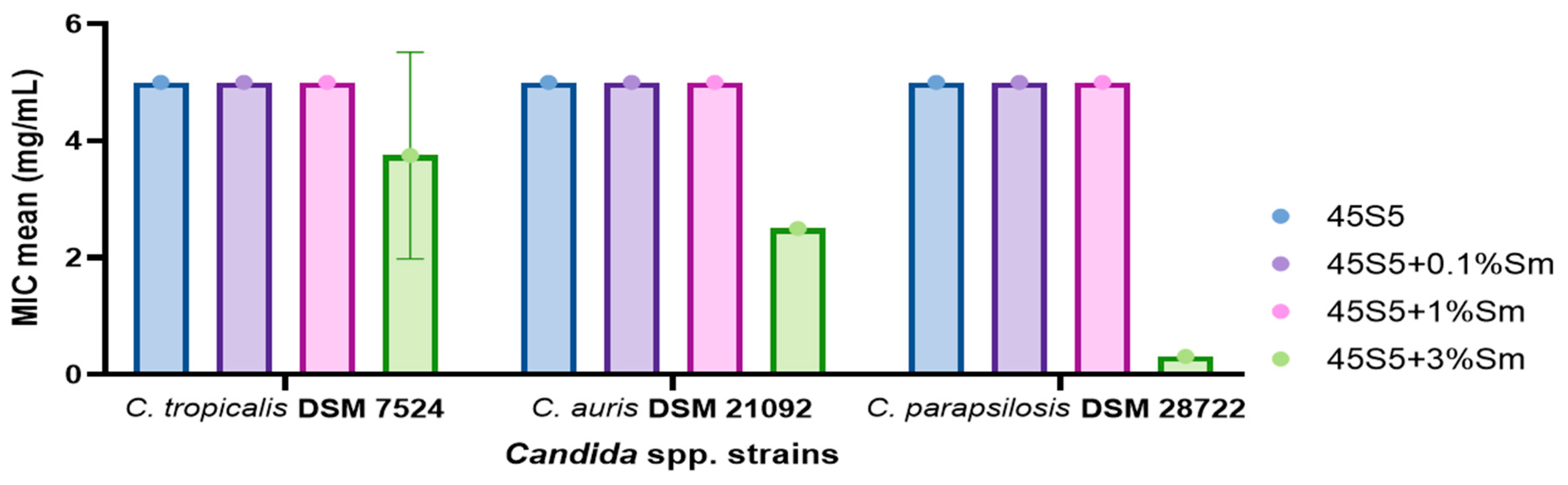
3.10.1. Quantitative Evaluation of the Antimicrobial Activity of the 45S5 Bioactive Glass Doped with Samarium Against Bacterial and Yeast Strains
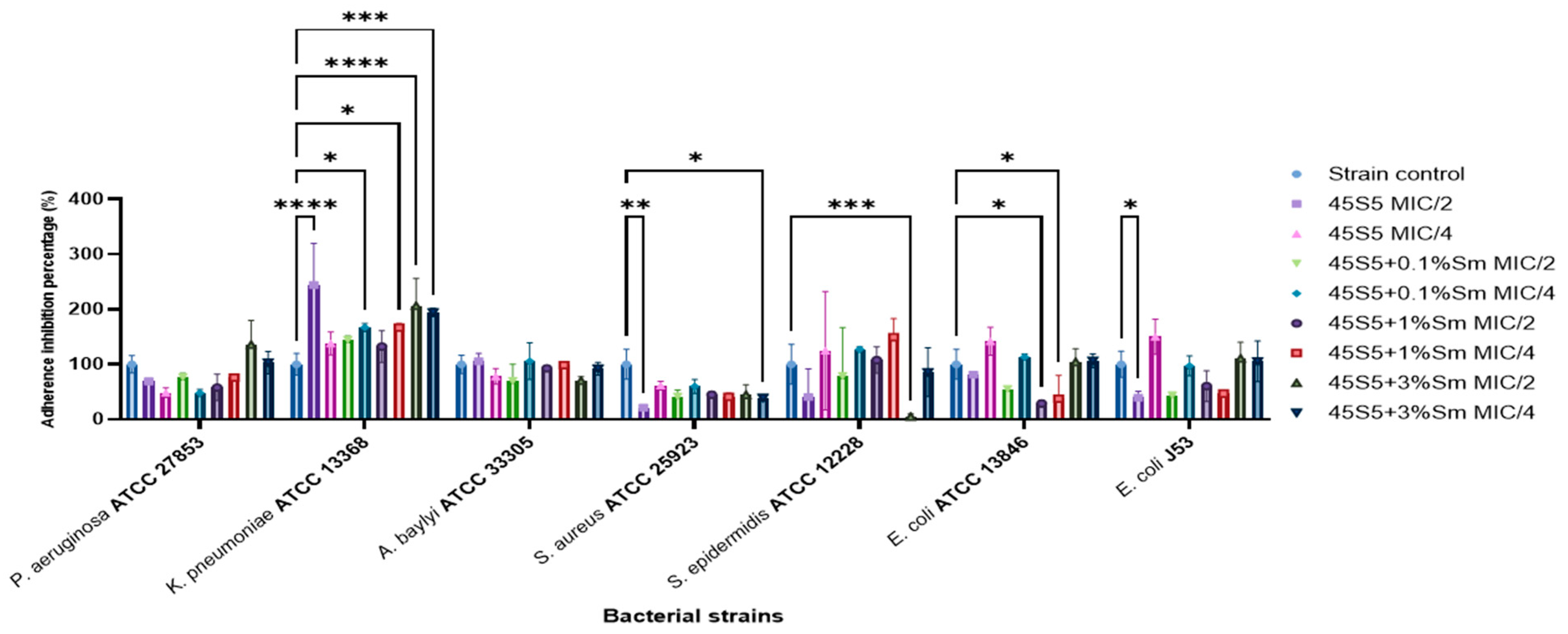
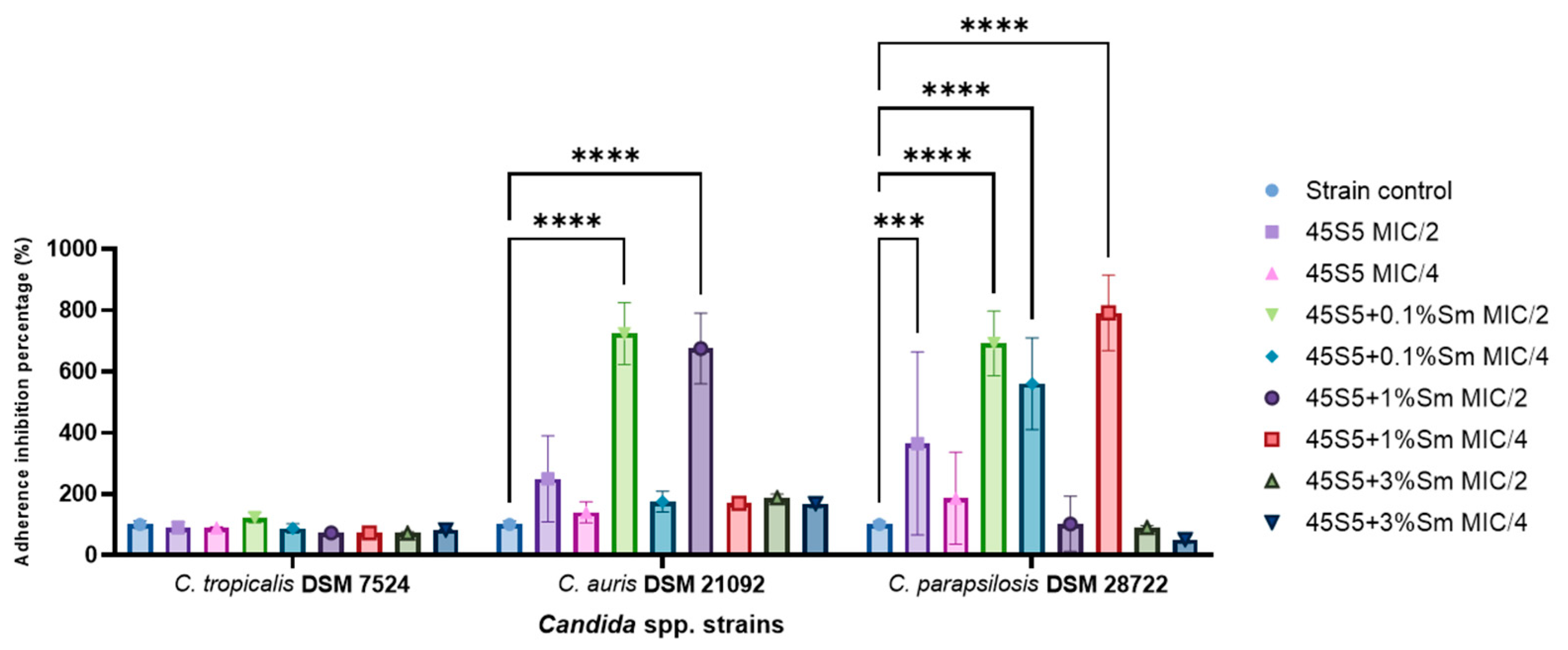
3.10.2. The Influence of the 45S5 Bioactive Glass Doped with Samarium on the Microbial Adherence Capacity to the Inert Substratum
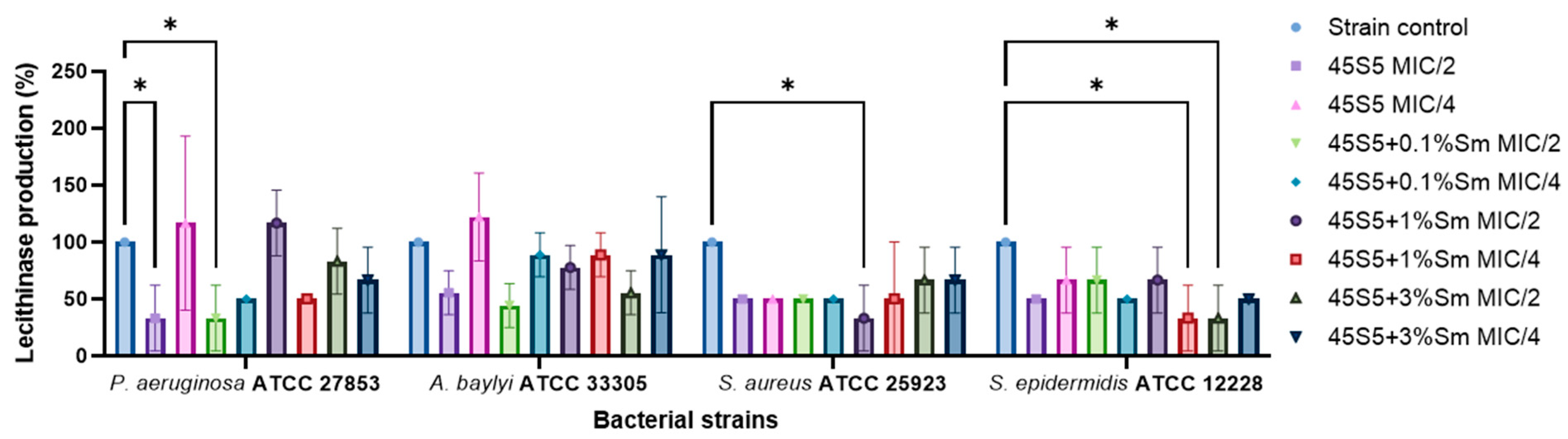
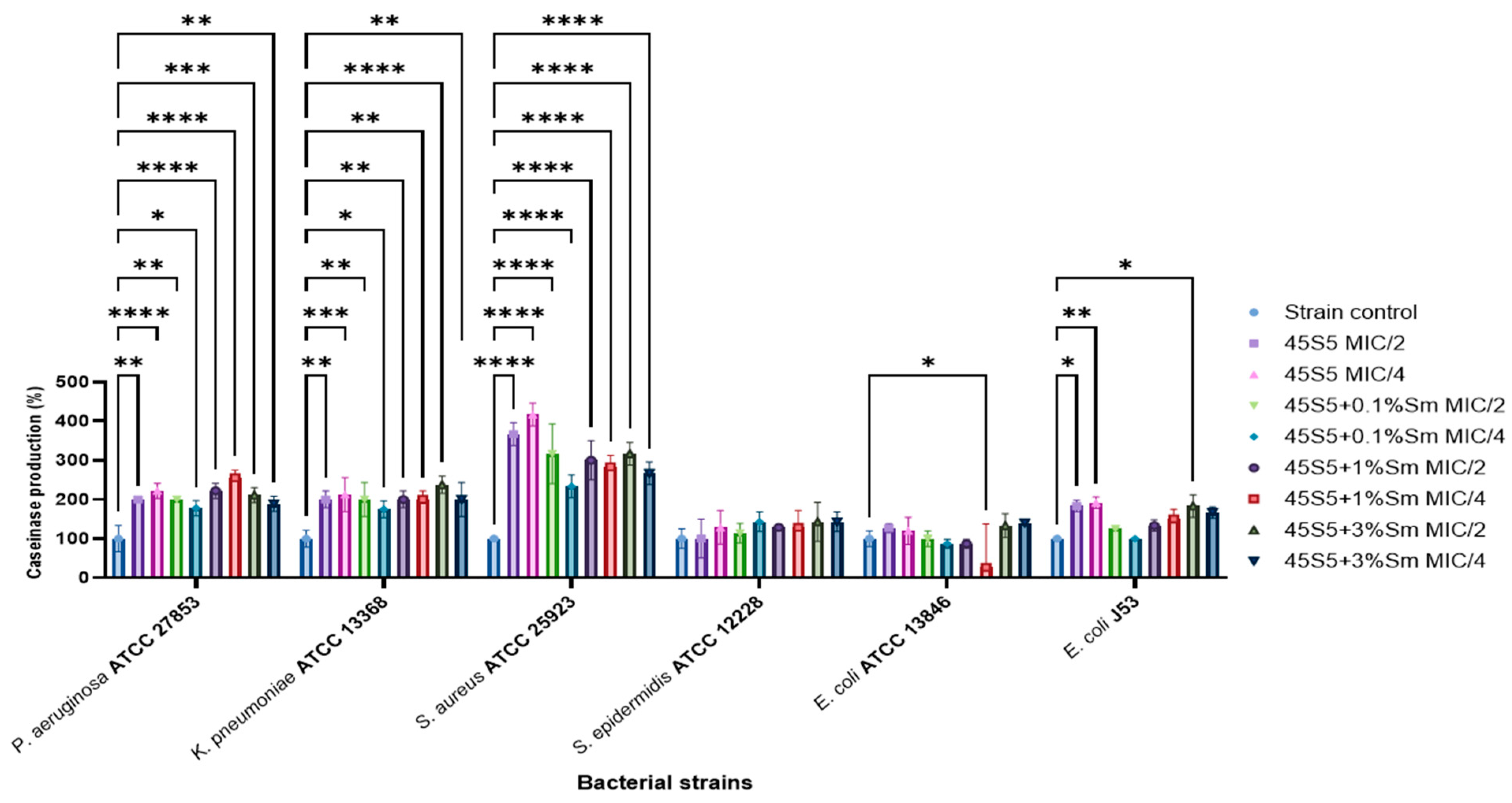
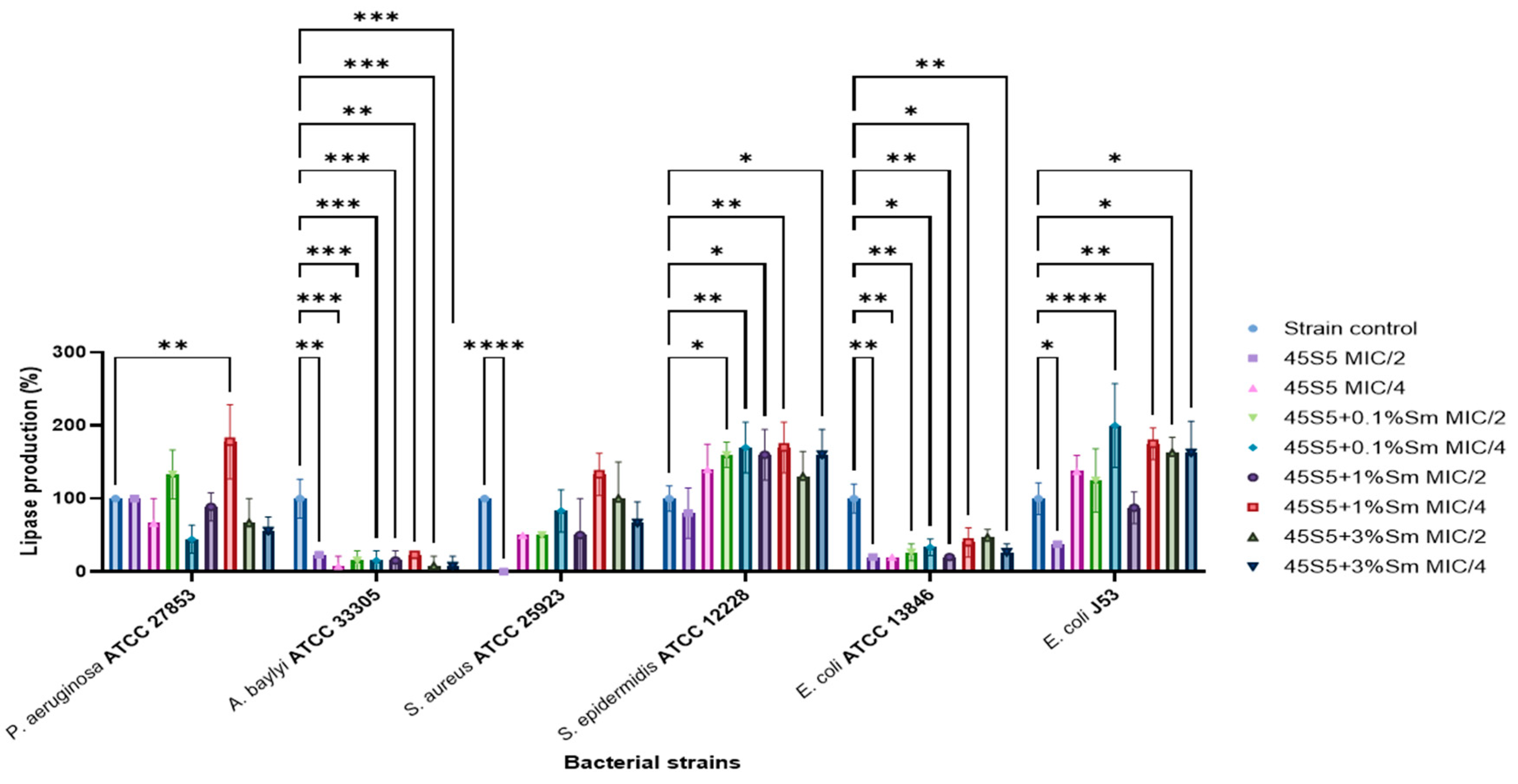
3.10.3. The Influence of 45S5 Bioactive Glass Doped with Samarium on the Soluble Virulence Factors Modulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hench, L.L.; Splinter, R.J.; Allen, W.C. Bonding Mechanisms at the Interface of Ceramic Prosthetic Materials. J. Biomed. Mater. Res. Symp. 1971, 2, 117–141. [Google Scholar]
- Mîrt, A.-L.; Ficai, D.; Oprea, O.-C.; Vasilievici, G.; Ficai, A. Current and Future Perspectives of Bioactive Glasses as Injectable Material. Nanomaterials 2024, 14, 1196. [Google Scholar] [CrossRef]
- Park, S.J.; Heogh, W.; Yang, J.; Kang, S.; Jeong, W.; Lee, H.; Jang, T.S.; Jung, H.D.; Jahazi, M.; Han, S.C.; et al. Meta-structure of amorphous-inspired 65.1Co28.2Cr5.3Mo lattices augmented by artificial intelligence. Adv. Compos. Hybrid Mater. 2024, 7, 224. [Google Scholar] [CrossRef]
- Negut, I.; Ristoscu, C.; Tozar, T.; Dinu, M.; Parau, A.C.; Grumezescu, V.; Hapenciuc, C.; Popa, M.; Stan, M.S.; Mihailescu, I.N.; et al. Implant Surfaces Containing Bioglasses and Ciprofloxacin as Platforms for Bone Repair and Improved Resistance to Microbial Colonization. Pharmaceutics 2022, 14, 1175. [Google Scholar] [CrossRef]
- Fiume, E.; Migneco, C.; Verné, E.; Baino, F. Comparison between Bioactive Sol-Gel and Melt-Derived Glasses/Glass-Ceramics Based on the Multicomponent SiO2–P2O5–CaO–MgO–Na2O–K2O System. Materials 2020, 13, 540. [Google Scholar] [CrossRef]
- Rizwan, M.; Hamdi, M.; Basirun, W.J. Bioglass® 45S5-based composites for bone tissue engineering and functional applications. J. Biomed. Mater. Res. Part A 2017, 105, 3197–3223. [Google Scholar] [CrossRef] [PubMed]
- Porwal, H.; Grasso, S.; Cordero-Arias, L.; Boccaccini, A.R.; Li, C.; Reece, M.J. Processing and bioactivity of 45S5 Bioglass®-graphene nanoplatelets composites. J. Mater. Sci. Mater. Med. 2014, 25, 1403–1413. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Morais, D.S.; Coelho, J.; Ferraz, M.P.; Gomes, P.S.; Fernandes, M.H.; Hussain, N.S.; Santosa, J.D.; Lopesa, M.A. Samarium doped glass-reinforced hydroxyapatite with enhanced osteoblastic performance and antibacterial properties for bone tissue regeneration. J. Mater. Chem. B 2014, 2, 5872. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 2022, 33, 3. [Google Scholar] [CrossRef]
- Chellan, P.; Sadler, P.J. The elements of life and medicines. Phil. Trans. R. Soc. A 2015, 373, 20140182. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, A.; Kochanowicz, M.; Żmojda, J.; Miluski, P.; Wajda, A.; Leśniak, M.; Dorosz, D. Biological properties of rare-earth doped bioactive glass. Opt. Fibers Their Appl. 2020, 11456, 10. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Ciobanu, S.C.; Chapon, P.; Predoi, D. Antimicrobial Properties of Samarium Doped Hydroxyapatite Suspensions and Coatings. Coatings 2020, 10, 1124. [Google Scholar] [CrossRef]
- Balas, M.; Badea, M.A.; Ciobanu, S.C.; Piciu, F.; Iconaru, S.L.; Dinischiotu, A.; Predoi, D. Biocompatibility and Osteogenic Activity of Samarium-Doped Hydroxyapatite—Biomimetic Nanoceramics for Bone Regeneration Applications. Biomimetics 2024, 9, 309. [Google Scholar] [CrossRef]
- Lavric, R.; Vreme, C.; Busuioc, C.; Isopencu, G.-O.; Nicoara, A.-I.; Oprea, O.-C.; Banciu, D.-D.; Constantinoiu, I.; Musat, A.-M.-R. The Effect of Silver and Samarium on the Properties of Bioglass Coatings Produced by Pulsed Laser Deposition and Spin Coating. J. Funct. Biomater. 2023, 14, 560. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Su, Y.; Chen, D.; Zhong, W. A doxorubicin delivery system: Samarium/mesoporous bioactive glass/alginate composite microspheres. Mater. Sci. Eng. C 2016, 67, 205–213. [Google Scholar] [CrossRef]
- Roberto, W.S.; Pereira, M.M.; Campos, T.P.R. Dosimetric Analysis and Characterization of Radioactive Seeds Produced by the Sol-Gel Method. Key Eng. Mater. 2003, 240–242, 579–582. [Google Scholar] [CrossRef]
- Roberto, W.S.; Pereira, M.M.; Campos, T.P.R. Structure and Dosimetric Analysis of Biodegradable Glasses for Prostate Cancer Treatment. Artif. Organs 2003, 27, 432–436. [Google Scholar] [CrossRef]
- Valente, E.S.; Campos, T.P.R. Gamma spectrometry and chemical characterization of ceramic seeds with samarium-153 and holmium-166 for brachytherapy proposal. Appl. Radiat. Isot. 2010, 68, 2157–2162. [Google Scholar] [CrossRef]
- Maini, C.L.; Bergomi, S.; Romano, L.; Sciuto, R. 153Sm-EDTMP for bone pain palliation in skeletal metastases. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 171–178. [Google Scholar] [CrossRef]
- Lam, M.G.E.H.; Dahmane, A.; Stevens, W.H.M.; Rijk, P.P.v.; Klerk, J.M.H.d.; Zonnenberg, B.A. Combined use of zoledronic acid and 153Sm-EDTMP in hormone-refractory prostate cancer patients with bone metastases. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 756–765. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Corbu, V.M.; Georgescu, A.-M.; Marinas, I.C.; Pericleanu, R.; Mogos, D.V.; Dumbravă, A.Ș.; Marinescu, L.; Pecete, I.; Vassu-Dimov, T.; Barbu, I.C.; et al. Phenotypic and Genotypic Characterization of Resistance and Virulence Markers in Candida spp. Isolated from Community-Acquired Infections in Bucharest, and the Impact of AgNPs on the Highly Resistant Isolates. J. Fungi 2024, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Corbu, V.M.; Gheorghe, I.; Marinaș, I.C.; Geană, E.I.; Moza, M.I.; Csutak, O.; Chifiriuc, M.C. Demonstration of Allium sativum Extract Inhibitory Effect on Biodeteriogenic Microbial Strain Growth, Biofilm Development, and Enzymatic and Organic Acid Production. Molecules 2021, 26, 7195. [Google Scholar] [CrossRef]
- Georgescu, M.; Gheorghe, I.; Curutiu, C.; Lazar, V.; Bleotu, C.; Chifiriuc, M.-C. Virulence and resistance features of Pseudomonas aeruginosa strains isolated from chronic leg ulcers. BMC Infect. Dis. 2016, 16, 92. [Google Scholar] [CrossRef]
- Truşcă, B.S.; Gheorghe-Barbu, I.; Manea, M.; Ianculescu, E.; Barbu, I.C.; Măruțescu, L.G.; Dițu, L.-M.; Chifiriuc, M.-C.; Lazăr, V. Snapshot of Phenotypic and Molecular Virulence and Resistance Profiles in Multidrug-Resistant Strains Isolated in a Tertiary Hospital in Romania. Pathogens 2023, 12, 609. [Google Scholar] [CrossRef]
- Gheorghe-Barbu, I.; Corbu, V.M.; Vrancianu, C.O.; Marinas, I.C.; Popa, M.; Dumbravă, A.Ș.; Niță-Lazăr, M.; Pecete, I.; Muntean, A.A.; Popa, M.I.; et al. Phenotypic and Genotypic Characterization of Recently Isolated Multidrug-Resistant Acinetobacter baumannii Clinical and Aquatic Strains and Demonstration of Silver Nanoparticle Potency. Microorganisms 2023, 11, 2439. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Graça, M.P.F.; Prezas, P.R.; Kumar, J.S.; Melo, B.M.G.; Sales, A.J.M.; Almeida, A.F.; Valente, M.A. Structural, thermal, morphological and dielectric investigations on 45S5 glass and glass-ceramics. J. Non-Cryst. Solids 2021, 562, 120780. [Google Scholar] [CrossRef]
- Lefebvre, L.; Chevalier, J.; Gremillard, L.; Zenati, R.; Thollet, G.; Bernache-Assolant, D.; Govin, A. Structural transformations of bioactive glass 45S5 with thermal treatments. Acta Mater. 2007, 55, 3305–3313. [Google Scholar] [CrossRef]
- Mecca, F.G.; Bellucci, D.; Cannillo, V. Effect of Thermal Treatments and Ion Substitution on Sintering and Crystallization of Bioactive Glasses: A Review. Materials 2023, 16, 4651. [Google Scholar] [CrossRef]
- Pirayesh, H.; Nychka, J.A. Sol–Gel Synthesis of Bioactive Glass-Ceramic 45S5 and its in vitro Dissolution and Mineralization Behavior. J. Am. Ceram. Soc. 2013, 96, 1643–1650. [Google Scholar] [CrossRef]
- Lombardi, M.; Gremillard, L.; Chevalier, J.; Lefebvre, L.; Cacciotti, I.; Bianco, A.; Montanaro, L. A Comparative Study between Melt-Derived and Sol-Gel Synthesized 45S5 Bioactive Glasses. Key Eng. Mater. 2013, 541, 15–30. [Google Scholar] [CrossRef]
- Faure, J.; Drevet, R.; Lemelle, A.; Jaber, N.B.; Tara, A.; Btaouri, H.E.; Benhayoune, H. A new sol–gel synthesis of 45S5 bioactive glass using an organic acid as catalyst. Mater. Sci. Eng. C 2015, 47, 407–412. [Google Scholar]
- Hum, J.; Boccaccini, A.R. Collagen as Coating Material for 45S5 Bioactive Glass-Based Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1807. [Google Scholar] [CrossRef]
- Ohsato, H.; Maki, I.; Takéuchi, Y. Structure of Na2CaSi2O6. Acta Crystallogr. Sect. C Struct. Chem. 1985, 41, 1575–1577. [Google Scholar] [CrossRef]
- Ihara, M.; Odani, K.; Yoshida, N.; Fukunaga, J.; Setoguchi, M.; Higashi, T. The crystal Stucture of Devitrite (Disodium Tricalcium Hexasilicate) Ca3Na2Si6O16. J. Ceram. Assoc. Jpn. 1984, 92, 373–378. [Google Scholar] [CrossRef]
- Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P. Rietveld refinement of the crystallographic structure of human dental enamel apatites. Am. Miner. 1999, 84, 1406–1414. [Google Scholar] [CrossRef]
- Ershad, M.; Vyas, V.K.; Prasad, S.; Ali, A.; Pyare, R. Effect of Sm2O3 substitution on mechanical and biological properties of 45S5 bioactive glass. J. Aust. Ceram. Soc. 2018, 54, 621–630. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef]
- Ungureanu, D.N.; Angelescu, N.; Tsakiris, V.; Marinescu, V. Investigations regarding chemical synthesis of calcium hydroxyapatite. Rom. J. Mater. 2012, 42, 52–60. [Google Scholar]
- Maximov, M.; Maximov, O.-C.; Craciun, L.; Ficai, D.; Ficai, A.; Andronescu, E. Bioactive Glass—An Extensive Study of the Preparation and Coating Methods. Coatings 2021, 11, 1386. [Google Scholar] [CrossRef]
- Nica, I.C.; Popa, M.; Marutescu, L.; Dinischiotu, A.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, D. Biocompatibility and Antibiofilm Properties of Samarium Doped Hydroxyapatite Coatings: An In Vitro Study. Coatings 2021, 11, 1185. [Google Scholar] [CrossRef]
- Araujo, M.S.; Silva, A.C.; Cabal, B.; Bartolomé, J.F.; Mello-Castanho, S. In vitro bioactivity and antibacterial capacity of 45S5 Bioglass®-based compositions containing alumina and strontium. J. Mater. Res. Technol. 2021, 13, 154–161. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Jakka, S.K.; Valente, M.A.; Graça, M.P.F.; Pádua, A.S.; Silva, J.C.; Sá-Nogueira, I.; Borges, J.P. Antibacterial Biomaterial Based on Bioglass Modified with Copper for Implants Coating. J. Funct. Biomater. 2023, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ru, Y.; You, J.; Lin, R.; Chen, S.; Qi, Y.; Li, D.; Zhang, C.; Qiu, Z. Antibacterial Properties of PCL@45s5 Composite Biomaterial Scaffolds Based on Additive Manufacturing. Polymers 2024, 16, 3379. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, F.; Ding, M.; Xiang, L.; Bai, J.; Li, Q.; Zhou, Y. Klebsiella pneumoniae BolA contributes to cell morphology, siderophore production, stresses challenge, cell adhesion and virulence. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhou, P.; Garcia, B.L.; Kotsakis, G.A. Comparison of antibacterial and antibiofilm activity of bioactive glass compounds S53P4 and 45S5. BMC Microbiol. 2022, 22, 212. [Google Scholar] [CrossRef]









| Name | Quantity Sm(NO3)3*6H2O (g) | Sm (Mass %) |
|---|---|---|
| 45S5 | - | - |
| 45S5 + 0.1% Sm | 0.075 g | 0.1% |
| 45S5 + 1.0% Sm | 0.75 g | 1.0% |
| 45S5 + 3.0% Sm | 2.25 g | 3.0% |
| Ions | Na+ | K+ | Ca2+ | Mg2+ | HCO3− | Cl− | HPO42− | SO42− |
| SBF | 142 | 5.0 | 2.5 | 1.5 | 4.2 | 148 | 1.0 | 0.5 |
| Human blood plasma | 142 | 5.0 | 2.5 | 1.5 | 27.0 | 103 | 1.0 | 0.5 |
| Sample | Combeite | Devitrite |
|---|---|---|
| 45S5 | 96.2% | 3.8% |
| 45S5 + 0.1% Sm | 98.7% | 1.3% |
| 45S5 + 1% Sm | 98.6% | 1.4% |
| 45S5 + 3% Sm | 94.5% | 4.5% |
| 45S5 + 3% Sm after 21 days in SBF | 97.5% | 1.3% |
| 45S5 | 45S5 + 0.1% Sm | 45S5 + 1% Sm | 45S5 + 3% Sm | |||||
|---|---|---|---|---|---|---|---|---|
| Combeite | Devitrite | Combeite | Devitrite | Combeite | Devitrite | Combeite | Devitrite | |
| a (Å) | 10.497 | 10.203 | 10.498 | 10.111 | 10.492 | 10.102 | 10.497 | 10.131 |
| b (Å) | 10.497 | 10.699 | 10.498 | 10.691 | 10.492 | 10.627 | 10.497 | 10.584 |
| c (Å) | 13.179 | 7.174 | 13.179 | 7.171 | 13.170 | 7.102 | 13.164 | 7.043 |
| α (°) | 90 | 110.86 | 90 | 110.8 | 90 | 109.90 | 90 | 109.13 |
| Β (°) | 90 | 97.78 | 90 | 98.2 | 90 | 98.10 | 90 | 98.13 |
| ɣ (°) | 120 | 78.10 | 120 | 78.01 | 120 | 78.16 | 120 | 78.18 |
| V/106 (pm3) | 1257.737 | 714.3854 | 1257.862 | 706.8171 | 1255.539 | 699.5542 | 1256.095 | 696.1263 |
| Sample | Combeite | Devitrite | Hydroxyapatite |
|---|---|---|---|
| 45S5 + 3% Sm | 94.5% | 4.5% | - |
| 45S5 + 3% Sm after 21 days in SBF | 97.5% | 1.3% | 1.2% |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| 45S5 | 2.690 | <LQ | 3.525 |
| 45S5 + 0.1% Sm | 7.387 | <LQ | 3.883 |
| 45S5 + 1.0% Sm | 1.370 | <LQ | 3.863 |
| 45S5 + 3.0% Sm | 2.060 | <LQ | 3.880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maximov, M.V.; Maximov, O.C.; Motelica, L.; Ficai, D.; Oprea, O.C.; Trușcă, R.D.; Balahura, L.-R.; Pericleanu, R.; Dumbravă, A.Ș.; Corbu, V.M.; et al. Comprehensive Evaluation of 45S5 Bioactive Glass Doped with Samarium: From Synthesis and Physical Properties to Biocompatibility and Antimicrobial Activity. Coatings 2025, 15, 404. https://doi.org/10.3390/coatings15040404
Maximov MV, Maximov OC, Motelica L, Ficai D, Oprea OC, Trușcă RD, Balahura L-R, Pericleanu R, Dumbravă AȘ, Corbu VM, et al. Comprehensive Evaluation of 45S5 Bioactive Glass Doped with Samarium: From Synthesis and Physical Properties to Biocompatibility and Antimicrobial Activity. Coatings. 2025; 15(4):404. https://doi.org/10.3390/coatings15040404
Chicago/Turabian StyleMaximov, Maxim V., Oana Cristina Maximov, Ludmila Motelica, Denisa Ficai, Ovidiu Cristian Oprea, Roxana Doina Trușcă, Liliana-Roxana Balahura (Stămat), Radu Pericleanu, Andreea Ștefania Dumbravă, Viorica Maria Corbu, and et al. 2025. "Comprehensive Evaluation of 45S5 Bioactive Glass Doped with Samarium: From Synthesis and Physical Properties to Biocompatibility and Antimicrobial Activity" Coatings 15, no. 4: 404. https://doi.org/10.3390/coatings15040404
APA StyleMaximov, M. V., Maximov, O. C., Motelica, L., Ficai, D., Oprea, O. C., Trușcă, R. D., Balahura, L.-R., Pericleanu, R., Dumbravă, A. Ș., Corbu, V. M., Surdu, V.-A., Vasilievici, G., Ficai, A., Dinescu, S., & Gheorghe-Barbu, I. (2025). Comprehensive Evaluation of 45S5 Bioactive Glass Doped with Samarium: From Synthesis and Physical Properties to Biocompatibility and Antimicrobial Activity. Coatings, 15(4), 404. https://doi.org/10.3390/coatings15040404












