Facile Synthesis of Sponge-like Microstructured CuO Anode Material for Rechargeable Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterization of the Synthesized Materials
2.3. Electrochemical Characterization Synthesized Materials
3. Results and Discussion
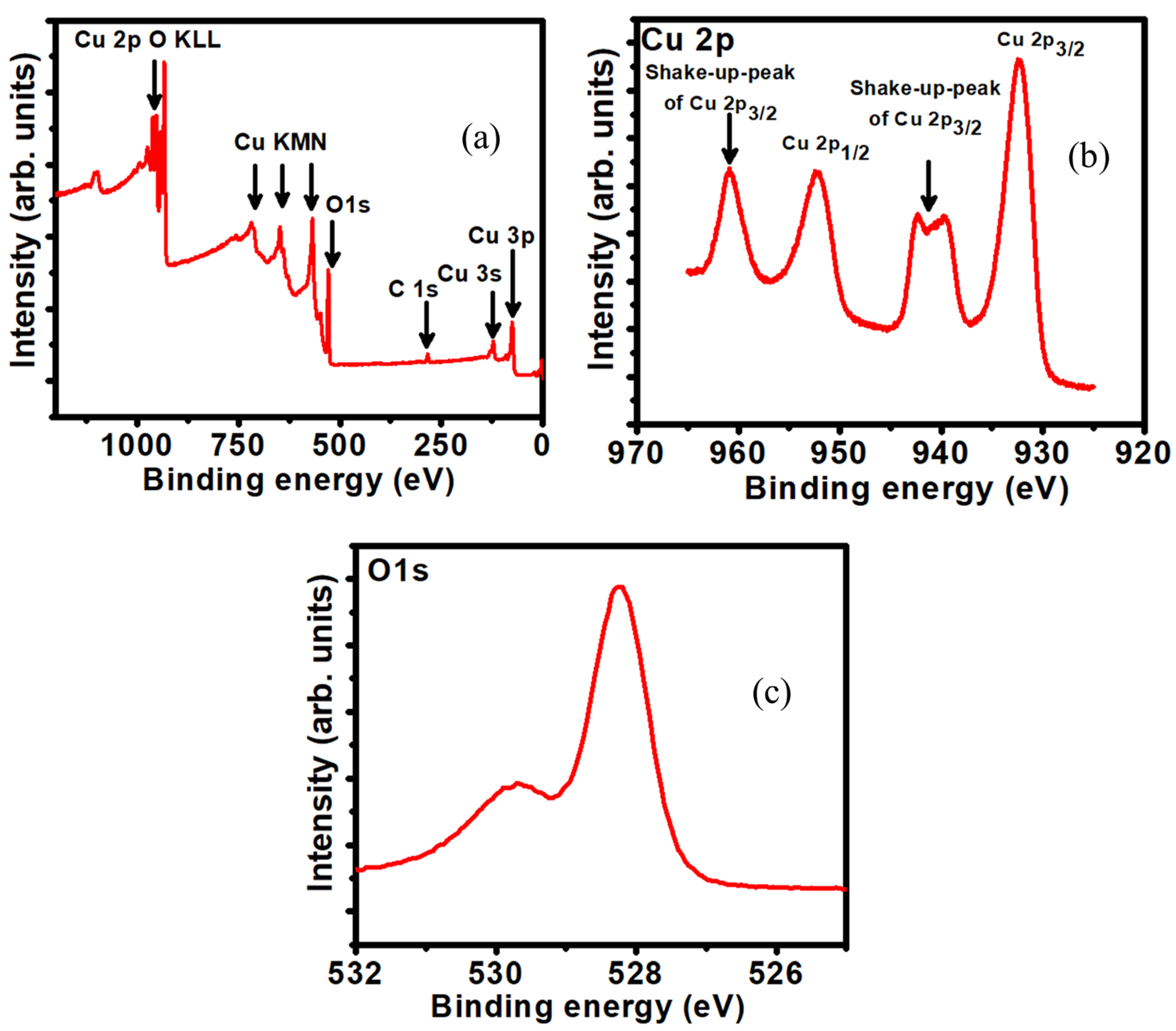
3.1. Material Characterization
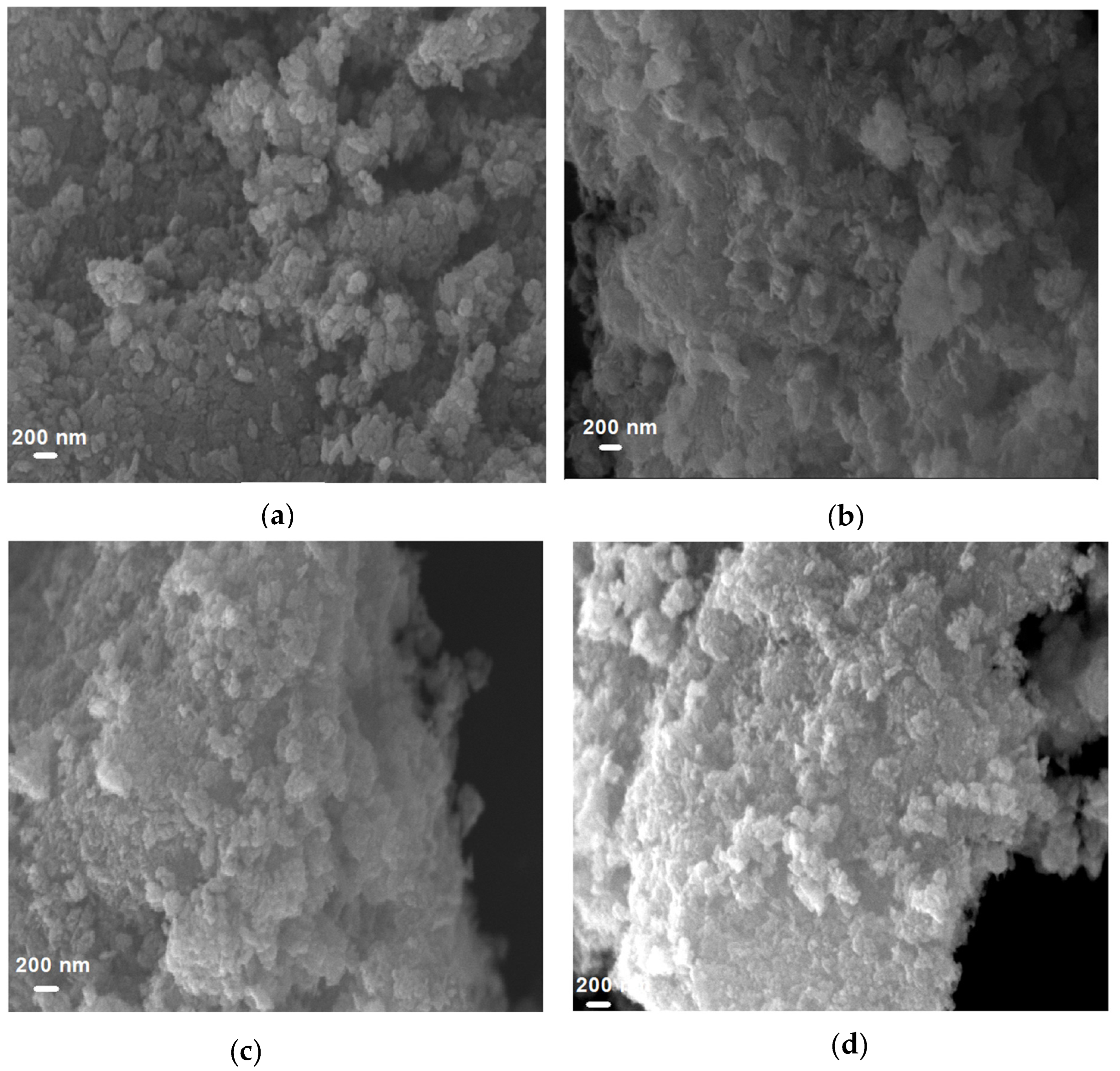
3.1.1. Surface Morphology of CuO
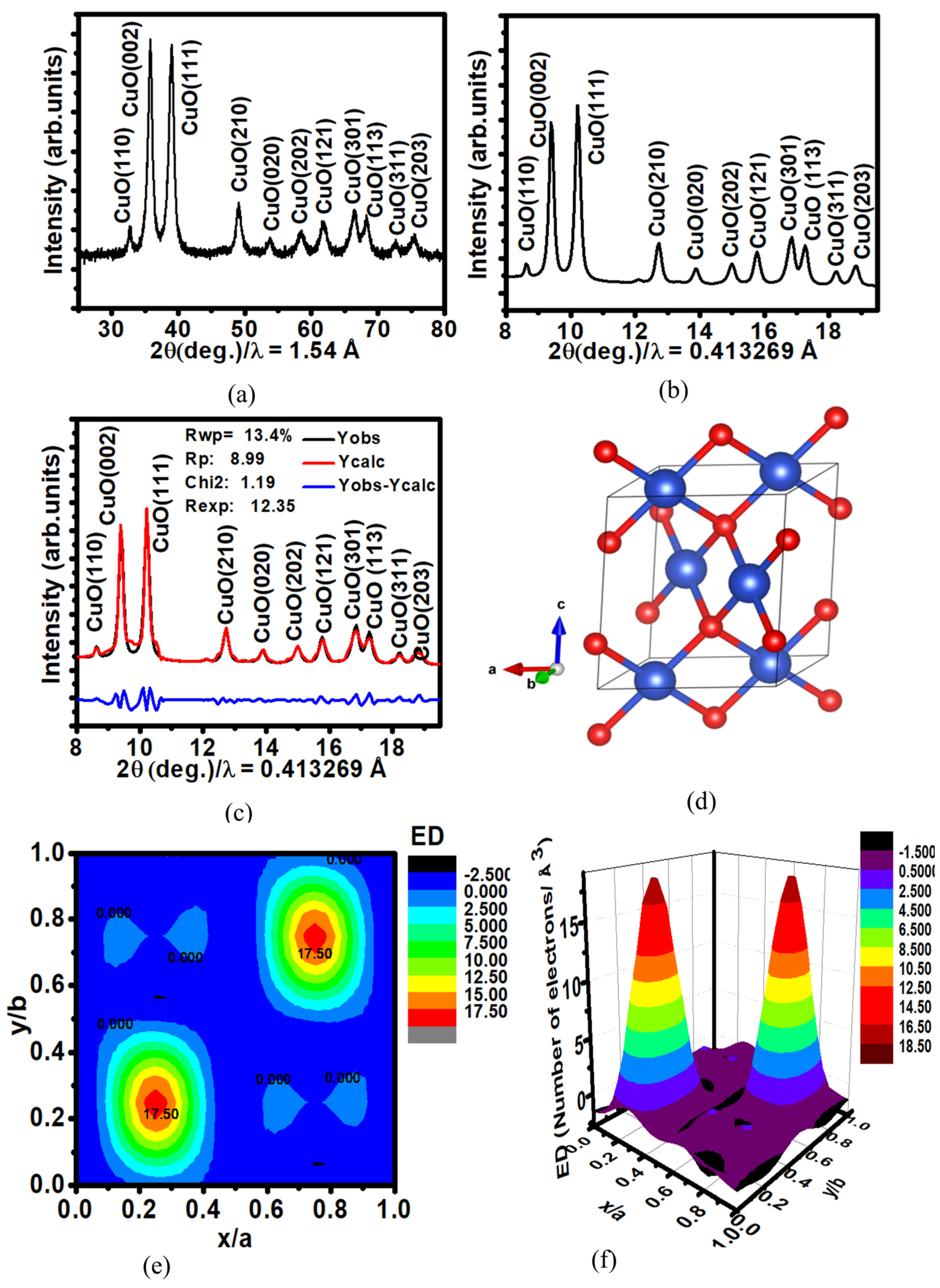
3.1.2. Crystallographic Properties
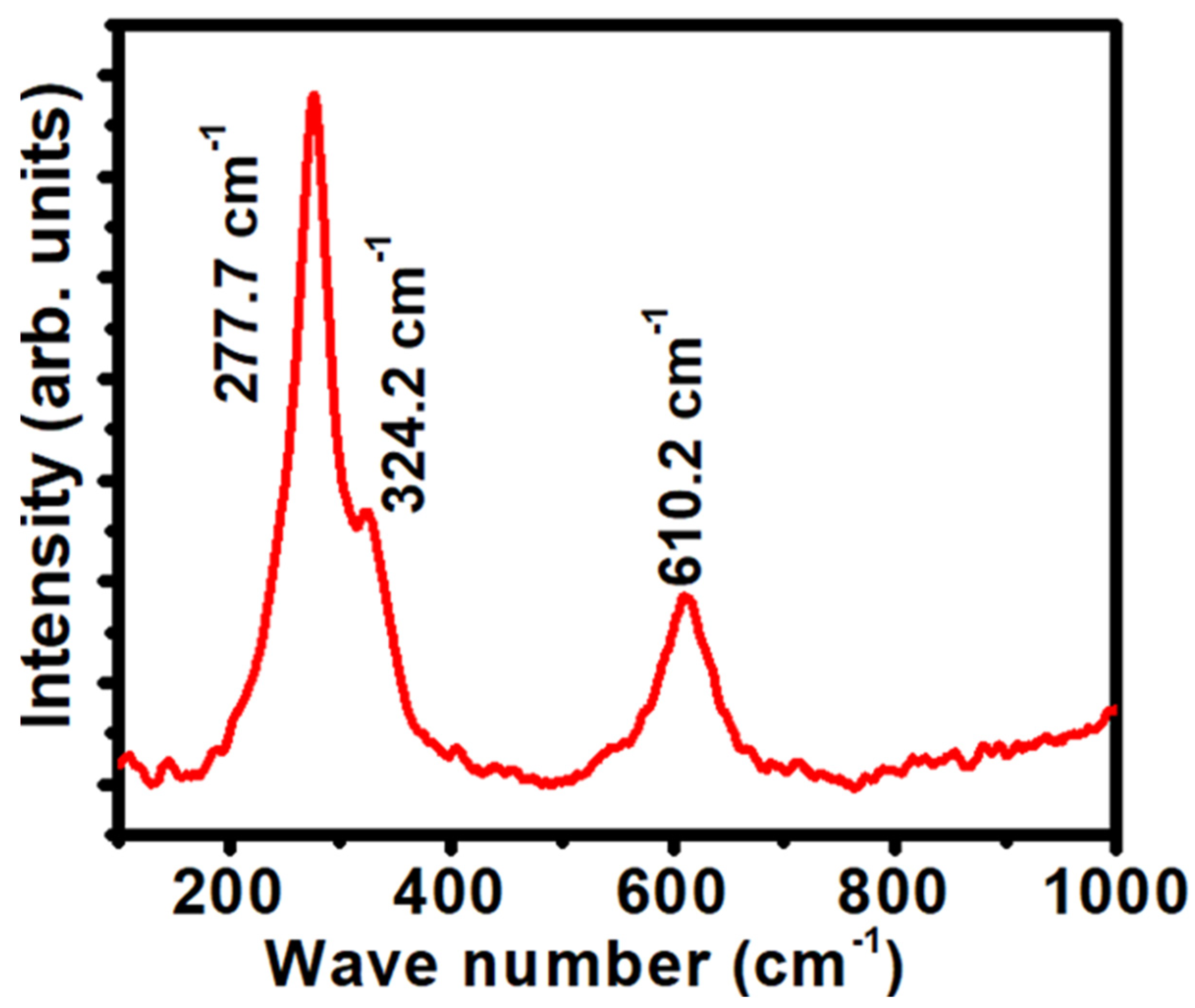
3.1.3. Optical Properties
3.1.4. Electrical Properties of CuO Anode Electrode
3.2. Electrochemical Performance
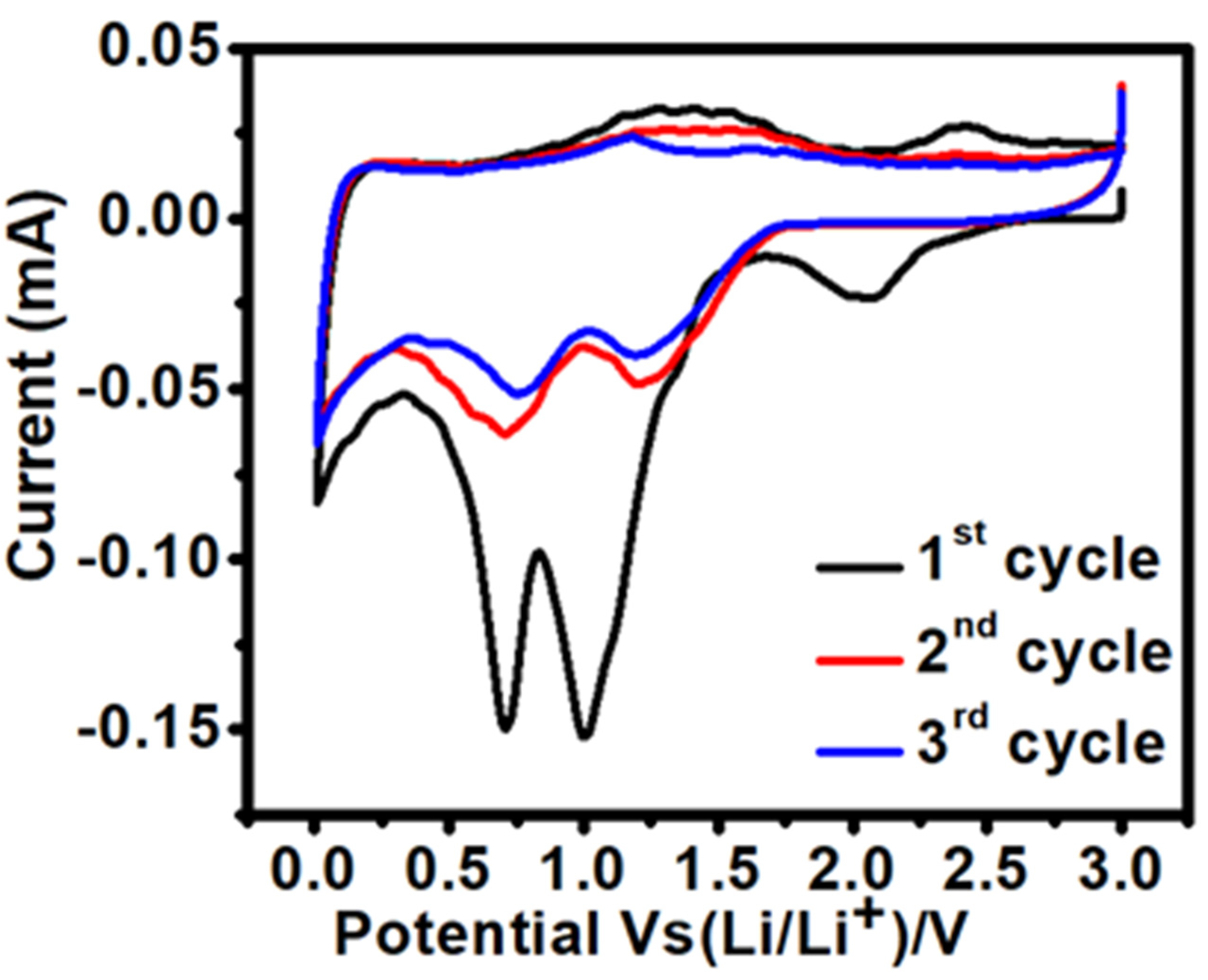
3.2.1. Cyclic Voltammetry (CV)
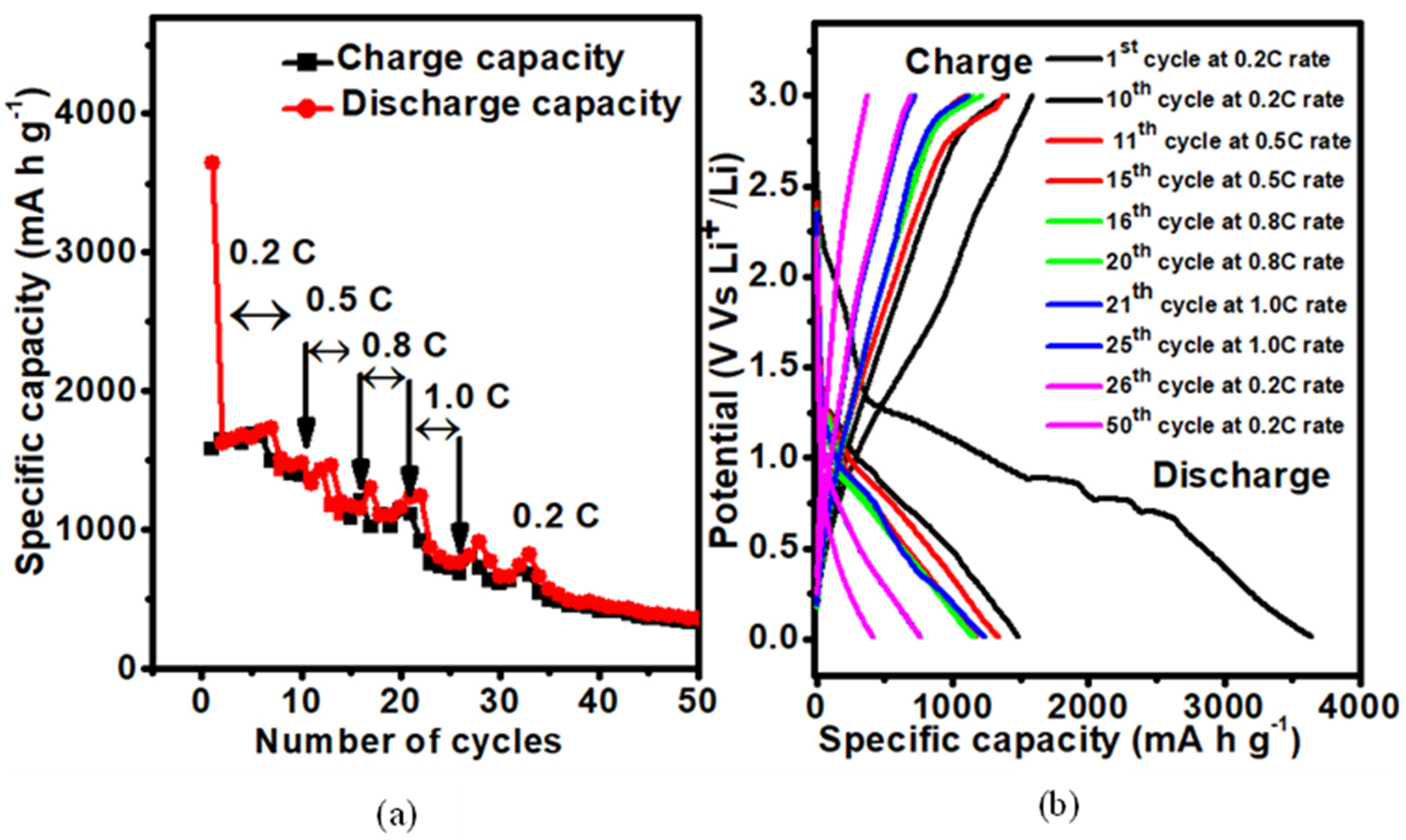
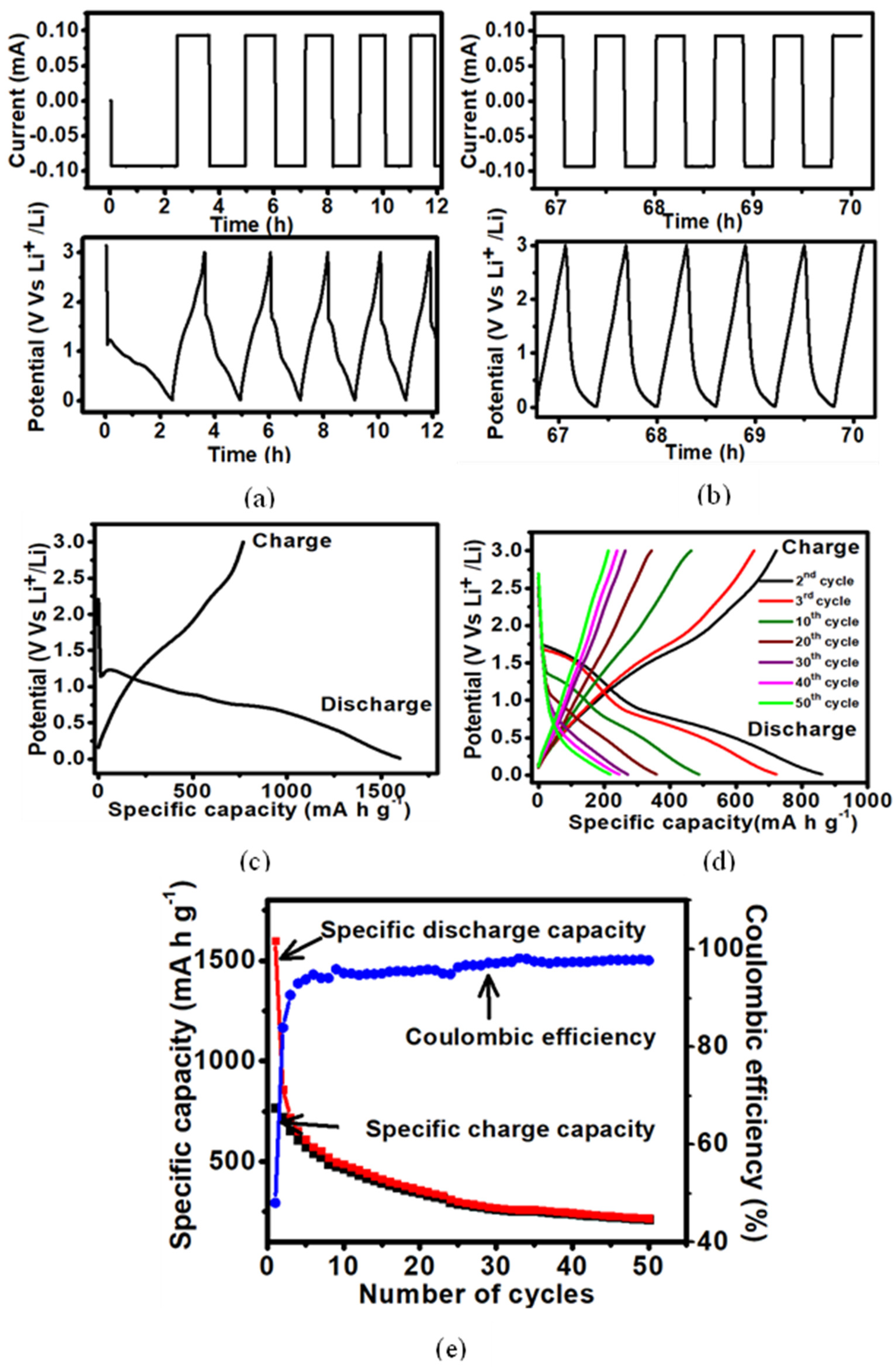
3.2.2. Galvanostatic Cycle Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schomburg, F.; Heidrich, B.; Wennemar, S.; Drees, R.; Roth, T.; Kurrat, M.; Heimes, H.; Jossen, A.; Winter, M.; Cheong, J.Y.; et al. Lithium-ion battery cell formation: Status and future directions towards a knowledge-based process design. Energy Environ. Sci. 2024, 17, 2686–2733. [Google Scholar] [CrossRef]
- Stephan, A.K. The Age of Li-Ion Batteries. Joule 2019, 3, 2583–2584. [Google Scholar] [CrossRef]
- Winslow, K.M.; Laux, S.J.; Townsend, T.G. A review on the growing concern and potential management strategies of waste lithium-ion batteries. Resour. Conserv. Recycl. 2018, 129, 263–277. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Winowatan, P.W.; Selwyn, S.; Priyono, B.; Syahrial, A.Z. Enhancing battery performance of Li4Ti5O12 nanorod synthesized by hydrothermal method with Sn addition as anode material for lithium-ion battery. J. King Saud Univ. Eng. Sci. 2021, 33, 396–403. [Google Scholar] [CrossRef]
- Yi, X.; Qi, G.; Liu, X.; Depcik, C.; Liu, L. Challenges and strategies toward anode materials with different lithium storage mechanisms for rechargeable lithium batteries. J. Energy Storage 2024, 95, 112480. [Google Scholar] [CrossRef]
- Subalakshmi, P.; Sivashanmugam, A. CuO nano hexagons, an efficient energy storage material for Li- ion battery appli-cation. J. Alloys Compd. 2017, 690, 523–531. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Zhang, Y.; Yang, Z.; Wang, Q.; Xia, J.; Yang, X. Facile microemulsion synthesis of porous CuO nanosphere film and its application in lithium ion batteries. Electrochimica Acta 2013, 113, 63–68. [Google Scholar] [CrossRef]
- Wong, W.T.K.S.; Zhuk, S.; Masudy, P.S.; Dalapati, G.K. Current Status and Future Prospects of Copper Oxide Heterojunction Solar Cells. Materials 2016, 9, 271. [Google Scholar] [CrossRef]
- Morariu, M.I.; Nicolaescu, M.; Hulka, I.; Duţeanu, N.; Orha, C.; Lăzău, C.; Bandas, C. Fabrication of Cu2O/CuO Nanowires by One-Step Thermal Oxidation of Flexible Copper Mesh for Supercapacitor Applications. Batteries 2024, 10, 246. [Google Scholar] [CrossRef]
- Renganathan, B.; Gopakumar, C.; Priya, A.K.; Rao, S.K.; Sastikumar, D.; Silambarasan, M.; Kannapiran, N. Optimizing Gas Sensing Performance of CuO Nanoparticles via Sol-Gel Synthesis Approach for Efficient Detection of Ammonia Gas. Mater. Res. Bull. 2023, 170, 112556. [Google Scholar] [CrossRef]
- Zedan, A.F.; Mohamed, A.T.; El-Shall, M.S.; AlQaradawi, S.Y.; AlJaber, A.S. Tailoring the reducibility and catalytic activity of CuO nanoparticles for low temperature CO oxidation. RSC Adv. 2018, 8, 19499–19511. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Ye, Y.; Yang, Y.; Zhang, X.; Pan, B.; Peng, Z.; Wu, M.; Qiu, Z.; Wang, C.; Yuan, Y.; et al. CuO nanoflowers/copper fiber felt integrated porous electrode for lithium-ion batteries. Sci. China Technol. Sci. 2020, 63, 2423–2434. [Google Scholar] [CrossRef]
- Deng, Z.; Ma, Z.; Li, Y.; Li, Y.; Chen, L.; Yang, X.; Wang, H.-E.; Su, B.-L. Boosting Lithium-Ion Storage Capability in CuO Nanosheets via Synergistic Engineering of Defects and Pores. Front. Chem. 2018, 6, 428. [Google Scholar] [CrossRef]
- Wang, P.; Gou, X.-X.; Xin, S.; Cao, F.-F. Facile synthesis of CuO nanochains as high-rate anode materials for lithium-ion batteries. New J. Chem. 2019, 43, 6535–6539. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, G.; Gu, H.; Yang, Z.; Cheng, H. A new lithium-ion battery: CuO nanorod array anode versus spinel LiNi0.5Mn1.5O4 cathode. J. Power Sources 2015, 273, 561–565. [Google Scholar] [CrossRef]
- Qian, Y.; Niehoff, P.; Zhou, D.; Adam, R.; Mikhailova, D.; Pyschik, M.; Börner, M.; Klöpsch, R.; Rafaja, D.; Schumacher, G.; et al. Investigation of nano-sized Cu(ii)O as a high capacity conversion material for Li-metal cells and lithium-ion full cells. J. Mater. Chem. A 2017, 5, 6556–6568. [Google Scholar] [CrossRef]
- Jose, A.S.; Gangaja, B.; Nair, S.; Santhanagopalan, D. Influence of cobalt concentration on CuO nanoplates morphology and its superior performance as Li–ion battery anode. Mater. Res. Express 2019, 6, 125543. [Google Scholar] [CrossRef]
- Meng, J.; Yang, Z.; Chen, L.; Qin, H.; Cui, F.; Jiang, Y.; Zeng, X. Energy storage performance of CuO as a cathode material for aqueous zinc ion battery. Mater. Today Energy 2020, 15, 100370. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Wang, S.; Li, S.; Jiao, S. Porous CuO microsphere architectures as high-performance cathode materials for aluminum-ion batteries. J. Mater. Chem. A 2018, 6, 3084–3090. [Google Scholar] [CrossRef]
- Gangaja, B.; Chandrasekharan, S.; Vadukumpully, S.; Nair, S.V.; Santhanagopalan, D. Surface chemical analysis of CuO nanofiber composite electrodes at different stages of lithiation/delithiation. J. Power Sources 2017, 340, 356–364. [Google Scholar] [CrossRef]
- Wang, H.; Liang, T.; Yu, X.; Zhao, W.; Xu, R.; Wang, D.; Liu, Y. Hydrothermal synthesis of well-crystallized CuO hierarchical structures and their direct application in high performance lithium-ion battery electrodes without further calcination. RSC Adv. 2016, 6, 96882–96888. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Z.; Li, H.; Yang, X.; Li, J.; Wang, P.; Jin, C.; Zhang, C. Three-dimensional clusters of peony-shaped CuO nanosheets as a high-rate anode for Li-ion batteries. RSC Adv. 2021, 11, 10760–10766. [Google Scholar] [CrossRef]
- Wang, C.; Higgins, D.; Wang, F.; Li, D.; Liua, R.; Liu, G.; Li, N.; Li, Q.; Xu, H.; Wu, G. Controlled synthesis of micro/nanostructured CuO anodes for lithium-ion batteries. Nano Energy 2014, 9, 334–344. [Google Scholar] [CrossRef]
- Xiang, J.; Tu, J.; Zhang, L.; Zhou, Y.; Wang, X.; Shi, S. Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium ion batteries. J. Power Sources 2010, 195, 313–319. [Google Scholar] [CrossRef]
- Patil, A.S.; Lohar, G.M.; Fulari, V.J. Structural, morphological, optical and photoelectrochemical cell properties of copper oxide using modified SILAR method. J. Mater. Sci. Mater Electron. 2016, 27, 9550–9557. [Google Scholar] [CrossRef]
- Xiao, A.; Zhou, S.; Zuo, C.; Zhuan, Y.; Ding, X. Improved electrochemical performances of CuO nanotube array prepared via electrodeposition as anode for lithium ion battery. Mater. Res. Bull. 2015, 70, 795–798. [Google Scholar] [CrossRef]
- Sahay, R.; Kumar, P.S.; Aravindan, V.; Sundaramurthy, J.; Ling, W.C.; Mhaisalkar, S.G.; Ramakrishna, S.; Madhavi, S. High Aspect Ratio Electrospun CuO Nanofibers as Anode Material for Lithium-Ion Batteries with Superior Cycleability. J. Phys. Chem. C 2012, 116, 18087–18092. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Batoo, K.M.; Kumari, S.; Kalia, R.; Kumar, R.; Hadi, M.; Raslan, E.H.; Imran, A. Structural and optical properties of copper oxide nanoparticles: A study of variation in structure and antibiotic activity. J. Mater. Res. 2021, 36, 1496–1509. [Google Scholar] [CrossRef]
- Dar, M.; Ahsanulhaq, Q.; Kim, Y.; Sohn, J.; Kim, W.; Shin, H. Versatile synthesis of rectangular shaped nanobat-like CuO nanostructures by hydrothermal method; structural properties and growth mechanism. Appl. Surf. Sci. 2009, 255, 6279–6284. [Google Scholar] [CrossRef]
- Rath, P.C.; Patra, J.; Saikia, D.; Mishra, M.; Tseng, C.-M.; Chang, J.-K.; Kao, H.-M. Comparative Study on the Morphology-Dependent Performance of Various CuO Nanostructures as Anode Materials for Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10876–10885. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Guo, H.; Tong, X. Synthesis of CuO nanowire arrays as high-performance electrode for lithium ion batteries. Mater. Lett. 2015, 139, 55–58. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef]
- Mohanty, D.; Chang, M.-J.; Hung, I.-M. The Effect of Different Amounts of Conductive Carbon Material on the Electrochemical Performance of the LiFePO4 Cathode in Li-Ion Batteries. Batteries 2023, 9, 515. [Google Scholar] [CrossRef]
- Tsai, S.-H.; Tsou, Y.-L.; Yang, C.-W.; Chen, T.-Y.; Lee, C.-Y. Applications of different nano-sized conductive materials in high energy density pouch type lithium ion batteries. Electrochimica Acta 2020, 362, 137166. [Google Scholar] [CrossRef]
- Hausbrand, R. Electronic energy levels at Li-ion cathode-liquid electrolyte interfaces: Concepts, experimental insights, and perspectives. J. Chem. Phys. 2020, 152, 180902. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Annathurai, S.; Chidambaram, S.; Baskaran, B.; Prasanna, V.G.K.D. Green Synthesis and Electrical Properties of p-CuO/n-ZnO Heterojunction Diodes. J. Inorg. Organomet. Polym. Mater. 2019, 29, 535–540. [Google Scholar] [CrossRef]
- Seo, S.-D.; Jin, Y.-H.; Lee, S.-H.; Shim, H.-W.; Kim, D.-W. Low-temperature synthesis of CuO-interlaced nanodiscs for lithium ion battery electrodes. Nanoscale Res. Lett. 2011, 6, 397. [Google Scholar] [CrossRef]
- Lee, J.; Choi, S.; Park, S. A Simple Fabrication of Interconnected CuO Nanotube Electrodes for High-Performance Lithium-Ion Batteries. Chem.–Asian J. 2013, 8, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Nair, S.V.; Santhanagopalan, D.; Rai, A.K. Nanoplate and mulberry-like porous shape of CuO as anode materials for secondary lithium ion battery. Electrochimica Acta 2016, 206, 217–225. [Google Scholar] [CrossRef]
- Wan, M.; Jin, D.; Feng, R.; Si, L.; Gao, M.; Yue, L. Pillow-shaped porous CuO as anode material for lithium-ion batteries. Inorg. Chem. Commun. 2011, 14, 38–41. [Google Scholar] [CrossRef]
- Wu, R.; Qian, X.; Yu, F.; Liu, H.; Zhou, K.; Wei, J.; Huang, Y. MOF-templated formation of porous CuO hollow octahedra for lithium-ion battery anode materials. J. Mater. Chem. A 2013, 1, 11126–11129. [Google Scholar] [CrossRef]



| Structural Parameters of CuO | |
|---|---|
| Space group | C12/c1 |
| a (Å) | 4.6829 |
| b (Å) | 3.4149 |
| c (Å) | 5.1346 |
| Volume (Å)3 | 81.0246 |
| α(deg.) | 90 |
| β(deg.) | 99.324 |
| γ(deg.) | 90 |
| Density (g/cm3) | 6.856 |
| Atom parameters of Cu | |
| x | 0.25000 |
| y | 0.25000 |
| z | 0.00000 |
| Atom parameters of O | |
| x | 0.00000 |
| y | 0.91556 |
| z | 0.75000 |
| Refinement details | |
| χ2 | 1.19 |
| Rp | 8.99 |
| Rwp | 13.4 |
| Rexp | 12.35 |
| Concentration | Discharge Capacity for the First Cycle (mA h g−1) | Charge Capacity for the First Cycle (mA h g−1) | Discharge Capacity for the 50th Cycle (mA h g−1) | Charge Capacity for the 50th Cycle (mA h g−1) |
|---|---|---|---|---|
| 0.001 M | 889.5 | 192.7 | 78.4 | 77.5 |
| 0.02 M | 1366 | 451.7 | 232.3 | 222.9 |
| 0.03 M | 3371.9 | 1369.7 | 442.9 | 431.5 |
| 0.04 M | 2739 | 1625.7 | 213.6 | 199.8 |
| 0.05 M | 2009.9 | 733.6 | 196.5 | 210.1 |
| 0.1 M | 842.2 | 105.8 | 209.8 | 198.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, W.T.R.S.; Amaraweera, T.H.N.G.; Jayathilaka, K.M.D.C.; Kumara, L.S.R.; Seo, O.; Osaka, K.; Sakata, O.; Wijesundera, R.P.; Wijayasinghe, H.W.M.A.C. Facile Synthesis of Sponge-like Microstructured CuO Anode Material for Rechargeable Lithium-Ion Batteries. Coatings 2025, 15, 467. https://doi.org/10.3390/coatings15040467
Fernando WTRS, Amaraweera THNG, Jayathilaka KMDC, Kumara LSR, Seo O, Osaka K, Sakata O, Wijesundera RP, Wijayasinghe HWMAC. Facile Synthesis of Sponge-like Microstructured CuO Anode Material for Rechargeable Lithium-Ion Batteries. Coatings. 2025; 15(4):467. https://doi.org/10.3390/coatings15040467
Chicago/Turabian StyleFernando, W. T. R. S., T. H. N. G. Amaraweera, K. M. D. C. Jayathilaka, L. S. R. Kumara, O. Seo, K. Osaka, O. Sakata, R. P. Wijesundera, and H. W. M. A. C. Wijayasinghe. 2025. "Facile Synthesis of Sponge-like Microstructured CuO Anode Material for Rechargeable Lithium-Ion Batteries" Coatings 15, no. 4: 467. https://doi.org/10.3390/coatings15040467
APA StyleFernando, W. T. R. S., Amaraweera, T. H. N. G., Jayathilaka, K. M. D. C., Kumara, L. S. R., Seo, O., Osaka, K., Sakata, O., Wijesundera, R. P., & Wijayasinghe, H. W. M. A. C. (2025). Facile Synthesis of Sponge-like Microstructured CuO Anode Material for Rechargeable Lithium-Ion Batteries. Coatings, 15(4), 467. https://doi.org/10.3390/coatings15040467







