Microstructure and Thermal Analysis Kinetics of Y2Hf2O7/Y3Al5O12 Composites Prepared by Solution Combustion Synthesis
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Sample Characterization
2.3. Kinetic Method for Thermal Analysis
2.3.1. Kissinger Method
2.3.2. Augis and Bennett Method
2.3.3. Mahadevan Method
3. Results and Discussion
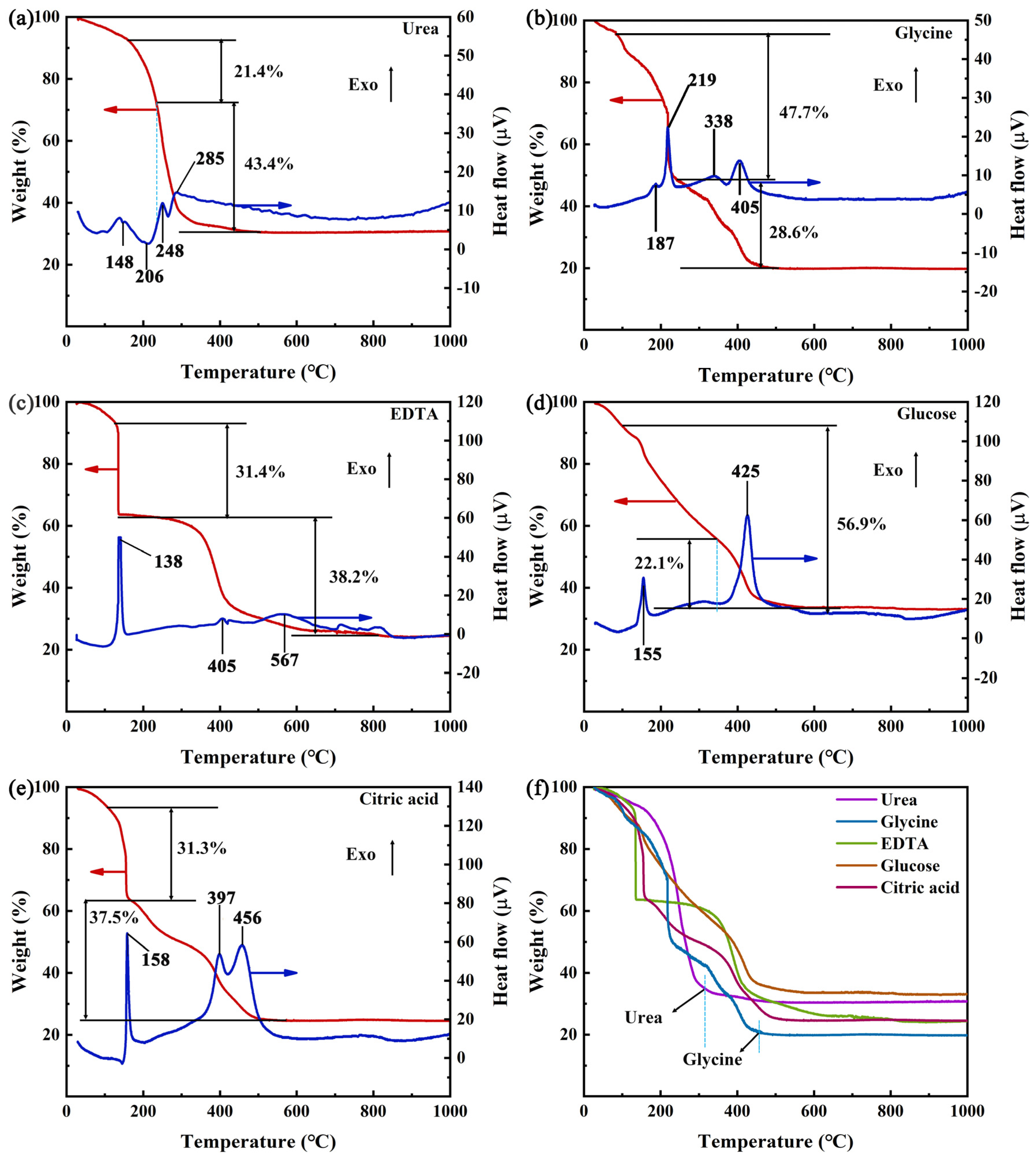
3.1. TG-DTA Analysis
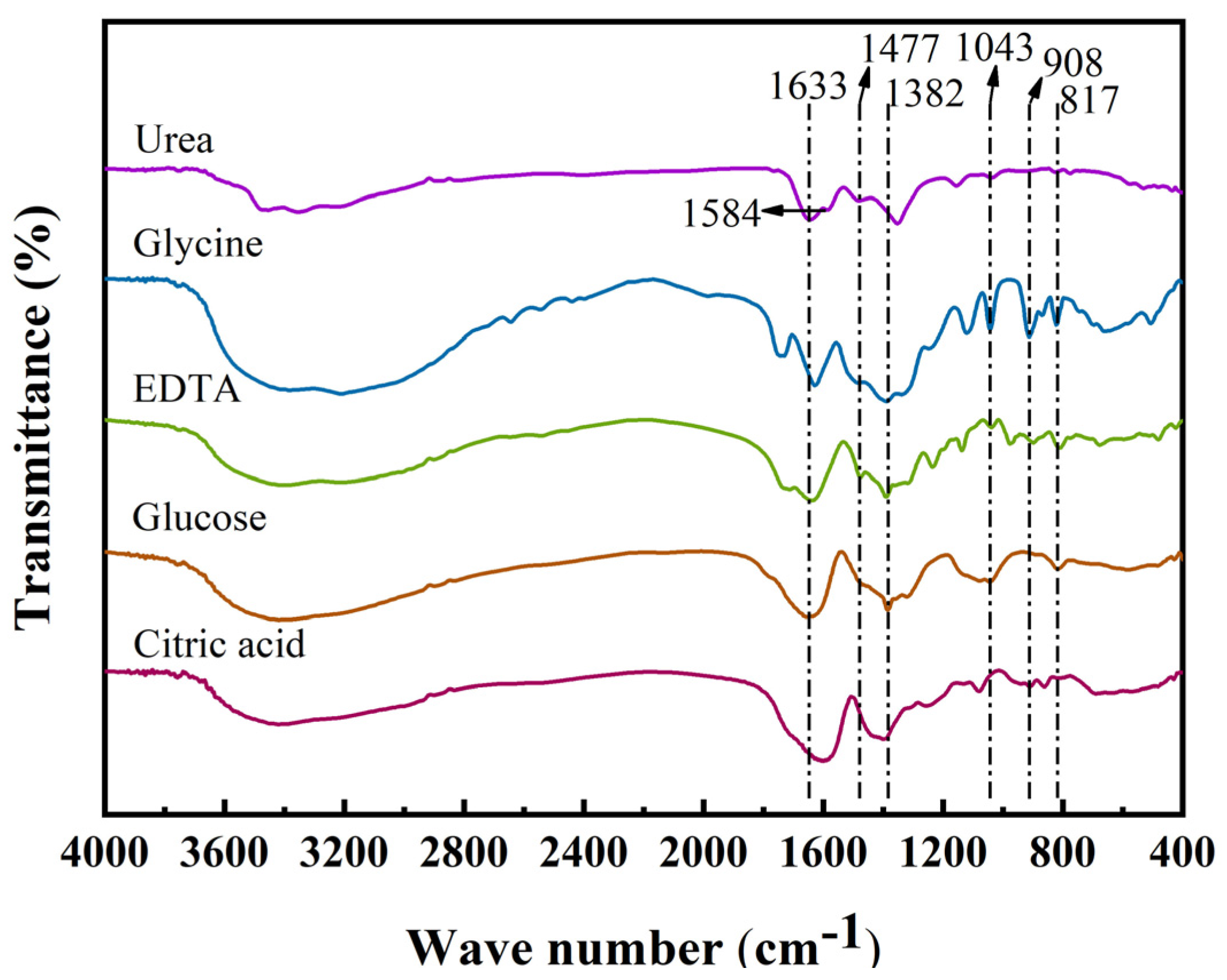
3.2. Analysis of Fourier-Transform Infrared Spectroscopy
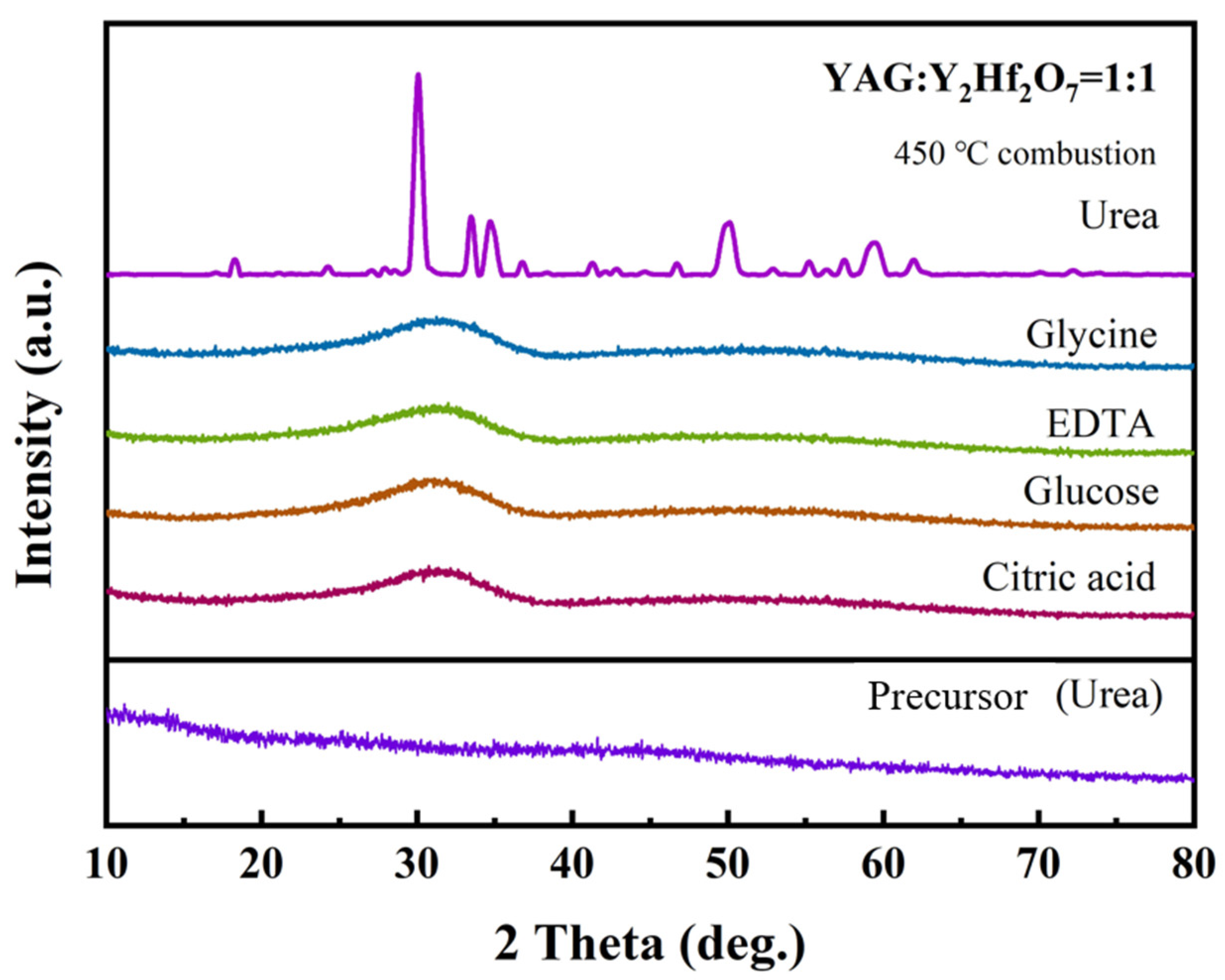
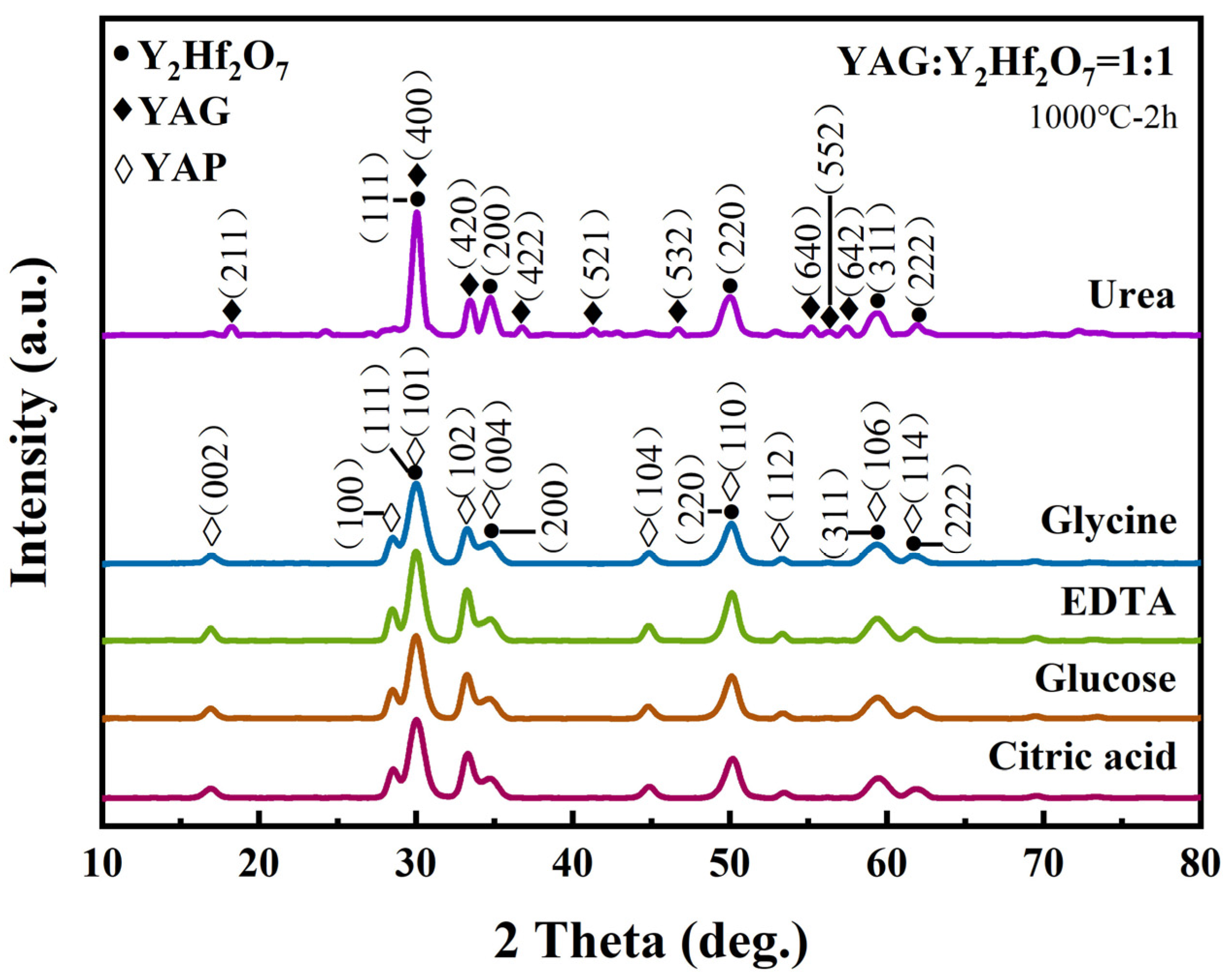
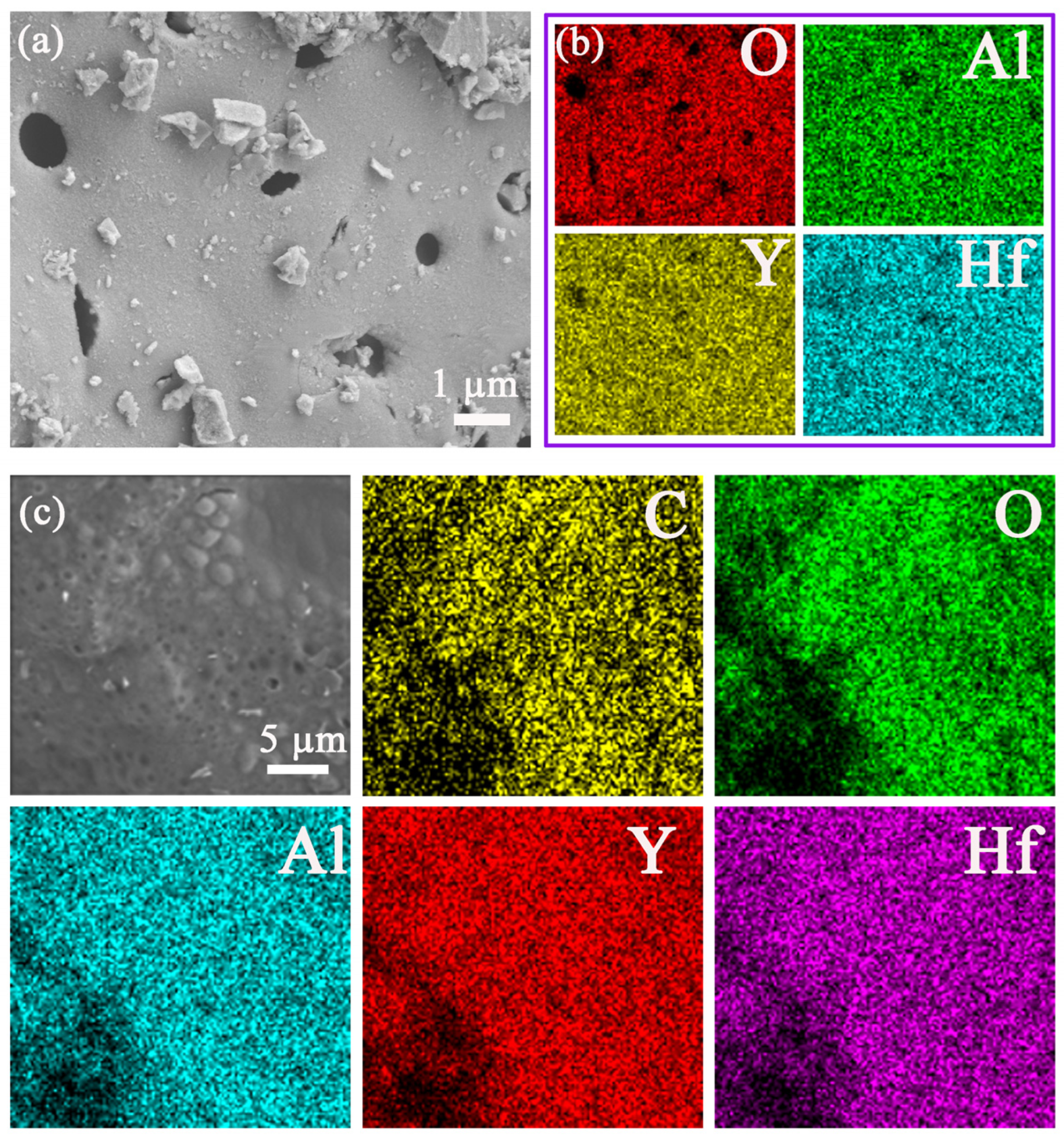
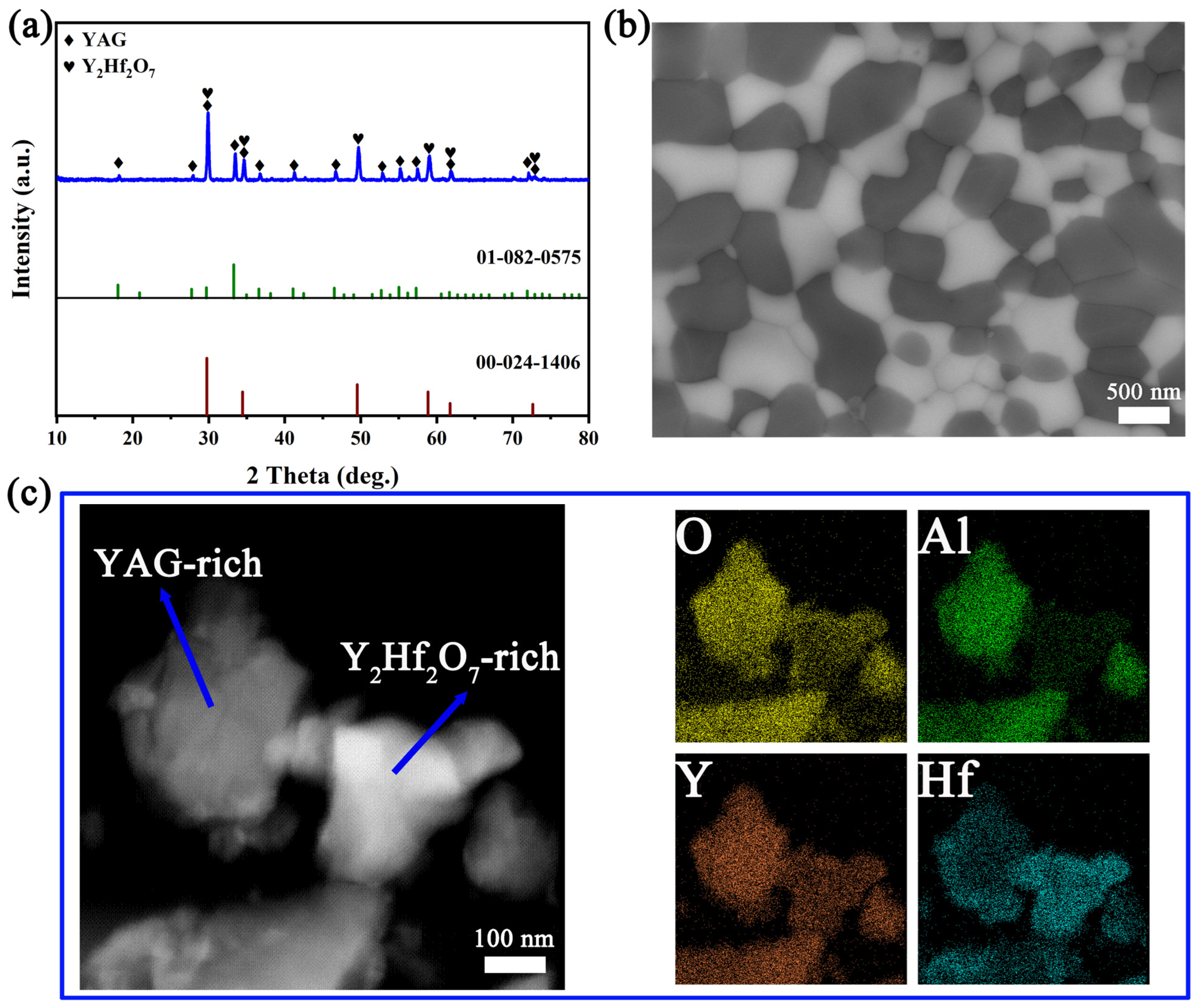
3.3. Analysis of Microstructure and Morphology
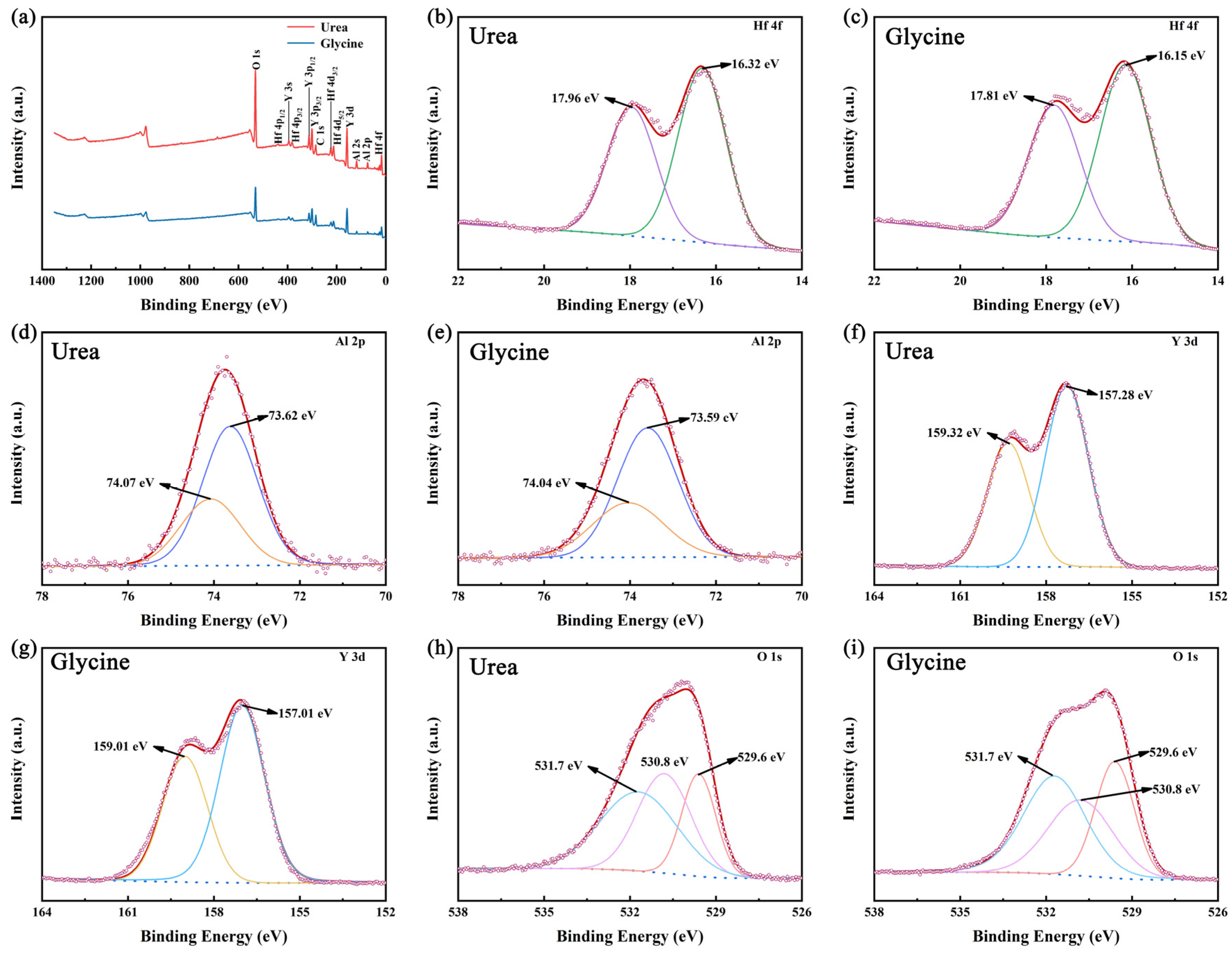
3.4. Analysis of X-Ray Photoelectron Spectroscopy
3.5. Determination of Activation Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Chen, Y.; Li, L.; Yang, D.; Liu, X.; Huang, A.; Zhang, X.; Lu, J.; Zhao, X. Unraveling the CMAS corrosion mechanism of APS high-yttria-stabilized zirconia thermal barrier coatings. J. Eur. Ceram. Soc. 2024, 44, 5154–5165. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, Z.; Liu, G.; He, L.; Mu, R.; Xu, Z. Sm-doped Gd2Zr2O7 thermal barrier coatings: Thermal expansion coefficient, structure and failure. Vacuum 2021, 190, 110314. [Google Scholar] [CrossRef]
- Yu, B.; Shen, Z.; Luo, Y.; Liu, G.; Mu, R.; He, L. Effects of A site content on the phase structure and thermal property of (La1−xGdx)2Zr2O7 ceramics. Ceram. Int. 2024, 51, 8192–8196. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Xu, J.; Liu, Y.; Feng, X.; Fan, F.; Wang, X.; Gao, F. Enhanced thermophysical and mechanical properties of gadolinium zirconate ceramics via non-stoichiometric design. J. Eur. Ceram. Soc. 2024, 44, 116790. [Google Scholar] [CrossRef]
- Kutty, K.G.; Rajagopalan, S.; Mathews, C.; Varadaraju, U. Thermal expansion behaviour of some rare earth oxide pyrochlores. Mater. Res. Bull. 1994, 29, 759–766. [Google Scholar] [CrossRef]
- Zhong, D.; Quansheng, W.; Zhuang, M.; Yunkai, L.; Yanbo, L. Thermal property of Y2Hf2O7 ceramic for thermal barrier coatings. Rare Met. Mater. Eng. 2011, 40, 268–270. [Google Scholar]
- Liao, Y.K.; Jiang, D.Y.; Ji, Y.M.; Shi, J.L. Combustion synthesis of nanosized Y2Hf2O7 and Lu2Hf2O7 powders. Key Eng. Mater. 2004, 280, 643–646. [Google Scholar]
- Gu, S.; Zhang, S.; Liu, F.; Li, W. New anti-ablation candidate for carbon/carbon composites: Preparation, composition and ablation behavior of Y2Hf2O7 coating under an oxyacetylene torch. J. Eur. Ceram. Soc. 2018, 38, 5082–5091. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, S.; Liu, F.; Liang, Y.; Li, W. Microstructure and thermal shock performance of Y2Hf2O7 coating deposited on SiC coated C/C composite. Appl. Surf. Sci. 2018, 455, 849–855. [Google Scholar] [CrossRef]
- Corman, G. Creep of yttrium aluminium garnet single crystals. J. Mater. Sci. Lett. 1993, 12, 379–382. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Jiang, B.; Zhou, J.; Zhang, W.; Wang, L.; Shen, Y.; Pan, Y.; Guo, J. Synthesis of nanocrystalline yttria powder and fabrication of Cr, Nd: YAG transparent ceramics. J. Alloys Compd. 2012, 515, 49–56. [Google Scholar] [CrossRef]
- Bučevac, D.; Omerašević, M.; Egelja, A.; Radovanović, Ž.; Kljajević, L.; Nenadović, S.; Krstić, V. Effect of YAG content on creep resistance and mechanical properties of Al2O3-YAG composite. Ceram. Int. 2020, 46, 15998–16007. [Google Scholar] [CrossRef]
- He, L.; Cao, S.; Li, W.; Dong, Q.; Sun, W. YAG/Al2O3/ZrO2 composite fibers of core-shell structure prepared by electrospinning. J. Asian Ceram. Soc. 2021, 9, 1046–1054. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.; Taheri-Nassaj, E.; Sarpoolaky, H. Synthesis of an alumina–YAG nanopowder via sol–gel method. J. Alloys Compd. 2008, 456, 282–285. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution combustion synthesis of nanoscale materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, K.; Mukasyan, A.; Varma, A. Direct synthesis of iron oxide nanopowders by the combustion approach: Reaction mechanism and properties. Chem. Mater. 2004, 16, 4896–4904. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Lee, J.H.; Ding, J.-R.; Kim, K.-S.; Manukyan, K.V.; Mukasyan, A.S. Combustion synthesis of zero-, one-, two-and three-dimensional nanostructures: Current trends and future perspectives. Prog. Energy Combust. Sci. 2017, 63, 79–118. [Google Scholar] [CrossRef]
- Sa-nguanprang, S.; Phuruangrat, A.; Thongtem, T.; Thongtem, S. Synthesis of ZnO nanoparticles by tartaric acid solution combustion and their photocatalytic properties. Russ. J. Inorg. Chem. 2020, 65, 1102–1110. [Google Scholar] [CrossRef]
- Marand, N.A.; Masoudpanah, S.; Bafghi, M.S. Solution combustion synthesis of nickel sulfide composite powders. Ceram. Int. 2018, 44, 17277–17282. [Google Scholar] [CrossRef]
- Kondo, A.; Kozawa, T.; Ishikawa, T.; Naito, M. Rapid synthesis of YAG phosphor by facile mechanical method. Int. J. Appl. Ceram. Technol. 2021, 19, 681–687. [Google Scholar] [CrossRef]
- Vallejos, S.; Di Maggio, F.; Shujah, T.; Blackman, C. Chemical vapour deposition of gas sensitive metal oxides. Chemosensors 2016, 4, 4. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Kang, J.; Liu, W.; Kuang, Y.; Tan, H.; Yu, Z.; Yang, L.; Yang, X.; Yu, K. Preparation of Ce-MnOx Composite Oxides via Coprecipitation and Their Catalytic Performance for CO Oxidation. Nanomaterials 2023, 13, 2158. [Google Scholar] [CrossRef]
- Sherikar, B.N.; Sahoo, B.; Umarji, A.M. Effect of fuel and fuel to oxidizer ratio in solution combustion synthesis of nanoceramic powders: MgO, CaO and ZnO. Solid State Sci. 2020, 109, 106426. [Google Scholar] [CrossRef]
- Sangeetha, A.; Ambli, A.; Nagabhushana, B.; Murugendrappa, M. Green color emitting pure cubic zirconia nano phosphor synthesized by solution combustion technique. J. Nanoparticle Res. 2024, 26, 18. [Google Scholar] [CrossRef]
- Heleena, M.; Kumar, S.S.; Balaji, N.; Aruna, S.T. Solution combustion synthesized micron sized yttrium aluminum garnet (Y3Al5O12) powder: A promising feedstock source for plasma spraying. Ceram. Int. 2022, 48, 35740–35749. [Google Scholar] [CrossRef]
- Amirkhanyan, N.; Kharatyan, S.; Manukyan, K.; Aprahamian, A. Thermodynamics and kinetics of solution combustion synthesis: Ni(NO3)2+fuels systems. Combust. Flame 2020, 221, 110–119. [Google Scholar] [CrossRef]
- Kumar, A.; Wolf, E.; Mukasyan, A. Solution combustion synthesis of metal nanopowders: Nickel—Reaction pathways. Am. Inst. Chem. Eng. J. 2011, 57, 2207–2214. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Popescu, C. Notes on workshop on kinetics/ESTAC-10, Rotterdam. J. Therm. Anal. Calorim. 2011, 105, 931. [Google Scholar] [CrossRef]
- Budrugeac, P. On the use of oxidative stability measurements for short-term thermal endurance characterization of polymeric materials. Polym. Degrad. Stab. 2000, 68, 289–293. [Google Scholar] [CrossRef]
- Bamford, C.H.; Tipper, C.F.H.; Compton, R.G. Comprehensive Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 1969. [Google Scholar]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Augis, J.; Bennett, J. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J. Therm. Anal. Calorim. 1978, 13, 283–292. [Google Scholar] [CrossRef]
- Mahadevan, S.; Giridhar, A.; Singh, A. Calorimetric measurements on as-sb-se glasses. J. Non-Crystalline Solids 1986, 88, 11–34. [Google Scholar] [CrossRef]
- Jain, D.; Sudarsan, V.; Patra, A.K.; Sastry, P.U.; Tyagi, A.K. Effect of local ordering around Th4+ ions in glycine-nitrate precursor gel on the powder characteristic of gel-combusted ThO2. J. Nucl. Mater. 2019, 527, 151826. [Google Scholar] [CrossRef]
- Subohi, O.; Shastri, L.; Kumar, G.S.; Malik, M.M.; Kurchania, R. Study of Maxwell–Wagner (M–W) relaxation behavior and hysteresis observed in bismuth titanate layered structure obtained by solution combustion synthesis using dextrose as fuel. Mater. Res. Bull. 2014, 49, 651–656. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Zhang, P.; Wang, Z.; Li, X.; Hu, M. Nano-sized plate-like alumina synthesis via solution combustion. Ceram. Int. 2019, 45, 9919–9925. [Google Scholar] [CrossRef]
- Rabbani, M.; Rahimi, R.; Ghadi, H.F. Photocatalytic application of BiFeO3 synthesized via a facile microwave-assisted solution combustion method. J. Sol-Gel Sci. Technol. 2018, 87, 340–346. [Google Scholar] [CrossRef]
- Golsheikh, M.M.; Arabi, A.M.; Afarani, M.S. Microwave assisted combustion synthesis of photolumiescent ZnAl2O4: Eu nano powders. Mater. Res. Express 2019, 6, 125052. [Google Scholar] [CrossRef]
- Terpugov, E.L.; Kondratyev, M.S.; Degtyareva, O.V. Light-induced effects in glycine aqueous solution studied by Fourier transform infrared-emission spectroscopy and ultraviolet-visible spectroscopy. J. Biomol. Struct. Dyn. 2020, 39, 108–117. [Google Scholar] [CrossRef]
- Grdadolnik, J.; Maréchal, Y. Urea and urea–water solutions—An infrared study. J. Mol. Struct. 2002, 615, 177–189. [Google Scholar] [CrossRef]
- Keuleers, R.; Desseyn, H.; Rousseau, B.; Van Alsenoy, C. Vibrational analysis of urea. J. Phys. Chem. A 1999, 103, 4621–4630. [Google Scholar] [CrossRef]
- Shang, Z.; Yu, Y.; Yang, H.; Yang, Z.; Xiao, Y.; Wang, X. One-step solution combustion synthesis of micro-nano-scale porous Cu/CeO2 with enhanced photocatalytic properties. J. Rare Earths 2023, 41, 250–258. [Google Scholar] [CrossRef]
- Tachiwaki, T.; Yoshinaka, M.; Hirota, K.; Ikegami, T.; Yamaguchi, O. Novel synthesis of Y3Al5O12 (YAG) leading to transparent ceramics. Solid State Commun. 2001, 119, 603–606. [Google Scholar] [CrossRef]
- Li, J.-G.; Lee, J.-H.; Mori, T.; Yajima, Y.; Takenouchi, S.; Ikegami, T. Crystal phase and sinterability of wet-chemically derived YAG powders. J. Ceram. Soc. Jpn. 2000, 108, 439–444. [Google Scholar] [CrossRef]
- Renault, O.; Samour, D.; Damlencourt, J.-F.; Blin, D.; Martin, F.; Marthon, S.; Barrett, N.; Besson, P. HfO2/SiO2 interface chemistry studied by synchrotron radiation X-ray photoelectron spectroscopy. Appl. Phys. Lett. 2002, 81, 3627–3629. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, D.; Li, S.; Liu, X.; Zhao, P.; Sun, N.; Ali, F.; Wang, J. Ferroelectric yttrium doped hafnium oxide films from all-inorganic aqueous precursor solution. Ceram. Int. 2018, 44, 13867–13872. [Google Scholar] [CrossRef]
- Talik, E.; Kruczek, M.; Zarek, W.; Kusz, J.; Wójcik, K.; Sakowska, H.; Szyrski, W. XPS characterization of YAlO3: Co single crystals. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2007, 42, 1341–1347. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, W.-H.; Oh, I.-K.; Kim, M.-K.; Lee, G.; Lee, C.-W.; Park, J.; Lansalot-Matras, C.; Noh, W.; Kim, H. Atomic layer deposition of Y2O3 and yttrium-doped HfO2 using a newly synthesized Y(iPrCp)2(N-iPr-amd) precursor for a high permittivity gate dielectric. Appl. Surf. Sci. 2014, 297, 16–21. [Google Scholar] [CrossRef]
- Tang, L.; Maruyama, H.; Han, T.; Nino, J.C.; Chen, Y.; Zhang, D. Resistive switching in atomic layer deposited HfO2/ZrO2 nanolayer stacks. Appl. Surf. Sci. 2020, 515, 146015. [Google Scholar] [CrossRef]
- Kainbayev, N.; Sriubas, M.; Bockute, K.; Virbukas, D.; Laukaitis, G. E-beam deposition of scandia-stabilized zirconia (ScSZ) thin films co-doped with Al. Coatings 2020, 10, 870. [Google Scholar] [CrossRef]




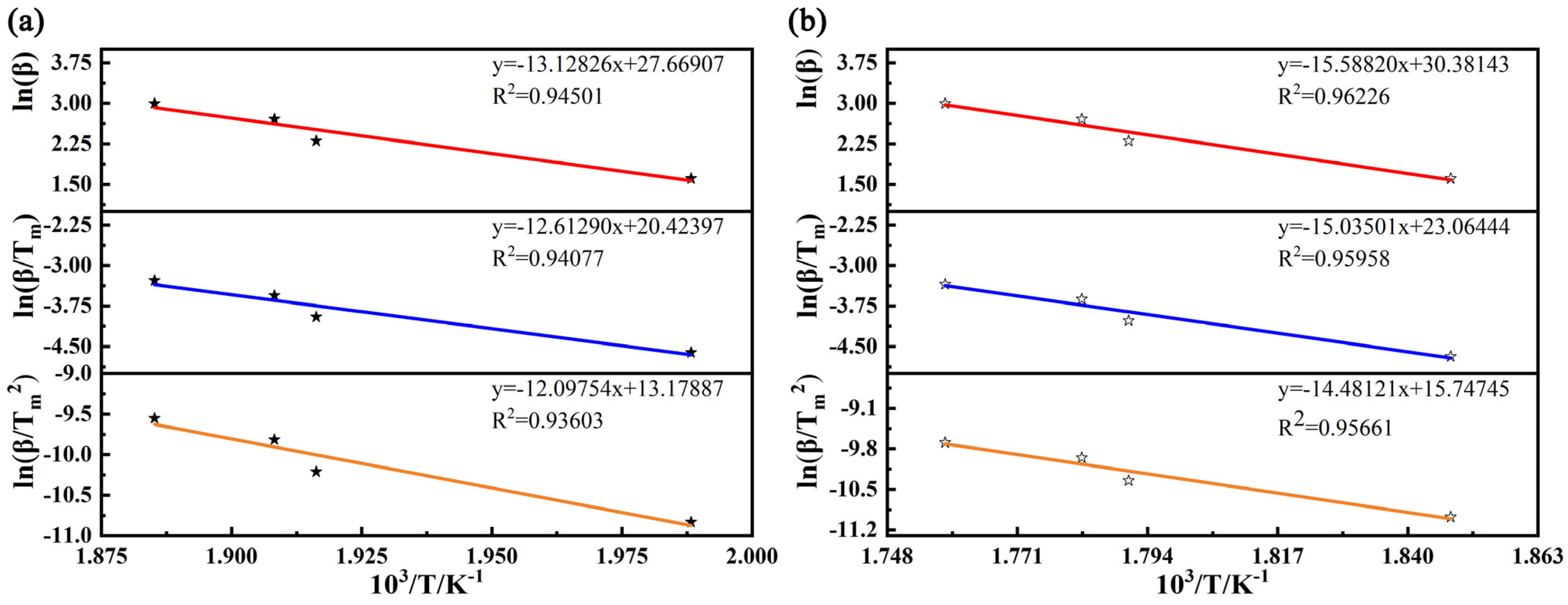
| β (K·min−1) | Tm (K) | 1/Tm (10−3·K−1) | ln(β/Tm2) | ln(β/Tm) | ln(β) | |
|---|---|---|---|---|---|---|
| Peak1 | 5 | 502.95 | 1.988 | −10.832 | −4.611 | 1.609 |
| 10 | 521.85 | 1.916 | −10.212 | −3.955 | 2.303 | |
| 15 | 524.05 | 1.908 | −9.815 | −3.554 | 2.708 | |
| 20 | 530.45 | 1.885 | −9.552 | −3.278 | 2.996 | |
| Peak2 | 5 | 541.25 | 1.848 | −10.978 | −4.684 | 1.609 |
| 10 | 558.45 | 1.791 | −10.348 | −4.023 | 2.303 | |
| 15 | 561.05 | 1.782 | −9.952 | −3.622 | 2.708 | |
| 20 | 568.75 | 1.758 | −9.691 | −3.348 | 2.996 |
| Methods | E1/(kJ·mol−1) | E2/(kJ·mol−1) |
|---|---|---|
| Kissinger | 100.579 | 120.397 |
| Augis and Bennett | 104.864 | 125.001 |
| Mahadevan | 109.148 | 129.600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Gu, S.; Guo, Y.; Xue, B.; Zhou, Q.; Yuan, R.; Cong, L.; Zhang, Y. Microstructure and Thermal Analysis Kinetics of Y2Hf2O7/Y3Al5O12 Composites Prepared by Solution Combustion Synthesis. Coatings 2025, 15, 470. https://doi.org/10.3390/coatings15040470
Li R, Gu S, Guo Y, Xue B, Zhou Q, Yuan R, Cong L, Zhang Y. Microstructure and Thermal Analysis Kinetics of Y2Hf2O7/Y3Al5O12 Composites Prepared by Solution Combustion Synthesis. Coatings. 2025; 15(4):470. https://doi.org/10.3390/coatings15040470
Chicago/Turabian StyleLi, Rui, Shengyue Gu, Yimin Guo, Bei Xue, Qian Zhou, Ruimei Yuan, Longkang Cong, and Yaming Zhang. 2025. "Microstructure and Thermal Analysis Kinetics of Y2Hf2O7/Y3Al5O12 Composites Prepared by Solution Combustion Synthesis" Coatings 15, no. 4: 470. https://doi.org/10.3390/coatings15040470
APA StyleLi, R., Gu, S., Guo, Y., Xue, B., Zhou, Q., Yuan, R., Cong, L., & Zhang, Y. (2025). Microstructure and Thermal Analysis Kinetics of Y2Hf2O7/Y3Al5O12 Composites Prepared by Solution Combustion Synthesis. Coatings, 15(4), 470. https://doi.org/10.3390/coatings15040470








