Potential of Cellulose After Oxidative Torrefaction for Fuel Enhancement and Utilization: Properties and Pyrolysis Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Oxidative Torrefaction Experiment
2.2. Sample Labeling and Characterization
2.3. Indicators
3. Results
3.1. Physicochemical Characteristics
3.1.1. Basic Properties of Cellulose Before and After Oxidative Torrefaction
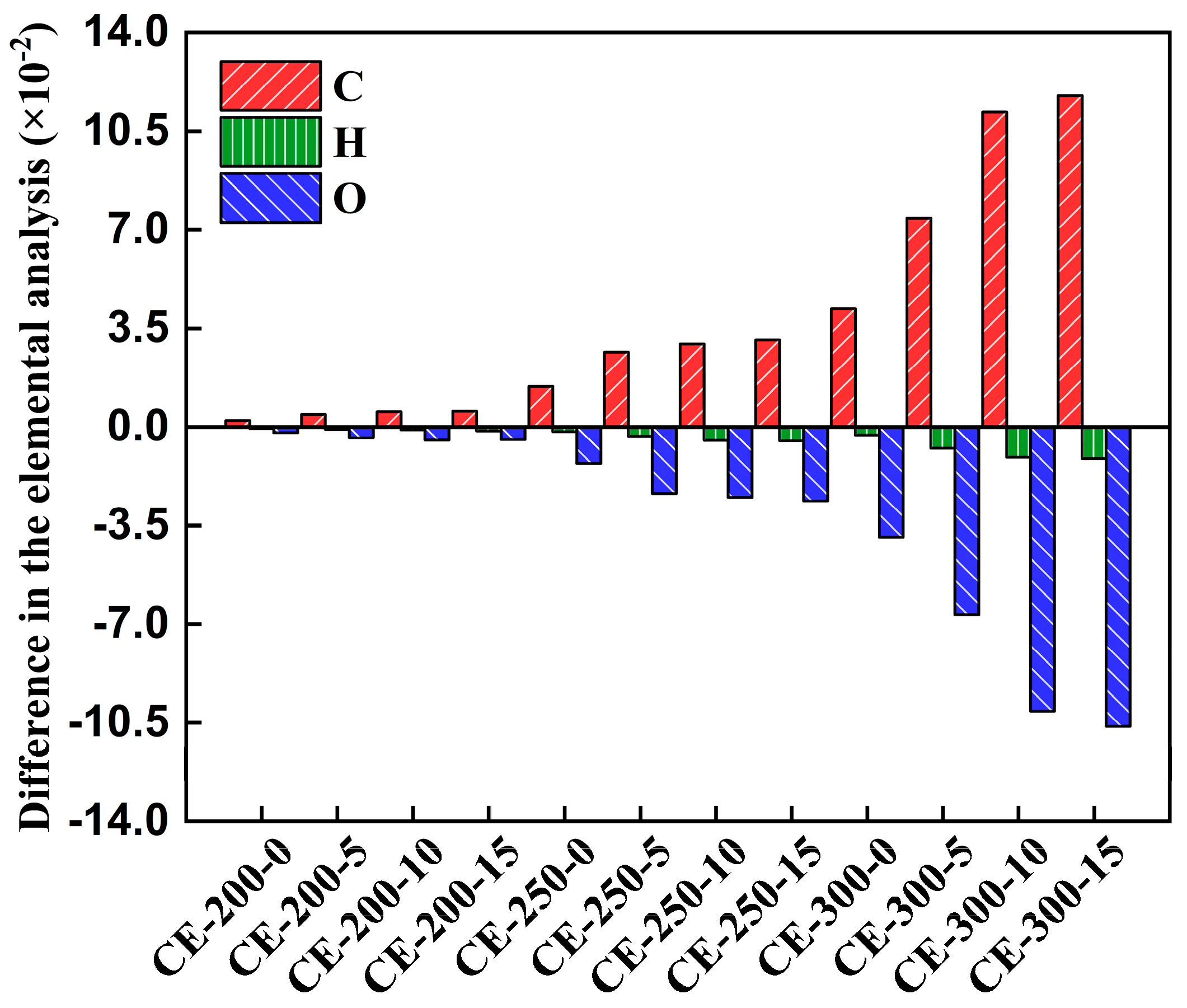
3.1.2. Changes in the Carbon, Hydrogen, and Oxygen Contents of Cellulose
3.1.3. Decarbonization, Dehydrogenation, and Deoxygenation
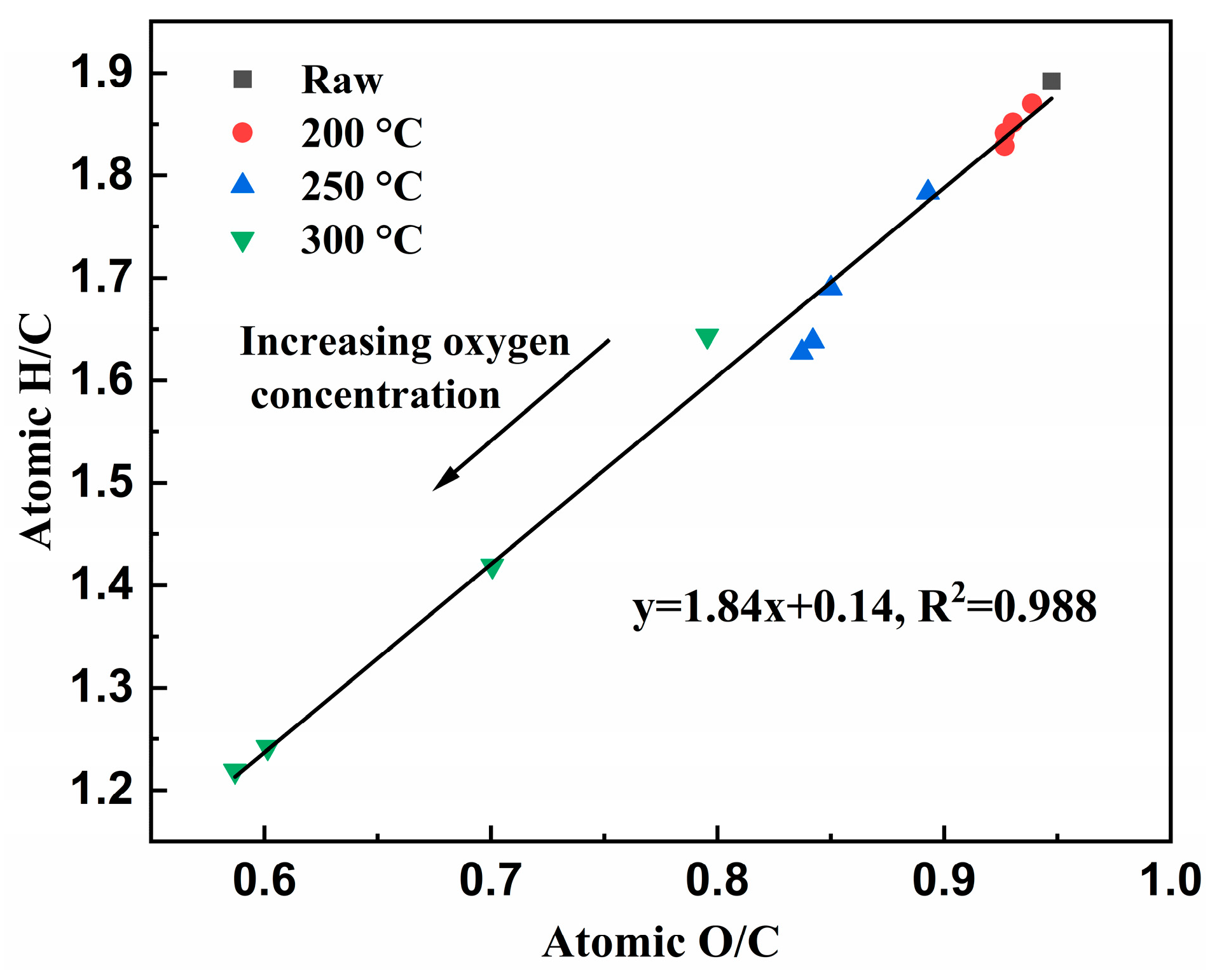
3.1.4. Van Krevelen Diagram of Cellulose
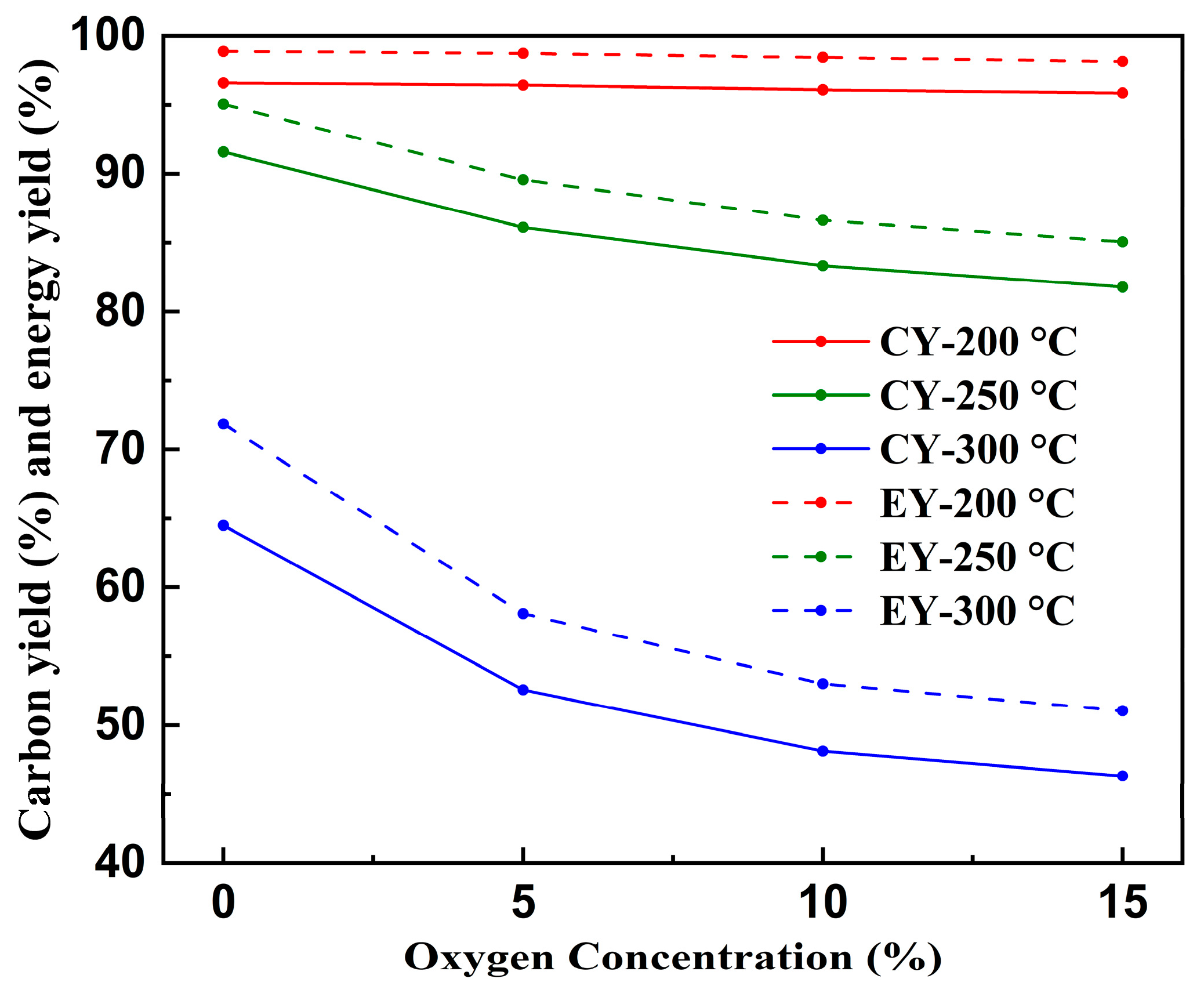
3.1.5. Carbon Yield and Energy Yield
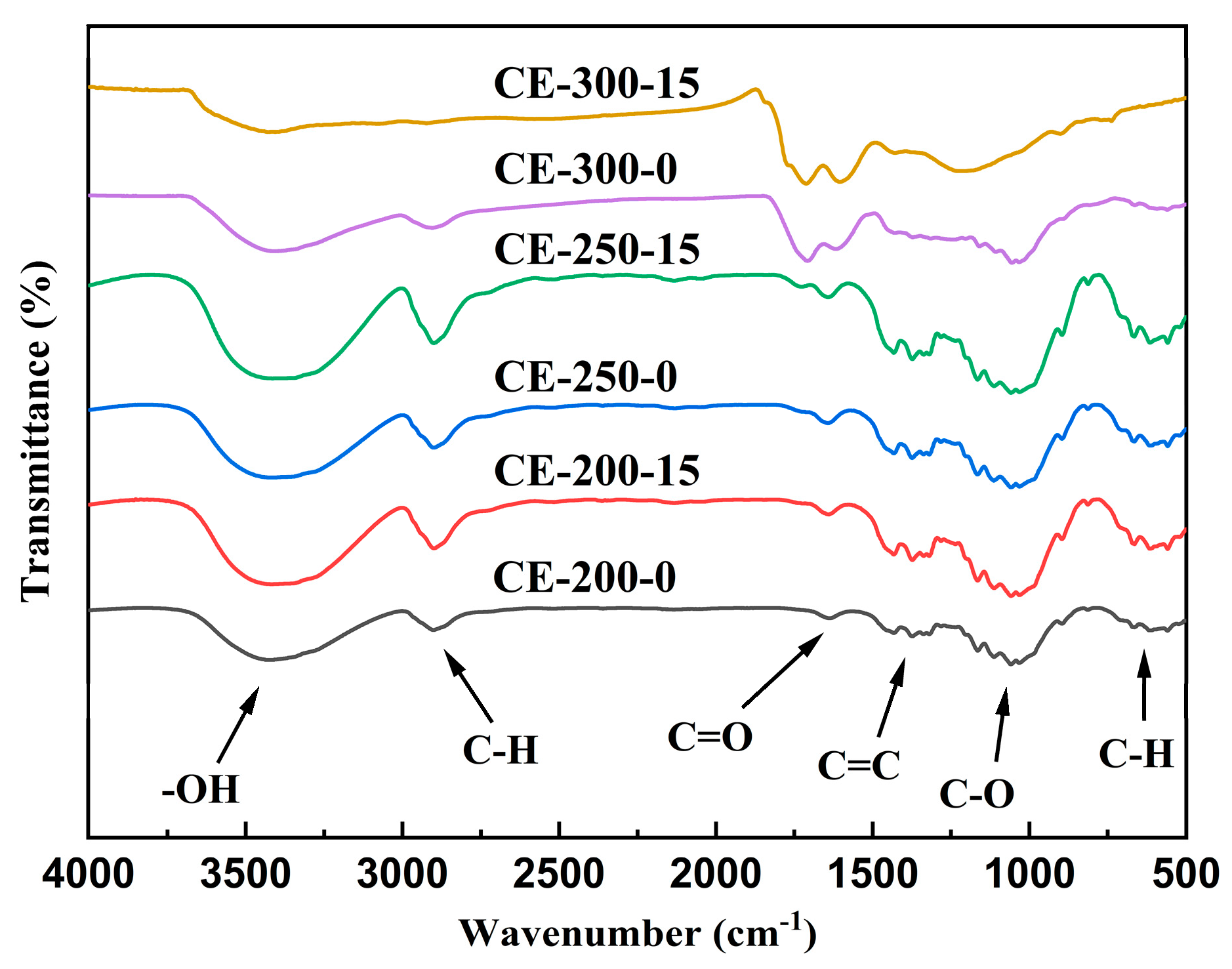
3.1.6. FTIR Analysis
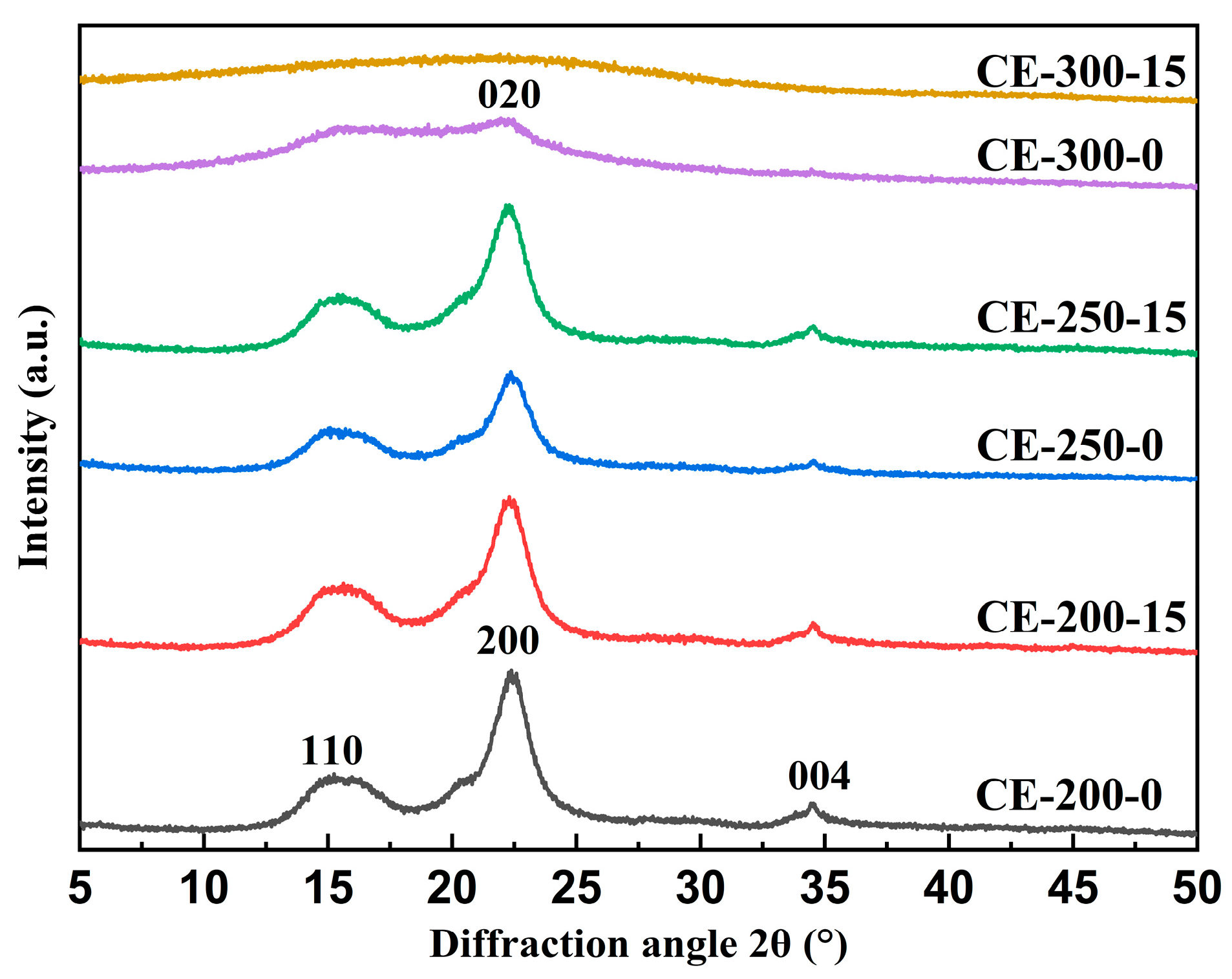
3.1.7. XRD Analysis
3.2. Pyrolysis Characteristics
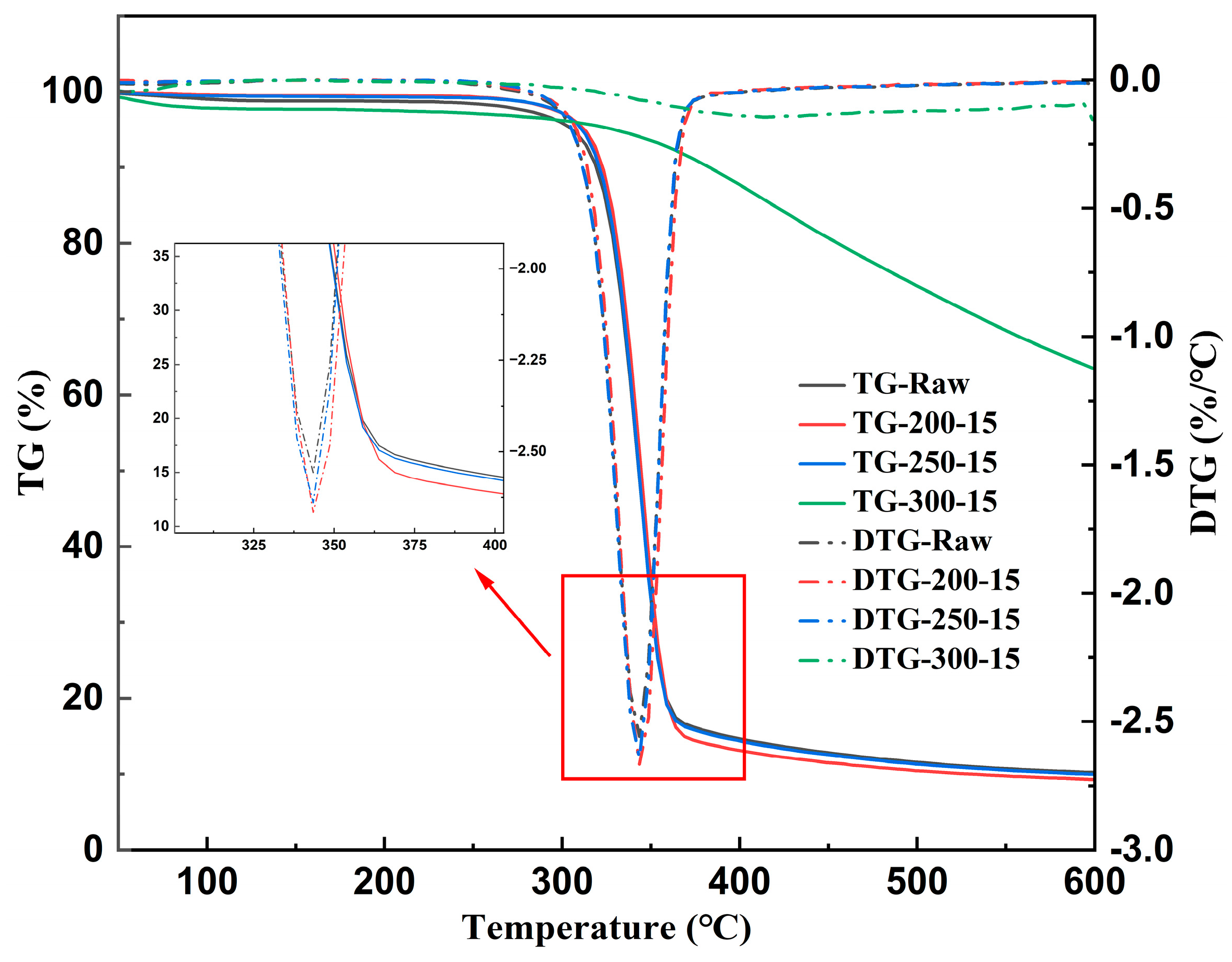
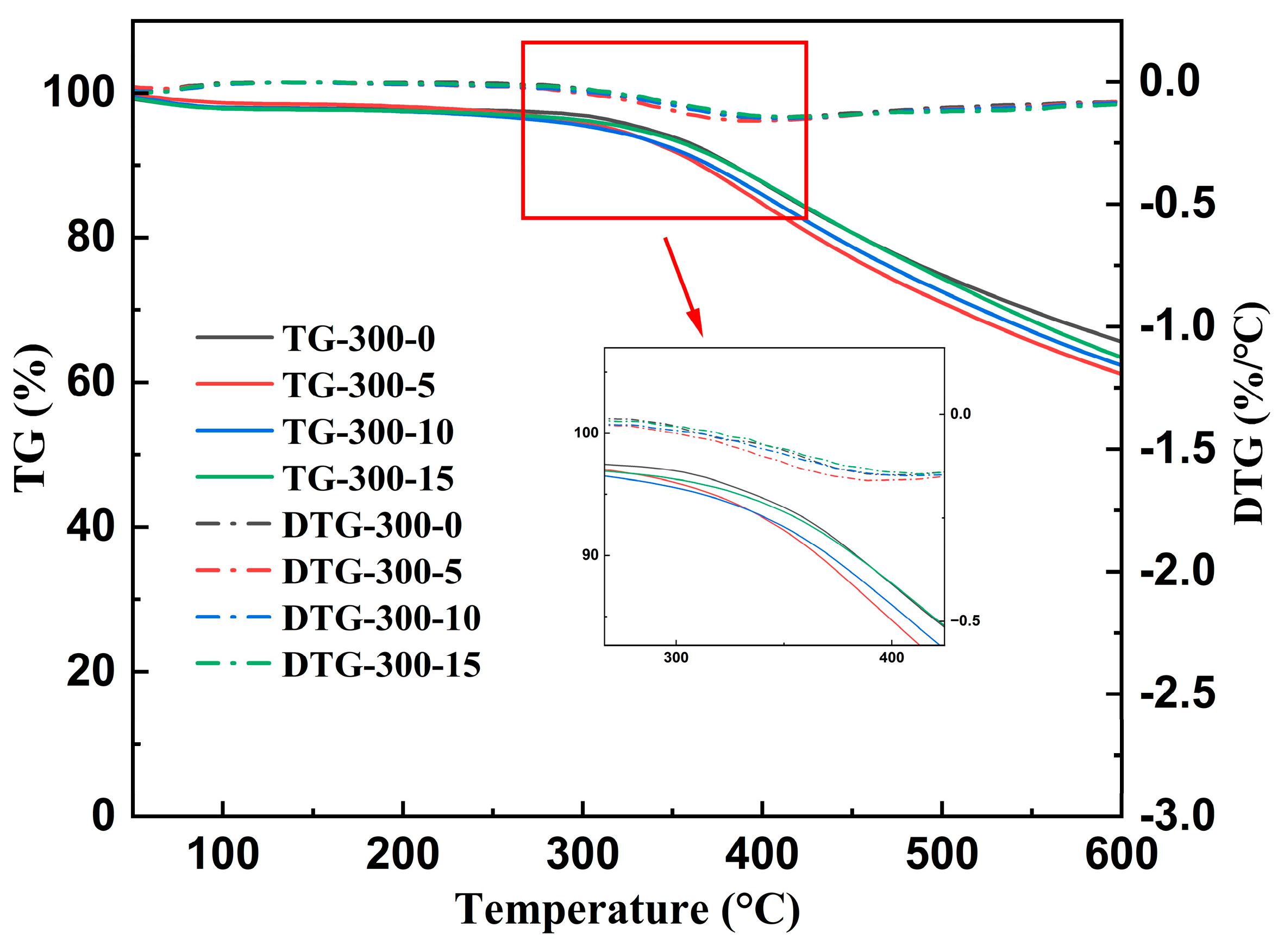
3.2.1. TG/DTG Analysis
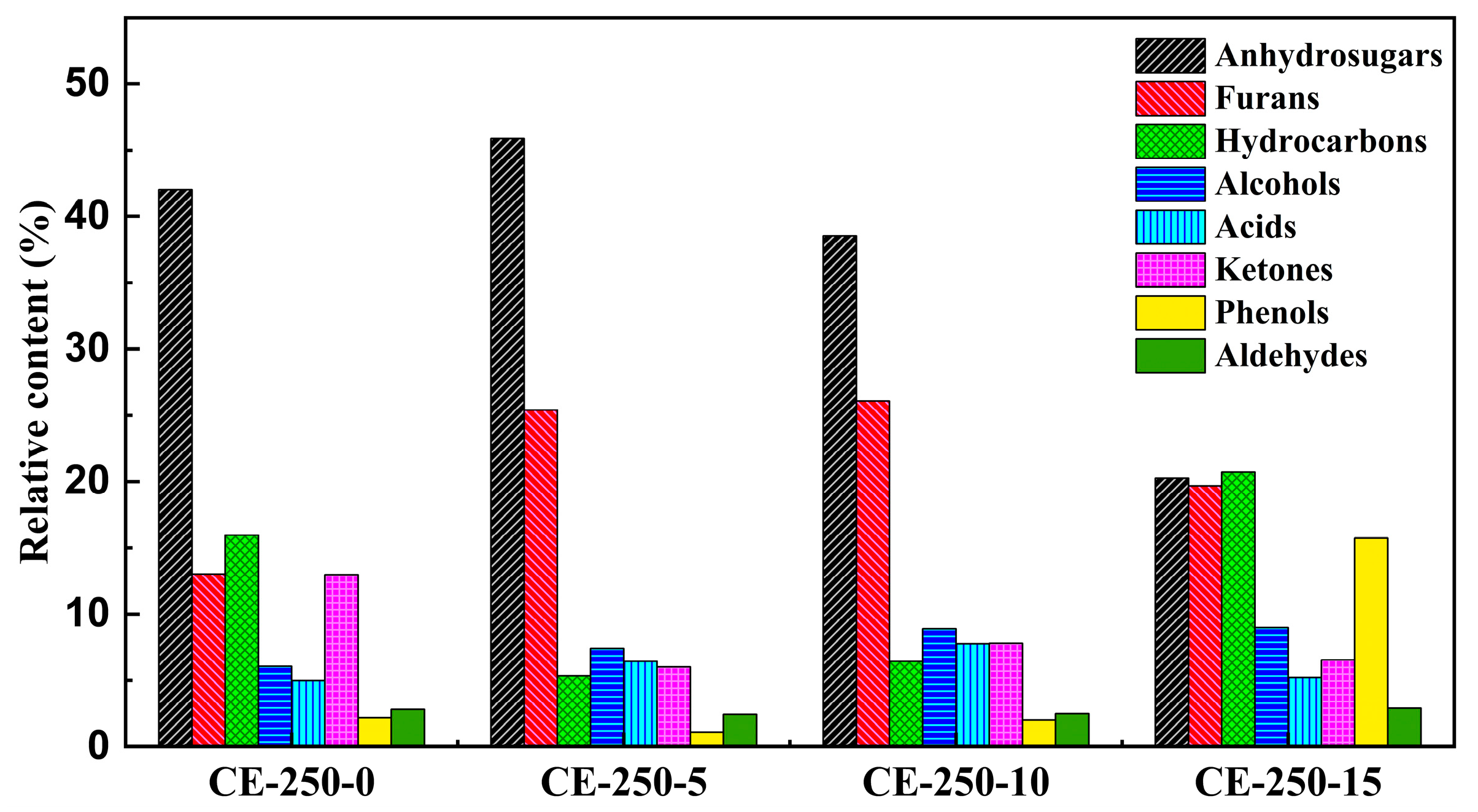
3.2.2. Analysis of the Pyrolysis Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasileiadou, A. From Organic Wastes to Bioenergy, Biofuels, and Value-Added Products for Urban Sustainability and Circular Economy: A Review. Urban Sci. 2024, 8, 121. [Google Scholar] [CrossRef]
- Poornima, S.; Manikandan, S.; Prakash, R.; Deena, S.R.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Biofuel and biochemical production Through Biomass Transformation Using Advanced Thermochemical and Biochemical Processes—A Review. Fuel 2024, 372, 132204. [Google Scholar]
- Zhang, L.H.; Xu, C.B.; Champagne, P. Overview of Recent Advances in Thermo-Chemical Conversion of Biomass. Energy Conv. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Tanger, P.; Field, J.L.; Jahn, C.E.; DeFoort, M.W.; Leach, J.E. Biomass for Thermochemical Conversion: Targets and Challenges. Front. Plant Sci. 2013, 4, 218. [Google Scholar]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass Pyrolysis: Past, Present, and Future. Environ. Eng. Manag. J. 2020, 22, 17–32. [Google Scholar]
- Boateng, A.A.; Mullen, C.A. Fast pyrolysis of Biomass Thermally Pretreated by Torrefaction. J. Anal. Appl. Pyrolysis 2013, 100, 95–102. [Google Scholar]
- Chen, C.S.; Qu, B.; Wang, W.X.; Wang, W.J.; Ji, G.Z.; Li, A.M. Rice Husk and Rice Straw Torrefaction: Properties and Pyrolysis Kinetics of Raw and Torrefied Biomass. Environ. Technol. Innov. 2021, 24, 101872. [Google Scholar] [CrossRef]
- Li, X.R.; Cen, K.H.; Wang, L.C.; Jia, D.X.; Zhu, X.F.; Chen, D.Y. Co-pyrolysis of Cellulose and Lignin: Effects of Pyrolysis Temperature, Residence Time, and Lignin Percentage on the Properties of Biochar Using Response Surface Methodology. Ind. Crops Prod. 2024, 219, 119071. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Godina, R.; Matias, J.C.D.; Nunes, L.J.R. Future Perspectives of Biomass Torrefaction: Review of the Current State-Of-The-Art and Research Development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar]
- Hu, Y.M.; Wang, S.; Wang, Q.; He, Z.X.; Abomohra, A.; Cao, B. Influence of Torrefaction Pretreatment on the Pyrolysis Characteristics of Seaweed Biomass. Cellulose 2019, 26, 8475–8487. [Google Scholar] [CrossRef]
- Neupane, S.; Adhikari, S.; Wang, Z.; Ragauskas, A.J.; Pu, Y. Effect of Torrefaction on Biomass Structure and Hydrocarbon Production from Fast Pyrolysis. Green Chem. 2015, 17, 2406–2417. [Google Scholar] [CrossRef]
- Zheng, A.Q.; Zhao, Z.L.; Chang, S.; Huang, Z.; He, F.; Li, H.B. Effect of Torrefaction Temperature on Product Distribution from Two-Staged Pyrolysis of Biomass. Energy Fuels 2012, 26, 2968–2974. [Google Scholar] [CrossRef]
- Zheng, Y.W.; Tao, L.; Yang, X.Q.; Huang, Y.B.; Liu, C.; Gu, J.Y.; Zheng, Z.F. Effect of the Torrefaction Temperature on the Structural Properties and Pyrolysis Behavior of Biomass. Bioresources 2017, 12, 3425–3447. [Google Scholar] [CrossRef]
- Alvarez, A.; Nogueiro, D.; Pizarro, C.; Matos, M.; Bueno, J.L. Non-Oxidative Torrefaction of Biomass to Enhance its Fuel Properties. Energy 2018, 158, 1–8. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, B.L.; Wu, S.; Guo, W.; Zhang, J.; Wu, Z.Q.; Wang, Z.L.; Lim, J.C. Effect of Torrefaction Pretreatment on the fast Pyrolysis Behavior Of Biomass: Product Distribution and Kinetic Analysis on Spruce-Pin-Fir Sawdust. J. Anal. Appl. Pyrolysis 2021, 158, 105259. [Google Scholar]
- He, Z.; Zhang, F.; Tu, R.; Jia, Z.W.; Cheng, S.C.; Sun, Y.; Wu, Y.J.; Shen, X.W.; Jiang, E.C.; Xu, X.W. The Influence of Torrefaction on Pyrolysed Biomass: The Relationship of Bio-Oil Composition with the Torrefaction Severity. Bioresour. Technol. 2020, 314, 123780. [Google Scholar]
- Devaraja, U.M.A.; Dissanayake, C.L.W.; Gunarathne, D.; Chen, W.H. Oxidative Torrefaction and Torrefaction-Based Biorefining of Biomass: A Critical Review. Biofuel Res. J. 2022, 9, 1672–1696. [Google Scholar]
- Xin, S.Z.; Mi, T.; Liu, X.Y.; Huang, F. Effect of Torrefaction on the Pyrolysis Characteristics of High Moisture Herbaceous Residues. Energy 2018, 152, 586–593. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ho, S.H.; Chen, W.H.; Fu, Y.J.; Chang, J.S.; Bi, X.T. Oxidative Torrefaction of Biomass Nutshells: Evaluations of Energy Efficiency as Well as Biochar Transportation and Storage. Appl. Energy 2019, 235, 428–441. [Google Scholar]
- Gao, H.L.; Zhang, D.L.; Liu, Z.F.; Wang, F.; Su, X.H.; Liu, W.; Yi, W.M. Effect of Pretreatment on the Pyrolysis Kinetics of Corn Stalk: Comparison of Inert, Oxidative, and Wet Torrefaction. BioEnergy Res. 2025, 18, 12. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Guil-Pedrosa, J.F.; García-Hernando, N.; Ghoniem, A.F. Evolution of Solid Residue Composition During Inert and Oxidative Biomass Torrefaction. Energy 2024, 312, 133486. [Google Scholar] [CrossRef]
- Ramos-Carmona, S.; Martínez, J.D.; Pérez, J.F. Torrefaction of Patula Pine Under Air Conditions: A Chemical and Structural Characterization. Ind. Crops Prod. 2018, 118, 302–310. [Google Scholar] [CrossRef]
- Huang, S.X.; Lei, C.; Qin, J.; Yi, C.; Chen, T.; Yao, L.L.; Li, B.; Wen, Y.J.; Zhou, Z.; Xia, M. Properties, Kinetics and Pyrolysis Products Distribution of Oxidative Torrefied Camellia Shell in Different Oxygen Concentration. Energy 2022, 251, 123941. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhou, X.C.; Wang, L.; Tan, M.J.; Huang, Z.L.; Li, C.Z.; Wu, Z.J.; Qin, X.L.; Li, H. Effect of Oxidative Torrefaction on the Pyrolysis of Clitocybe Maxima Stipe: Pyrolysis Behaviour, and Products’ Properties. J. Anal. Appl. Pyrolysis 2024, 177, 106311. [Google Scholar] [CrossRef]
- Jiang, S.L.; Tan, M.J.; Huang, Z.L.; Hu, J.U.; Li, C.Z.; Lei, T.Z.; Zhang, X.; Wu, Z.J.; Huang, J.; Qin, X.L.; et al. Combining Oxidative Torrefaction and Pyrolysis of Phragmites australis: Improvement of the Adsorption Capacity of Biochar for Tetracycline. Front. Energy Res. 2021, 9, 673758. [Google Scholar] [CrossRef]
- Gao, X.F.; Tan, M.J.; Jiang, S.L.; Huang, Z.L.; Li, C.Z.; Lei, T.Z.; Wang, Z.W.; Zhu, Y.; Li, H. Pyrolysis of Torrefied Rice Straw from Gas-Pressurized and Oxidative Torrefaction: Pyrolysis Kinetic Analysis and the Properties Of Biochars. J. Anal. Appl. Pyrolysis 2021, 157, 105238. [Google Scholar] [CrossRef]
- Wang, S.R.; Dai, G.X.; Ru, B.; Zhao, Y.; Wang, X.L.; Xiao, G.; Luo, Z.Y. Influence of Torrefaction on the Characteristics and Pyrolysis Behavior of Cellulose. Energy 2017, 120, 864–871. [Google Scholar] [CrossRef]
- Chen, W.H.; Eng, C.F.; Lin, Y.Y.; Bach, Q.V.; Ashokkumar, V.; Show, P.L. Two-Step Thermodegradation Kinetics of Cellulose, Hemicelluloses, and Lignin Under Isothermal Torrefaction Analyzed by Particle Swarm Optimization. Energy Conv. Manag. 2021, 238, 114116. [Google Scholar] [CrossRef]
- Bartarajnai, E.; Czégény, Z.; Sebestyén, Z.; May, Z.; Bozi, J.; Jakab, E. Thermal Decomposition of Black Locust and Wheat Straw Under Torrefaction. In Proceedings of the 8th International Symposium on Feedstock Recycling of Polymeric Materials (8th ISFR 2015), Leoben, Austria, 7–10 September 2015. [Google Scholar]
- Zheng, A.Q.; Li, L.W.; Zhao, Z.; Tian, Y.Y.; Li, H.B. Effect of Torrefaction Pretreatment on Chemical Structure and Pyrolysis Behaviors of Cellulose. In Proceedings of the 5th International Conference on Renewable Energy and Environmental Protection (ICREEP), Shenzhen, China, 23–25 October 2020; p. 012014. [Google Scholar]
- Lv, P.; Almeida, G.; Perré, P. Torrefaction of cellulose: Validity and limitation of the temperature/duration equivalence. Bioresources 2012, 7, 3720–3731. [Google Scholar] [CrossRef]
- Li, D.Q.; Li, C.; Fan, M.J.; Shao, Y.W.; Sun, Y.F.; Zhang, L.J.; Zhang, S.; Huang, Y.; Li, B.; Wang, S.; et al. Investigation of Property of Biochar in Staged Pyrolysis of Cellulose. J. Anal. Appl. Pyrolysis 2023, 172, 105999. [Google Scholar] [CrossRef]
- Chen, J.Z.; Lu, Z.M.; Bao, Z.Y.; Cai, J.F.; Wei, Y.X.; Yao, S.C. Char Formation During Pyrolysis of Torrefied Cellulose: Role of Potassium Catalysis and Torrefaction Pretreatment. J. Anal. Appl. Pyrolysis 2024, 181, 106644. [Google Scholar]
- Bennadji, H.; Khachatryan, L.; Lomnicki, S.M. Kinetic Modeling of Cellulose Fractional Pyrolysis. Energy Fuels 2018, 32, 3436–3446. [Google Scholar]
- Kawamoto, H. Reactions and Molecular Mechanisms of Cellulose Pyrolysis. Mokuzai Gakkaishi 2015, 61, 1–24. [Google Scholar]
- Ni, X.Y.; Cen, K.H.; Li, X.R.; Cui, D.X.; Liu, M.; Zhu, L.Z.; Chen, D.Y. Cellulose Upgradation by Torrefaction Pretreatment and Effect of Torrefaction Severity on the Quality of Pyrolysis Products. Ind. Crops Prod. 2025, 225, 120516. [Google Scholar] [CrossRef]
- GB/T 28731-2012; Proximate Analysis of Solid Biofuels. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; Standardization Administration of the People’s Republic of China: Beijing, China, 2012.
- Chen, D.Y.; Cen, K.H.; Gan, Z.Y.; Zhuang, X.Z.; Ba, Y.P. Comparative Study of Electric-Heating Torrefaction and Solar-Driven Torrefaction of Biomass: Characterization of Property Variation and Energy Usage with Torrefaction Severity. Appl. Energ. Combust. Sci. 2021, 9, 100051. [Google Scholar]
- Chen, Y.C.; Chen, W.H.; Lin, B.J.; Chang, J.S.; Ong, H.C. Impact of Torrefaction on the Composition, Structure and Reactivity of a Microalga Residue. Appl. Energy 2016, 181, 110–119. [Google Scholar]
- Chen, D.Y.; Gao, A.J.; Cen, K.H.; Zhang, J.; Cao, X.B.; Ma, Z.Q. Investigation of Biomass Torrefaction based on Three Major Components: Hemicellulose, Cellulose, and Lignin. Energy Conv. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Chen, D.Y.; Cen, K.H.; Zhuang, X.Z.; Gan, Z.Y.; Zhou, J.B.; Zhang, Y.M.; Zhang, H. Insight into Biomass Pyrolysis Mechanism based on Cellulose, Hemicellulose, and Lignin: Evolution of Volatiles and Kinetics, Elucidation of Reaction Pathways, and Characterization of Gas, Biochar and Bio-Oil. Combust. Flame 2022, 242, 112142. [Google Scholar]
- Mei, Y.Y.; Yang, Q.; Yang, H.P.; Li, J.S.; Zeng, K.; Chen, Y.Q.; Zhang, S.H.; Chen, H.P. Impact of cellulose Deoxidization Temperature on the Composition of Liquid Products Obtained by Subsequent Pyrolysis. Fuel Process. Technol. 2019, 184, 73–79. [Google Scholar]
- Martínez, M.G.; Couce, A.A.; Dupont, C.; Perez, D.D.; Thiéry, S.; Meyer, X.M.; Gourdon, C. Torrefaction of cellulose, hemicelluloses and lignin extracted from woody and agricultural biomass in TGA-GC/MS: Linking Production Profiles of Volatile Species to Biomass Type and Macromolecular Composition. Ind. Crops Prod. 2022, 176, 114350. [Google Scholar] [CrossRef]
- Uemura, Y.; Saadon, S.; Osman, N.; Mansor, N.; Tanoue, K. Torrefaction of oil Palm Kernel Shell in the Presence of Oxygen and Carbon Dioxide. Fuel 2015, 144, 171–179. [Google Scholar]
- Wang, S.R.; Dai, G.X.; Ru, B.; Zhao, Y.; Wang, X.L.; Zhou, J.S.; Luo, Z.Y.; Cen, K.F. Effects of Torrefaction on Hemicellulose Structural Characteristics And Pyrolysis Behaviors. Bioresour. Technol. 2016, 218, 1106–1114. [Google Scholar]
- Chen, W.H.; Zhuang, Y.Q.; Liu, S.H.; Juang, T.T.; Tsai, C.M. Product Characteristics from the Torrefaction of Oil Palm Fiber Pellets in Inert and Oxidative Atmospheres. Bioresour. Technol. 2016, 199, 367–374. [Google Scholar]
- Ramos, R.T.; Salas, B.V.; Alpírez, G.M.; Ortega, M.A.C.; Alvarez, M.A.C.; Camacho, O.T.; Cabarcas, M.T.B. Torrefaction under Different Reaction Atmospheres to Improve the Fuel Properties of Wheat Straw. Processes 2023, 11, 1971. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and Assessment of Methods for Cellulose Crystallinity Determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- Cao, X.B.; Zhang, J.; Cen, K.H.; Chen, F.; Chen, D.Y.; Li, Y.J. Investigation of the Relevance Between Thermal Degradation Behavior and Physicochemical Property of Cellulose Under Different Torrefaction Severities. Biomass Bioenerg. 2021, 148, 106061. [Google Scholar]
- Su, Y.H.; Zhang, S.P.; Liu, L.Q.; Xu, D.; Xiong, Y.Q. Investigation of Representative Components of Flue Gas used as Torrefaction Pretreatment Atmosphere and Its Effects on Fast Pyrolysis Behaviors. Bioresour. Technol. 2018, 267, 584–590. [Google Scholar] [CrossRef]

| Sample | Ultimate Analysis (wt.%, db) | O/C | H/C | HHV (MJ/kg) | ||||
|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | ||||
| CE-Raw | 41.29 | 6.51 | 52.16 | 0.02 | 0.02 | 0.95 | 1.89 | 15.22 |
| CE-200-0 | 41.52 | 6.47 | 51.97 | 0.02 | 0.02 | 0.94 | 1.87 | 15.59 |
| CE-200-5 | 41.74 | 6.44 | 51.78 | 0.02 | 0.02 | 0.93 | 1.85 | 15.59 |
| CE-200-10 | 41.84 | 6.42 | 51.70 | 0.02 | 0.02 | 0.93 | 1.84 | 15.60 |
| CE-200-15 | 41.86 | 6.38 | 51.72 | 0.02 | 0.02 | 0.93 | 1.83 | 15.59 |
| CE-250-0 | 42.73 | 6.35 | 50.87 | 0.03 | 0.02 | 0.89 | 1.78 | 15.80 |
| CE-250-5 | 43.95 | 6.19 | 49.81 | 0.03 | 0.02 | 0.85 | 1.69 | 15.83 |
| CE-250-10 | 44.24 | 6.04 | 49.67 | 0.03 | 0.02 | 0.84 | 1.64 | 15.82 |
| CE-250-15 | 44.39 | 6.02 | 49.55 | 0.03 | 0.01 | 0.84 | 1.63 | 15.83 |
| CE-300-0 | 45.49 | 6.23 | 48.25 | 0.02 | 0.01 | 0.80 | 1.64 | 16.95 |
| CE-300-5 | 48.71 | 5.76 | 45.5 | 0.02 | 0.01 | 0.70 | 1.42 | 16.82 |
| CE-300-10 | 52.47 | 5.43 | 42.08 | 0.01 | 0.01 | 0.60 | 1.24 | 16.76 |
| CE-300-15 | 53.06 | 5.39 | 41.53 | 0.01 | 0.01 | 0.59 | 1.22 | 16.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Cen, K.; Ni, X.; Liu, M.; Chen, D. Potential of Cellulose After Oxidative Torrefaction for Fuel Enhancement and Utilization: Properties and Pyrolysis Characteristics. Coatings 2025, 15, 407. https://doi.org/10.3390/coatings15040407
Zhu L, Cen K, Ni X, Liu M, Chen D. Potential of Cellulose After Oxidative Torrefaction for Fuel Enhancement and Utilization: Properties and Pyrolysis Characteristics. Coatings. 2025; 15(4):407. https://doi.org/10.3390/coatings15040407
Chicago/Turabian StyleZhu, Lingzhi, Kehui Cen, Xiayu Ni, Mian Liu, and Dengyu Chen. 2025. "Potential of Cellulose After Oxidative Torrefaction for Fuel Enhancement and Utilization: Properties and Pyrolysis Characteristics" Coatings 15, no. 4: 407. https://doi.org/10.3390/coatings15040407
APA StyleZhu, L., Cen, K., Ni, X., Liu, M., & Chen, D. (2025). Potential of Cellulose After Oxidative Torrefaction for Fuel Enhancement and Utilization: Properties and Pyrolysis Characteristics. Coatings, 15(4), 407. https://doi.org/10.3390/coatings15040407






