Influence of Different Chemical Methods Used for the Deposition of CdSe/ZnO Layers
Abstract
:1. Introduction
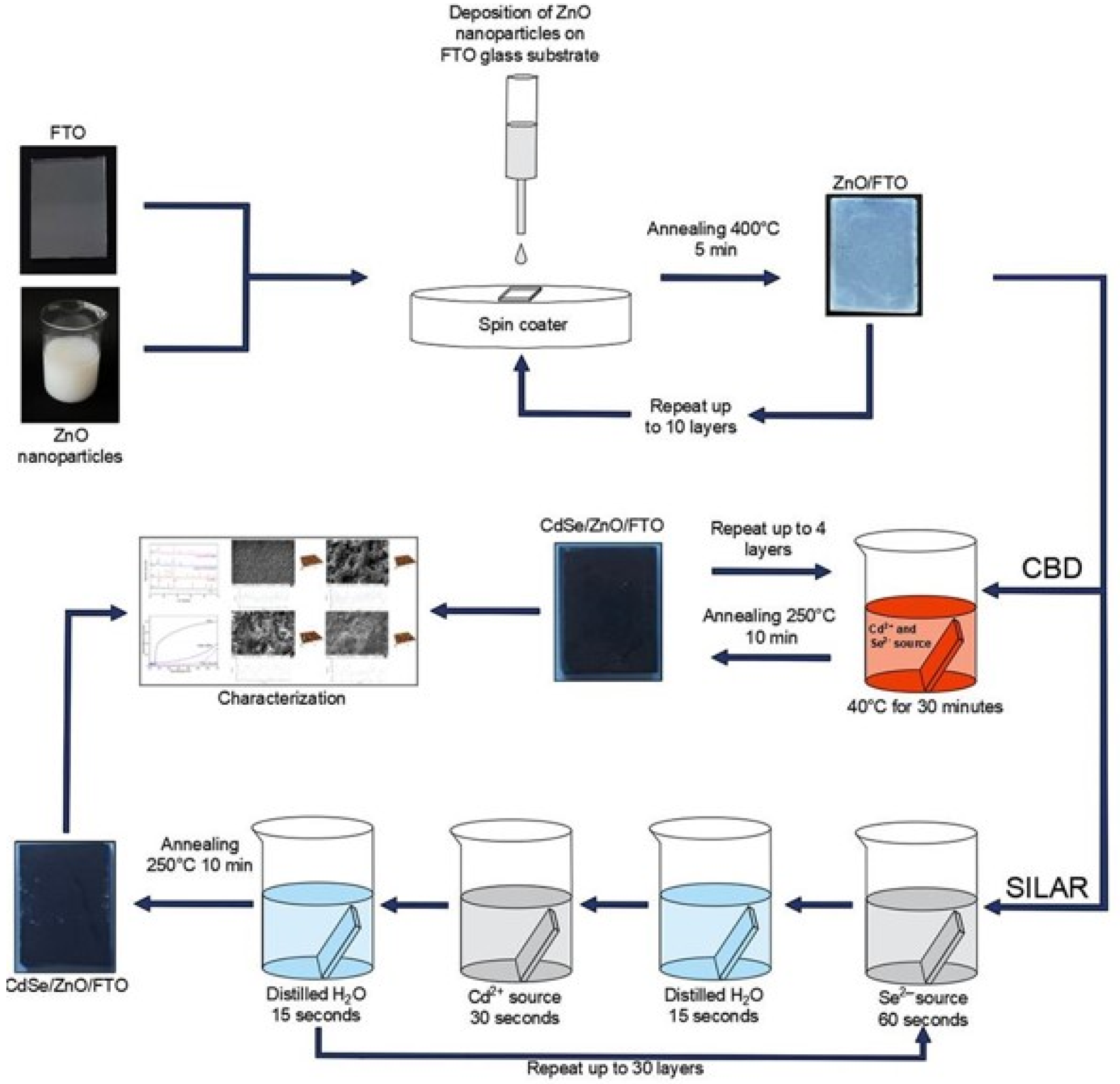
2. Materials and Methods
2.1. Materials
2.2. Formation of ZnO Layers on FTO Substrates
2.3. Formation of CdSe/ZnO Thin Films on FTO Substrates Using CBD and SILAR
2.4. Characterization of CdSe/ZnO Structure
3. Results and Discussion
3.1. Structural Studies
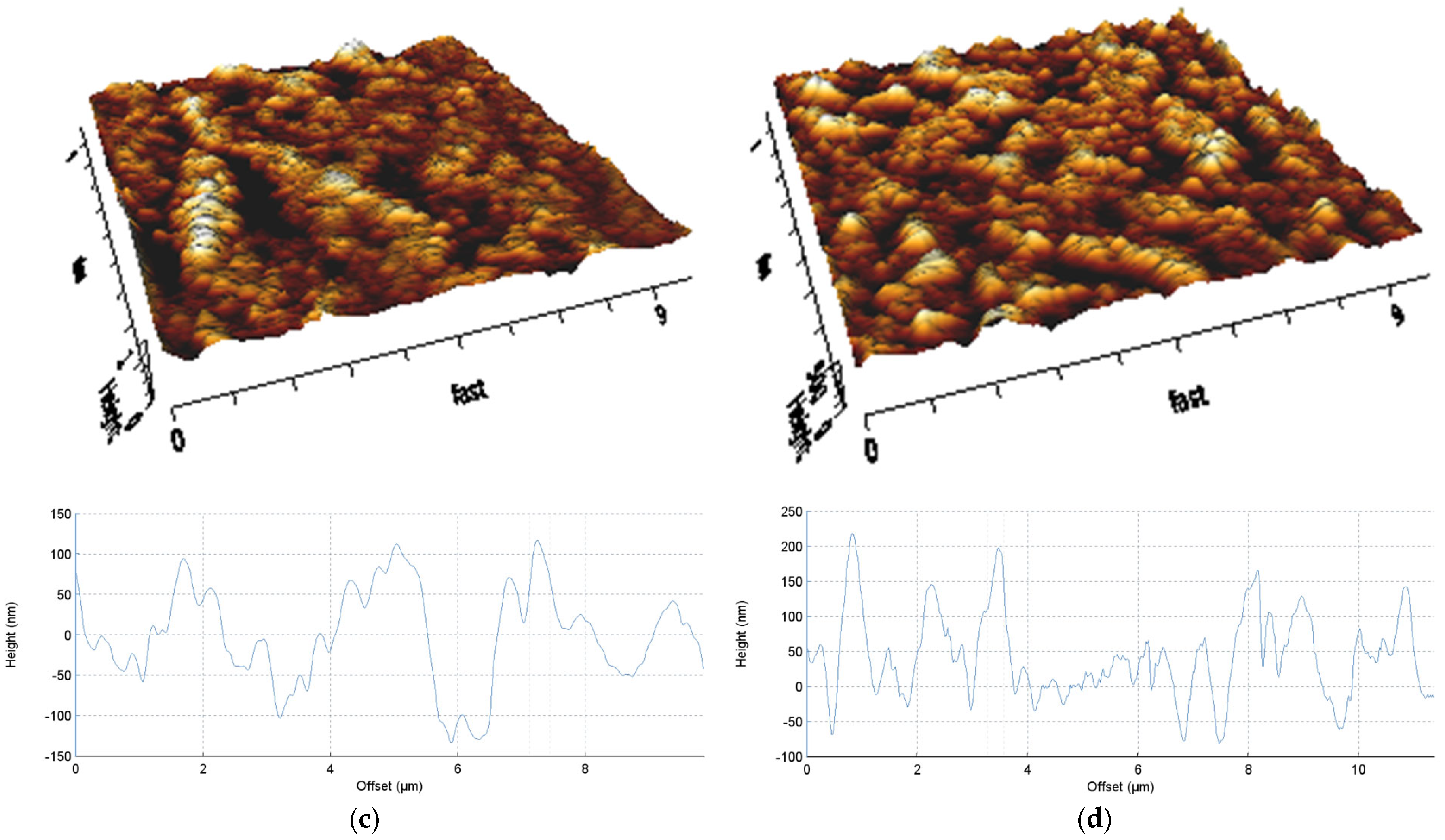
3.2. Morphological Analysis
3.3. Energy Dispersive X-Ray Analysis Spectroscopy
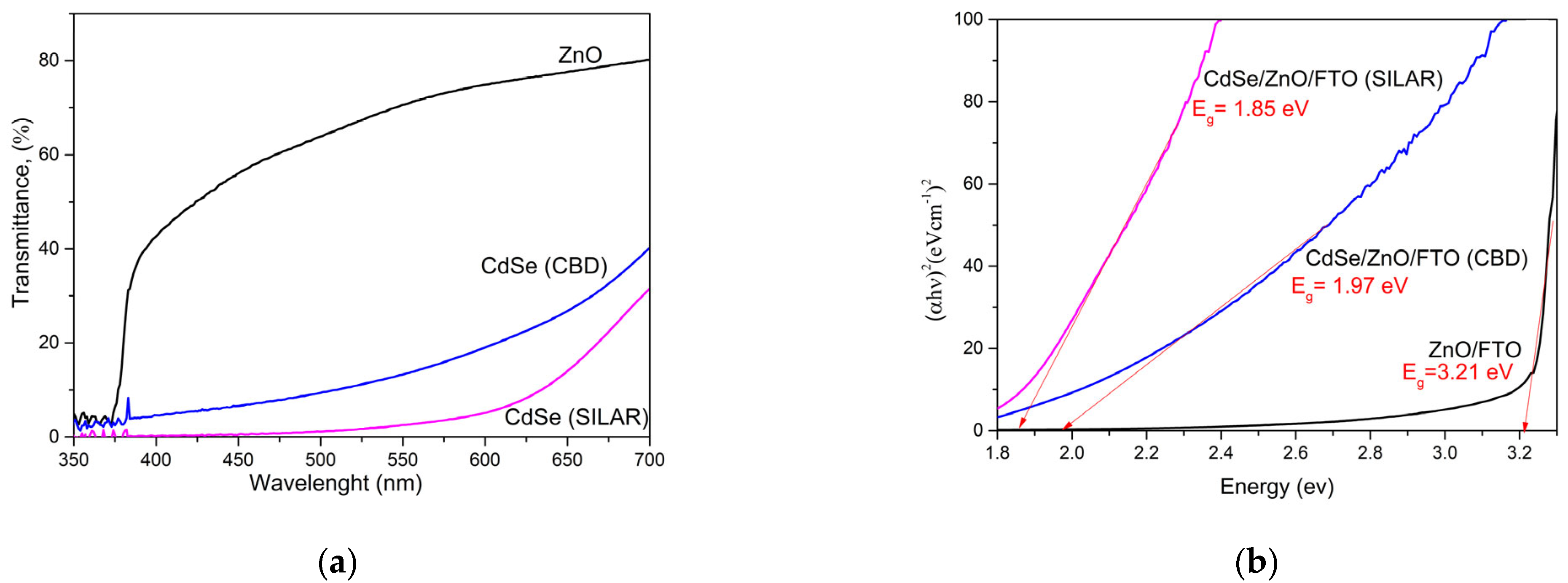
3.4. Optical Analysis
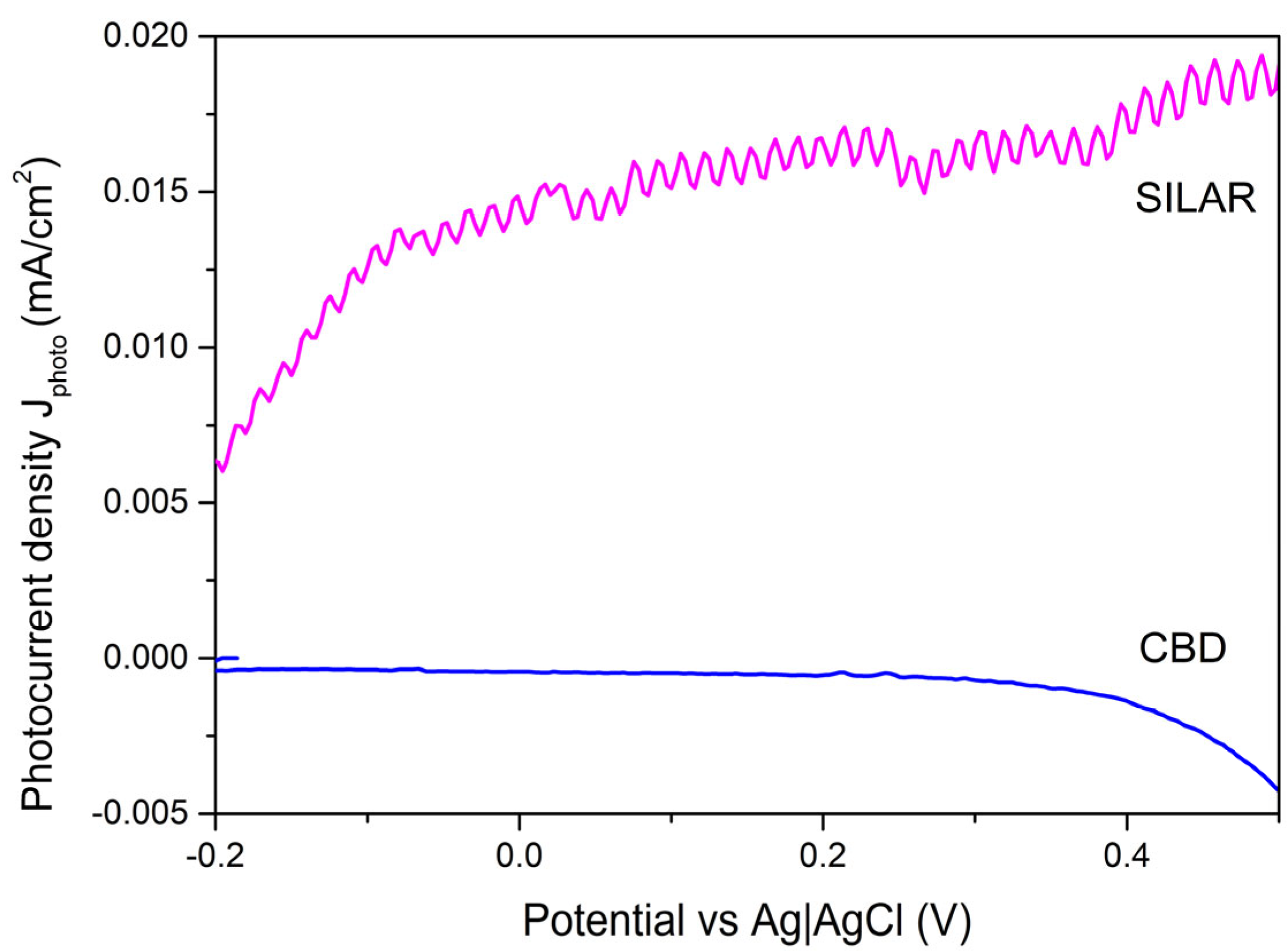
3.5. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oloruntoba, S.O.; Zainal, Z.; Sulaiman, Y.; Zawawi, R.M.; Tan, H.J. Annealing Temperature Effect on the Characteristics of CdSe/ZnO Nanorod Heterostructures Fabricated by SILAR-Technique for Improved Photoelectrochemical Cell Performance. Opt. Mater. 2025, 160, 116636. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical Devices for Solar Water Splitting—Materials and Challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, Z.; Mao, X.; Dong, Y.; Peng, L.; Sun-Waterhouse, D.; Kennedy, J.V.; Waterhouse, G.I.N.; Liu, J.; Luo, Z.; et al. Recent Advances in Self-Supported Semiconductor Heterojunction Nanoarrays as Efficient Photoanodes for Photoelectrochemical Water Splitting. Small 2022, 18, 2204553. [Google Scholar] [CrossRef]
- Baviskar, P.K.; Dubal, D.P.; Majumder, S.; Ennaoui, A.; Sankapal, B.R. “Basic Idea, Advance Approach”: Efficiency Boost by Sensitization of Blended Dye on Chemically Deposited ZnO Films. J. Photochem. Photobiol. A Chem. 2016, 318, 135–141. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Lei, W.; Song, J.L.; Deng, W.Q.; Sun, X.W. Co-Sensitized Quantum Dot Solar Cell Based on ZnO Nanowire. Appl. Surf. Sci. 2010, 256, 7438–7441. [Google Scholar] [CrossRef]
- Qiu, X.; Que, W.; Yin, X.; Zhang, J.; Chen, J. ZnO/CdS/CdSe Core/Double Shell Nanorod Arrays Derived by a Successive Ionic Layer Adsorption and Reaction Process for Quantum Dot-Sensitized Solar Cells. Semicond. Sci. Technol. 2011, 26, 095028. [Google Scholar] [CrossRef]
- Nikam, P.R.; Baviskar, P.K.; Majumder, S.; Sali, J.V.; Sankapal, B.R. SILAR Controlled CdSe Nanoparticles Sensitized ZnO Nanorods Photoanode for Solar Cell Application: Electrolyte Effect. J. Colloid Interface Sci. 2018, 524, 148–155. [Google Scholar] [CrossRef]
- Güell, F.; Galdámez-Martínez, A.; Martínez-Alanis, P.R.; Catto, A.C.; da Silva, L.F.; Mastelaro, V.R.; Santana, G.; Dutt, A. ZnO-Based Nanomaterials Approach for Photocatalytic and Sensing Applications: Recent Progress and Trends. Mater. Adv. 2023, 4, 3685–3707. [Google Scholar] [CrossRef]
- Li, K.; Lin, X.; Song, B.; Kondrotas, R.; Wang, C.; Lu, Y.; Yang, X.; Chen, C.; Tang, J. Rapid Thermal Evaporation for Cadmium Selenide Thin-Film Solar Cells. Front. Optoelectron. 2021, 14, 482–490. [Google Scholar] [CrossRef]
- Mahato, S.; Shakti, N.; Kar, A.K. Annealing Temperature Dependent Structural and Optical Properties of Electrodeposited CdSe Thin Films. Mater. Sci. Semicond. Process. 2015, 39, 742–747. [Google Scholar] [CrossRef]
- Bera, S.; Singh, S.B.; Ray, S.K. Green Route Synthesis of High Quality CdSe Quantum Dots for Applications in Light Emitting Devices. J. Solid State Chem. 2012, 189, 75–79. [Google Scholar] [CrossRef]
- Mahato, S.; Kar, A.K. The Effect of Annealing on Structural, Optical and Photosensitive Properties of Electrodeposited Cadmium Selenide Thin Films. J. Sci. Adv. Mater. Devices 2017, 2, 165–171. [Google Scholar] [CrossRef]
- Inbanathan, F.P.N.; Kumar, P.; Dasari, K.; Katiyar, R.S.; Chen, J.; Jadwisienczak, W.M. Ellipsometry Study of CdSe Thin Films Deposited by PLD on ITO Coated Glass Substrates. Materials 2021, 14, 3307. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, D.; Kc, D.; Khattri, K.B.; Adhikari, D.R.; Gaib, R.A.; Shah, D.K. Numerical Assessment of Optoelectrical Properties of ZnSe–CdSe Solar Cell-Based with ZnO Antireflection Coating Layer. Sci. Rep. 2023, 13, 12193. [Google Scholar] [CrossRef]
- Xie, Y.; Madel, M.; Li, Y.; Jie, W.; Neuschl, B.; Feneberg, M.; Thonke, K. Polarity-Controlled Ultraviolet/Visible Light ZnO Nanorods/p-Si Photodetector. J. Appl. Phys. 2012, 112, 123111. [Google Scholar] [CrossRef]
- Zhu, H.; Shan, C.X.; Yao, B.; Li, B.H.; Zhang, J.Y.; Zhao, D.X.; Shen, D.Z.; Fan, X.W. High Spectrum Selectivity Ultraviolet Photodetector Fabricated from an N-ZnO/p-GaN Heterojunction. J. Phys. Chem. C 2008, 112, 20546–20548. [Google Scholar] [CrossRef]
- Leschkies, K.S.; Divakar, R.; Basu, J.; Enache-Pommer, E.; Boercker, J.E.; Carter, C.B.; Kortshagen, U.R.; Norris, D.J.; Aydil, E.S. Photosensitization of ZnO Nanowires with CdSe Quantum Dots for Photovoltaic Devices. Nano Lett. 2007, 7, 1793–1798. [Google Scholar] [CrossRef]
- Haque, A.; Zechel, F.; Vretenár, V.; Roy, M.; Sýkora, M. Type-II CdSe/ZnO Core/Shell Nanorods: Nanoheterostructures with A Tunable Dual Emission in Visible and Near-Infrared Spectral Ranges. Adv. Funct. Mater. 2024, 34, 2305296. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, S.N.; Kumar, M. Synthesis and Properties of CdSe Quantum Dot Sensitized ZnO Nanocomposites. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 44, 555–564. [Google Scholar] [CrossRef]
- Ramírez-Amador, R.; Flores-Carrasco, G.; Alcántara-Iniesta, S.; Rodríguez González, J.; García-Teniza, O.; Mercado-Agular, E.; Vásquez-Ortiz, A.B. Structural, Morphological, Optical, and Electrical Characterization of Fluorine Doped Tin Oxide (FTO) Thin Films Synthesized by PSP. Solid State Phenom. 2019, 286, 64–71. [Google Scholar]
- Sudhagar, P.; Chandramohan, S.; Kumar, R.S.; Sathyamoorthy, R.; Hong, C.H.; Kang, Y.S. Fabrication and Charge-Transfer Characteristics of CdS QDs Sensitized Vertically Grown Flower-like ZnO Solar Cells with CdSe Cosensitizers. Phys. Status Solidi (A) Appl. Mater. Sci. 2011, 208, 474–479. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, L.; Zhang, G.; Jiao, J. Open Structure ZnO/CdSe Core/Shell Nanoneedle Arrays for Solar Cells. Nanoscale Res. Lett. 2012, 7, 1–6. [Google Scholar] [CrossRef]
- Zarębska, K.; Łęcki, T.; Skompska, M. Synthesis of CdSe on FTO-Supported ZnO Nanorods by SILAR and Electrochemical Methods and Comparison of Photoelectrochemical Properties of FTO/ZnO/CdSe Systems in Aqueous S2−/Sn2− Electrolyte. J. Electroanal. Chem. 2018, 819, 459–468. [Google Scholar] [CrossRef]
- Pan, S.; Ebrahim, S.; Soliman, M.; Qiao, Q. Seed-Mediated Direct Growth of CdSe Nanoclusters on Substrates. J. Nanoparticle Res. 2013, 15, 1420. [Google Scholar] [CrossRef]
- Majidi, H.; Baxter, J.B. Electrodeposition of CdSe Coatings on ZnO Nanowire Arrays for Extremely Thin Absorber Solar Cells. Electrochim. Acta 2011, 56, 2703–2711. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. The Reflection of X-Rays by Crystals. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1913, 88, 428–438. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-Ray Diffraction Analysis by Williamson-Hall, Halder-Wagner and Size-Strain Plot Methods of CdSe Nanoparticles- a Comparative Study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi (b) 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hassabo, A.G.; Mohamed, A.L.; Shaheen, T.I. Surface Modification of SiO2 Coated ZnO Nanoparticles for Multifunctional Cotton Fabrics. J. Colloid Interface Sci. 2017, 498, 413–422. [Google Scholar] [CrossRef]
- Nishinaka, H.; Kamada, Y.; Kameyama, N.; Fujita, S. Epitaxial ZnO Thin Films on A-Plane Sapphire Substrates Grown by Ultrasonic Spray-Assisted Mist Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2009, 48, 121103. [Google Scholar] [CrossRef]
- Chen, X.; Li, J. Assembly and Photocatalytic Degradation Activity of Spherical ZnO/CdSe Heterostructures on Flexible Carbon Cloth Substrates. Nanomaterials 2022, 12, 1898. [Google Scholar] [CrossRef] [PubMed]
- Aldeek, F.; Mustin, C.; Balan, L.; Medjahdi, G.; Roques-Carmes, T.; Arnoux, P.; Schneider, R. Enhanced Photostability from CdSe(S)/ZnO Core/Shell Quantum Dots and Their Use in Biolabeling. Eur. J. Inorg. Chem. 2011, 2011, 794–801. [Google Scholar] [CrossRef]
- Raghunath Sankapal, B.; Zhang, J.; Yoshida, T.; Minoura, H. Electrochemical Fabrication of Nanoporous ZnO/Q-CdSe Photovoltaic Device. Trans. Mater. Res. Soc. Japan 2006, 31, 429–432. [Google Scholar]
- Qian, L.; Yang, J.; Zhou, R.; Tang, A.; Zheng, Y.; Tseng, T.K.; Bera, D.; Xue, J.; Holloway, P.H. Hybrid Polymer-CdSe Solar Cells with a ZnO Nanoparticle Buffer Layer for Improved Efficiency and Lifetime. J. Mater. Chem. 2011, 21, 3814–3817. [Google Scholar] [CrossRef]
- Barceló, I.; Lana-Villarreal, T.; Gómez, R. Efficient Sensitization of ZnO Nanoporous Films with CdSe QDs Grown by Successive Ionic Layer Adsorption and Reaction (SILAR). J. Photochem. Photobiol. A Chem. 2011, 220, 47–53. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. On the Optical Band Gap of Zinc Oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Hodes, G.; Grunbaum, E.; Feldman, Y.; Bastide, S.; Lévy-Clément, C. Variable Optical Properties and Effective Porosity of CdSe Nanocrystalline Films Electrodeposited from Selenosulfate Solutions. J. Electrochem. Soc. 2005, 152, G917. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, F.; Guo, D.; Gao, Z.; Wu, D.; Jiang, K. Synthesis of ZnO/CdSe Hierarchical Heterostructure with Improved Visible Photocatalytic Efficiency. Appl. Surf. Sci. 2013, 274, 39–44. [Google Scholar] [CrossRef]

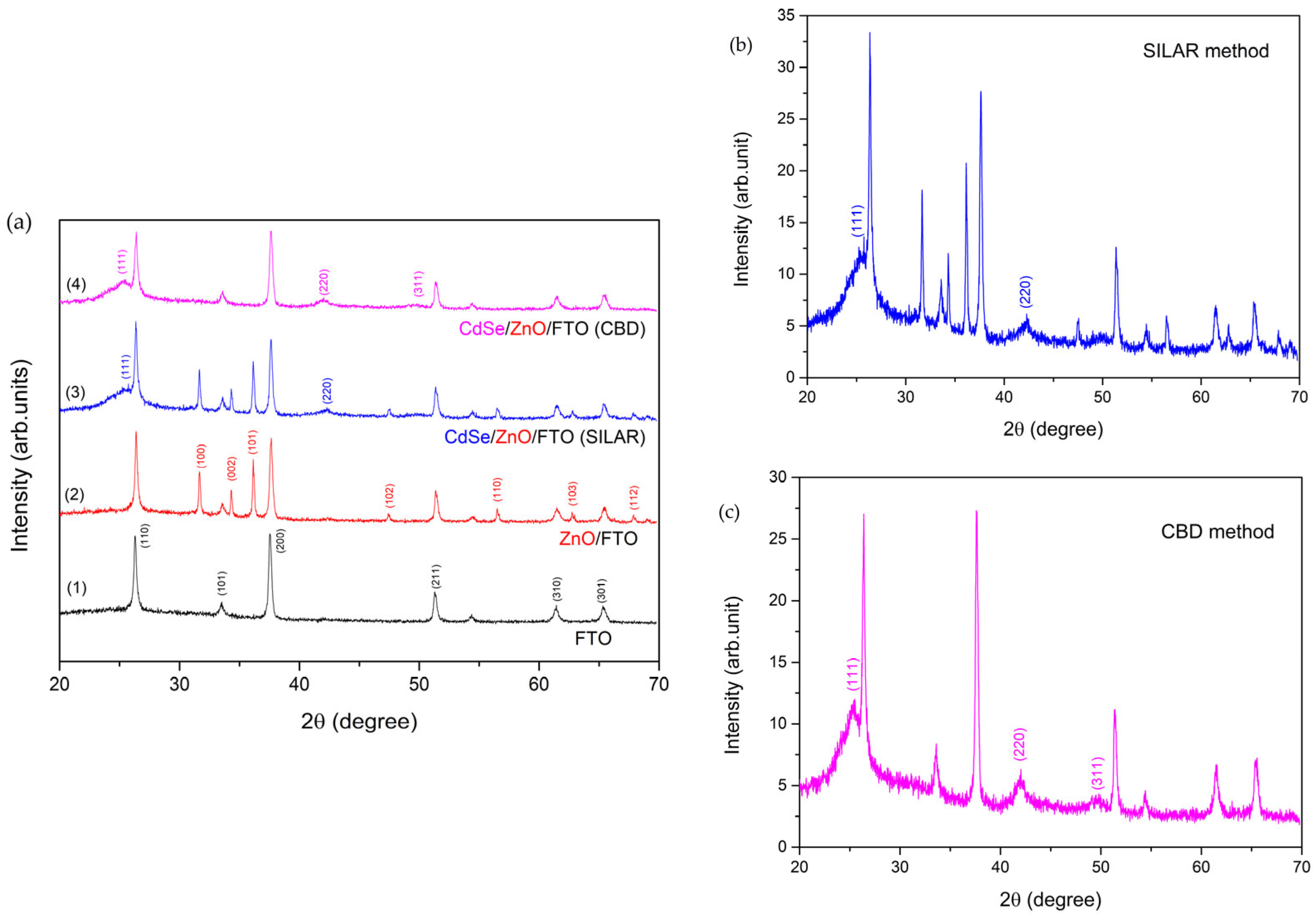



| Peaks Assigned to Materials | Analysis Results | JCPDS Data | ||||
|---|---|---|---|---|---|---|
| 2θ | d, Ǻ | d, Ǻ | Miller Indexes (hkl) | PDF No. | Crystalline Phase | |
| FTO | 26.78 | 3.33 | 3.35 | (110) | 71-0652 | Cassiterite SnO2 tetragonal |
| 33.96 | 2.64 | 2.64 | (101) | |||
| 37.98 | 2.37 | 2.37 | (200) | |||
| 51.75 | 1.77 | 1.76 | (211) | |||
| 61.99 | 1.50 | 1.49 | (310) | |||
| 65.82 | 1.42 | 1.42 | (301) | |||
| ZnO | 31.94 | 2.80 | 2.81 | (100) | 36-1451 | Wurtzite ZnO hexagonal |
| 34.61 | 2.59 | 2.60 | (002) | |||
| 36.42 | 2.46 | 2.48 | (101) | |||
| 47.94 | 1.89 | 1.91 | (102) | |||
| 57.05 | 1.61 | 1.62 | (110) | |||
| 63.29 | 1.47 | 1.47 | (103) | |||
| 68.37 | 1.37 | 1.38 | (112) | |||
| CdSe | 25.37 | 3.51 | 3.37 | (111) | 19-191 | CdSe cubic |
| 42.03 | 2.15 | 2.02 | (220) | |||
| 49.76 | 1.83 | 1.68 | (311) | |||
| Parameters | FTO | ZnO/FTO | CdSe/ZnO/FTO | CdSe/ZnO/FTO |
|---|---|---|---|---|
| - | - | - | SILAR | CBD |
| Average roughness, Ra, nm | 12.61 | 11.49 | 58.19 | 50.64 |
| RMS roughness, Rq, nm | 15.85 | 14.49 | 78.67 | 63.95 |
| Maximum surface roughness, Rt, nm | 143.30 | 124.80 | 1012.00 | 531.40 |
| CdSe Layer Formation Method | Zn, at% | O, at% | Cd, at% | Se, at% | Cd/Se |
|---|---|---|---|---|---|
| SILAR | 13.93 | 38.69 | 10.44 | 9.00 | 1.16 |
| CBD | 0.05 | 24.58 | 13.26 | 12.13 | 1.09 |
| CdSe Forming Method | Sample Surface Area A, cm2 | Current Density juv Value Achieved Using UV Radiation, mA/cm2 | Current Density jdark Value Achieved in the Dark, mA/cm2 | juv/jdark | Photocurrent jphoto, mA/cm2 |
|---|---|---|---|---|---|
| SILAR | 1.95 | 0.0232 | 0.0038 | 6.063 | 0.0938 |
| CBD | 1.05 | 0.0011 | 0.0055 | 0.2 | −0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubauskas, G.; Paluckiene, E.; Usoviene, E.; Petrasauskiene, N. Influence of Different Chemical Methods Used for the Deposition of CdSe/ZnO Layers. Coatings 2025, 15, 415. https://doi.org/10.3390/coatings15040415
Jakubauskas G, Paluckiene E, Usoviene E, Petrasauskiene N. Influence of Different Chemical Methods Used for the Deposition of CdSe/ZnO Layers. Coatings. 2025; 15(4):415. https://doi.org/10.3390/coatings15040415
Chicago/Turabian StyleJakubauskas, Gediminas, Edita Paluckiene, Egle Usoviene, and Neringa Petrasauskiene. 2025. "Influence of Different Chemical Methods Used for the Deposition of CdSe/ZnO Layers" Coatings 15, no. 4: 415. https://doi.org/10.3390/coatings15040415
APA StyleJakubauskas, G., Paluckiene, E., Usoviene, E., & Petrasauskiene, N. (2025). Influence of Different Chemical Methods Used for the Deposition of CdSe/ZnO Layers. Coatings, 15(4), 415. https://doi.org/10.3390/coatings15040415







