Fabrication of PVTF/COL Composite Films and Its Impact on Osteogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PVTF/COL Composite Film
2.3. Characterization of Materials
2.4. Cell Culture
2.5. Cell Vitality Assays
2.6. Alkaline Phosphatase (ALP) Assay
2.7. Statistical Analysis
3. Results and Discussion
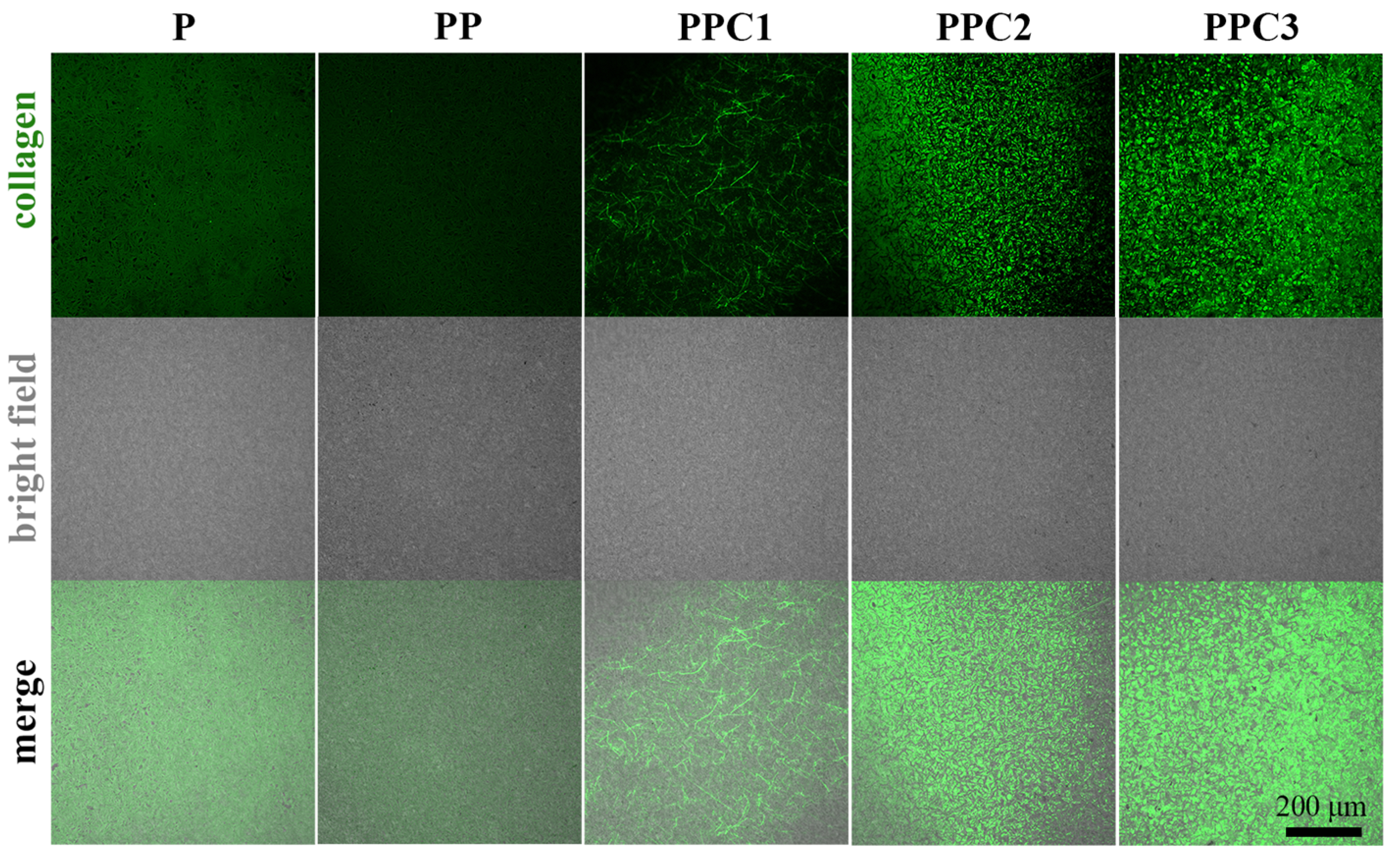
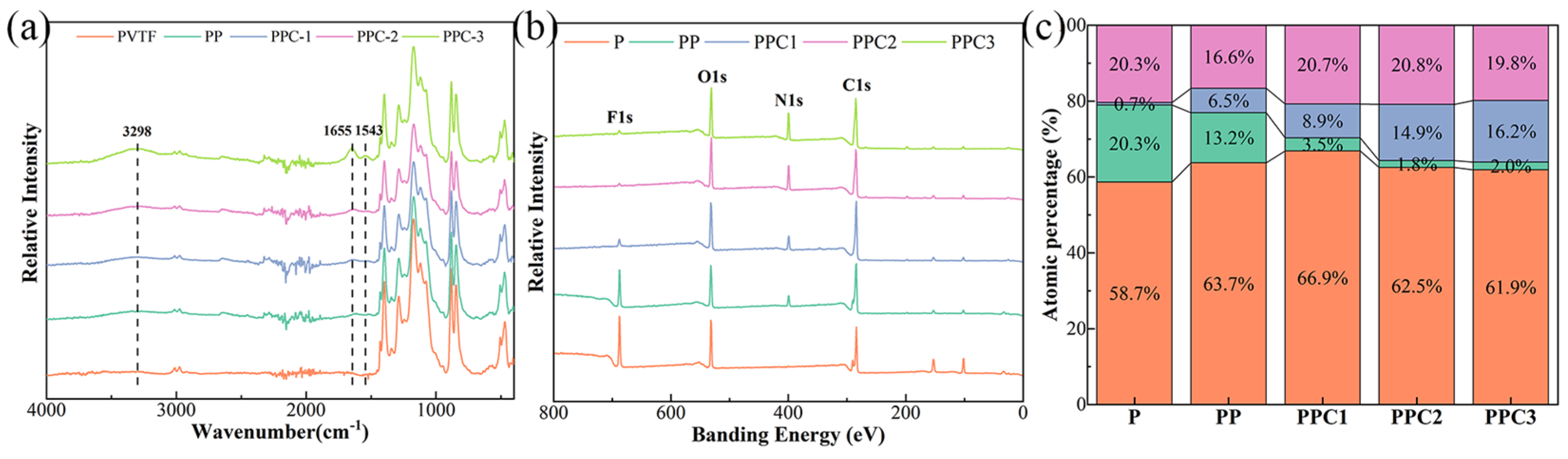
3.1. The Morphology and Composition of PVTF/COL Composite Film
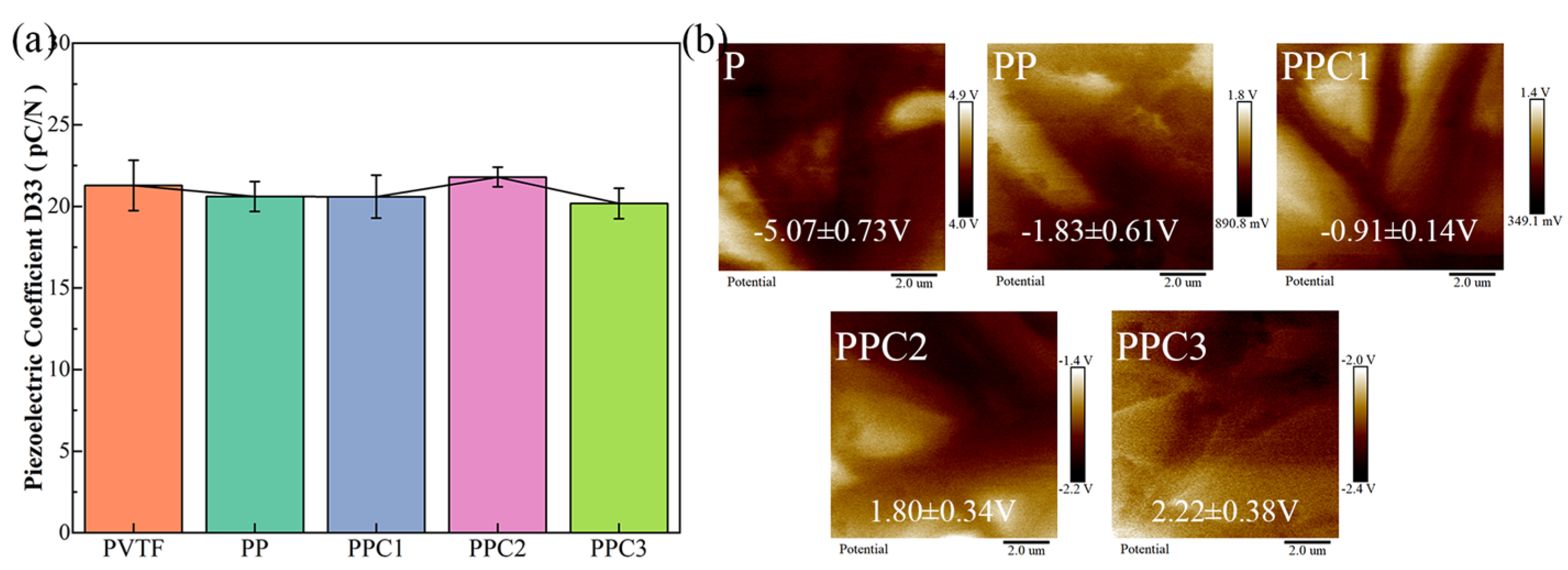
3.2. The Surface Potential and Hydrophilicity of PVTF/COL Composite Film
3.3. The Tensile Strength and Puncture Strength of PVTF/COL Composite Film
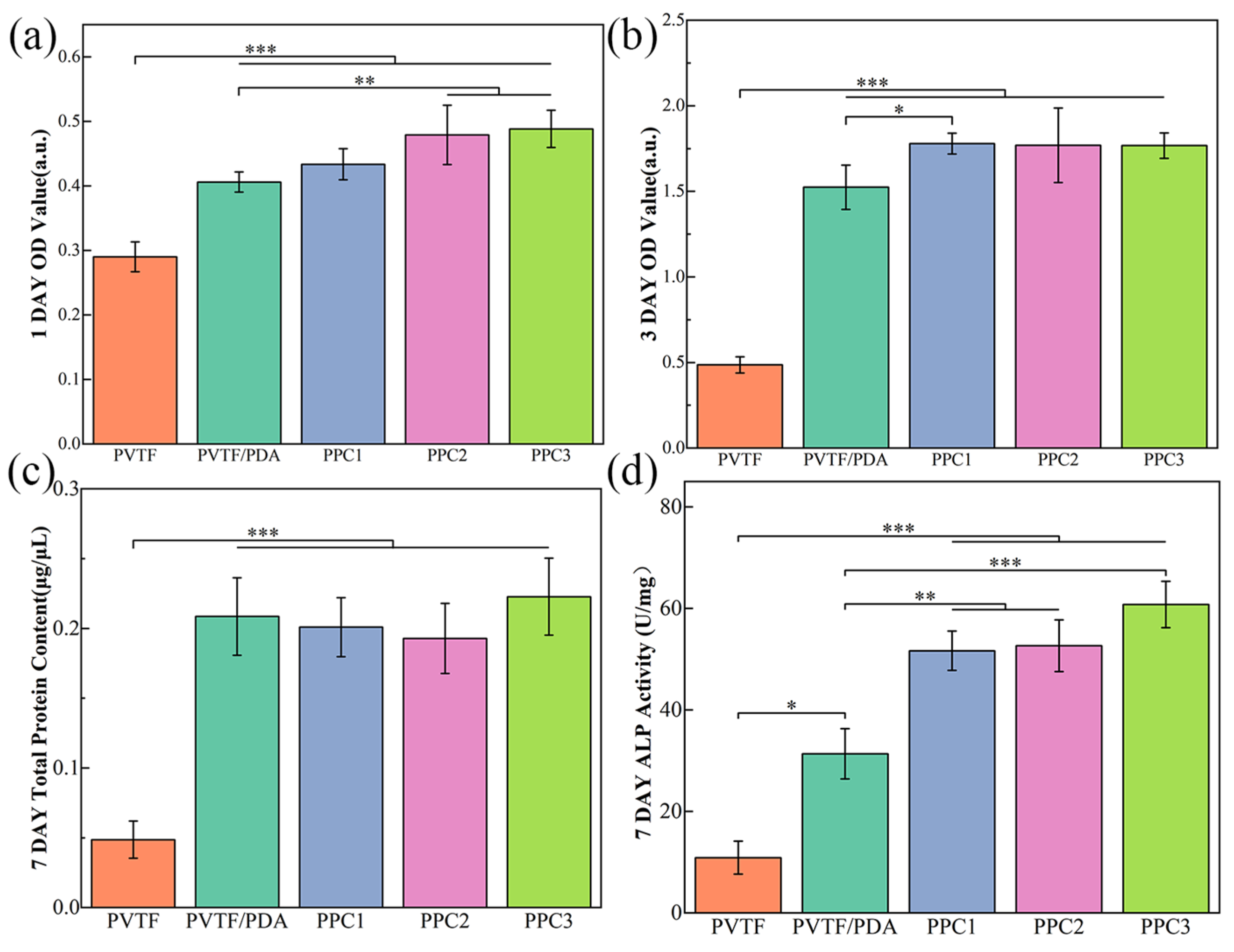
3.4. The Biocompatibility and Mechanical Properties of PVTF
4. Conclusions
- (1)
- Polydopamine effectively facilitated the composite of collagen on the surface of PVTF films, with varying composite amounts achieved by adjusting the concentration of the collagen solution. Although the surface morphology of the composite film showed no significant changes, its hydrophilicity was notably improved, with the water contact angle decreasing from 98.9° for pure PVTF to 33.2° for PPC3;
- (2)
- The prepared composite film exhibited good mechanical strength, with a tensile strength of 24.94 MPa, 1.9 times higher than that of the Jason collagen membrane. And the puncture strength of composite film was 0.54 N/mm2, which falls between the values of commercial collagen membranes and titanium. The surface potential of the film gradually shifted toward the positive potential direction as collagen recombined, achieving a Kelvin potential of 2.22 V on the PPC3 surface;
- (3)
- The prepared composite film demonstrates good biocompatibility and bone-promoting effects, with the PPC3 sample showing the best overall performance. Compared to the PVTF sample, the adhesion of cells to the PPC3 sample was 1.7 times higher after 1 day, cell proliferation was 3.6 times higher after 3 days, and ALP activity was 5.6 times higher after 7 days.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Deas, D.E.; Moritz, A.J.; Sagun, R.S., Jr.; Gruwell, S.F.; Powell, C.A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol. 2000 2016, 71, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontol. 2000 2017, 75, 152–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Su, Y.; Kucine, A.J.; Cheng, K.; Zhu, D. Guided Bone Regeneration Using Barrier Membrane in Dental Applications. ACS Biomater. Sci. Eng. 2023, 9, 5457–5478. [Google Scholar] [CrossRef]
- Donos, N.; Mardas, N.; Chadha, V. Clinical outcomes of implants following lateral bone augmentation: Systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J. Clin. Periodontol. 2008, 35, 173–202. [Google Scholar] [CrossRef]
- Chiapasco, M.; Zaniboni, M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. 4), 113–123. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Halbritter, S.; Harnisch, H.; Weber, H.P.; Buser, D. A retrospective analysis of patients referred for implant placement to a specialty clinic: Indications, surgical procedures, and early failures. Int. J. Oral Maxillofac. Implant. 2008, 23, 1109–1116. [Google Scholar]
- Alqahtani, A.M. Guided Tissue and Bone Regeneration Membranes: A Review of Biomaterials and Techniques for Periodontal Treatments. Polymers 2023, 15, 3355. [Google Scholar] [CrossRef]
- Lei, M.; Wan, H.; Song, J.; Lu, Y.; Chang, R.; Wang, H.; Zhou, H.; Zhang, X.; Liu, C.; Qu, X. Programmable Electro-Assembly of Collagen: Constructing Porous Janus Films with Customized Dual Signals for Immunomodulation and Tissue Regeneration in Periodontitis Treatment. Adv. Sci. 2024, 11, 2305756. [Google Scholar] [CrossRef]
- Yan, F.; Yu, M.; He, Y.; Wang, F.; Yang, F.; Zhao, X.; Zheng, Y.; Liu, Y.; Xia, D.; Liu, Y. Hierarchical Mineralized Collagen Coated Zn Membrane to Tailor Cell Microenvironment for Guided Bone Regeneration. Adv. Funct. Mater. 2025, 35, 2412695. [Google Scholar] [CrossRef]
- Zeller, A.-N.; Schenk, R.; Bonsmann, M.; Stockbrink, G.; Becher, S.; Pabst, A. Complication rates of guided bone regeneration using titanium-reinforced PTFE membranes: A retrospective analysis. Clin. Oral Investig. 2024, 28, 616. [Google Scholar] [CrossRef]
- Kohutiar, M.; Kakošová, L.; Krbata, M.; Janík, R.; Fekiač, J.J.; Breznická, A.; Eckert, M.; Mikuš, P.; Timárová, Ľ. Comprehensive Review: Technological Approaches, Properties, and Applications of Pure and Reinforced Polyamide 6 (PA6) and Polyamide 12 (PA12) Composite Materials. Polymers 2025, 17, 442. [Google Scholar] [CrossRef]
- Durán-Rey, D.; Brito-Pereira, R.; Ribeiro, C.; Ribeiro, S.; Sánchez-Margallo, J.A.; Crisóstomo, V.; Irastorza, I.; Silván, U.; Lanceros-Méndez, S.; Sánchez-Margallo, F.M. Development and evaluation of different electroactive poly(vinylidene fluoride) architectures for endothelial cell culture. Front. Bioeng. Biotechnol. 2022, 10, 1044667. [Google Scholar] [CrossRef]
- Purushothaman, S.M.; Tronco, M.F.; Kottathodi, B.; Royaud, I.; Ponçot, M.; Kalarikkal, N.; Thomas, S.; Rouxel, D. A review on electrospun PVDF-based nanocomposites: Recent trends and developments in energy harvesting and sensing applications. Polymer 2023, 283, 126179. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly(vinylidene fluoride) (PVDF). Adv. Compos. Hybrid. Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Jolly, B.; Ullah, W.; Saxena, N.; Sharma, R.; Janyani, V. Structural and microstructural analysis of spin-coated PVDF thin films. Ferroelectrics 2021, 583, 151–161. [Google Scholar] [CrossRef]
- Mohammadpourfazeli, S.; Arash, S.; Ansari, A.; Yang, S.; Mallick, K.; Bagherzadeh, R. Future prospects and recent developments of polyvinylidene fluoride (PVDF) piezoelectric polymer; fabrication methods, structure, and electro-mechanical properties. RSC Adv. 2023, 13, 370–387. [Google Scholar] [CrossRef]
- Zhang, J.; He, X.; Zhou, Z.; Chen, X.; Shao, J.; Huang, D.; Dong, L.; Lin, J.; Wang, H.; Weng, W.; et al. The osteogenic response to chirality-patterned surface potential distribution of CFO/P(VDF-TrFE) membranes. Biomater. Sci. 2022, 10, 4576–4587. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, Z.; Chen, Z.; Xu, K.; Wu, C.; Duan, X.; Dong, L.; Chen, Z.; Weng, W.; Cheng, K. Accelerated Bone Regeneration on the Metal Surface through Controllable Surface Potential. ACS Appl. Mater. Interfaces 2023, 15, 46493–46503. [Google Scholar] [CrossRef]
- Heng, B.C.; Bai, Y.; Li, X.; Lim, L.W.; Li, W.; Ge, Z.; Zhang, X.; Deng, X. Electroactive Biomaterials for Facilitating Bone Defect Repair under Pathological Conditions. Adv. Sci. 2023, 10, 2204502. [Google Scholar] [CrossRef]
- Liu, Z.; Wan, X.; Wang, Z.L.; Li, L. Electroactive Biomaterials and Systems for Cell Fate Determination and Tissue Regeneration: Design and Applications. Adv. Mater. 2021, 33, 2007429. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Lee, P.S.; Hess, R.; Haenchen, V.; Basu, B.; Scharnweber, D. Interplay of Substrate Conductivity, Cellular Microenvironment, and Pulsatile Electrical Stimulation toward Osteogenesis of Human Mesenchymal Stem Cells in vitro. ACS Appl. Mater. Interfaces 2015, 7, 23015–23028. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Taira, T.; Noishiki, Y. Collagen engineering for biomaterial use. Clin. Mater. 1992, 9, 139–148. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Ding, H.; Li, P.; Qiao, H.; Liang, E.; Jia, Y.; Guan, S. Interaction Mechanism of RGD Tripeptide on Different Surfaces of Mg and Mg Alloys: A First-Principles Study. Coatings 2022, 12, 1814. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, B.; Zhuang, J.; Wang, Q.; Dong, L.; Cheng, K.; Weng, W. Surface potential-governed cellular osteogenic differentiation on ferroelectric polyvinylidene fluoride trifluoroethylene films. Acta Biomater. 2018, 74, 291–301. [Google Scholar] [CrossRef]
- Wang, Z.; He, X.; Tang, B.; Chen, X.; Dong, L.; Cheng, K.; Weng, W. Polarization behavior of bone marrow-derived macrophages on charged P(VDF-TrFE) coatings. Biomater. Sci. 2021, 9, 874–881. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, J.; He, G.; Dang, D.; Xie, Y.; Wang, Q. Fluorous effect-induced emission of azido substituted poly(vinylidene fluoride) with high photostability and film formation. Polym. Chem. 2020, 11, 1307–1313. [Google Scholar] [CrossRef]
- Gholami, S.; Llacuna, J.L.; Vatanpour, V.; Dehqan, A.; Paziresh, S.; Cortina, J.L. Impact of a new functionalization of multiwalled carbon nanotubes on antifouling and permeability of PVDF nanocomposite membranes for dye wastewater treatment. Chemosphere 2022, 294, 133699. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Cheng, B.; Du, X.; Su, J. Preparation of Collagen/Aspartic Acid Nanocomposite Fibers and Their Self-Assembly Behaviors. J. Nat. Fibers 2022, 19, 8830–8841. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.a.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Ho, C.-C.; Ding, S.-J. The pH-controlled nanoparticles size of polydopamine for anti-cancer drug delivery. J. Mater. Sci. Mater. Med. 2013, 24, 2381–2390. [Google Scholar] [CrossRef]
- Tejido-Rastrilla, R.; Ferraris, S.; Goldmann, W.H.; Grünewald, A.; Detsch, R.; Baldi, G.; Spriano, S.; Boccaccini, A.R. Studies on Cell Compatibility, Antibacterial Behavior, and Zeta Potential of Ag-Containing Polydopamine-Coated Bioactive Glass-Ceramic. Materials 2019, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lyu, H.; Zhang, X.; Xiao, Y.; Li, A.; Ma, Z.; Guo, C.; Pei, Y. Revealing the aggregation behaviors of mesostructured collagen by the evaluation of reconstituted collagen performance. Food Hydrocoll. 2022, 130, 107700. [Google Scholar] [CrossRef]
- Gonapinuwala, S.T.; Jones, J.R.; Kirk, S.; De Croos, M.; Bronlund, J.E. Collagen Fibrils with Native D-periodicity from Fish Skin: A Simplified Fibrillogenesis Method. Food Bioprocess. Technol. 2024, 18, 3834–3847. [Google Scholar] [CrossRef]
- De Oliveira, S.; Miklosic, G.; Veziers, J.; Grastilleur, S.; Coradin, T.; Le Visage, C.; Guicheux, J.; D’este, M.; Hélary, C. Optimizing the physical properties of collagen/hyaluronan hydrogels by inhibition of polyionic complexes formation at pH close to the collagen isoelectric point. Soft Matter 2023, 19, 9027–9035. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xue, F.; Ding, E. Preparation of polyacrylamide grafted collagen extracted from leather wastes and their application in kaolin flocculation. J. Appl. Polym. Sci. 2015, 132, 41556. [Google Scholar] [CrossRef]
- Li, Y.; Asadi, A.; Monroe, M.R.; Douglas, E.P. pH effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Mater. Sci. Eng. C 2009, 29, 1643–1649. [Google Scholar] [CrossRef]
- Ten Brink, T.; Damanik, F.; Rotmans, J.I.; Moroni, L. Unraveling and Harnessing the Immune Response at the Cell–Biomaterial Interface for Tissue Engineering Purposes. Adv. Healthc. Mater. 2024, 13, 2301939. [Google Scholar] [CrossRef]
- Parisi, L.; Ghezzi, B.; Bianchi, M.G.; Toffoli, A.; Rossi, F.; Bussolati, O.; Macaluso, G.M. Titanium dental implants hydrophilicity promotes preferential serum fibronectin over albumin competitive adsorption modulating early cell response. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111307. [Google Scholar] [CrossRef]
- Zhang, K.R.; Gao, H.L.; Pan, X.F.; Zhou, P.; Xing, X.; Xu, R.; Pan, Z.; Wang, S.; Zhu, Y.M.; Hu, B.; et al. Multifunctional Bilayer Nanocomposite Guided Bone Regeneration Membrane. Matter 2019, 1, 770–781. [Google Scholar] [CrossRef]
- Chu, X.; Dai, Y.L.; Fu, Z.D.; Wang, J.; Song, J.M.; Dong, Z.B.; Tang, Z.J.; Yan, Y.; Yu, K. Mechanical properties, biodegradation behavior and biocompatibility of novel Zn-based alloy membranes prepared by powder metallurgy for guided bone regeneration. Mater. Today Commun. 2025, 42, 111367. [Google Scholar] [CrossRef]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical qualification of collagen membranes used in dentistry. Ann. Ist. Super. Sanita 2015, 51, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, F.; Behrens, E.; Kern, M.; Gassling, V.; Wiltfang, J. Mechanical properties of collagen membranes: Are they sufficient for orbital floor reconstructions? J. Craniomaxillofac. Surg. 2015, 43, 260–263. [Google Scholar] [CrossRef]
- Fekiač, J.J.; Krbata, M.; Kohutiar, M.; Janík, R.; Kakošová, L.; Breznická, A.; Eckert, M.; Mikuš, P. Comprehensive Review: Optimization of Epoxy Composites, Mechanical Properties, & Technological Trends. Polymers 2025, 17, 271. [Google Scholar] [CrossRef]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the cell–material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar] [CrossRef]
- Li, Z.; Du, T.; Ruan, C.; Niu, X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 2021, 6, 1491–1511. [Google Scholar] [CrossRef]



| Sample | Biodegradability | Mechanical Properties | Bioactivity | Expected Therapeutic Effect |
|---|---|---|---|---|
| Collagen | + | − | ++ | ++ |
| PVTF | − | + | + | + |
| PVTF/COL | − | + | ++ | ++(+) |
| PTFE | − | + | − | + |
| Titanium | − | ++ | − | + |
| Sample | Water Contact Angle (Degree) |
|---|---|
| PVTF | 98.9 ± 3.2 |
| PP | 59.8 ± 5.8 |
| PPC1 | 49.6 ± 8.2 |
| PPC2 | 45.6 ± 8.3 |
| PPC3 | 33.2 ± 8.9 |
| Sample | Tensile Strength (MPa) | Puncture Strength (N/mm2) | Surface Potential (V) | 1D Cell Viability (a.u.) | 3D Cell Viability (a.u.) | 7D ALP Activity (U/mg) |
|---|---|---|---|---|---|---|
| PVTF | - | - | −5.07 ± 0.73 | 0.29 ± 0.02 | 0.49 ± 0.05 | 10.89 ± 3.24 |
| PP | - | - | −1.83 ± 0.61 | 0.41 ± 0.02 | 1.52 ± 0.13 | 31.36 ± 4.95 |
| PPC1 | - | - | −0.91 ± 0.14 | 0.43 ± 0.02 | 1.78 ± 0.06 | 51.67 ± 3.86 |
| PPC2 | - | - | 1.80 ± 0.34 | 0.48 ± 0.05 | 1.77 ± 0.22 | 52.66 ± 5.12 |
| PPC3 | 24.94 ± 0.95 | 0.54 ± 0.02 | 2.22 ± 0.38 | 0.49 ± 0.03 | 1.77 ± 0.07 | 60.80 ± 4.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wu, C.; Lin, W.; Chen, X.; Weng, W.; Yu, X.; Cheng, K. Fabrication of PVTF/COL Composite Films and Its Impact on Osteogenic Differentiation. Coatings 2025, 15, 416. https://doi.org/10.3390/coatings15040416
Liu H, Wu C, Lin W, Chen X, Weng W, Yu X, Cheng K. Fabrication of PVTF/COL Composite Films and Its Impact on Osteogenic Differentiation. Coatings. 2025; 15(4):416. https://doi.org/10.3390/coatings15040416
Chicago/Turabian StyleLiu, Haoqing, Chengwei Wu, Weimin Lin, Xiaoyi Chen, Wenjian Weng, Xingyan Yu, and Kui Cheng. 2025. "Fabrication of PVTF/COL Composite Films and Its Impact on Osteogenic Differentiation" Coatings 15, no. 4: 416. https://doi.org/10.3390/coatings15040416
APA StyleLiu, H., Wu, C., Lin, W., Chen, X., Weng, W., Yu, X., & Cheng, K. (2025). Fabrication of PVTF/COL Composite Films and Its Impact on Osteogenic Differentiation. Coatings, 15(4), 416. https://doi.org/10.3390/coatings15040416








