Composite Edible Coating from Arabic Gum and Mango Peel Hydrocolloids Enriched with Mango Seed Extracts for the Preservation of Grapes (Vitis vinifera) During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Obtention of Mango Peel Hydrocolloids
2.4. Obtention of Mango Seed Extracts
2.5. Determination of Total Phenolic Content (TPC) and Antioxidant Activity
2.6. CG-MS Analysis
2.7. Preparation of Composite Edible Coating Solutions
2.8. Rheological Analysis
2.9. Application of the Coating to Grapes (Vitis vinifera) and Quality Parameters
2.9.1. Fungal Decay Percentage
2.9.2. Determination of Weight Loss
2.9.3. Determination of pH and Titratable Acidity
2.9.4. Color Analysis
2.10. Statistical Analysis
3. Results
3.1. Results of Mango Seed Extracts
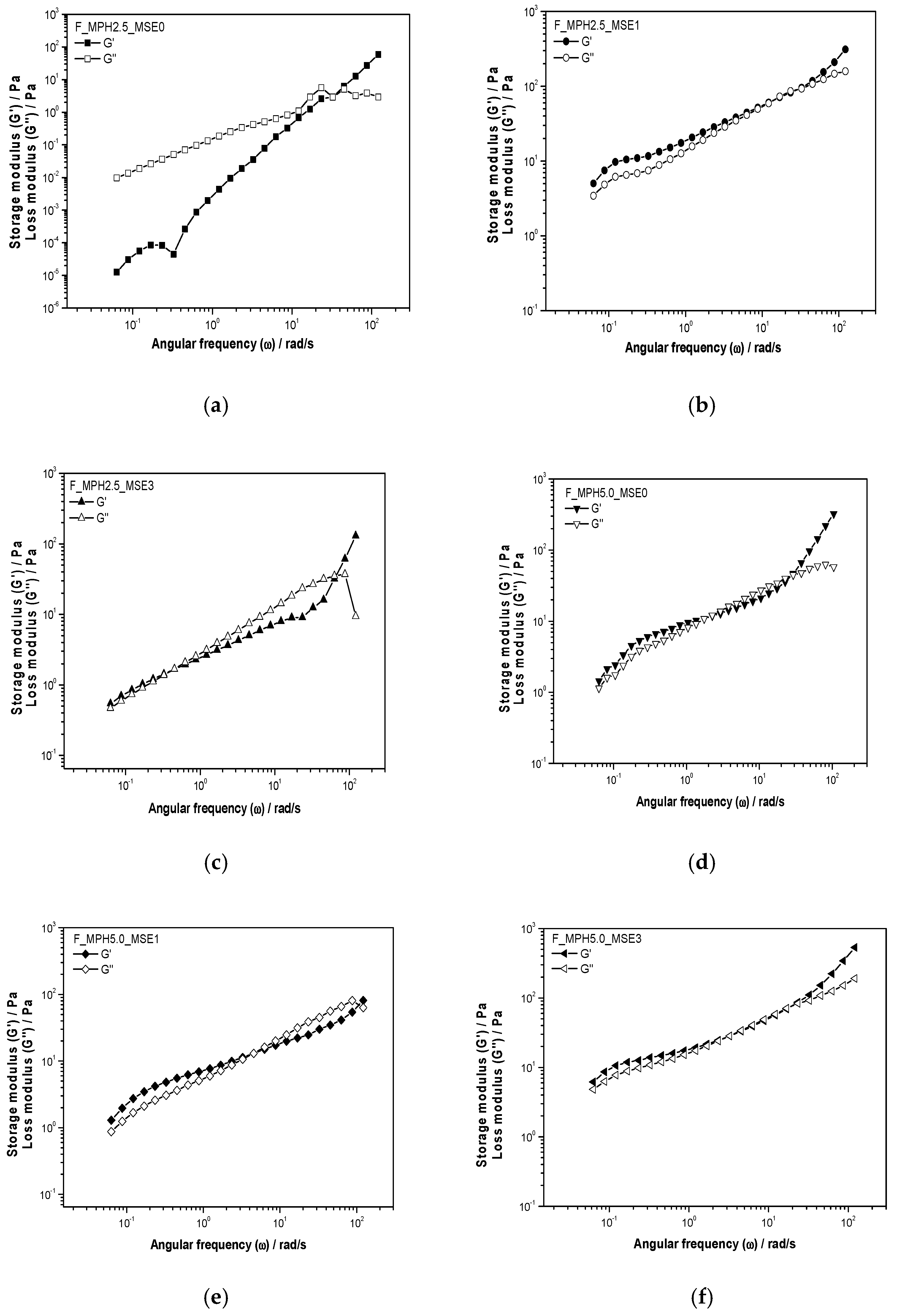
3.2. Rheology of Edible Coating Solutions
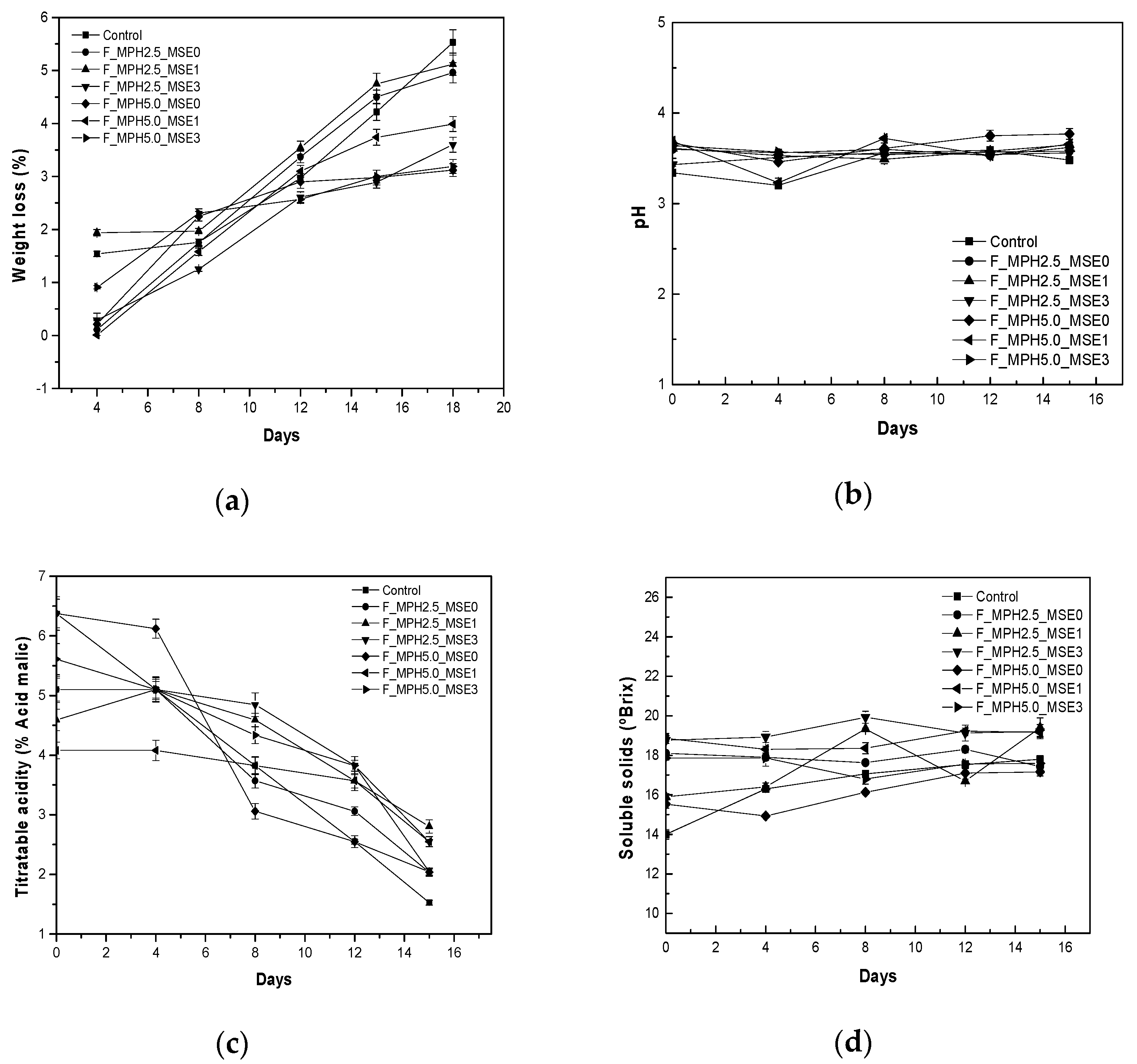
3.3. Application of Edible Coating Solution for the Preservation of Grapes (Vitis vinifera)
4. Discussion
4.1. Mango Seed Extracts
4.1.1. Total Phenolic Compounds and Antioxidant Capacity
4.1.2. CG-MS
4.2. Rheological Properties of Edible Coating Solution
4.2.1. Stationary Behavior
4.2.2. Dynamic Behavior
4.3. Application of Edible Coating Solution for the Preservation of Grapes (Vitis vinifera)
4.3.1. Physicochemical Properties
4.3.2. Loss of Weight
4.3.3. pH and Titratable Acidity (TA)
4.3.4. Soluble Solids
4.3.5. Color Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Glen, L.; Creasy, L.L.C. Grapes, 2nd ed.; CABI: New York, NY, USA, 2018. [Google Scholar]
- Dhekney, S.A. Grapes. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 261–265. [Google Scholar] [CrossRef]
- de Sousa, L.L.; de Andrade, S.C.A.; Athayde, A.J.A.A.; de Oliveira, C.E.V.; de Sales, C.V.; Madruga, M.S.; de Souza, E.L. Efficacy of Origanum vulgare L. and Rosmarinus officinalis L. Essential Oils in Combination to Control Postharvest Pathogenic Aspergilli and Autochthonous Mycoflora in Vitis labrusca L. (Table Grapes). Int. J. Food Microbiol. 2013, 165, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Castelo Branco Melo, N.F.; de MendonçaSoares, B.L.; Marques Diniz, K.; Ferreira Leal, C.; Canto, D.; Flores, M.A.P.; Henrique da Costa Tavares-Filho, J.; Galembeck, A.; Montenegro Stamford, T.L.; Montenegro Stamford-Arnaud, T.; et al. Effects of Fungal Chitosan Nanoparticles as Eco-Friendly Edible Coatings on the Quality of Postharvest Table Grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Bourtoom, T. Review Article Edible Films and Coatings: Characteristics and Properties. J. Int. Food Res. 2008, 15, 237–248. [Google Scholar]
- Debeaufort, F.; Quezada-Gallo, J.A.; Voilley, A. Edible Barriers: A Solution to Control Water Migration in Foods. ACS Symp. Ser. 2000, 753, 9–16. [Google Scholar] [CrossRef]
- Han, J.H. Edible Films and Coatings: A Review. In Innovations in Food Packaging, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 213–255. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the Functionality of Chitosan- and Alginate-Based Active Edible Coatings/Films for the Preservation of Fruits and Vegetables: A Review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Tiamiyu, Q.O.; Adebayo, S.E.; Yusuf, A.A. Gum Arabic Edible Coating and Its Application in Preservation of Fresh Fruits and Vegetables: A Review. Food Chem. Adv. 2023, 2, 100251. [Google Scholar] [CrossRef]
- Debeaufort, F.; Voilley, A. Lipid-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 135–168. [Google Scholar]
- Shit, S.; Shah, P. Edible Polymers: Challenges and Opportunities. J. Polym. 2014, 2014, 427259. [Google Scholar] [CrossRef]
- Brody, S.D.; Davis, S.E.; Highfield, W.E.; Bernhardt, S.P. A Spatial-Temporal Analysis of Section 404 Wetland Permitting in Texas and Florida: Thirteen Years of Impact along the Coast. Wetlands 2008, 28, 107–116. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. The Use of Packaging Techniques to Maintain Freshness in Fresh-Cut Fruits and Vegetables: A Review. Int. J. Food Sci. Technol. 2009, 44, 875–889. [Google Scholar] [CrossRef]
- Alali, A.A.; Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A. Postharvest Gum Arabic and Salicylic Acid Dipping Affect Quality and Biochemical Changes of ‘Grand Nain’ Bananas during Shelf Life. Sci Hortic 2018, 237, 51–58. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of Gum Arabic and Aloe Vera Gel Based Edible Coatings in Combination with Plant Extracts on Postharvest Quality and Storability of ‘Gola’ Guava Fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Ali, A.; Khan Cheong, C.; Zahid, N. Composite Effect of Propolis and Gum Arabic to Control Postharvest Anthracnose and Maintain Quality of Papaya during Storage. Int. J. Agric. Biol 2013, 16, 11171122. [Google Scholar]
- Khaliq, G.; Muda Mohamed, M.T.; Ghazali, H.M.; Ding, P.; Ali, A. Influence of Gum Arabic Coating Enriched with Calcium Chloride on Physiological, Biochemical and Quality Responses of Mango (Mangifera indica L.) Fruit Stored under Low Temperature Stress. Postharvest Biol. Technol. 2016, 111, 362–369. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ali, S.; Canan, İ. Postharvest Application of Gum Arabic Edible Coating Delays Ripening and Maintains Quality of Persimmon Fruits during Storage. J. Food Process Preserv. 2020, 44, e14583. [Google Scholar] [CrossRef]
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P.G. Gum Arabic as a Novel Edible Coating for Enhancing Shelf-Life and Improving Postharvest Quality of Tomato (Solanum lycopersicum L.) Fruit. Postharvest Biol. Technol. 2010, 58, 42–47. [Google Scholar] [CrossRef]
- Ali, S.; Akbar Anjum, M.; Nawaz, A.; Naz, S.; Ejaz, S.; Shahzad Saleem, M.; Tul-Ain Haider, S.; Ul Hasan, M. Effect of Gum Arabic Coating on Antioxidative Enzyme Activities and Quality of Apricot (Prunus armeniaca L.) Fruit during Ambient Storage. J. Food Biochem. 2021, 45, e13656. [Google Scholar] [CrossRef]
- Huang, Q.; Wan, C.; Zhang, Y.; Chen, C.; Chen, J. Gum Arabic Edible Coating Reduces Postharvest Decay and Alleviates Nutritional Quality Deterioration of Ponkan Fruit During Cold Storage. Front. Nutr. 2021, 8, 717596. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and Characterization of Composite Edible Films Based on Sodium Alginate and Pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, Mechanical and Barrier Properties of Bio-Based Composite Films Based on Chitosan and Whey Protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and Active Films and Coatings as Carriers of Natural Antioxidants for Lipid Food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in Fruits and Vegetables - the Millennium’s Health. Int J Food Sci Technol 2008, 36, 703–725. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 450160. [Google Scholar] [CrossRef] [PubMed]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K. Physicochemical Characteristics, Bioactive Compounds and Industrial Applications of Mango Kernel and Its Products: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2421–2446. [Google Scholar] [CrossRef]
- Marsiglia-Fuentes, R.; Quintana, S.E.; García Zapateiro, L.A. Novel Hydrocolloids Obtained from Mango (Mangifera Indica) Var. Hilaza: Chemical, Physicochemical, Techno-Functional, and Structural Characteristics. Gels 2022, 8, 354. [Google Scholar] [CrossRef]
- Quintana, S.E.; Llalla, O.; García-Zapateiro, L.A.; García-Risco, M.R.; Fornari, T. Preparation and Characterization of Licorice-Chitosan Coatings for Postharvest Treatment of Fresh Strawberries. Appl. Sci. 2020, 10, 8431. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Quintana, S.E.; Villanueva-Bermejo, D.; Reglero, G.; García-Risco, M.R.; Fornari, T. Supercritical Antisolvent Particle Precipitation and Fractionation of Rosemary (Rosmarinus officinalis L.) Extracts. J. CO2 Util. 2019, 34, 479–489. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of Chitosan Coatings on the Physicochemical Characteristics of Eksotika II Papaya (Carica papaya L.) Fruit during Cold Storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Mieles-Gómez, L.; Lastra-Ripoll, S.E.; Torregroza-Fuentes, E.; Quintana, S.E.; García-Zapateiro, L.A. Rheological and Microstructural Properties of Oil-in-Water Emulsion Gels Containing Natural Plant Extracts Stabilized with Carboxymethyl Cellulose/Mango (Mangiferaindica) Starch. Fluids 2021, 6, 312. [Google Scholar] [CrossRef]
- Vetal, M.D.; Chavan, R.S.; Rathod, V.K. Microwave Assisted Extraction of Ursolic Acid and Oleanolic Acid from Ocimum Sanctum. Biotechnol. Bioprocess. Eng. 2014, 19, 720–726. [Google Scholar] [CrossRef]
- Martínez-Olivo, A.O.; Zamora-Gasga, V.M.; Medina-Torres, L.; Pérez-Larios, A.; Sánchez-Burgos, J.A. Formulation of Double Emulsions of Mango Seed Extract (Mangifera indica L.) “Ataulfo” Incorporated into a Mango by-Product Flour Drink: Release Kinetics, Antioxidant Capacity, and Inhibition of Cyclooxygenases. Food Hydrocoll. Health 2023, 3, 100120. [Google Scholar] [CrossRef]
- Ashoush, I.S.; Abdel-Razik, M.M.; Yassin, N.M.N. Characteristics of Mango Seed Kernel Butter and Its Effects on Quality Attributes of Muffins. Alex. J. Food Sci. Technol. 2012, 9, 1–9. [Google Scholar]
- Pereira Farias, N.N.; Freitas, E.R.; Nepomuceno, R.C.; Marques Gomes, H.; Herik Souza, D.; Costa, M.K.d.O.; Soares da Costa, H.; Fernandes, D.R.; Santos Araújo, L.R.; Jerônimo do Nascimento, G.A.; et al. Ethanolic Extract of Mango Seed in Broiler Feed: Effect on Productive Performance, Segments of the Digestive Tract and Blood Parameters. Anim. Feed. Sci. Technol. 2021, 279, 114999. [Google Scholar] [CrossRef]
- Odabaş, H.İ.; Koca, I. Application of Response Surface Methodology for Optimizing the Recovery of Phenolic Compounds from Hazelnut Skin Using Different Extraction Methods. Ind. Crops Prod. 2016, 91, 114–124. [Google Scholar] [CrossRef]
- Luo, X.; Cui, J.; Zhang, H.; Duan, Y.; Zhang, D.; Cai, M.; Chen, G. Ultrasound Assisted Extraction of Polyphenolic Compounds from Red Sorghum (Sorghum bicolor L.) Bran and Their Biological Activities and Polyphenolic Compositions. Ind. Crops Prod. 2018, 112, 296–304. [Google Scholar] [CrossRef]
- Castañeda-Valbuena, D.; Ayora-Talavera, T.; Luján-Hidalgo, C.; Álvarez-Gutiérrez, P.; Martínez-Galero, N.; Meza-Gordillo, R. Ultrasound Extraction Conditions Effect on Antioxidant Capacity of Mango By-Product Extracts. Food Bioprod. Process. 2021, 127, 212–224. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Ayala-Zavala, J.F.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; Miranda, M.R.; Silva-Espinoza, B.A. Using Sensory Evaluation to Determine the Highest Acceptable Concentration of Mango Seed Extract as Antibacterial and Antioxidant Agent in Fresh-Cut Mango. Foods 2018, 7, 120. [Google Scholar] [CrossRef]
- Kumar Nagendla, N.; Muralidharan, K.; Raju, M.; Harshvardhan, M.; Selvakumar, P.; Mohan Bhandi, M.; Krishna Reddy Mudiam, M.; Ramalingam, V. Comprehensive Metabolomic Analysis of Mangifera Indica Leaves Using UPLC-ESI-Q-TOF-MSE for Cell Differentiation: An in Vitro and in Vivo Study. Food Res. Int. 2023, 171, 112993. [Google Scholar] [CrossRef]
- Ragheb, A.Y.; El-Ansari, M.A.; Heikal, O.A.; Galal, A.F.; Salama, A.A.A.; Kassem, M.E.S.; Saleh, N.A.M. Phenolic Glycosides and Bioactive Mangifera indica L. Kernel Extract as Neuroprotective Agents against LPS-Induced Alzheimer’s Disease in Rats. S. Afr. J. Bot. 2023, 157, 37–43. [Google Scholar] [CrossRef]
- Prabhu, K.; Prasathkumar, M.; Sivaraman, J.; Sadhasivam, S.; Gajdács, M.; Gasimov, E.K.; Umar Khayam Sahibzada, M.; Almehmadi, M.; Abdulaziz, O. Phytochemical Characterization, Antibacterial, and Anti-Biofilm Efficacy of Mangifera Indica Seed Kernel: A Preliminary Study Using in Vitro and in Silico Approaches. J. King Saud. Univ. Sci. 2023, 35, 102688. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gurjar, P.S.; Beer, K.; Pongener, A.; Ravi, S.C.; Singh, S.; Verma, A.; Singh, A.; Thakur, M.; Tripathy, S.; et al. A Review on Valorization of Different Byproducts of Mango (Mangifera indica L.) for Functional Food and Human Health. Food Biosci. 2022, 48, 101783. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional Composition and Bioactive Compounds in Three Different Parts of Mango Fruit. Int. J. Environ. Res. Public. Health 2021, 18, 741. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kuo, W.-S.; Lai, L.-S. Rheological and Physical Characterization of Film-Forming Solutions and Edible Films from Tapioca Starch/Decolorized Hsian-Tsao Leaf Gum. Food Hydrocoll. 2009, 23, 2132–2140. [Google Scholar] [CrossRef]
- Jiang, Y.; Reddy, C.K.; Huang, K.; Chen, L.; Xu, B. Hydrocolloidal Properties of Flaxseed Gum/Konjac Glucomannan Compound Gel. Int. J. Biol. Macromol. 2019, 133, 1156–1163. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, E.; Rousta, E. Shelf-Life Extension of Tomato (Solanum lycopersicum L.) Using an Edible Coating of Bitter Almond Gum-Fish Gelatin Conjugates. Prog. Org. Coat. 2022, 170, 106980. [Google Scholar] [CrossRef]
- Isopencu, G.O.; Stoica-Guzun, A.; Busuioc, C.; Stroescu, M.; Deleanu, I.M. Development of Antioxidant and Antimicrobial Edible Coatings Incorporating Bacterial Cellulose, Pectin, and Blackberry Pomace. Carbohydr. Polym. Technol. Appl. 2021, 2, 100057. [Google Scholar] [CrossRef]
- Rodrigues, M.Á.V.; Bertolo, M.R.V.; Marangon, C.A.; Martins, V.d.C.A.; Plepis, A.M.d.G. Chitosan and Gelatin Materials Incorporated with Phenolic Extracts of Grape Seed and Jabuticaba Peel: Rheological, Physicochemical, Antioxidant, Antimicrobial and Barrier Properties. Int. J. Biol. Macromol. 2020, 160, 769–779. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Polyphenol-Rich Extract from Murta Leaves on Rheological Properties of Film-Forming Solutions Based on Different Hydrocolloid Blends. J. Food Eng. 2014, 140, 28–38. [Google Scholar] [CrossRef]
- Cofelice, M.; Cuomo, F.; Lopez, F. Rheological Properties of Alginate–Essential Oil Nanodispersions. Colloids Interfaces 2018, 2, 48. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: East Lansing, MI, USA, 1996; ISBN 0963203614. [Google Scholar]
- Salazar, D.; Arancibia, M.; Lalaleo, D.; Rodríguez-Maecker, R.; López-Caballero, M.E.; Montero, M.P. Physico-Chemical Properties and Filmogenic Aptitude for Edible Packaging of Ecuadorian Discard Green Banana Flours (Musa acuminanta AAA). Food Hydrocoll. 2022, 122, 107048. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Thermophysical Properties of Chitosan, Chitosan–Starch and Chitosan–Pullulan Films near the Glass Transition. Carbohydr. Polym. 2002, 48, 179–190. [Google Scholar] [CrossRef]
- Pereira, J.; Malairaj, S.; Brohi, S.A.; Boateng, E.F.; Zhang, W. Impact of Unripe Banana Flour on Water States, Rheological Behaviour and Structural Properties of Myofibrillar Protein Composite Gel. LWT 2020, 125, 109276. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Souki, N.P.D.B.G.; Moraes, I.C.F.; Pinho, S.C. Rheological and Mechanical Characterization of Curcumin-Loaded Emulsion-Filled Gels Produced with Whey Protein Isolate and Xanthan Gum. LWT 2017, 86, 166–173. [Google Scholar] [CrossRef]
- Vargas, M.; Chiralt, A.; Albors, A.; González-Martínez, C. Effect of Chitosan-Based Edible Coatings Applied by Vacuum Impregnation on Quality Preservation of Fresh-Cut Carrot. Postharvest Biol. Technol. 2009, 51, 263–271. [Google Scholar] [CrossRef]
- Yousuf, B.; Srivastava, A.K. A Novel Approach for Quality Maintenance and Shelf Life Extension of Fresh-Cut Kajari Melon: Effect of Treatments with Honey and Soy Protein Isolate. LWT-Food Sci. Technol. 2017, 79, 568–578. [Google Scholar] [CrossRef]
- Kanetis, L.; Exarchou, V.; Charalambous, Z.; Goulas, V. Edible Coating Composed of Chitosan and Salvia fruticosa Mill. Extract for the Control of Grey Mould of Table Grapes. J. Sci. Food Agric. 2017, 97, 452–460. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Pastor, C.; Vargas, M.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of Hydroxypropylmethylcellulose and Chitosan Coatings with and without Bergamot Essential Oil on Quality and Safety of Cold-Stored Grapes. Postharvest Biol. Technol. 2011, 60, 57–63. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of Chitosan Coating on Postharvest Life and Quality of Guava (Psidium guajava L.) Fruit during Cold Storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z.; Rahmati, E. Effect of Edible Coatings on the Shelf-Life of Fresh Strawberries: A Comparative Study Using TOPSIS-Shannon Entropy Method. NFS J. 2021, 23, 17–23. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Amraie, M.; Salehi, M.; Mohseni, M.; Aloui, H. Effect of Chitosan-Based Coatings Enriched with Savory and/or Tarragon Essential Oils on Postharvest Maintenance of Kumquat (Fortunella sp.) Fruit. Food Sci. Nutr. 2019, 7, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tesfay, S.Z.; Magwaza, L.S. Evaluating the Efficacy of Moringa Leaf Extract, Chitosan and Carboxymethyl Cellulose as Edible Coatings for Enhancing Quality and Extending Postharvest Life of Avocado (Persea americana Mill.) Fruit. Food Packag. Shelf Life 2017, 11, 40–48. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of Chitosan–Aloe Vera Coating on Postharvest Quality of Blueberry (Vaccinium corymbosum) Fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Mendes Aroucha, E.; Vilson Alves, G.; De Lima, R.H.; Aroucha Santos, M.; Souza Sobreira, M. Acidez em frutas e hortaliças. Rev. Verde De Agroecol. E Desenvolv. Sustentáve 2010, 5, 1–4. [Google Scholar]
- Oz, A.T.; Ulukanli, Z. Application of Edible Starch-Based Coating Including Glycerol plus Oleum Nigella on Arils from Long-Stored Whole Pomegranate Fruits. J. Food Process Preserv. 2012, 36, 81–95. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Nawaz, A.; Anjum, M.A.; Naz, S.; Ejaz, S.; Hussain, S. Aloe Vera Gel Coating Delays Postharvest Browning and Maintains Quality of Harvested Litchi Fruit. Postharvest Biol. Technol. 2019, 157, 110960. [Google Scholar] [CrossRef]
- El-Anany, A.M.; Hassan, G.F.A.; Ali, F.M.R. Effects of Edible Coatings on the Shelf-Life and Quality of Anna Apple (Malus Domestica Borkh) during Cold Storage. J. Food Technol. 2009, 7, 5–11. [Google Scholar]
- Antunes, M.D.C.; Correia, M.P.; Miguel, M.G.; Martins, M.A.; Neves, M.A. The Effect of Calcium Chloride Postharvest Application on Fruit Storage Ability and Quality of ‘Beliana’ and ‘Lindo’ Apricot (Prunus armeniaca L.) Cultivars. Acta Hortic. 2003, 604, 721–726. [Google Scholar] [CrossRef]
- Verma, M.G.T.A.-M.G.T.A.-R.R.S.A.-S.S.A.-M.K. Effect of Edible Coatings on ‘Misty’ Blueberry (Vaccinium corymbosum) Fruits Stored at Low Temperature. Acta Physiol. Plant 2019, 41, 183. [Google Scholar] [CrossRef]
- Haider, S.T.-A.; Ahmad, S.; Khan, A.S.; Basra, M.A. Comparison of Different Fruit Coatings to Enhance the Shelflife of Kinnow Mandarin. Pak. J. Agric. Sci. 2017, 54. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Sardar, H.; Saddiq, B. Tragacanth Gum Coating Modulates Oxidative Stress and Maintains Quality of Harvested Apricot Fruits. Int. J. Biol. Macromol. 2020, 163, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- Bhan, C.; Asrey, R.; Meena, N.K.; Rudra, S.G.; Chawla, G.; Kumar, R.; Kumar, R. Guar Gum and Chitosan-Based Composite Edible Coating Extends the Shelf Life and Preserves the Bioactive Compounds in Stored Kinnow Fruits. Int. J. Biol. Macromol. 2022, 222, 2922–2935. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vázquez, R.; Stinco, C.M.; Meléndez-Martínez, A.J.; Heredia, F.J.; Vicario, I.M. Visual and Instrumental Evaluation of Orange Juice Color: A Consumers’preference Study. J. Sens. Stud. 2011, 26, 436–444. [Google Scholar]
- Rodríguez-Pulido, F.J.; Gómez-Robledo, L.; Melgosa, M.; Gordillo, B.; González-Miret, M.L.; Heredia, F.J. Ripeness Estimation of Grape Berries and Seeds by Image Analysis. Comput. Electron. Agric. 2012, 82, 128–133. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Quality of Cold-Stored Strawberries as Affected by Chitosan–Oleic Acid Edible Coatings. Postharvest Biol. Technol. 2006, 41, 164–171. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, Y.; Wang, D.; Zhang, L.; Sun, J.; Sun, L.; Zhang, B. Effect of Alginate Coating Combined with Yeast Antagonist on Strawberry (Fragaria×ananassa) Preservation Quality. Postharvest Biol. Technol. 2009, 53, 84–90. [Google Scholar] [CrossRef]
- Neto, F.J.D.; Tecchio, M.A.; Pimentel, A.; Vedoato, B.T.F.; Lima, G.P.P.; Roberto, S.R. Effect of ABA on Colour of Berries, Anthocyanin Accumulation and Total Phenolic Compounds of ‘Rubi’ table Grape (‘Vitis vinifera’). Aust. J. Crop Sci. 2017, 11, 199–205. [Google Scholar] [CrossRef]
- Chamizo-González, F.; Estévez, I.G.; Gordillo, B.; Manjón, E.; Escribano-Bailón, M.T.; Heredia, F.J.; González-Miret, M.L. First Insights into the Binding Mechanism and Colour Effect of the Interaction of Grape Seed 11S Globulin with Malvidin 3-O-Glucoside by Fluorescence Spectroscopy, Differential Colorimetry and Molecular Modelling. Food Chem. 2023, 413, 135591. [Google Scholar] [CrossRef]
- Shahab, M.; Roberto, S.R.; Ahmed, S.; Colombo, R.C.; Silvestre, J.P.; Koyama, R.; de Souza, R.T. Relationship between Anthocyanins and Skin Color of Table Grapes Treated with Abscisic Acid at Different Stages of Berry Ripening. Sci. Hortic. 2020, 259, 108859. [Google Scholar]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J. Evolution of Colour and Phenolic Compounds during Garnacha Tintorera Grape Raisining. Food Chem. 2013, 141, 3230–3240. [Google Scholar]
- Serratosa, M.P.; Lopez-Toledano, A.; Merida, J.; Medina, M. Changes in Color and Phenolic Compounds during the Raisining of Grape Cv. Pedro Ximenez. J. Agric. Food Chem. 2008, 56, 2810–2816. [Google Scholar] [PubMed]

| No. | Code Sample | Mango Peel Hydrocolloid % | Mango Seed Extracts % | Arabic Gum % | Chitosan % | Glycerol % |
|---|---|---|---|---|---|---|
| 1. | F_MPH2.5_MSE0 | 2.5 | 0 | 1 | 1 | 1 |
| 2. | F_MPH2.5_MSE1 | 2.5 | 1 | 1 | 1 | 1 |
| 3. | F_MPH2.5_MSE3 | 2.5 | 3 | 1 | 1 | 1 |
| 4. | F_MPH5.0_MSE0 | 5.0 | 0 | 1 | 1 | 1 |
| 5. | F_MPH5.0_MSE1 | 5.0 | 1 | 1 | 1 | 1 |
| 6. | F_MPH5.0_MSE3 | 5.0 | 3 | 1 | 1 | 1 |
| No. | Code Sample | Power W | Yield % | Total Phenolic Compounds mg GAE/g of Extract | Antioxidant Capacity µmol Trolox/g of Extract |
|---|---|---|---|---|---|
| 1. | MSE180 | 180 | 25.20 ± 1.26 a | 25,024.97 ± 937.21 a | 404.94 ± 37.22 a |
| 2. | MSE360 | 360 | 26.66 ± 1.33 a | 24,640.14 ± 589.99 a | 428.32 ± 45.07 a |
| 3. | MSE540 | 540 | 20.40 ± 1.02 b | 16,057.71 ± 625.76 b | 425.21 ± 49.47 a |
| Code Sample | k | ||
|---|---|---|---|
| F_MPH2.5_MSE0 | 0.24 ± 0.01 a | 0.7.8 ± 0.01 a | 0.99 |
| F_MPH2.5_MSE1 | 4.49 ± 0.03 b | 0.55 ± 0.01 b | 0.99 |
| F_MPH2.5_MSE3 | 5.86 ± 0.12 c | 0.47 ± 0.01 c | 0.95 |
| F_MPH5.0_MSE0 | 3.51 ± 0.07 d | 0.68 ± 0.01 d | 0.98 |
| F_MPH5.0_MSE1 | 4.81 ± 0.02 e | 0.57 ± 0.01 e | 0.99 |
| F_MPH5.0_MSE3 | 26.20 ± 0.19 f | 0.50 ± 0.01 f | 0.99 |
| Code Sample | Day 0 | Day 4 | Day 8 | Day 12 | Day 15 |
|---|---|---|---|---|---|
| a* | |||||
| Control | 1.70 ± 0.26 a | 0.86 ± 0.05 ac | 0.60 ± 0.30 ab | 1.60 ± 0.40 ad | 1.73 ± 0.20 ab |
| F_MPH2.5_MSE0 | 0.46 ± 0.05 de | 1.00 ± 0.17 c | 1.83 ± 0.23 d | 1.96 ± 0.55 d | 2.02 ± 0.17 b |
| F_MPH2.5_MSE1 | 1.30 ± 0.26 b | 1.53 ± 0.25 d | 2.10 ± 0.10 d | 2.86 ± 0.37 e | 0.96 ± 0.30 c |
| F_MPH2.5_MSE3 | 0.36 ± 0.05 e | 0.66 ± 0.11 a | 1.20 ± 0.02 c | 1.40 ± 0.20 ab | 1.56 ± 0.18 a |
| F_MPH5.0_MSE0 | 0.76 ± 0.05 c | 0.78 ± 0.07 ac | 0.86 ± 0.20 b | 0.94 ± 0.05 b | 0.98 ± 0.12 c |
| F_MPH5.0_MSE1 | 0.63 ± 0.05 cde | 0.10 ± 0.00 b | 0.43 ± 0.15 a | 0.33 ± 0.05 c | 0.26 ± 0.02 d |
| F_MPH5.0_MSE3 | 0.73 ± 0.15 cd | 1.43 ± 0.30 d | 0.70 ± 0.00 ab | 1.40 ± 0.20 ab | 0.33 ± 0.23 d |
| b* | |||||
| Control | −2.13 ± 0.40 a | −1.23 ± 0.15 ab | −1.43 ± 0.25 a | −0.46 ± 0.05 a | −0.30 ± 0.10 ab |
| F_MPH2.5_MSE0 | −0.96 ± 0.30 c | −0.90 ± 0.28 b | −0.73 ± 0.05 c | −0.15 ± 0.07 b | −0.13 ± 0.05 a |
| F_MPH2.5_MSE1 | −1.33 ± 0.60 bc | −0.96 ± 0.57 ab | −1.00 ± 0.10 b | −1.30 ± 0.36 e | −1.20 ± 0.10 c |
| F_MPH2.5_MSE3 | −1.50 ± 0.17 bc | −1.43 ± 0.20 a | −1.23 ± 0.11 ab | −0.56 ± 0.05 ac | −0.39 ± 0.04 b |
| F_MPH5.0_MSE0 | −1.16 ± 0.05 bc | −1.40 ± 0.26 ab | −0.53 ± 0.11 cd | −0.96 ± 0.11 d | −1.02 ± 0.07 c |
| F_MPH5.0_MSE1 | −1.40 ± 0.36 bc | −0.93 ± 0.23 ab | −1.23 ± 0.25 ab | −0.80 ± 0.17 cd | −0.30 ± 0.23 ab |
| F_MPH5.0_MSE3 | −1.56 ± 0.15 ab | −0.93 ± 0.15 ab | −0.43 ± 0.15 d | −0.40 ± 0.17 ab | −0.37 ± 0.07 b |
| L* | |||||
| Control | 7.66 ± 0.11 ab | 5.33 ± 0.05 a | 5.56 ± 0.25 a | 6.63 ± 0.11 a | 9.23 ± 0.32 a |
| F_MPH2.5_MSE0 | 7.36 ± 0.15 a | 11.70 ± 0.20 f | 10.40 ± 0.36 e | 9.66 ± 0.32 c | 8.44 ± 0.21 c |
| F_MPH2.5_MSE1 | 9.20 ± 1.34 c | 7.06 ± 0.47 b | 8.36 ± 0.05 bc | 8.40 ± 0.34 b | 7.46 ± 0.32 d |
| F_MPH2.5_MSE3 | 8.43 ± 0.37 bc | 9.50 ± 0.45 d | 8.06 ± 0.05 b | 7.06 ± 0.30 a | 6.84 ± 0.22 e |
| F_MPH5.0_MSE0 | 8.50 ± 0.20 bc | 8.70 ± 0.45 c | 8.60 ± 0.26 c | 8.30 ± 0.53 b | 8.13 ± 0.21 c |
| F_MPH5.0_MSE1 | 11.26 ± 0.32 d | 10.23 ± 0.20 e | 9.73 ± 0.32 d | 10.43 ± 0.15 d | 11.2 ± 0.26 b |
| F_MPH5.0_MSE3 | 9.36 ± 0.20 c | 11.83 ± 0.15 f | 10.63 ± 0.25 e | 10.33 ± 0.40 d | 9.67 ± 0.34 a |
| Control | - | 2.63 ± 0.10 a | 2.47 ± 0.09 ab | 1.96 ± 0.08 c | 2.41 ± 0.09 b |
| F_MPH2.5_MSE0 | - | 4.37 ± 0.17 a | 3.34 ± 0.16 b | 2.86 ± 0.13 c | 2.07 ± 0.10 d |
| F_MPH2.5_MSE1 | - | 2.18 ± 0.08 a | 1.20 ± 0.03 c | 1.75 ± 0.05 b | 1.77 ± 0.07 b |
| F_MPH2.5_MSE3 | - | 1.11 ± 0.03 a | 0.95 ± 0.02 b | 1.96 ± 0.06 c | 2.28 ± 0.08 d |
| F_MPH5.0_MSE0 | - | 0.31 ± 0.01 a | 0.64 ± 0.02 b | 0.33 ± 0.01 a | 0.45 ± 0.01 c |
| F_MPH5.0_MSE1 | - | 1.25 ± 0.03 a | 1.55 ± 0.05 b | 1.06 ± 0.04 c | 1.16 ± 0.03 d |
| F_MPH5.0_MSE3 | - | 2.64 ± 0.08 a | 1.70 ± 0.06 b | 1.65 ± 0.05 b | 1.29 ± 0.04 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Ortiz, L.; Quintana, S.E.; García-Zapateiro, L.A. Composite Edible Coating from Arabic Gum and Mango Peel Hydrocolloids Enriched with Mango Seed Extracts for the Preservation of Grapes (Vitis vinifera) During Storage. Coatings 2025, 15, 435. https://doi.org/10.3390/coatings15040435
López-Ortiz L, Quintana SE, García-Zapateiro LA. Composite Edible Coating from Arabic Gum and Mango Peel Hydrocolloids Enriched with Mango Seed Extracts for the Preservation of Grapes (Vitis vinifera) During Storage. Coatings. 2025; 15(4):435. https://doi.org/10.3390/coatings15040435
Chicago/Turabian StyleLópez-Ortiz, Luisa, Somaris E. Quintana, and Luis A. García-Zapateiro. 2025. "Composite Edible Coating from Arabic Gum and Mango Peel Hydrocolloids Enriched with Mango Seed Extracts for the Preservation of Grapes (Vitis vinifera) During Storage" Coatings 15, no. 4: 435. https://doi.org/10.3390/coatings15040435
APA StyleLópez-Ortiz, L., Quintana, S. E., & García-Zapateiro, L. A. (2025). Composite Edible Coating from Arabic Gum and Mango Peel Hydrocolloids Enriched with Mango Seed Extracts for the Preservation of Grapes (Vitis vinifera) During Storage. Coatings, 15(4), 435. https://doi.org/10.3390/coatings15040435









