Abstract
This work aimed to investigate the structure and corrosion resistance of Al2O3/ZnO multilayer coatings deposited by ALD on the standard surgical scalpel blades made of carbon steel. The surface topography of the coatings was examined using a scanning electron microscope (SEM), revealing the significant effect of the number of deposited Al2O3/ZnO bilayers on the morphology of the multilayer coatings. The XRD method was used for the phase analysis, allowing to confirm the presence of ZnO and ZnAl2O4 phases. The presence of the ZnAl2O4 structure was also confirmed using a Raman spectrometer. A qualitative analysis of the chemical composition of the obtained coatings was performed using the energy dispersive spectrometry (EDX) method. In order to determine the corrosion resistance, potentiodynamic tests were performed using Ringer’s solution at a temperature of 37 °C. The beneficial effect of increasing the number of deposited Al2O3/ZnO bilayers on the corrosion resistance was confirmed, with the lowest corrosion current density value of 2.05 μA/cm2 and the highest polarization resistance of 12.15 kΩ obtained in the case of the N72 coating.
1. Introduction
Nowadays, the vapor deposition methods are being widely used to improve the functional properties of metallic materials—in particular, for enhancing corrosion and wear resistance. To ensure a proper corrosion protection of a substrate, the applied coatings should provide a cathodic protection or, otherwise, be characterized by low defect density. Nevertheless, thin films deposited by using physical vapor deposition (PVD) or chemical vapor deposition (CVD) usually exhibit numerous defects, diminishing their protective abilities. For example, in case of the PVD processes, the characteristics of the vapor flux transfer contribute to the occurrence of pinholes, droplets and other discontinuities in the deposited coatings. Also, the columnar structure, which usually characterizes these coatings, deteriorates their insulating abilities, as the grain boundaries facilitate the penetration of electrolyte to the substrate [1,2,3].
In recent years, the atomic layer deposition (ALD) emerged as a promising method in the field of corrosion protection. The possibility of depositing conformal, virtually defect-free layers makes this technique intrinsically suitable for the deposition of anticorrosive coatings. The atomic layer deposition (ALD) belongs to the group of chemical vapor deposition (CVD) methods. In this method, in contrast to other CVD processes, the reactants are pulsed into the working chamber in an alternate manner, one at time; simultaneously, between consecutive pulses, the chamber is purged using an inert gas, most popularly nitrogen. A distinctive feature of the ALD is the self-limiting growth mechanism, allowing for the simple and accurate control of the thickness of the film being deposited. Furthermore, it also translates into the possibility of the even coating of complex-shaped objects and good large-area uniformity. Simultaneously, another advantage is the possibility to accurately control the chemical composition of the film being deposited [4,5,6,7].
The oxides are among the most commonly used coating materials for corrosion-protective ALD thin films. Owing to their nanometric thickness and chemical composition, ALD oxide coatings can be treated as an imitation of the passive layers, which form spontaneously on the surface of some metals under exposure to the environment. Naturally formed passive oxide layers, particularly in case of the titanium and tantalum, contribute to their high corrosion resistance, even in the most aggressive media. Their protective abilities derive from chemical and thermal stability, good adhesion to the substrate, conformability and low porosity. The ALD oxide layers with similar characteristics can be used for enhancing the corrosion resistance of metallic materials, particularly in cases when the substrate alloy does not exhibit the ability to passivate or the protection given by the passive layer is found insufficient [7,8].
Until now, aluminum and titanium oxides have been the most frequently studied coating materials. In particular, high resistivity and the possibility of obtaining coatings with an amorphous structure are conducive to the application of an aluminum oxide. Furthermore, the most popular deposition process of aluminum oxide—utilizing trimethylaluminum (TMA) and water—has advantageous characteristics. The high reactivity of TMA contribute to more complete surface reactions, resulting in decreased impurity content. However, the low chemical stability of Al2O3 limits its use in aggressive environments [8,9,10].
The titanium oxide is being another frequently used material for ALD protective coatings. Depending on the deposition temperature, TiO2 can exhibit both amorphous or polycrystalline structure—in the form of anatase or rutile. In the case of polycrystalline thin films, their ability to insulate the substrate from the corrosive environment is diminished, as the corrosive media can penetrate through the grain boundaries into the substrate. However, in order to obtain an amorphous structure, lower deposition temperatures are required, which usually results in higher impurity content. Comparing the polycrystalline TiO2 thin films, it was found that coatings with the anatase structure exhibit higher corrosion protection ability than those comprising rutile—which is attributed to the lower defect density. The formation of anatase occurs for deposition temperatures ranging between 200 °C and 300 °C, whereas for obtaining rutile, temperatures above 300 °C are required [7,9].
In turn, for biomedical applications, the application of zinc oxide coatings is considered a promising solution. The atomic layer deposited ZnO can provide adequate corrosion protection, simultaneously reducing risk of infection—in effect of its antibacterial properties. It was proven in earlier works, that the application of zinc oxide coating increases the corrosion resistance of stainless steel immersed in simulated body fluids. Furthermore, it was shown that its application contributes to reducing the bacterial adhesion and their proliferation rate. The antibacterial activity of ZnO coatings is attributed to the several factors—among which, as one of greatest importance, is the generation of reactive oxide species (ROS) under the influence of UV irradiation, such as hydrogen peroxide, superoxide, hydroxyl radical and singlet oxygen. The other factors contributing to the bactericidal effect involve the release of Zn2+ ions, resulting from the dissolution of zinc oxide in an aqueous environment, and the specific surface topography of ZnO coatings, hindering the bacterial adhesion. Consequently, zinc oxide coatings can be used in applications requiring corrosion resistance and bactericidal properties, such as surgical instruments [11,12,13,14]. To compare the corrosion resistance of selected ALD oxide coatings, the values of the corrosion current density parameter available in the literature were summarized in Table 1.

Table 1.
Comparison of corrosion resistance of different ALD oxide coatings, based on corrosion current density parameter.
The corrosion resistance of ALD protective coatings generally increases with their thickness—which is mainly attributed to the decrease in residual porosity. However, the increase in a coating’s thickness connects with the rise of internal stresses, resulting in the deterioration of the delamination resistance. In turn, the utilization of a multilayer coating architecture can contribute to improvement of both corrosion protection efficiency and delamination resistance. It is widely assumed that the improvement in coating adhesion to the substrate is caused by the relaxation of internal stresses, occurring at the interfaces of subsequent layers. Furthermore, multilayer coatings are characterized by enhanced corrosion resistance, which is related to their lower porosity—as the subsequent layers seal the discontinuities existing in those previously deposited [8,9,17].
The use of the multilayer coatings allows one to obtain the most beneficial property combination of the individual coating constituents—as in the case of one of the most frequently studied configurations, comprising of alternately deposited layers of aluminum and titanium oxides. An amorphous Al2O3 layer contributes to good sealing properties, while the TiO2 layer hinders the dissolution of chemically unstable Al2O3, thus ensuring adequate durability of the coating. Furthermore, as the nucleation of Al2O3 occurs more effectively in comparison with other oxides, it can be used as the first layer to be deposited on the substrate, in order to provide proper adhesion of subsequent layers [9,17].
The coatings used on surgical instruments should meet the requirements regarding their corrosion resistance and biocompatibility, concurrently allowing to enhance their wear resistance. In the case of sharp tools, important consideration is also to reduce the friction and provide the long-lasting sharpness of the cutting edge. The sharpness of the cutting edge affects the quality of the performed incision and consequently the subsequent healing process. In order to minimalize bleeding and achieve a shorter healing time, the regularity and uniformity of the incision are required. Standard martensitic scalpel blades rapidly lose their sharpness, which results in the necessity of using multiple blades, even in the case of a single incision. One of the solutions is the application of the protective ceramic coatings, which can increase their wear resistance and extend the lifespan of the cutting edge [18,19,20]. For example, nitride- and carbide-based coatings, such as TiN, (Ti,Al)N and Ti(C,N), are among the commonly used. Although offering good biocompatibility, improved wear resistance and antireflective properties, exhibit also several limitations, such as inherent defects typical for the PVD coatings, negatively affecting their corrosion resistance [1,2,18]. A promising approach, which enables one to overcome these limitations, involves the use of the atomic layer deposition process, allowing for the application of a precise, conformal thin film with uniform, well-defined chemical composition. Another advantageous feature is the relatively low process temperature, enabling the coating of quenched and tempered steel substrates, such as martensitic scalpel blades [2,6].
Recently, there has been a rising trend in the development of coatings exhibiting antibacterial activity, in order to diminish the risk of postoperative infections associated with medical instruments [14,21,22]. The application of the ALD zinc oxide coating was previously shown as an effective method to reduce bacterial adhesion [13]. The results of the corrosion tests undertaken confirmed also its beneficial effect on corrosion resistance. A promising route of further development is to use a multilayer architecture of the coating, allowing for the enhancement in the sealing properties of the coating, simultaneously improving its adhesion to the substrate. The advantageous properties of aluminum oxide, alongside with its biocompatible behavior, make it a suitable coating material for this application [22]. Aluminum oxide thin films, beside their advantageous corrosion resistance, are characterized by high hardness [23] and wear resistance [24,25]. Furthermore, it was shown that the application of Al2O3 coating can contribute to reducing residual bioburden after cleaning, which is of great importance in terms of reusability of the surgical instruments [26]. An interesting feature is also the possibility of interference coloring through the application of ALD coatings of suitable thickness, allowing for the quick distinction of blades with different geometry [27]. Accordingly, this work proposes the application of ALD Al2O3/ZnO multilayer coatings as a method to enhance the corrosion resistance of surgical instruments and a possible pathway to develop the reusable surgical scalpel blades.
2. Materials and Methods
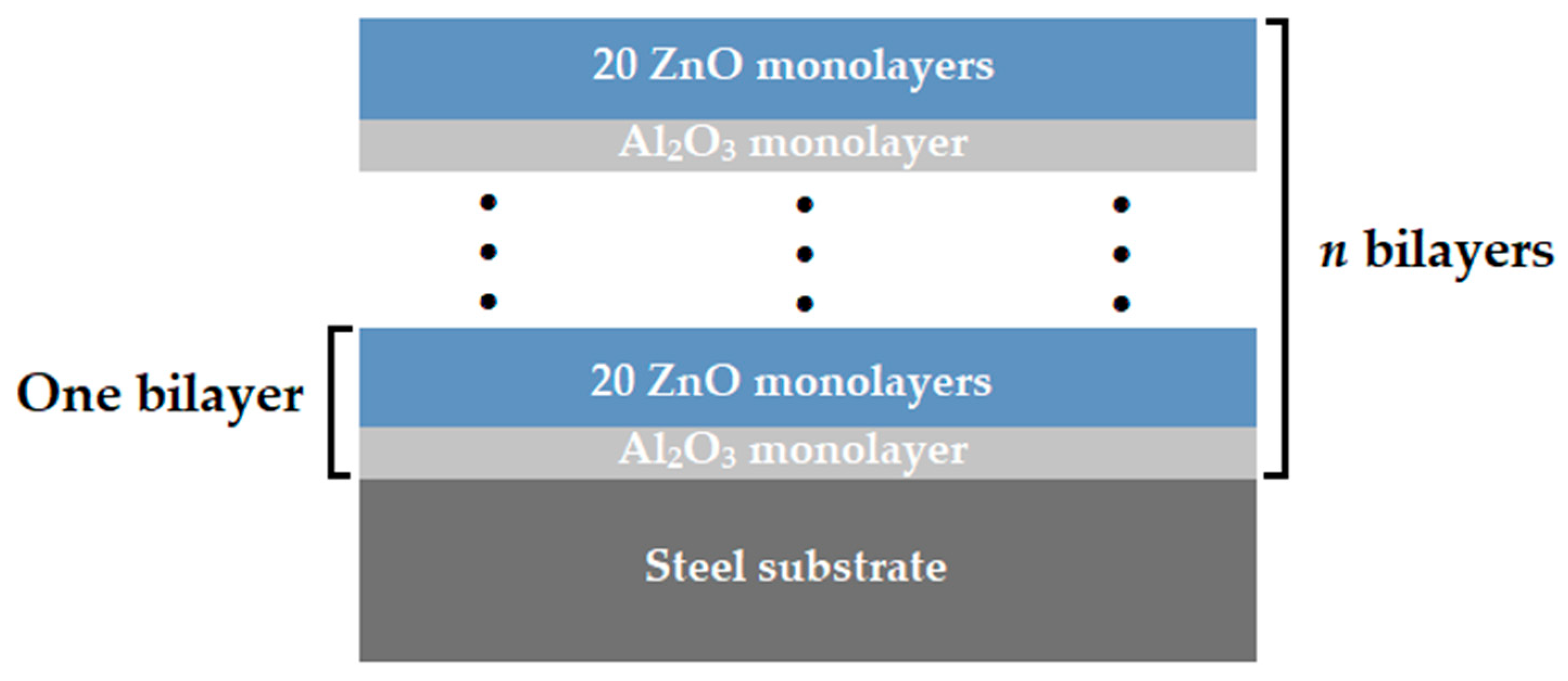
As a substrate material, the disposable surgical scalpel blades made of carbon steel C75 (1.1248) (InterMed Company, Shenzhen, China) were used. The multilayer coatings of Al2O3/ZnO were applied by the atomic layer deposition method, using an R-200 Standard system from Picosun (Espoo, Finland). Diethylzinc (DEZ) (stream, Bischheim, France) and trimethylaluminum (TMA) (stream, Bischheim, France) were used as a zinc oxide and aluminum oxide precursors, while deionized water was used as a reactant in both cases. The dosing time for both the reagents and precursors was equal to 0.1 s. The deposition process was conducted at a temperature of 200 °C, using nitrogen as a transporting gas and to purge the chamber between the dosing of subsequent reagents. The purging time was 4 s for both precursors and 5 s for water, at a nitrogen flow equal to 200 sccm. The conditions were selected on the basis of previous works, as optimal for the deposition of ZnO [11,12]. Concurrently, this allows to the deposition of amorphous Al2O3 thin films characterizing with good quality [28]. Multilayer coatings were obtained by the alternate deposition of aluminum oxide and zinc oxide layers. Each deposition cycle of aluminum oxide corresponded to 20 deposition cycles of zinc oxide. This Al2O3/ZnO bilayer deposition sequence was repeated 24, 48, and 72 times, resulting in the final structure and layer thickness. The obtained multilayer coatings were denoted as N24, N48 and N72, respectively. Figure 1 shows the schematic diagram of the deposited multilayer coating configurations.
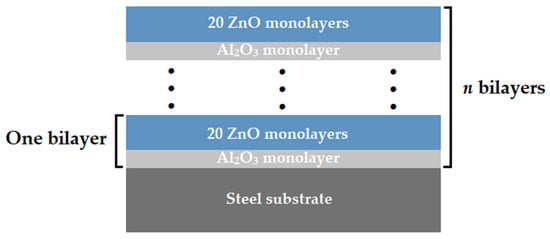
Figure 1.
Architecture of deposited multilayer coatings.
The thickness and colors of the deposited layers were determined using an FR-pRo-UV/VIS spectroscopic reflectometer (ThetaMetrisis SA., Peristeri, Greece). Measurements were taken in reflectance mode.
The surface morphology of the deposited coatings was examined using a Zeiss Supra 35 scanning electron microscope (SEM) (Zeiss, Oberkochen, Germany), equipped with the EDAX spectrometer from Thermo Scientific (Mahwah, NJ, USA). The detection of secondary electrons (by the In-Lens detector) was used for the imaging of the observed surfaces. The applied accelerating voltage was equal to 3 kV. The observations were carried out at magnification ranging from 100,000× to 150,000×. Qualitative studies of chemical composition were performed using energy dispersive spectrometry (EDX) on the basis of the images obtained with the SEM using secondary electrons detection, at 20 kV accelerating voltage, at magnification ranging from 5000× to 15,000×. The phase composition of the deposited coatings was analyzed with the use of the X-ray diffraction method (XRD) on the Panalytical X’Pert X-ray apparatus (EA, Almelo, The Netherlands) using filtered cobalt anode radiation (Co λ = 1.7909 Å).
The structural analysis of the samples was conducted using a Renishaw inVia Reflex Raman spectrometer (New Mills, UK), which was equipped with a 514.5 nm (green) excitation laser. The recorded spectra spanned a broad Raman shift range from 50 to 3100 cm−1. Raman spectroscopy facilitates the detection of active Raman phonon modes in crystalline structures and active Raman vibrational modes in particulate matter. This technique enables the acquisition of distinctive spectral fingerprints of various crystalline polymorphic materials, as well as spectral data from molecules present at the measurement site.
Additionally, the structural analysis of the ZnO/Al2O3 thin films was performed using transmission electron microscopy (TEM). The measurements were carried out using a high-resolution S/TEM Titan 80–300 microscope (FEI, Hillsboro, OR, USA), equipped with a super-twin lens and an annular dark-field detector, operating at an accelerating voltage of 300 kV. Observations were conducted in both conventional TEM and scanning transmission electron microscopy (STEM) modes. The instrument is equipped with three coaxial detectors dedicated to STEM imaging: bright field (BF), annular dark field (ADF) and high-angle annular dark field (HAADF). Selected area electron diffraction (SAED) was also performed to obtain crystallographic information.
To evaluate the corrosion resistance of the coatings, potentiodynamic investigations were performed. In order to provide the conditions corresponding to those prevailing in tissues and the physiological liquid environment, tests were carried out in Ringer’s solution (NaCl—8.6g/L; KCl—0.3 g/L; CaCl2∙6H2O—0.48 g/L) (250 mL), supplied by Baxter, at a temperature of 37 °C and pH = 7 ± 0.2. An Autolab 302N potentiostat (Metrohm-Autolab, Utrecht, The Netherlands) equipped with a three-electrode measuring system was used for the study. A saturated calomel electrode (SCE) (Hanna Instruments, Padua, Italy) was used as the reference electrode, with platinum wire being the counter electrode and the material tested as the working electrode. In order to evaluate the corrosion resistance, changes in open-circuit potential (EOCP) were recorded—within the stabilization time of 1800 s—followed by measurements of polarization in the range of −250 mV to 250 mV, with scan rate of 0.001 V/s. On the basis of the obtained results, corrosion potential (Ecorr) and corrosion current density (icorr) were determined with the use of the Tafel extrapolation method.
3. Results and Discussion
The results of the conducted thickness measurements of the deposited thin films were summarized in the Table 2. The AZO deposited with a cycle number of 504 have a thickness of approximately 80.7 nm. The AZO layers deposited with a cycle number of 1008 have a thickness of about 161.3 nm. The thickest layer deposited after 1512 cycles has a thickness of 244.6 nm. On the basis of the determined thicknesses, it was possible to determined the average speed of layer growth during one ALD cycle. For AZO, it is approximately 0.16 nm per cycle.

Table 2.
The results of the thickness measurements of the deposited thin films.
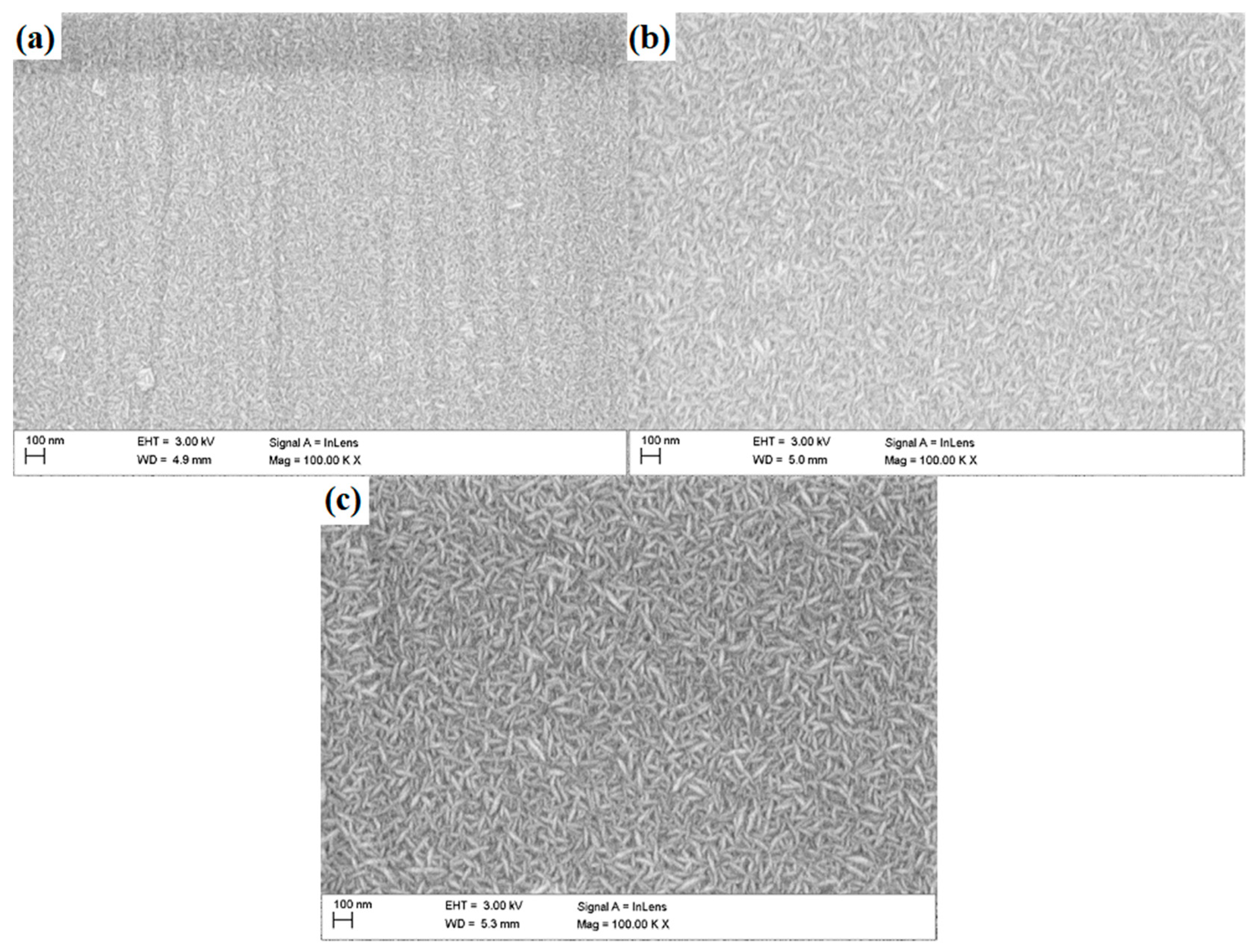
The observations performed with the use of the SEM revealed the granular morphology of the deposited coatings (Figure 2)—with grains of elongated shape, characteristic for zinc oxide, which represents the outer layer of each multilayer configuration. The grains were distributed randomly, with no dominant direction of their arrangement. With increasing number of layers deposited, an increase in grain size can be observed.
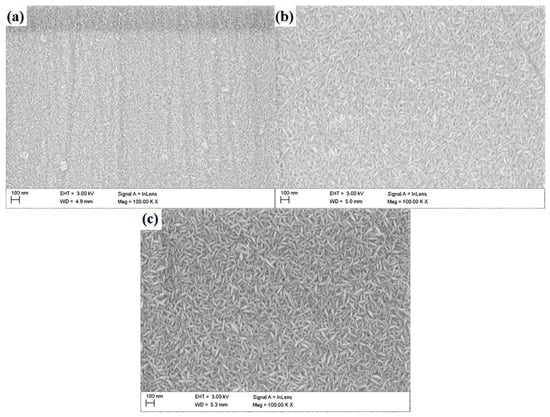
Figure 2.
Surface morphology of N24 (a), N48 (b) and N72 (c) coatings deposited on steel substrate.
In case of the N24 coating, the presence of surface inhomogeneities—such as grooves and macroparticles can be observed. The presence of grooves can be related to the occurrence of scratches on the substrates surface, which were subsequently reproduced by the deposited coatings. Characteristic features of the atomic layer deposition and the nanometric thickness of the coatings process makes them reproduce the topographical features of the substrate. The quantity of visible impurities decreases significantly with the coatings thickness; simultaneously, the grooves present on coatings surface became less pronounced.
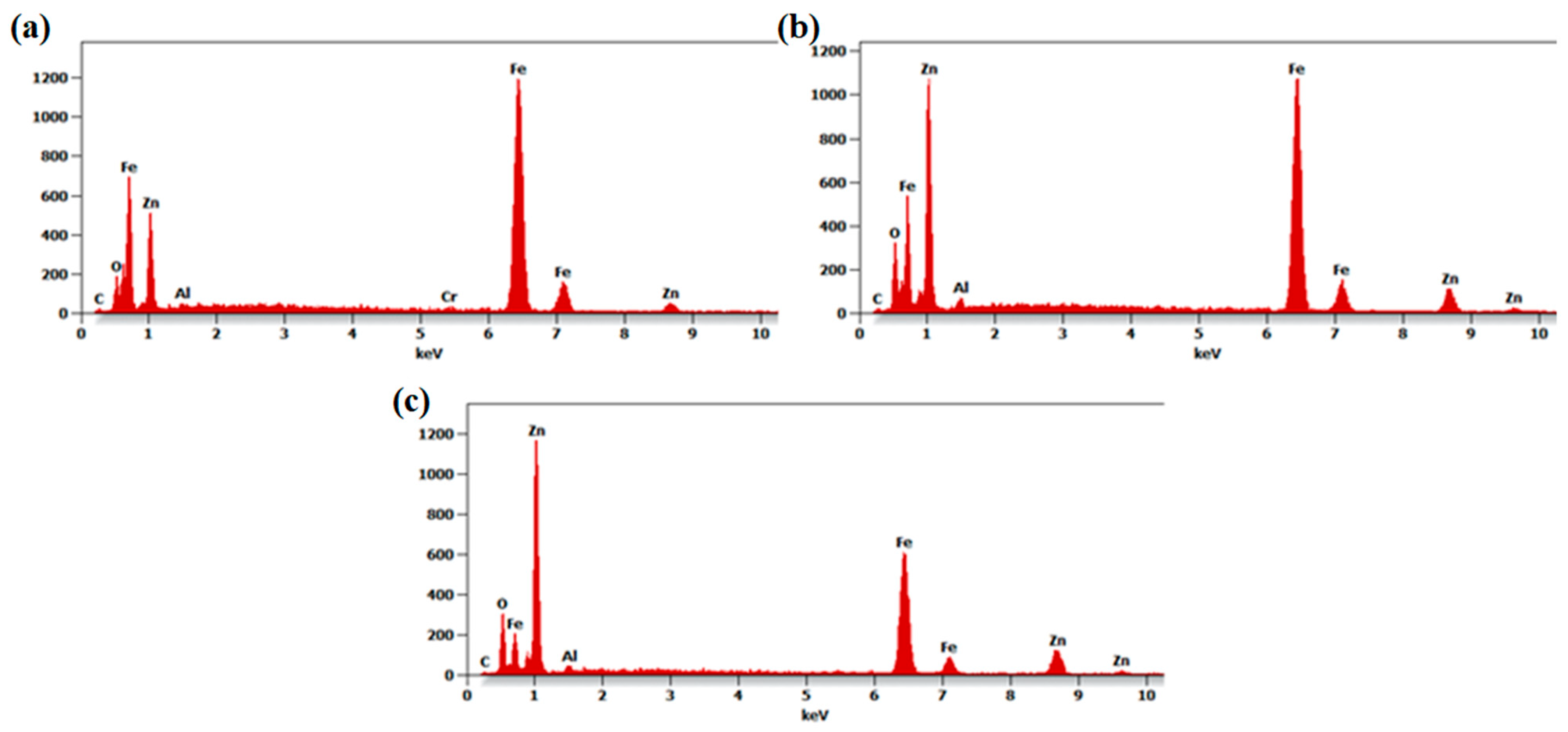
The conducted chemical composition analysis confirmed the presence of zinc, aluminum and oxygen, suitable for the deposited coatings (Figure 3). As the thickness of the deposited coatings is much smaller than the emission depth of characteristic X-rays, reflections deriving from the substrate constituents such as iron and chromium (in the case of the N24 coating) were also recorded. Peaks recorded at around 1.01 keV and 8.64 keV can be attributed to zinc; it can be observed that their intensity increases with the number of deposited layers. Concurrently, reflections deriving from the substrate constituents became less pronounced.
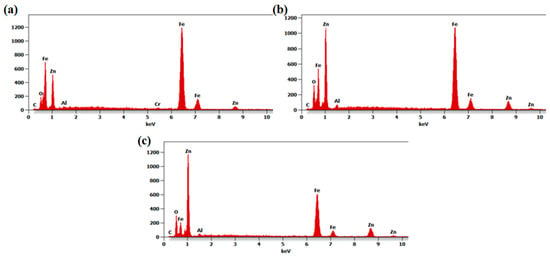
Figure 3.
The EDX spectra of N24 (a), N48 (b) and N72 (c) coatings deposited on steel substrates.
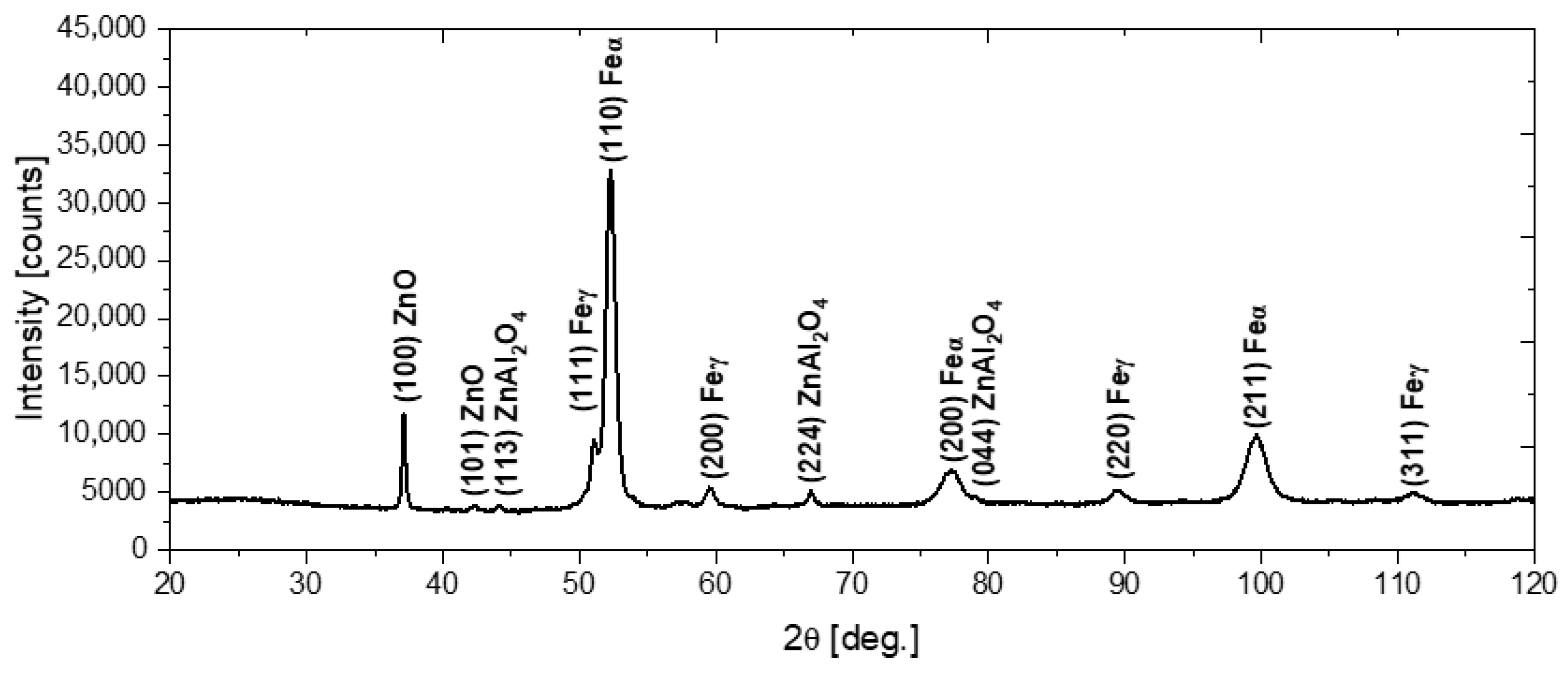
In order to characterize the structure of the deposited coatings, phase analysis using the X-ray diffraction method was performed, with the use of Co K-α radiation. Figure 4 shows the X-ray diffraction pattern obtained for the N72 coating deposited on the steel substrate. The diffraction peaks at 37.15° and 42.39° correspond to the (100) and (101) crystal planes of the hexagonal wurtzite-type ZnO (zincite, space group P63mc) [29,30]. Concurrently, the diffraction peaks at 44.27°, 67.01° and 79.13° can be assigned to the (114), (224) and (044) crystal planes of cubic ZnAl2O4 (gahnite, space group Fdm) [31,32]. As the penetration depth of X-rays into the material exceeds the thickness of the deposited coatings, the presence of α-Fe and γ-Fe peaks deriving from the steel substrate can also be observed [33,34].
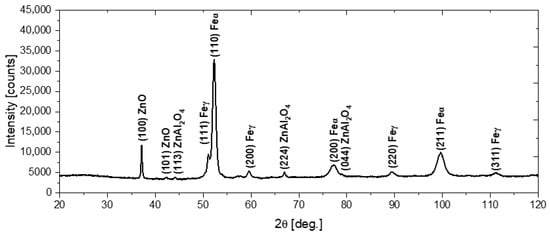
Figure 4.
The diffraction pattern of the N72 coating deposited on the steel substrate.
The preferential orientation of the ZnO was along the (100) plane. Previous studies have shown that the crystalline growth direction of the hexagonal ZnO was substantially affected by the deposition temperature. It was reported that in the case of coatings deposited in the temperature range 155–200 °C, the (100)-oriented crystals were dominant, whereas for lower deposition temperatures, both the (002) and (100) represent preferred crystal orientations [35,36,37]. The (002) plane, which grows in the direction, consists of alternate planes of O2− and Zn2+ ions and consequently can be negatively or positively charged, depending on surface termination. It was stated that negatively charged hydrocarbon ligands—which can form as a result of premature dissociation of DEZ, could adhere to a positively charged polar surface (terminated by Zn2+ ions), suppressing the growth in direction. Consequently, the switching of crystal orientation to (100) dominance was observed [36,37]. Furthermore, Banerjee et al. [35] reported that doping with aluminum can also contribute to the preferential growth of the (100)-oriented crystals. Concurrently, the appearance of ZnAl2O4, related to the diffusion of aluminum from Al2O3 layers, was also observed in earlier works [32,38]. The solid solubility limit of aluminum in zinc oxide was reported to be below 3 at.%; consequently, at higher concentrations, the segregation of excess Al atoms and the phase separation of ZnAl2O4 is likely to occur [38].
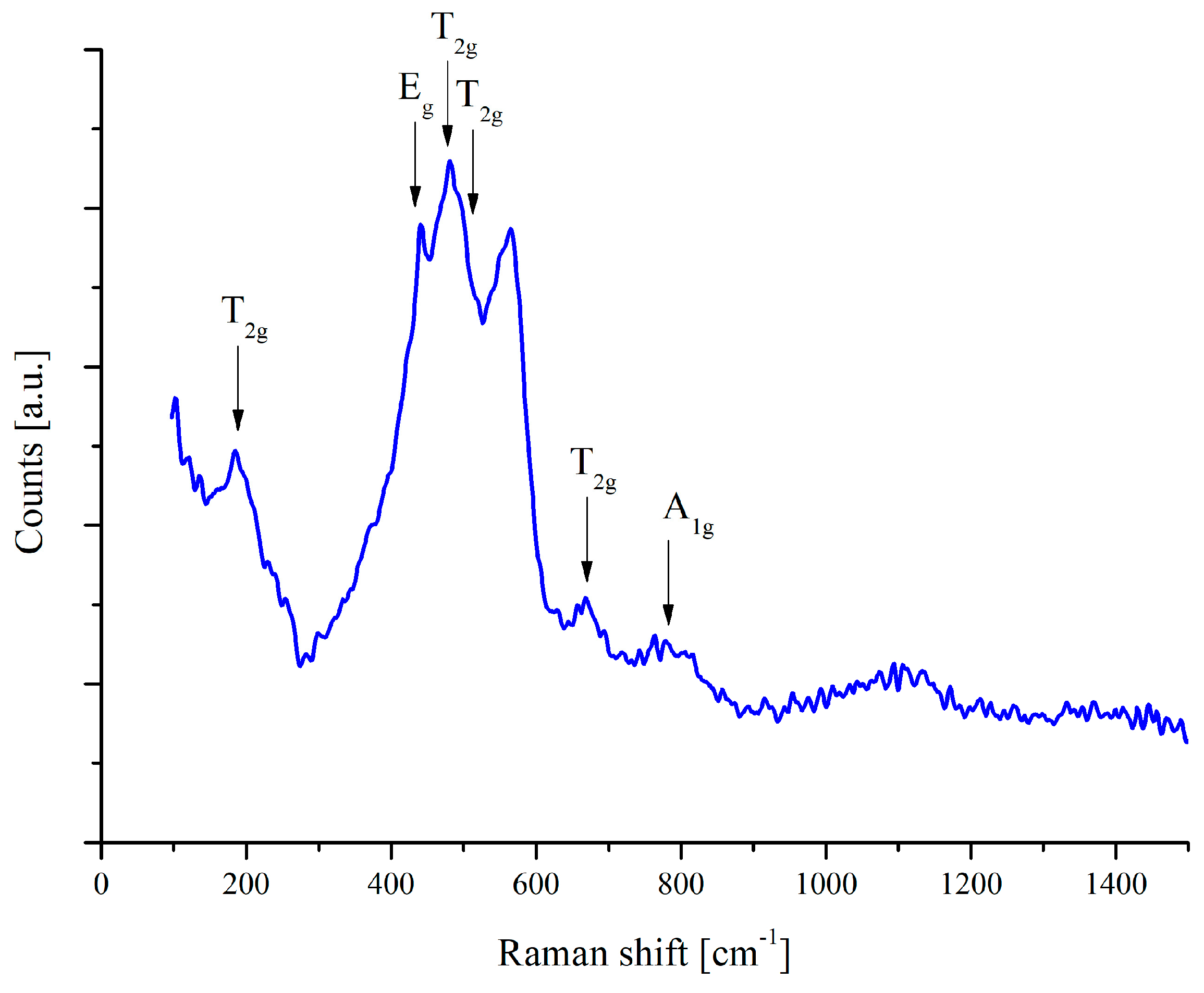
The presence of the ZnAl2O4 structure was also confirmed using a Raman spectrometer. The Raman spectrum of the ZnAl2O4 spinel, presented in Figure 5, exhibits characteristic vibrational modes corresponding to the cubic spinel structure (space group Fdm). According to group theory, the expected Raman-active modes for spinel-type compounds include one A mode, one E mode and three T modes. In the recorded spectrum, several distinct peaks are identified and assigned to their respective phonon modes. The T mode at approximately 200 cm−1 corresponds to lattice vibrations involving Zn and Al cations. The peak around 420 cm−1 is assigned to the E mode, which represents symmetrical oxygen displacement within the tetrahedral and octahedral sublattices. Additionally, the T modes appear in the range of 500–650 cm−1, reflecting the motion of oxygen atoms associated with AlO6 octahedra. The peak at approximately 760 cm−1 is attributed to the A symmetric stretching mode of Al–O bonds within the octahedral sites. The obtained Raman spectrum aligns well with previously reported data for ZnAl2O4, confirming the spinel structure and the presence of well-defined vibrational modes. The absence of additional peaks suggests the phase purity of the deposited material, as no secondary phases, such as ZnO or Al2O3, were detected.
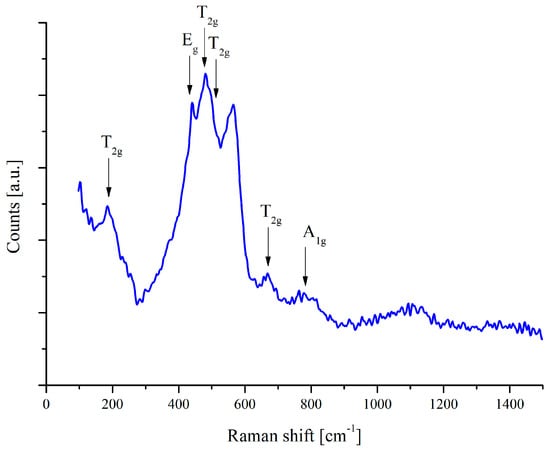
Figure 5.
Raman spectrum of ZnAl2O4 thin film on polished steel substrate.
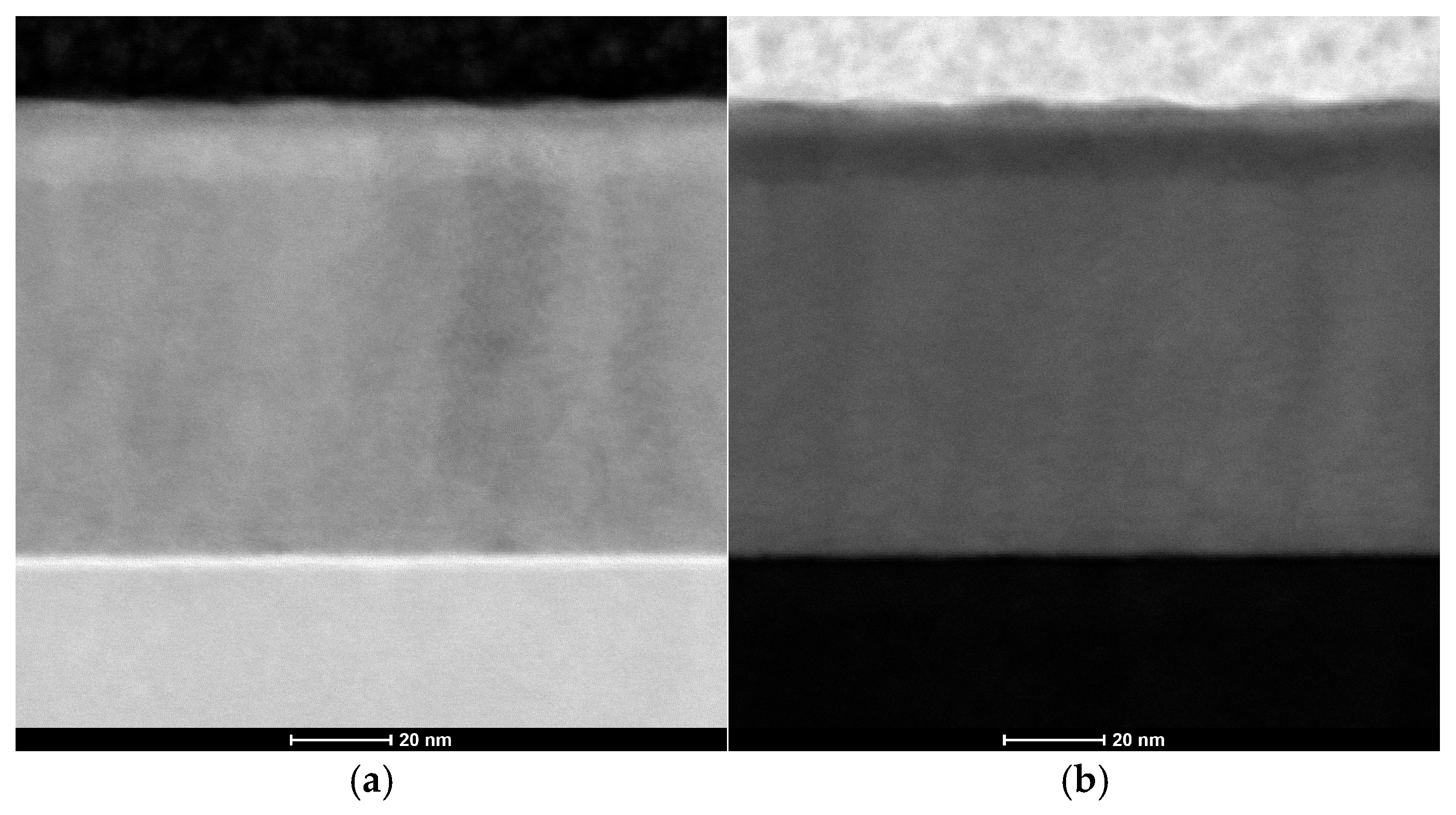
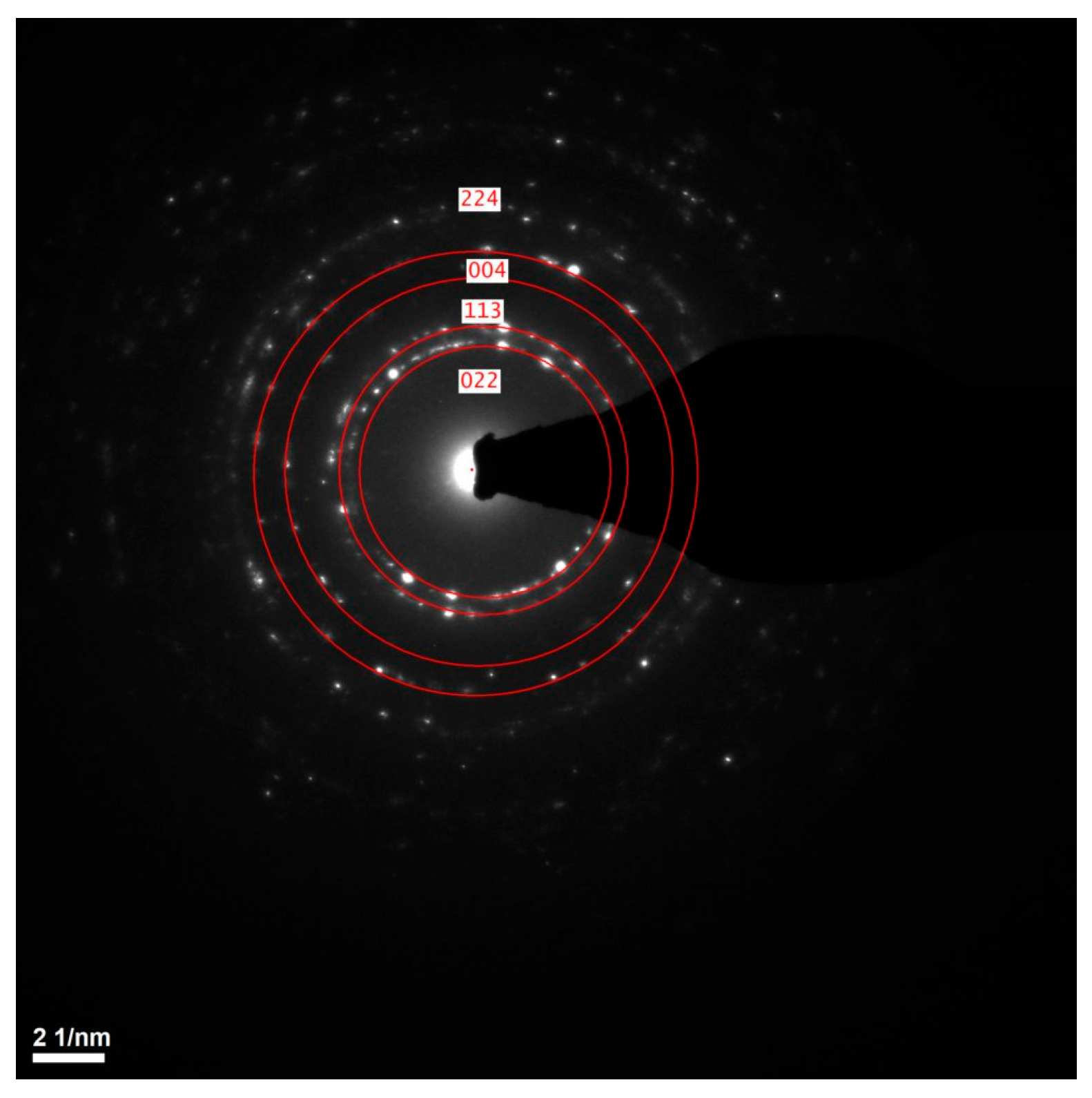
STEM and TEM analyses confirmed the structural uniformity of the ALD-deposited cubic ZnAl2O4 layer. Figure 6a presents bright-field (BF) and Figure 6b presents high-angle annular dark-field (HAADF) images of the cross-sectional lamella, revealing a homogeneous and continuous film without visible microcracks, voids or structural defects. Additionally, the selected area electron diffraction (SAED) pattern shown in Figure 7 exhibits well-defined diffraction rings, corresponding to the (224), (004), (113) and (022) planes of cubic ZnAl2O4, further confirming the crystalline nature of the coating.
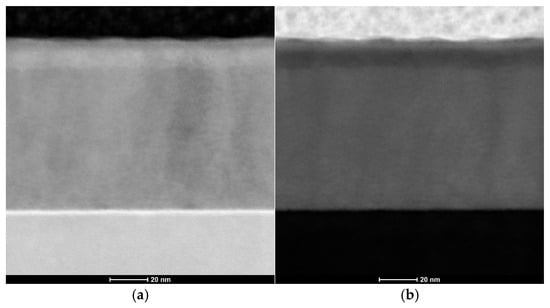
Figure 6.
Images of the synthesized zinc oxide nanoparticles: (a) STEM-BF; (b) STEM-HAADF.
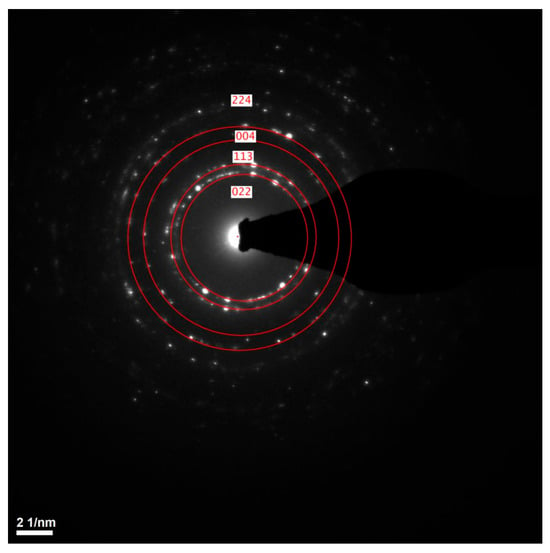
Figure 7.
The SAED pattern of cubic ZnAl2O4 thin film.
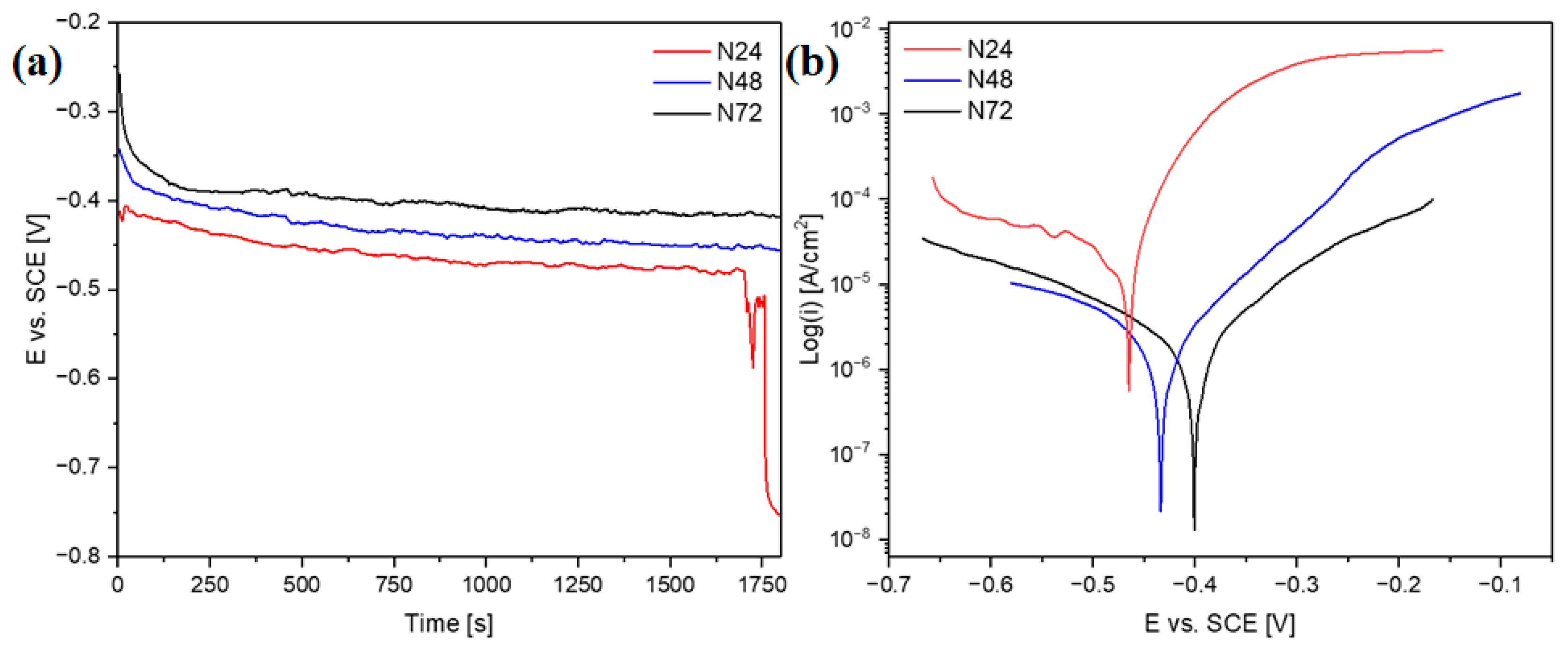
The corrosion resistance of the coatings was determined with the use of electrochemical tests. Figure 8a shows the changes of open-circuit potential as a function of time, whereas Figure 8b shows the recorded polarization curves. The open-circuit potentials for the samples N48 and N72 stabilized at approximately −420 mV and −450 mV vs. SCE, respectively. In the case of sample N48, a rapid decrease in potential during the first 130 s of measurement was observed—which can be related to the dissolution of impurities adsorbed on the surface. For sample N24, after 1700 s of measurement, there was a sharp decrease in potential, indicating that the continuity of the coating was breached; at the end of measurement, the potential reached a value of −750 mV. The characteristic corrosion resistance parameters obtained in effect of conducted measurements were summarized in Table 3.
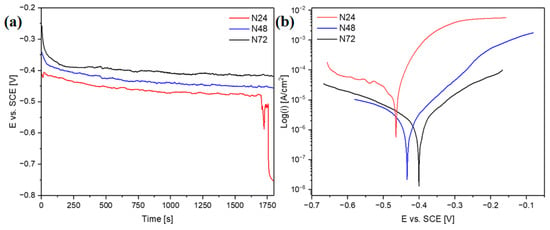
Figure 8.
Changes of open-circuit potential as a function of time (a) and potentiodynamic polarization curves (b) in Ringer’s solution at 37 °C.

Table 3.
Results of potentiodynamic polarization measurements conducted in Ringer’s solution after the 30 min of stabilization of working electrode.
The decrease in corrosion current density with the increase in the number of Al2O3/ZnO bilayers can be observed. In particular, significantly lower corrosion current density values can be noticed for the N48 and N72 coatings—as compared to the sample coated with the thinnest coating (N24). More effective corrosion protection of substrate, provided by the two thicker coatings, is further evidenced by a positive shift in corrosion potential—in comparison with the N24 coating. Concurrently, there is also a substantial rise in polarization resistance for the N48 and N72 coatings. In comparison with the previously investigated ZnO thin films [11], a substantial improvement of corrosion resistance can be noticed for all studied multilayer coatings—evidenced by the decrease in corrosion current density values and the positive shift in corrosion potential. In particular, for the N48 and N72 coatings, the recorded values of corrosion current density were at least two orders of magnitude lower than in the case of ZnO thin films. These two coating configurations show also more favorable corrosion properties, in terms of corrosion current density and polarization resistance, as-compared to the ZnO coatings deposited on the AISI 316LVM substrates after different pre-treatments described in the work [39]. Earlier works also describe the corrosion resistance of the ALD aluminum oxide coatings. In the article [40], the effect of different deposition temperatures on the corrosion resistance of Al2O3 in 3.5% NaCl solution at 25 was investigated. The most advantageous parameters characterize the Al2O3 coating deposited at 300 °C, exhibiting the lowest corrosion current density (50 μA/cm2) and the highest polarization resistance value (352.6 Ωcm2). Although the results obtained in case of the N24 coating are less favorable, significantly lower corrosion current density values and higher polarization resistance values were obtained in our study for the N48 and N72 coatings, despite their substantially lower thickness. The beneficial effect of application of the multilayer architecture on the corrosion resistance of the ALD coatings was confirmed in earlier works [8,17,30]. In the work [17], the advantageous properties of Al2O3/TiO2 multilayer coatings were related to the synergistic effect of the combination of an amorphous Al2O3 layer with good sealing properties and a crystalline TiO2 layer providing better chemical durability. Similarly, in this work, the utilization of the multilayer architecture involving the amorphous Al2O3 allowed to enhance the coatings’ protective abilities.
4. Conclusions
The proper surface modification of the surgical instruments allows to enhance their functional properties. The application of well-suited coatings not only enhances their corrosion resistance—which is intrinsically of great importance, as these tools are exposed to chemically active environments—but can also contribute to reducing the risk of infection by reducing bacterial adhesion and proliferation. The ALD zinc oxide layers have been proven to effectively improve the corrosion resistance of the standard scalpel blades while exhibiting antibacterial properties. To further improve the protective abilities of coatings and reduce their susceptibility to delamination, the use of the multilayer coatings consisting of alternate layers of Al2O3 and ZnO was proposed. The multilayer architecture of coatings enables to obtain the most beneficial property combination of its individual constituents. In this case, the utilization of aluminum oxide improves the corrosion behavior, while the zinc oxide layer provides the expected antibacterial activity.
The Al2O3/ZnO multilayer coatings were deposited successfully on a commercial scalpel blade made of carbon steel. The presence of the ZnAl2O4 structure was confirmed using a XRD and a Raman spectrometer. The results of the performed electrochemical tests revealed that the corrosion resistance increase with the number of Al2O3/ZnO bilayers, which was evidenced by a decrease in corrosion current density and an increase in polarization resistance values. The comparison with the previously investigated ZnO thin films indicated the superior corrosion resistance of the multilayer coatings. Therefore, the application of the multilayer ALD coatings can be considered as a promising method to enhance the durability of surgical instruments made of carbon steel, such as scalpel blades, potentially making them reusable. Future work will further investigate the effect of the use a multilayer architecture on the bactericidal properties and wear resistance of coatings.
Author Contributions
Conceptualization, M.M.S. and M.S.; methodology, M.M.S., M.S., K.M. and J.B.; software, M.M.S., M.S., J.B. and K.M.; validation, M.M.S. and M.S.; formal analysis, M.M.S., M.S., J.B. and K.M.; investigation, M.M.S., M.S. and J.B.; resources, M.M.S. and M.S.; data curation, M.M.S., M.S., J.B. and K.M.; writing—original draft preparation, M.M.S., M.S. and J.B.; writing—review and editing, M.M.S. and M.S.; visualization, M.M.S., M.S. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fenker, M.; Balzer, M.; Kappl, H. Corrosion protection with hard coatings on steel: Past approaches and current research efforts. Surf. Coat. Technol. 2014, 257, 182–205. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Dobrzańska-Danikiewicz, A.D. Obróbka Powierzchni Materiałów Inżynierskich; Open Access Library: Gliwice, Poland, 2011; Volume 5, pp. 1–480. [Google Scholar]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M.; Bončina, T.; Kek Merl, D. Influence of Growth Defects on the Corrosion Resistance of Sputter-Deposited TiAlN Hard Coatings. Coatings 2019, 9, 511. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar]
- Ritala, M.; Leskelä, M. Atomic Layer Deposition. In Handbook of Thin Films Materials: Deposition and Processing of Thin Films; Nalwa, H.S., Ed.; Academic Press: Cambridge, MA, USA, 2002; Volume 1, pp. 103–159. [Google Scholar]
- Santinacci, L. Atomic layer deposition: An efficient tool for corrosion protection. Curr. Opin. Colloid Interface Sci. 2023, 63, 101674. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Andreatta, F.; Lekka, M.; Guzman, L.; Lorenzo Fedrizzi, L. Atomic layer deposition: State-of-the-art and research/industrial perspectives. Corros. Rev. 2011, 29, 191–208. [Google Scholar]
- Marin, E.; Fedrizzi, L. Atomic layer deposition an innovative technology to improve corrosion and surface functionalities of alloys. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–89. [Google Scholar]
- Pehkonen, S.O.; Yuan, S. The inorganic film coatings for corrosion protection. In Tailored Thin Coatings for Corrosion Inhibition Using a Molecular Approach, 1st ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 185–255. [Google Scholar]
- Matero, R.; Ritala, M.; Leskelä, M.; Salo, T.; Aromaa, J.; Forsén, O. Atomic layer deposited thin films for corrosion protection. J. Phys. IV 1999, 9, 493–499. [Google Scholar]
- Szindler, M.M.; Szindler, M.; Basiaga, M.; Łoński, W.; Kaim, P. Application of ALD thin films on the surface of the surgical scalpel blade. Coatings 2021, 11, 1096. [Google Scholar] [CrossRef]
- Staszuk, M.; Pakuła, D.; Reimann, Ł.; Król, M.; Basiaga, M.; Mysłek, D.; Kříž, A. Structure and properties of ZnO coatings obtained by atomic layer deposition (ALD) method on a Cr-Ni-Mo steel substrate type. Materials 2020, 13, 4223. [Google Scholar] [CrossRef]
- Basiaga, M.; Paszenda, Z.; Lisoń, J.; Taratuta, A.; Kazek-Kęsik, A.; Krok-Borkowic, M.; Nuckowsk, P.; Szindler, M.; Staszuk, M.; Major, Ł.; et al. Microstructure and antibacterial properties of a ZnO coating on a biomaterial Surface. Arch. Civ. Mech. Eng. 2022, 22, 93. [Google Scholar] [CrossRef]
- Nazarov, D.; Kozlova, L.; Rogacheva, E.; Kraeva, L.; Maximov, M. Atomic Layer Deposition of Antibacterial Nanocoatings: A Review. Antibiotics 2023, 12, 1656. [Google Scholar] [CrossRef]
- Staszuk, M. Investigations of CrN+Cr2O3/TiO2 coatings obtained in a PVD/ALD hybrid method on austenitic 316L steel substrate. Vacuum 2023, 207, 111653. [Google Scholar]
- Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Normand, B.; Härkönen, E.; Marcus, P. Electrochemical and time-of-flight secondary ion mass spectrometry analysis of ultra-thin metal oxide (Al2O3 and Ta2O5) coatings deposited by atomic layer deposition on stainless steel. Electrochim. Acta 2011, 56, 10516–10523. [Google Scholar]
- Marin, E.; Guzman, L.; Lanzutti, A.; Ensinger, W.; Fedrizzi, L. Multilayer Al2O3/TiO2 Atomic Layer Deposition coatings for the corrosion protection of stainless steel. Thin Solid Film. 2012, 522, 283288. [Google Scholar] [CrossRef]
- Pini, S.; Groppetti, R.; Mucchino, C.; Geretto, V. Evaluation of DLC, WC/C, and TiN coatings on martensitic stainless steel and yttria-stabilized tetragonal zirconia polycrystal substrates for reusable surgical scalpels. ISRN Ceram. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Tsai, P.; Lin, Y.; Li, J.; Jian, S.; Jang, J.; Li, C.; Chu, J.; Huang, J.C.C. Sharpness improvement of surgical blade by means of ZrCuAlAgSi metallic glass and metallic glass thin film coating. Intermetallics 2012, 31, 127–131. [Google Scholar] [CrossRef]
- Chu, J.P.; Diyatmika, W.; Tseng, Y.-J.; Liu, Y.-K.; Liao, W.-C.; Chang, S.-H.; Chen, M.-J.; Lee, J.-W.; Jang, J.S.C. Coating Cutting Blades with Thin-Film Metallic Glass to Enhance Sharpness. Sci. Rep. 2019, 9, 15558. [Google Scholar]
- Alias, R.; Rizwan, M.; Mahmoodian, R.; Vellasamy, K.M.; Hamdi, M. Physico-chemical and antimicrobial properties of Ag/Ta2O5 nanocomposite coatings. Ceram. Int. 2021, 47, 24139–24148. [Google Scholar]
- Świeczko-Żurek, B. Biomateriały, 1st ed.; Wydawnictwo Politechniki Gdańskiej: Gdańsk, Poland, 2009; pp. 1–151. [Google Scholar]
- Tripp, K.M.; Stampfer, C.; Miller, D.C.; Helbling, T.; Herrmann, C.F.; Hierold, C.; Gall, K.; George, S.M.; Bright, V.M. The mechanical properties of atomic layer deposited alumina for use in micro- and nano-electromechanical systems. Sens. Actuators A Phys. 2006, 130–131, 419–429. [Google Scholar]
- Hsain, Z.; Zeng, G.; Strandwitz, N.C.; Brandon, A.; Krick, B.A. Wear behavior of annealed atomic layer deposited alumina. Wear 2017, 372–373, 139–144. [Google Scholar] [CrossRef]
- Lorenzo-Martin, C.; Ajayi, O.O.; Hartman, K.; Bhattacharya, S.; Yacout, A. Effect of Al2O3 coating on fretting wear performance of Zr alloy. Wear 2019, 426–427 Pt A, 219–227. [Google Scholar]
- Cloud, A.N.; Kumar, S.; Kavdia, M.; Abu-Safe, H.H.; Gordon, M.H. Protein adsorption on low temperature alpha alumina films for surgical instruments. Surf. Coat. Technol. 2008, 203, 913–917. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Xing, T.; He, A.; Huang, Z.; Liu, X.; Chen, F.; Xu, W.; Zhao, S.; Liu, Y.; et al. Full-Color Tunable and Highly Fire-Retardant Colored Carbon Fibers. Adv. Fiber Mater. 2023, 5, 1618–1631. [Google Scholar]
- Ylivaara, O.M.E.; Liu, X.; Kilpi, L.; Lyytinen, J.; Schneider, S.; Laitinen, M.; Julin, J.; Ali, S.; Sintonen, S.; Berdova, M.; et al. Aluminum oxide from trimethylaluminum and water by atomic layer deposition: The temperature dependence of residual stress, elastic modulus, hardness and adhesion. Thin Solid Film. 2014, 552, 124–135. [Google Scholar] [CrossRef]
- Karvonen, L.; Säynätjoki, A.; Chen, Y.; Jussila, H.; Rönn, J.; Ruoho, M.; Alasaarela, T.; Kujala, S.; Norwood, R.A.; Peyghambarian, N.; et al. Enhancement of the third-order optical nonlinearity in ZnO/Al2O3 nanolaminates fabricated by atomic layer deposition. Appl. Phys. Lett. 2013, 103, 031903. [Google Scholar] [CrossRef]
- Osorio, D.; Lopez, J.; Tiznado, H.; Farias, M.H.; Hernandez-Landaverde, M.A.; Ramirez-Cardona, M.; Yañez-Limon, J.M.; Gutierrez, J.O.; Caicedo, J.C.; Zambrano, G. Structure and Surface Morphology Effect on the Cytotoxicity of [Al2O3/ZnO]n/316L SS Nanolaminates Growth by Atomic Layer Deposition (ALD). Crystals 2020, 10, 620. [Google Scholar] [CrossRef]
- Levy, D.; Pavese, A.; Sani, A.; Pischedda, V. Structure and Compressibility of Synthetic ZnAl2O4 (Gahnite) Under High-Pressure Conditions, from Synchrotron X-Ray Powder Diffraction. Phys. Chem. Miner. 2001, 28, 612–618. [Google Scholar] [CrossRef]
- Yoshioka, S.; Oba, F.; Huang, R.; Tanaka, I.; Mizoguchi, T.; Yamamoto, T. Atomic structures of supersaturated ZnO–Al2O3 solid solutions. J. Appl. Phys. 2008, 103, 014309. [Google Scholar]
- Zhang, Y.; Lai, P.; Jia, H.; Ju, X.; Cui, G. Investigation of Test Parameters on EBSD Analysis of Retained Austenite in TRIP and Pipeline Steels. Metals 2019, 9, 94. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Y.; Cui, G.; Jia, H.; Ju, X.; Jiang, Z.; Ma, Z. Analysis of retained austenite in TRIP590 steel by electron backscattered diffraction method. J. Iron Steel Res. Int. 2020, 27, 355–365. [Google Scholar] [CrossRef]
- Banerjee, P.; Lee, W.-J.; Bae, K.-R.; Lee, S.B.; Rubloff, G.W. Structural, electrical, and optical properties of atomic layer deposition Al-doped ZnO films. J. Appl. Phys. 2010, 108, 043504. [Google Scholar]
- Pung, S.-Y.; Choy, K.-L.; Hou, X.; Shan, C. Preferential growth of ZnO thin films by the atomic layer deposition technique. Nanotechnology 2008, 19, 435609. [Google Scholar] [CrossRef] [PubMed]
- Lujala, V.; Skarp, J.; Tammenmaa, M.; Suntola, T. Atomic layer epitaxy growth of doped zinc oxide thin films from organometals. Appl. Surf. Sci. 1994, 82–83, 34–40. [Google Scholar] [CrossRef]
- Cheng, Y. Effects of post-deposition rapid thermal annealing on aluminum-doped ZnO thin films grown by atomic layer deposition. Appl. Surf. Sci. 2011, 258, 604–607. [Google Scholar]
- Basiaga, M.; Walke, W.; Antonowicz, M.; Kajzer, W.; Szewczenko, J.; Domanowska, A.; Michalewicz, A.; Szindler, M.; Staszuk, M.; Czajkowski, M. Impact of Surface Treatment on the Functional Properties Stainless Steel for Biomedical Applications. Materials 2020, 13, 4767. [Google Scholar] [CrossRef]
- Boryło, P.; Lukaszkowicz, K.; Szindler, M.; Kubacki, J.; Balin, K.; Basiaga, M.; Szewczenko, J. Structure and properties of Al2O3 thin films deposited by ALD process. Vacuum 2016, 131, 319–326. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).