Enhanced Methanol Electro-Oxidation in Hierarchical Au-Pt Dendrites Supported on Graphene-like Substrate
Abstract
1. Introduction
2. Experimental
2.1. Reagents
2.2. Characterization
2.3. Deposition of Hierarchical Dendritic PtNPs/AuDNs Microstructures on EPLE
2.4. Electrochemical Measurements
3. Results and Discussions
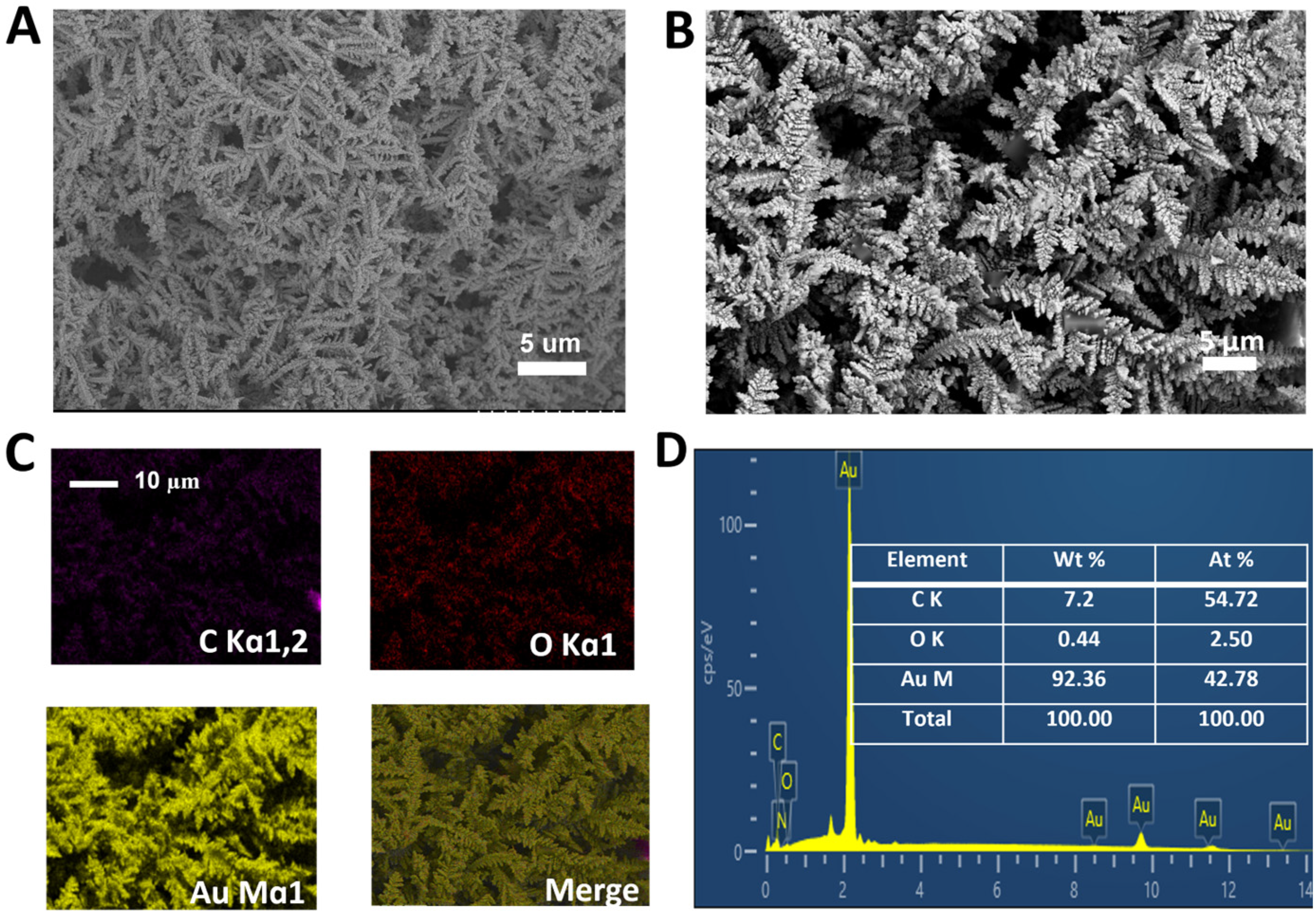
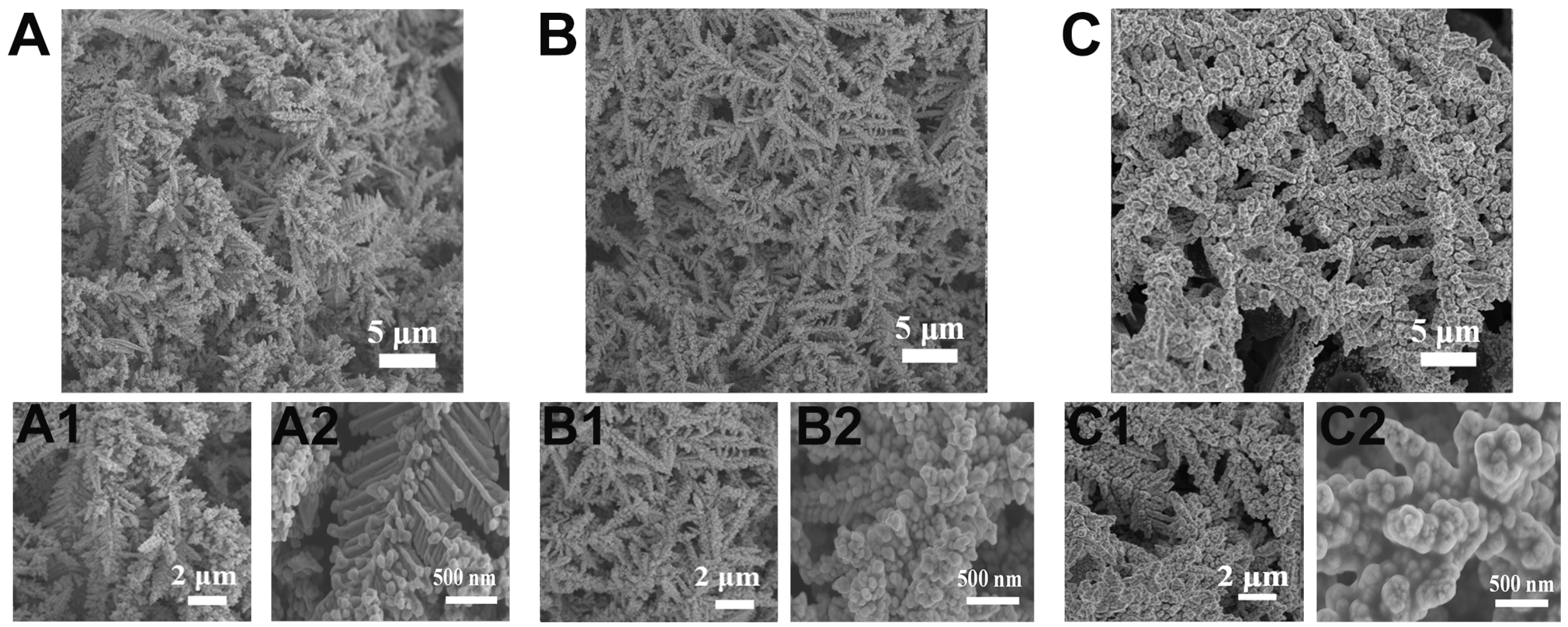
3.1. Preparation and Characterization of AuDNs/EPLE
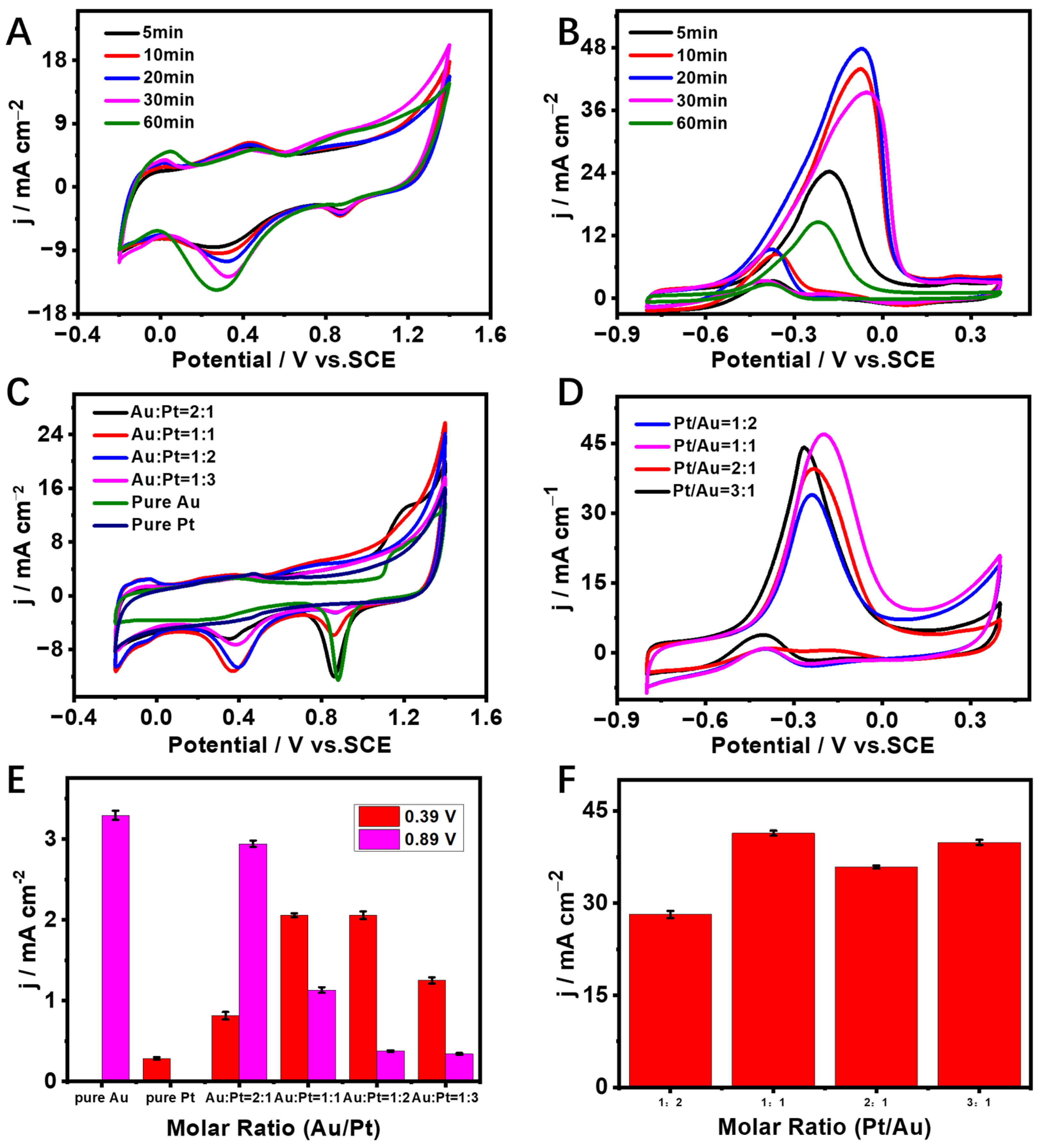
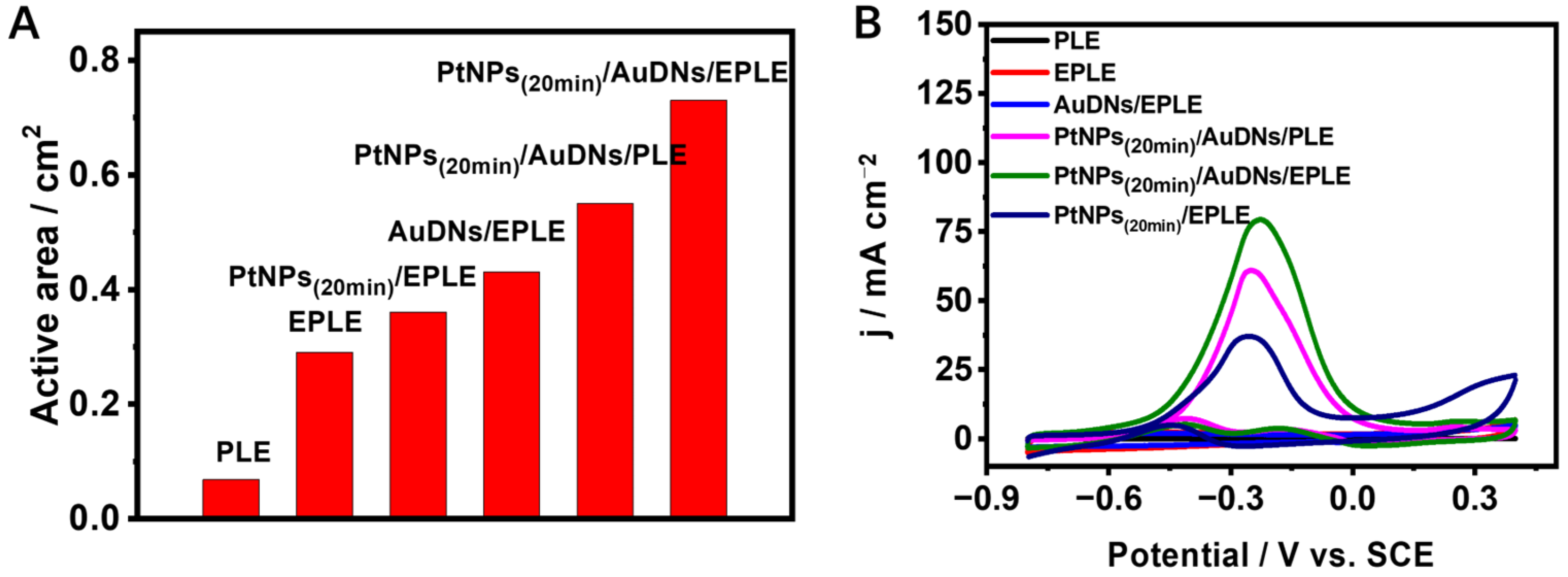
3.2. Optimization of Electrodeposition Conditions of Pt Particles on AuDNs/EPLE
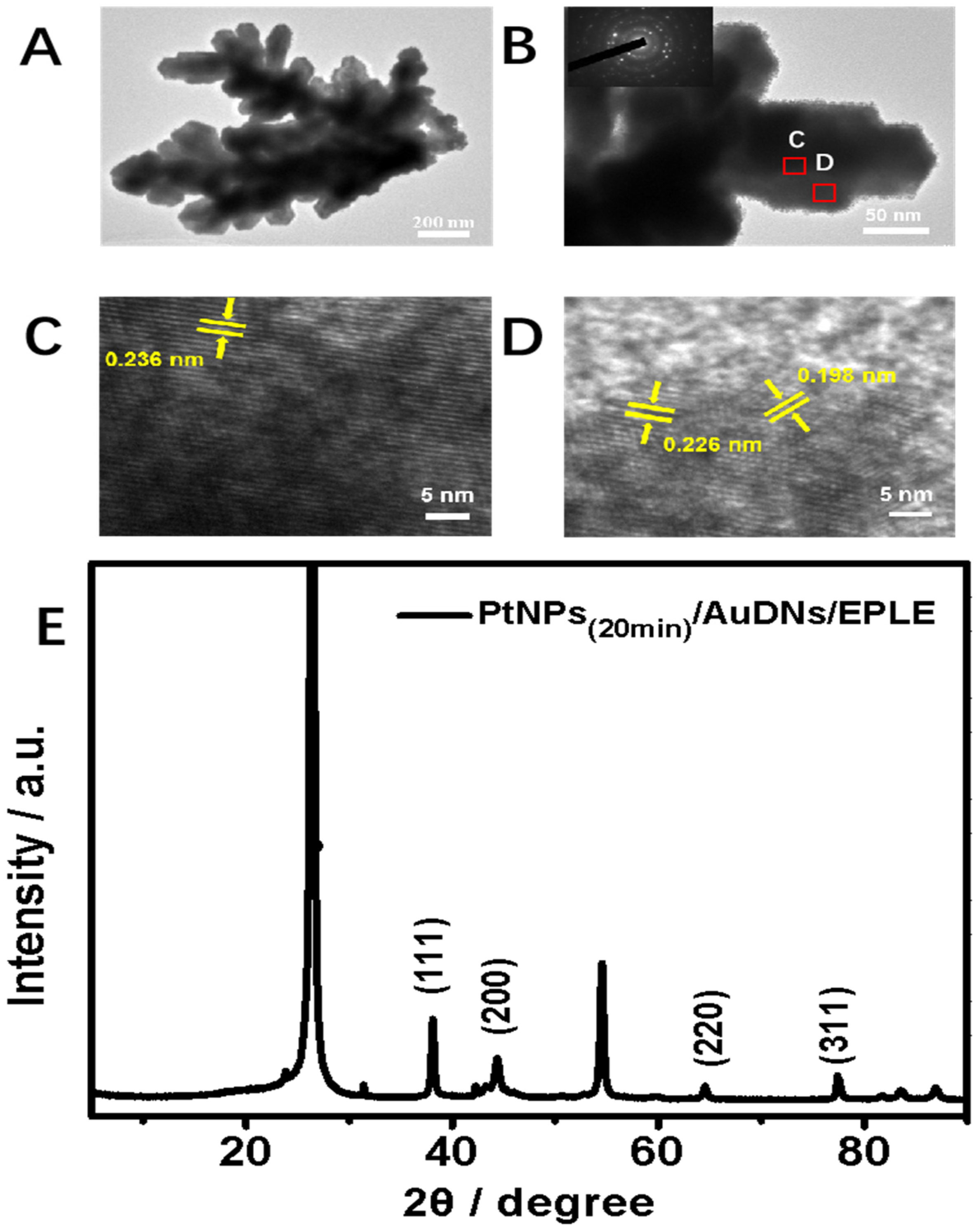
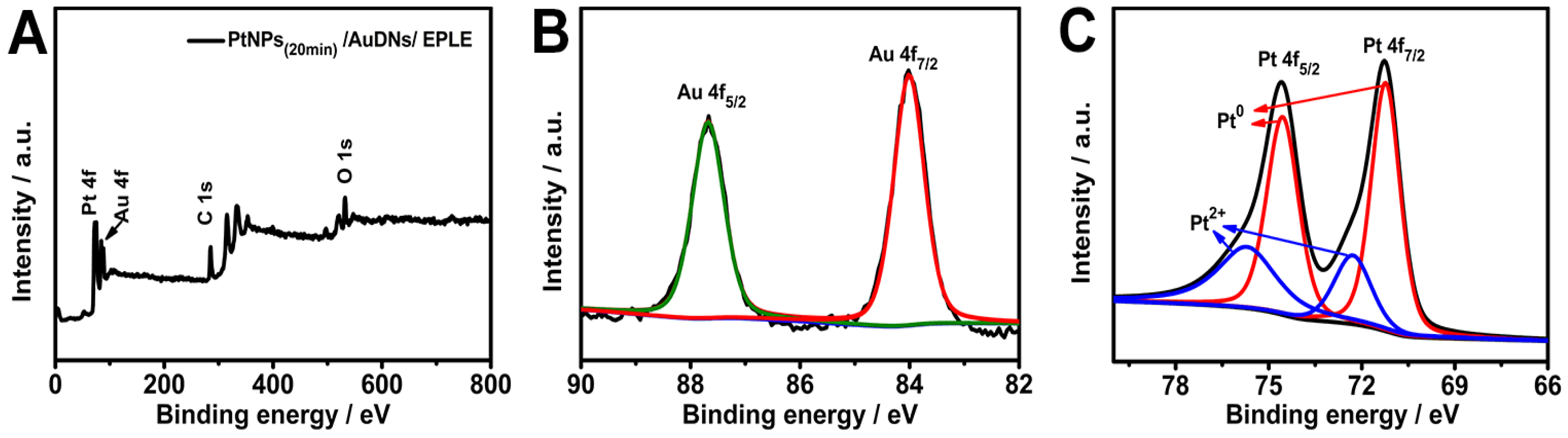
3.3. Characterization of PtNPs(20min)/AuDNs/EPLE
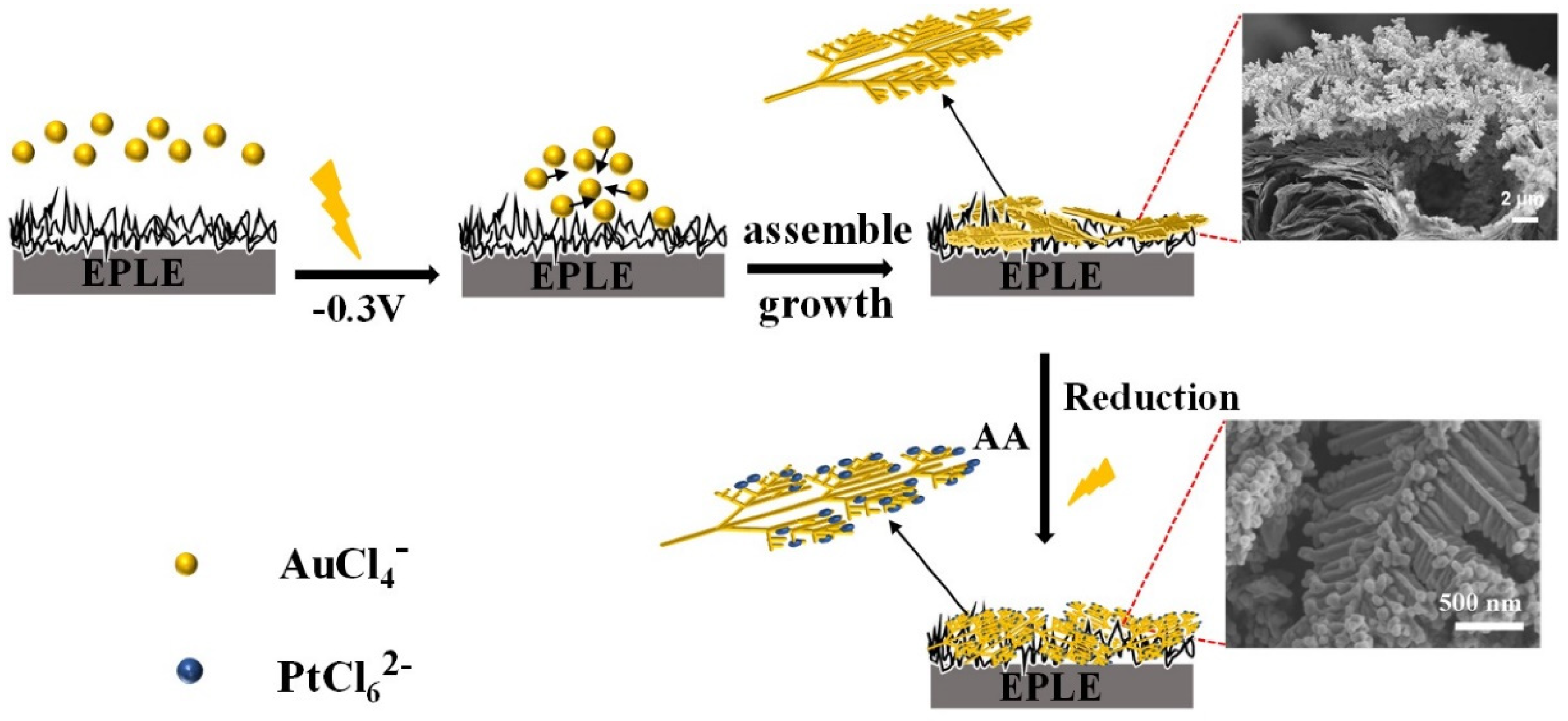
3.4. Formation Mechanism of PtNPs(20min)/AuDNs/EPLE
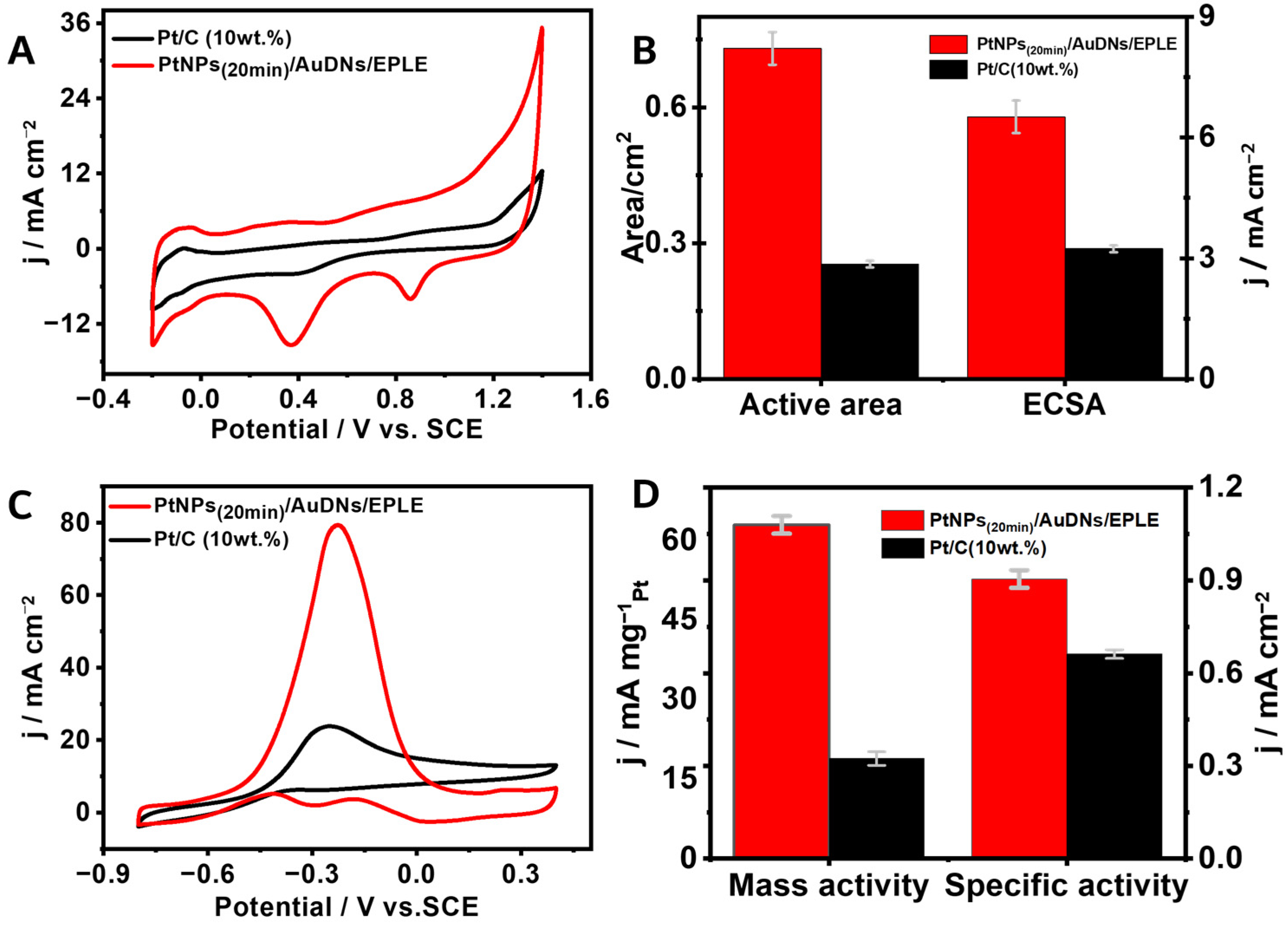
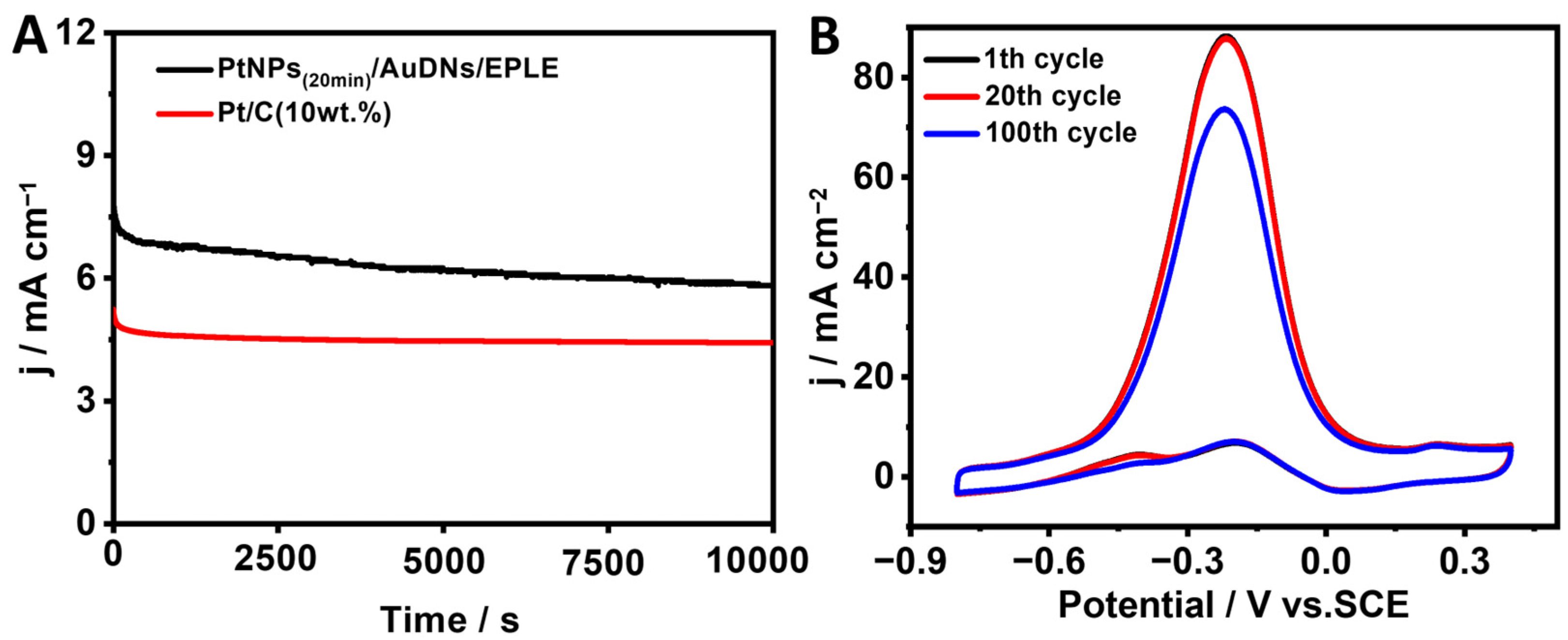
3.5. The Catalytic Activity Toward the Oxidation of Methanol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Niu, H.; Xia, C.; Li, F.-M.; Shahid, Z.; Xia, B.Y. Integration Construction of Hybrid Electrocatalysts for Oxygen Reduction. Adv. Mater. 2024, 36, 2404773. [Google Scholar] [CrossRef]
- Din, M.A.U.; Idrees, M.; Jamil, S.; Irfan, S.; Nazir, G.; Mudassir, M.A.; Saleem, M.S.; Batool, S.; Cheng, N.P.; Saidur, R. Advances and challenges of methanol-tolerant oxygen reduction reaction electrocatalysts for the direct methanol fuel cell. J. Energy Chem. 2023, 77, 499–513. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Guo, W.; Wang, L.; Chen, J.; Pan, H.; Sun, W. Toward Electrocatalytic Methanol Oxidation Reaction: Longstanding Debates and Emerging Catalysts. Adv. Mater. 2023, 35, 2211099. [Google Scholar] [CrossRef]
- Burhan, H.; Arikan, K.; Alma, M.H.; Nas, M.S.; Karimi-Maleh, H.; Sen, F.; Karimi, F.; Vasseghian, Y. Highly efficient carbon hybrid supported catalysts using nano-architecture as anode catalysts for direct methanol fuel cells. Int. J. Hydrogen Energy 2023, 48, 6657–6665. [Google Scholar] [CrossRef]
- Li, J.S.; Li, L.M.; Wang, J.; Cabot, A.; Zhu, Y.F. Boosting Hydrogen Evolution by Methanol Oxidation Reaction on Ni-Based Electrocatalysts: From Fundamental Electrochemistry to Perspectives. ACS Energy Lett. 2024, 9, 853–879. [Google Scholar] [CrossRef]
- Shen, Q.; Jiang, L.; Zhang, H.; Min, Q.; Hou, W.; Zhu, J.-J. Three-dimensional Dendritic Pt Nanostructures: Sonoelectrochemical Synthesis and Electrochemical Applications. J. Phys. Chem. C 2008, 112, 16385–16392. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, X.; Luque, R.; Eid, K. Structure-activity relationship of tri-metallic Pt-based nanocatalysts for methanol oxidation reaction. Coord. Chem. Rev. 2023, 493, 215280. [Google Scholar] [CrossRef]
- Liang, J.; Cheng, H.; Zhao, B.; Hu, Q.; Xing, Z.; Zhang, Y.; Niu, L. Boosting the Methanol Oxidation Reaction Activity of Pt-Ru Clusters via Resonance Energy Transfer. Small 2023, 19, e2302149. [Google Scholar] [CrossRef]
- Dong, K.Y.; Dai, H.Z.; Pu, H.K.; Zhang, T.; Wang, Y.Y.; Deng, Y.J. Synthesis of alloyed PtPd nanowires and study on their electro-catalytic performance to methanol oxidation reaction. Int. J. Hydrogen Energy 2023, 48, 35240–35249. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, S.L.; Deng, Y.X.; Luan, S.H.; Wang, C.K.; Ding, L.F.; Jiang, X.; Sun, D.M.; Tang, Y.W. PtPdAg nanotrees with low Pt content for high CO tolerance within formic acid and methanol electrooxidation. Rare Met. 2025, 44, 300–310. [Google Scholar] [CrossRef]
- Li, X.L.; Huang, Y.Q.; Chen, Z.Y.; Hu, S.Q.; Zhu, J.L.; Tsiakaras, P.; Shen, P.K. Novel PtNi nanoflowers regulated by a third element (Rh, Ru, Pd) as efficient multifunctional electrocatalysts for ORR, MOR and HER. Chem. Eng. J. 2023, 454, 140131. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, G.; Singh, P.P.; Kaushal, S. Supported bimetallic nanoparticles as anode catalysts for direct methanol fuel cells: A review. Int. J. Hydrogen Energy 2021, 46, 15820–15849. [Google Scholar] [CrossRef]
- Pilapil, B.K.; van Drunen, J.; Makonnen, Y.; Beauchemin, D.; Jerkiewicz, G.; Gates, B.D. Ordered Porous Electrodes by Design: Toward Enhancing the Effective Utilization of Platinum in Electrocatalysis. Adv. Funct. Mater. 2017, 27, 1703171. [Google Scholar] [CrossRef]
- Lou, W.H.; Ali, A.; Shen, P.K. Recent development of Au arched Pt nanomaterials as promising electrocatalysts for methanol oxidation reaction. Nano Res. 2022, 15, 18–37. [Google Scholar] [CrossRef]
- Mymoona, P.; Shibu, E.S.; Jeyabharathi, C. Adsorbed Carbon Monoxide-Enabled Self-Terminated Au-Grafting on Pt6 Nanoclusters for Enhanced Methanol Electrooxidation. Small 2024, 20, e2401998. [Google Scholar] [CrossRef]
- Reddy, G.V.; Sekhar, Y.C.; Raghavendra, P.; Reddy, M.N.; Chandana, P.S.; Sarma, L.S. Controlled synthesis of reduced graphene oxide-supported bimetallic Pt-Au nanoparticles for enhanced electrooxidation of methanol. Solid State Sci. 2024, 149, 107469. [Google Scholar] [CrossRef]
- Wei, K.; Lin, H.; Zhao, X.; Zhao, Z.; Marinkovic, N.; Morales, M.; Huang, Z.; Perlmutter, L.; Guan, H.; Harris, C.; et al. Au/Pt Bimetallic Nanowires with Stepped Pt Sites for Enhanced C–C Cleavage in C2+ Alcohol Electro-oxidation Reactions. J. Am. Chem. Soc. 2023, 145, 19076–19085. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, T.; Sato, K.; Yamamoto, T.; Tamura, M.; Iida, T.; Shoji, T.; Tsuboi, Y.; Torimoto, T. Promoting Oxygen Reduction Reaction by Excitation of Localized Surface Plasmon of Shape- and Facet-Controlled Octahedral Au@Pt Core-Shell Nanocrystals. Chemelectrochem 2023, 10, e202300182. [Google Scholar] [CrossRef]
- Li, C.J.; Liu, S.L.; Yin, S.L.; Yu, H.J.; Wang, Z.Q.; Xu, Y.; Li, X.N.; Wang, L.; Wang, H.J. Facile preparation of Pt-based cage-bell structured nanoarchitectures for enhanced methanol oxidation electrocatalysis. Int. J. Hydrogen Energy 2020, 45, 2478–2485. [Google Scholar] [CrossRef]
- Liang, W.; Wang, Y.; Zhao, L.; Guo, W.; Li, D.; Qin, W.; Wu, H.; Sun, Y.; Jiang, L. 3D Anisotropic Au@Pt–Pd Hemispherical Nanostructures as Efficient Electrocatalysts for Methanol, Ethanol, and Formic Acid Oxidation Reaction. Adv. Mater. 2021, 33, 2100713. [Google Scholar] [CrossRef]
- Fidiani, E.; Thirunavukkarasu, G.; Li, Y.; Chiu, Y.L.; Du, S.F. Au integrated AgPt nanorods for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Mater. Chem. A 2021, 9, 5578–5587. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lee, S.; Haddadnezhad, M.; Oh, M.J.; Zhao, Q.; Yoo, S.; Liu, L.; Jung, I.; Park, S. Multi-Layered PtAu Nanoframes and Their Light-Enhanced Electrocatalytic Activity via Plasmonic Hot Spots. Small 2023, 19, e2206377. [Google Scholar] [CrossRef] [PubMed]
- Rey-Raap, N.; dos Santos-Gomez, L.; Arenillas, A. Carbons for fuel cell energy generation. Carbon 2024, 228, 119291. [Google Scholar] [CrossRef]
- Huang, X.J.; Yarimaga, O.; Kim, J.H.; Choi, Y.K. Substrate surface roughness-dependent 3-D complex nanoarchitectures of gold particles from directed electrodeposition. J. Mater. Chem. 2009, 19, 478–483. [Google Scholar] [CrossRef]
- Yu, M.; Chen, J.; Liu, J.; Li, S.; Ma, Y.; Zhang, J.; An, J. Mesoporous NiCo2O4 nanoneedles grown on 3D graphene-nickel foam for supercapacitor and methanol electro-oxidation. Electrochim. Acta 2015, 151, 99–108. [Google Scholar] [CrossRef]
- Samad, S.; Loh, K.S.; Wong, W.Y.; Lee, T.K.; Sunarso, J.; Chong, S.T.; Daud, W.R.W. Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int. J. Hydrogen Energy 2018, 43, 7823–7854. [Google Scholar] [CrossRef]
- Parvez, K.; Wu, Z.-S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Muellen, K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef]
- Xiang, X.; Feng, S.; Chen, J.; Feng, J.; Hou, Y.; Ruan, Y.; Weng, X.; Milcovich, G. Gold Nanoparticles/Electrochemically Expanded Graphite Composite: A Bifunctional Platform toward Glucose Sensing and SERS Applications. J. Electroanal. Chem. 2019, 851, 113471. [Google Scholar] [CrossRef]
- Yu, L.; Lv, M.-Y.; Zhang, T.; Zhou, Q.; Zhang, J.; Weng, X.; Ruan, Y.; Feng, J. In Situ Growth of Self-Supported CuO Nanorods from Cu-MOFs for Glucose Sensing and Elucidation of the Sensing Mechanism. Anal. Methods 2024, 16, 731–741. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real surface area measurements in electrochemistry. J. Electroanal. Chem. 1992, 327, 353–376. [Google Scholar] [CrossRef]
- Habibi, B.; Ghaderi, S. Synthesis, characterization and electrocatalytic activity of Co@Pt nanoparticles supported on carbon-ceramic substrate for fuel cell applications. Int. J. Hydrogen Energy 2015, 40, 5115–5125. [Google Scholar] [CrossRef]
- Nagar, B.; Balsells, M.; de la Escosura-Muñiz, A.; Gomez-Romero, P.; Merkoçi, A. Fully Printed One-Step Biosensing Device Using Graphene/AuNPs Composite. Biosens. Bioelectron. 2019, 129, 238–244. [Google Scholar] [CrossRef]
- Li, J.; Jilani, S.Z.; Lin, H.; Liu, X.; Wei, K.; Jia, Y.; Zhang, P.; Chi, M.; Tong, Y.J.; Xi, Z.; et al. Ternary CoPtAu Nanoparticles as a General Catalyst for Highly Efficient Electro-oxidation of Liquid Fuels. Angew. Chem.-Int. Ed. 2019, 58, 11527–11533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Wang, X. Nanoporous bimetallic Pt-Au alloy nanocomposites with superior catalytic activity towards electro-oxidation of methanol and formic acid. Nanoscale 2011, 3, 1663–1674. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Sinduja, B.; Shankar, S.; John, S.A. Displacement reduction routed Au-Pt bimetallic nanoparticles: A highly durable electrocatalyst for methanol oxidation and oxygen reduction. Sustain. Energy Fuels 2018, 2, 1588–1599. [Google Scholar] [CrossRef]
- Chen, C.-S.; Pan, F.-M.; Yu, H.-J. Electrocatalytic activity of Pt nanoparticles on a karst-like Ni thin film toward methanol oxidation in alkaline solutions. Appl. Catal. B-Environ. 2011, 104, 382–389. [Google Scholar] [CrossRef]
- Ren, F.; Zhai, C.; Zhu, M.; Wang, C.; Wang, H.; Bin, D.; Guo, J.; Yang, P.; Du, Y. Facile synthesis of PtAu nanoparticles supported on polydopamine reduced and modified graphene oxide as a highly active catalyst for methanol oxidation. Electrochim. Acta 2015, 153, 175–183. [Google Scholar] [CrossRef]
- Ji, Z.; Zhu, G.; Shen, X.; Zhou, H.; Wu, C.; Wang, M. Reduced graphene oxide supported FePt alloy nanoparticles with high electrocatalytic performance for methanol oxidation. New J. Chem. 2012, 36, 1774–1780. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, J. Methanol dehydrogenation reaction at Au@Pt catalysts: Insight into the methanol electrooxidation. Electrochim. Acta 2018, 283, 11–17. [Google Scholar] [CrossRef]
- Jia, H.; Chang, G.; Shu, H.; Xu, M.; Wang, X.; Zhang, Z.; Liu, X.; He, H.; Wang, K.; Zhu, R.; et al. Pt nanoparticles modified Au dendritic nanostructures: Facile synthesis and enhanced electrocatalytic performance for methanol oxidation. Int. J. Hydrogen Energy 2017, 42, 22100–22107. [Google Scholar] [CrossRef]
- Kang, J.; Nam, S.; Oh, Y.; Choi, H.; Wi, S.; Lee, B.; Hwang, T.; Hong, S.; Park, B. Electronic Effect in Methanol Dehydrogenation on Pt Surfaces: Potential Control during Methanol Electrooxidation. J. Phys. Chem. Lett. 2013, 4, 2931–2936. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y.; Zhou, Y.; Zhao, S.; Zhang, C.; Zhang, H.; Sheng, X. In-situ formation of supported Au nanoparticles in hierarchical yolk-shell CeO2 /mSiO2 structures as highly reactive and sinter-resistant catalysts. J. Colloid Interface Sci. 2017, 488, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, J.; Liu, B.; Ling, S.; Long, R.; Xiong, Y. Turning Au Nanoclusters Catalytically Active for Visible-Light-Driven CO2 Reduction through Bridging Ligands. J. Am. Chem. Soc. 2018, 140, 16514–16520. [Google Scholar] [CrossRef]
- van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Observing the oxidation of platinum. Nat. Commun. 2017, 8, 429. [Google Scholar] [CrossRef]
- Huang, D.; Qi, Y.; Bai, X.; Shi, L.; Jia, H.; Zhang, D.; Zheng, L. One-Pot Synthesis of Dendritic Gold Nanostructures in Aqueous Solutions of Quaternary Ammonium Cationic Surfactants: Effects of the Head Group and Hydrocarbon Chain Length. ACS Appl. Mater. Interfaces 2012, 4, 4665–4671. [Google Scholar] [CrossRef] [PubMed]
- Imai, H. Self-Organized Formation of Hierarchical Structures. In Biomineralization I; Springer: Berlin/Heidelberg, Germany, 2007; pp. 43–72. [Google Scholar] [CrossRef]
- Ye, W.; Yan, J.; Ye, Q.; Zhou, F. Template-Free and Direct Electrochemical Deposition of Hierarchical Dendritic Gold Microstructures: Growth and Their Multiple Applications. J. Phys. Chem. 2010, 114, 15617–15624. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem.-Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Feng, L.; Wu, X.; Ren, L.; Xiang, Y.; He, W.; Zhang, K.; Zhou, W.; Xie, S. Crystal Growth & Design of Au@Pt Nanostructures by Gold-Nanorod-Seeded Growth. Chem.-A Eur. J. 2008, 14, 9764–9771. [Google Scholar] [CrossRef]
- Fu, T.; Fang, J.; Wang, C.; Zhao, J. Hollow porous nanoparticles with Pt skin on a Ag-Pt alloy structure as a highly active electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 8803–8811. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Nemoto, Y.; Tateyama, Y.; Yamauchi, Y. On the Role of Ascorbic Acid in the Synthesis of Single-Crystal Hyperbranched Platinum Nanostructures. Cryst. Growth Des. 2010, 10, 3454–3460. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Zhigalina, O.M.; Khmelenin, D.N.; Ponomarev, I.I.; Vtyurina, E.S.; Cherkovskiy, E.N.; Basu, V.G.; Modestov, A.D. Deposition of Pt Nanoparticles by Ascorbic Acid on Composite Electrospun Polyacrylonitrile-Based Carbon Nanofiber for HT-PEM Fuel Cell Cathodes. Catalysts 2022, 12, 891. [Google Scholar] [CrossRef]
- Chen, S.; Wu, H.; Tao, J.; Xin, H.; Zhu, Y.; Chen, J. Pt-Ni Seed-Core-Frame Hierarchical Nanostructures and Their Conversion to Nanoframes for Enhanced Methanol Electro-Oxidation. Catalysts 2019, 9, 39. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhao, Y.; Ruan, Y.; Weng, X.; Milcovich, G. Enhanced Methanol Electro-Oxidation in Hierarchical Au-Pt Dendrites Supported on Graphene-like Substrate. Coatings 2025, 15, 458. https://doi.org/10.3390/coatings15040458
Zhu Z, Zhao Y, Ruan Y, Weng X, Milcovich G. Enhanced Methanol Electro-Oxidation in Hierarchical Au-Pt Dendrites Supported on Graphene-like Substrate. Coatings. 2025; 15(4):458. https://doi.org/10.3390/coatings15040458
Chicago/Turabian StyleZhu, Zifeng, Yiming Zhao, Yongming Ruan, Xuexiang Weng, and Gesmi Milcovich. 2025. "Enhanced Methanol Electro-Oxidation in Hierarchical Au-Pt Dendrites Supported on Graphene-like Substrate" Coatings 15, no. 4: 458. https://doi.org/10.3390/coatings15040458
APA StyleZhu, Z., Zhao, Y., Ruan, Y., Weng, X., & Milcovich, G. (2025). Enhanced Methanol Electro-Oxidation in Hierarchical Au-Pt Dendrites Supported on Graphene-like Substrate. Coatings, 15(4), 458. https://doi.org/10.3390/coatings15040458






