Co- and Sn-Doped YMnO3 Perovskites for Electrocatalytic Water-Splitting and Photocatalytic Pollutant Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Electrochemical Setup and Testing
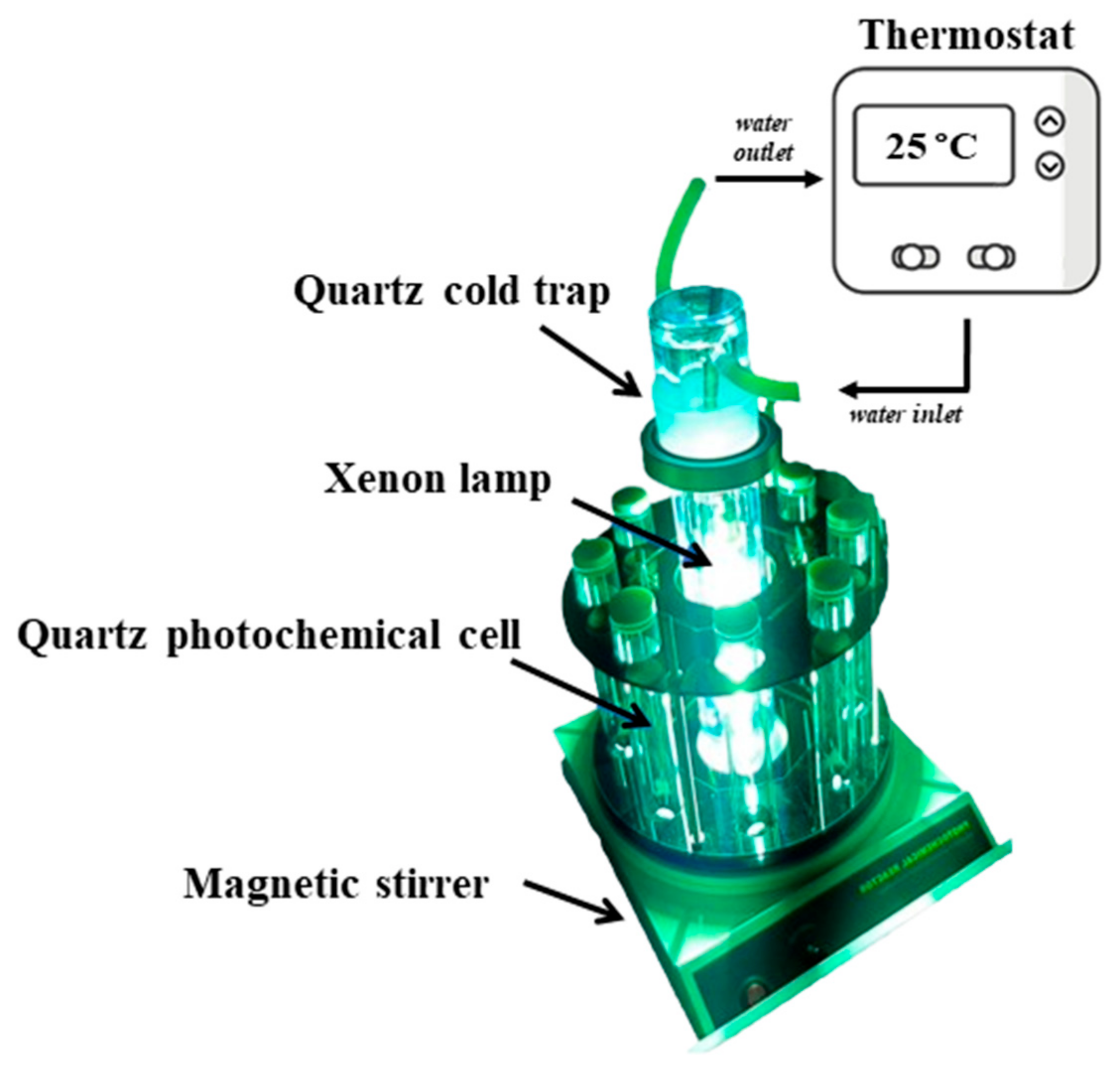
2.3. Sample Preparation and Photocatalytic Activity Experiments
3. Results and Discussion
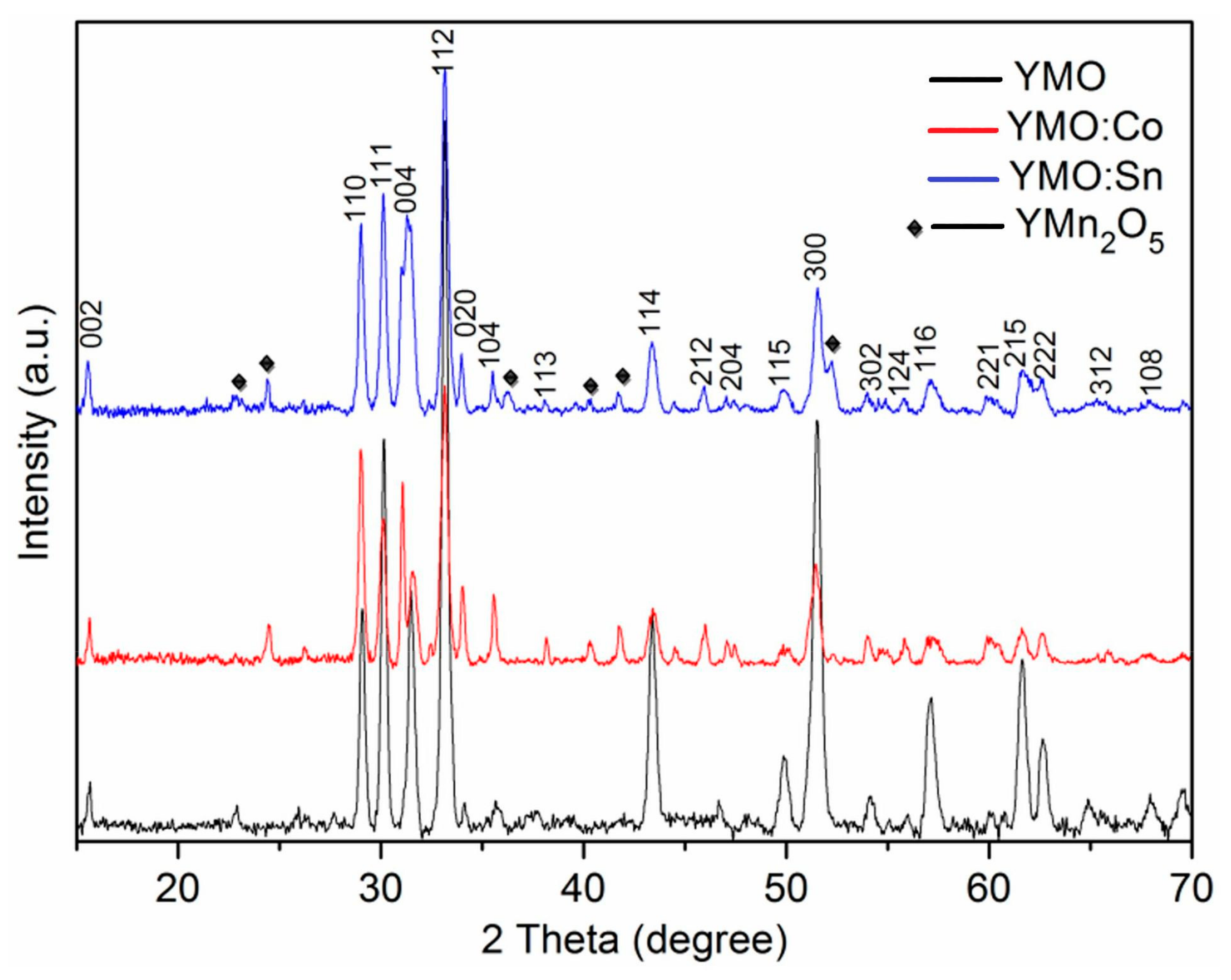
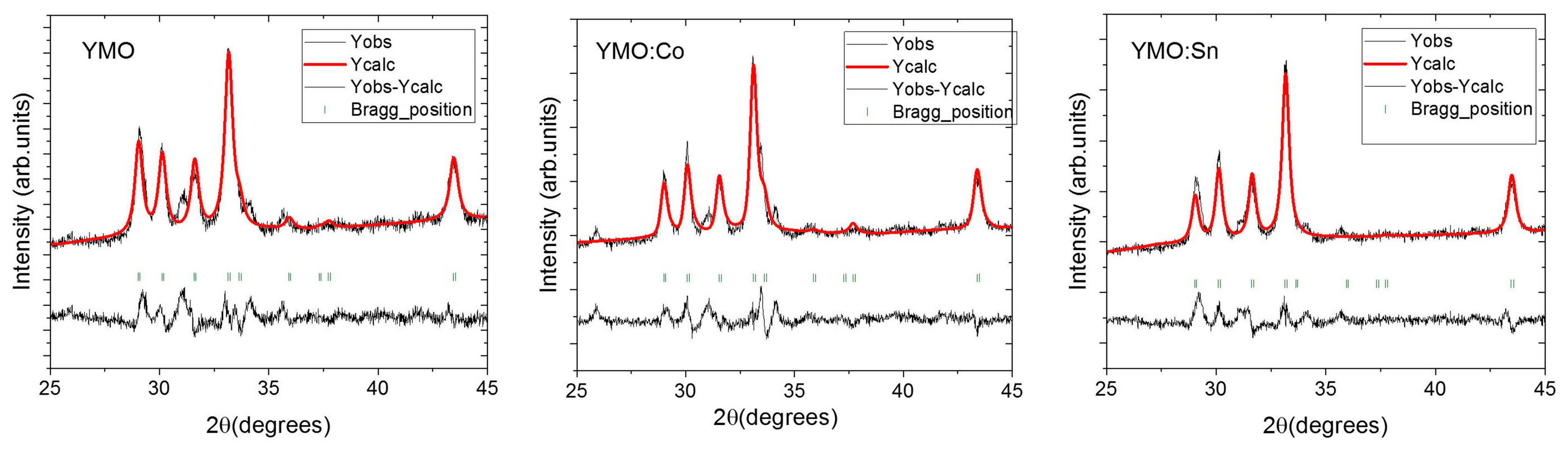
3.1. XRD Analysis
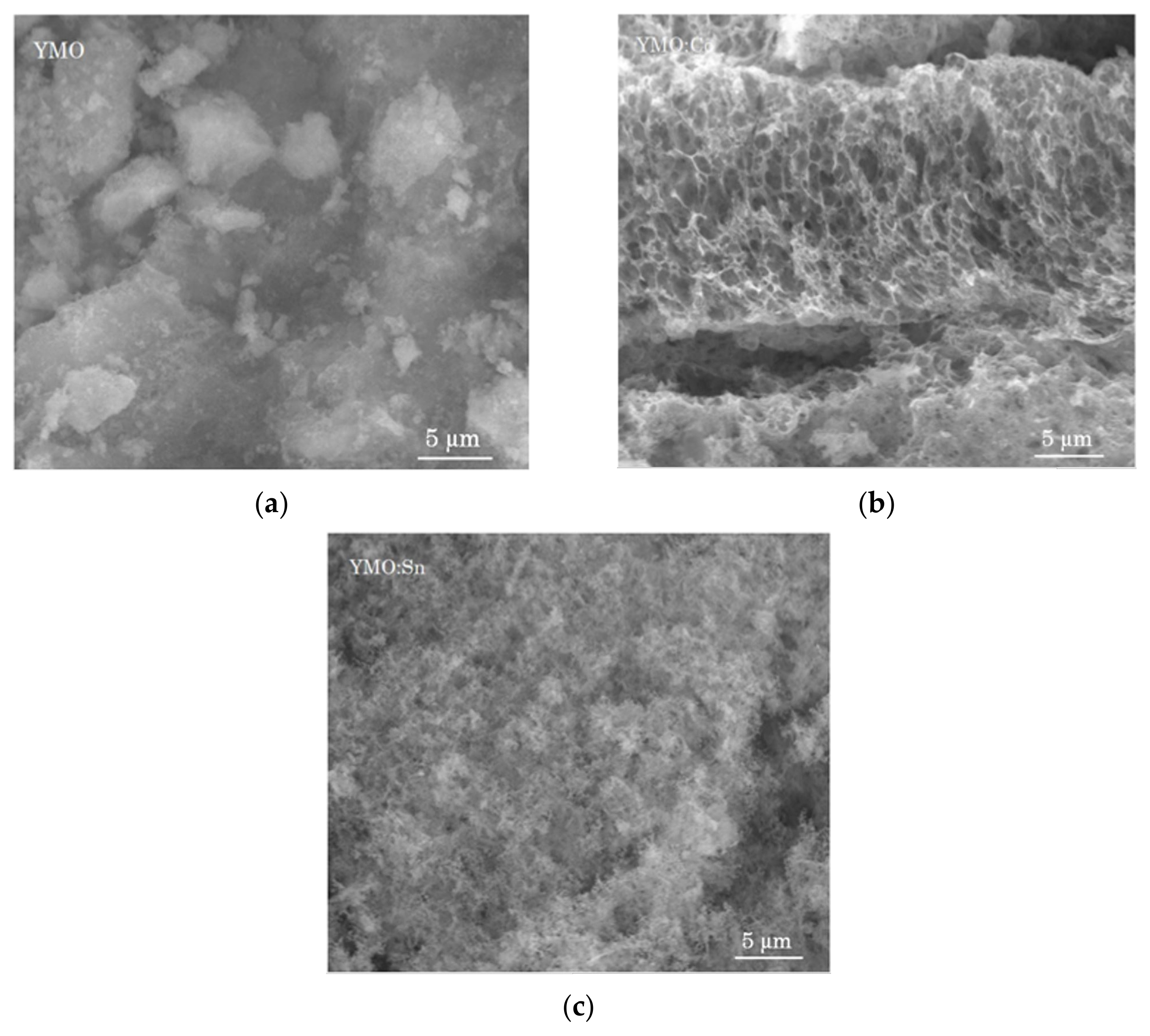
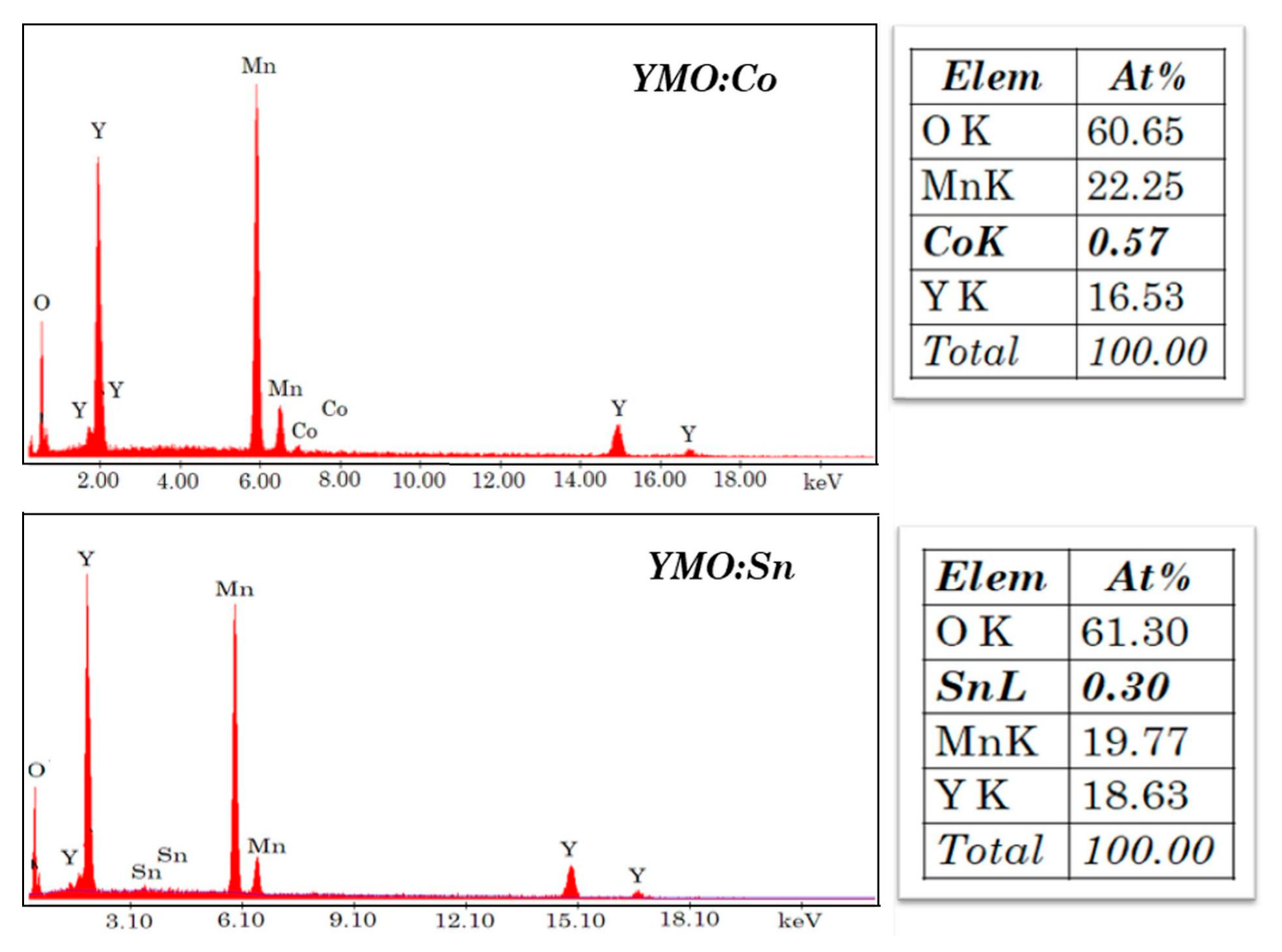
3.2. SEM Micrographs and EDX Analysis
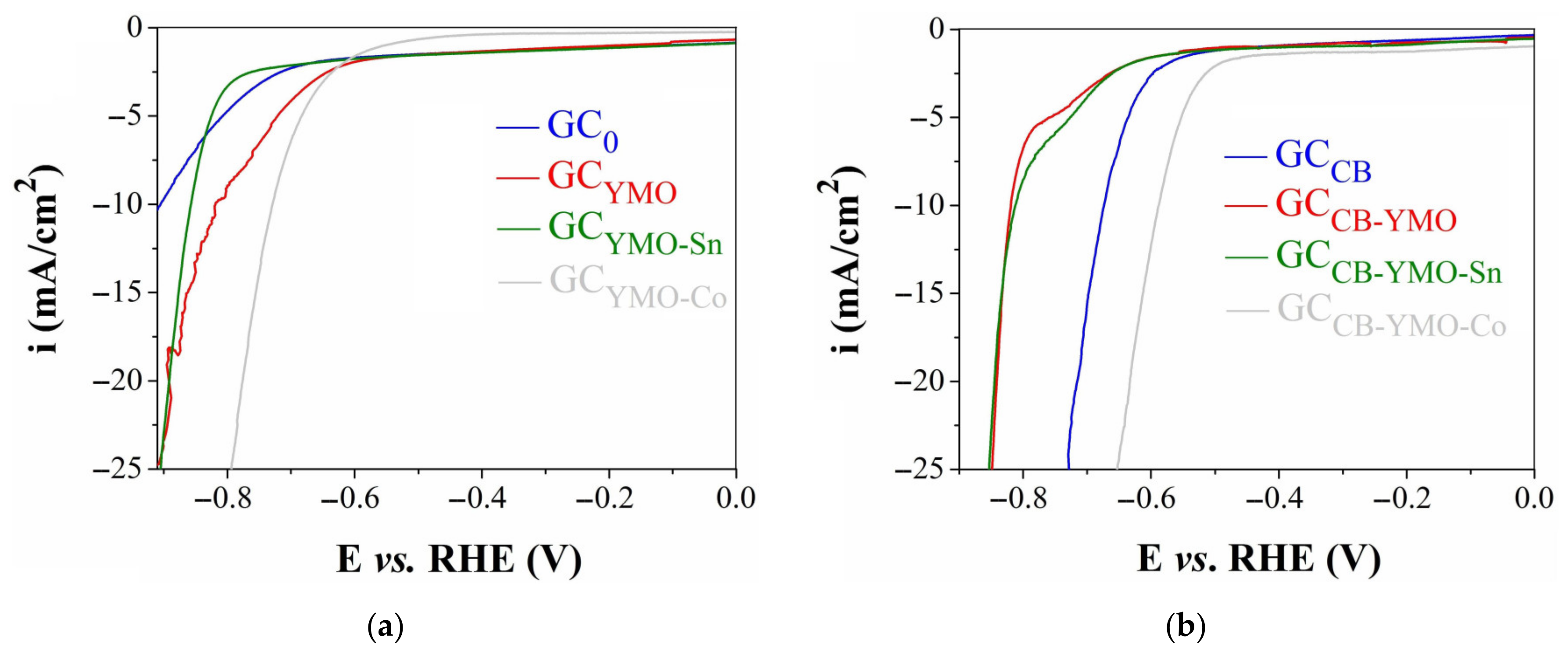
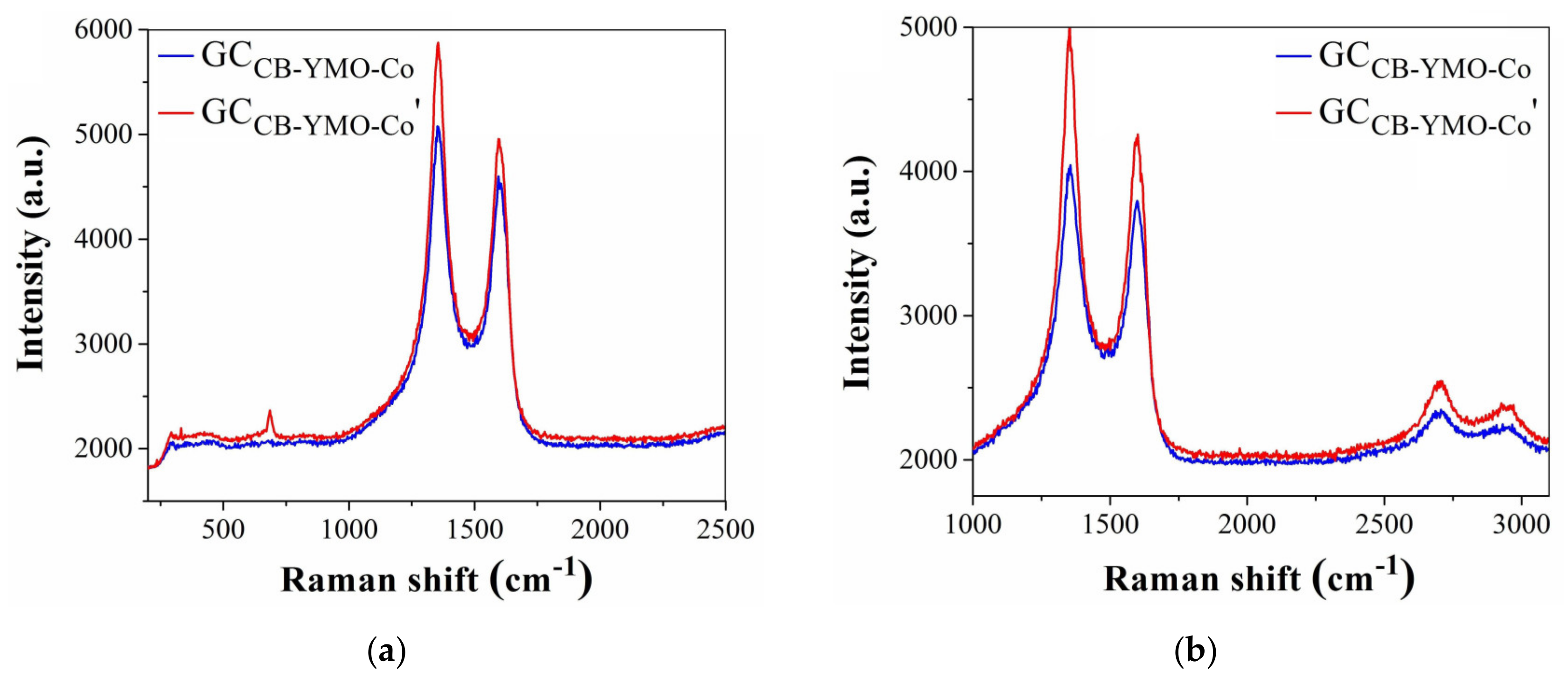
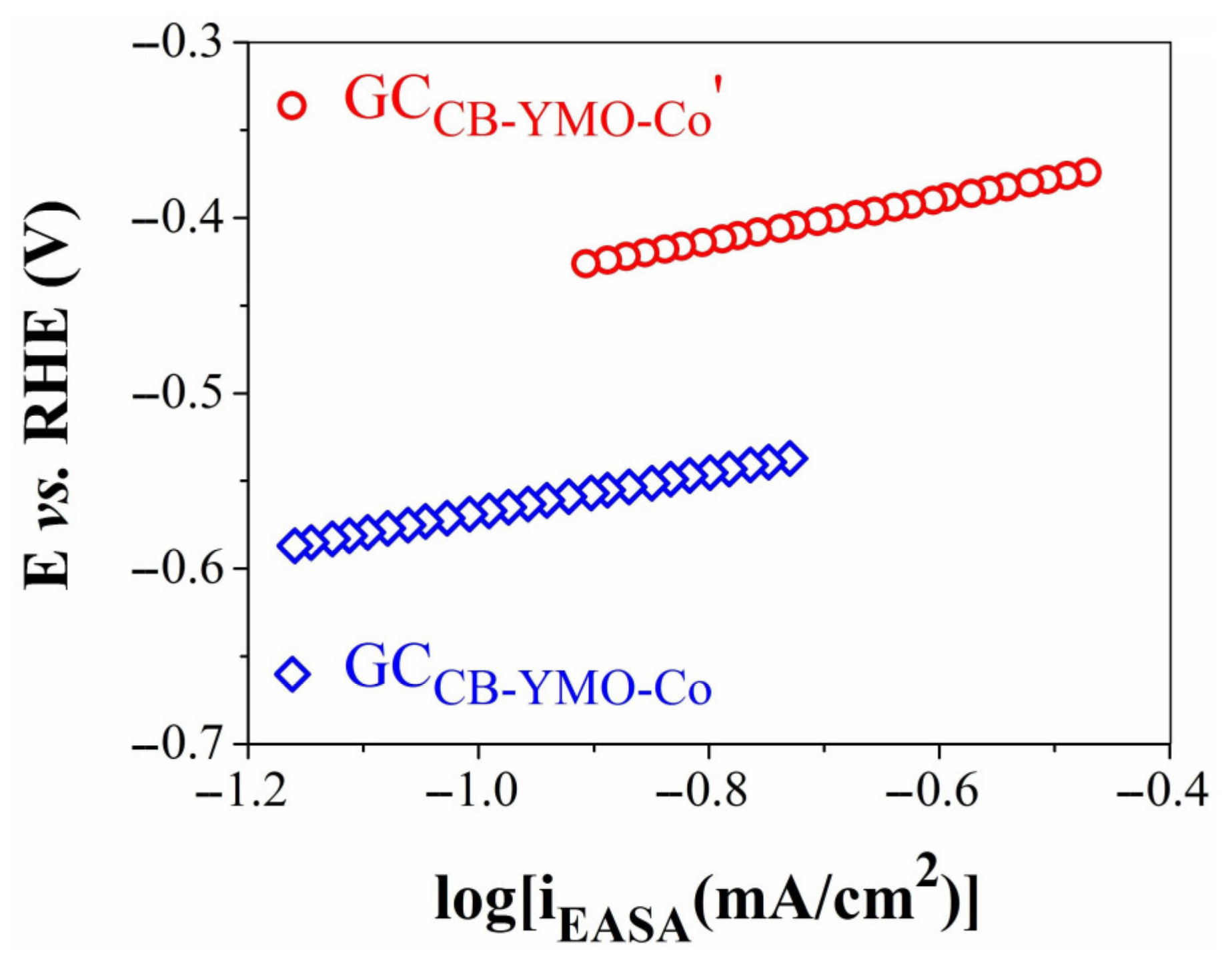
3.3. Electrochemical Studies
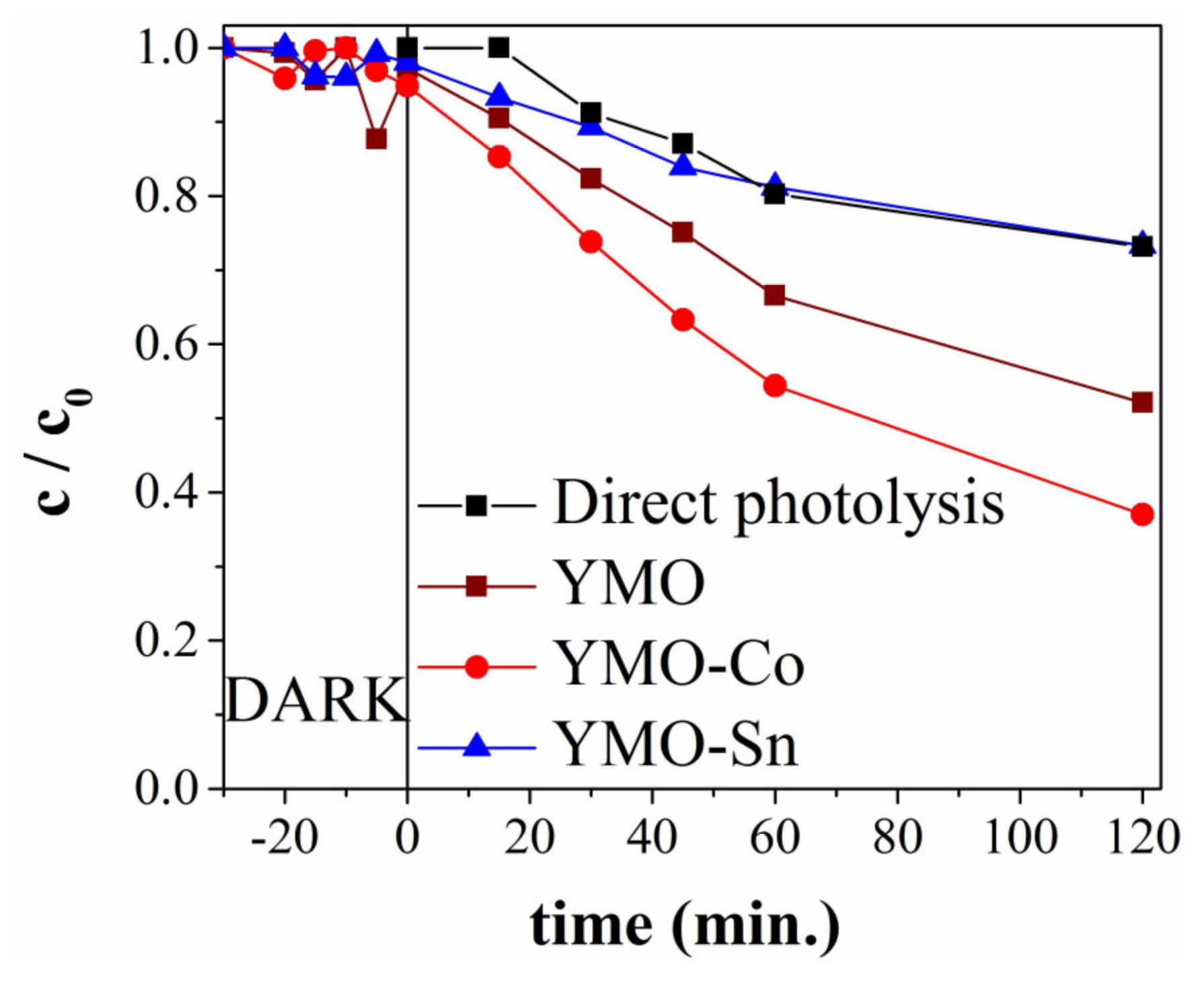
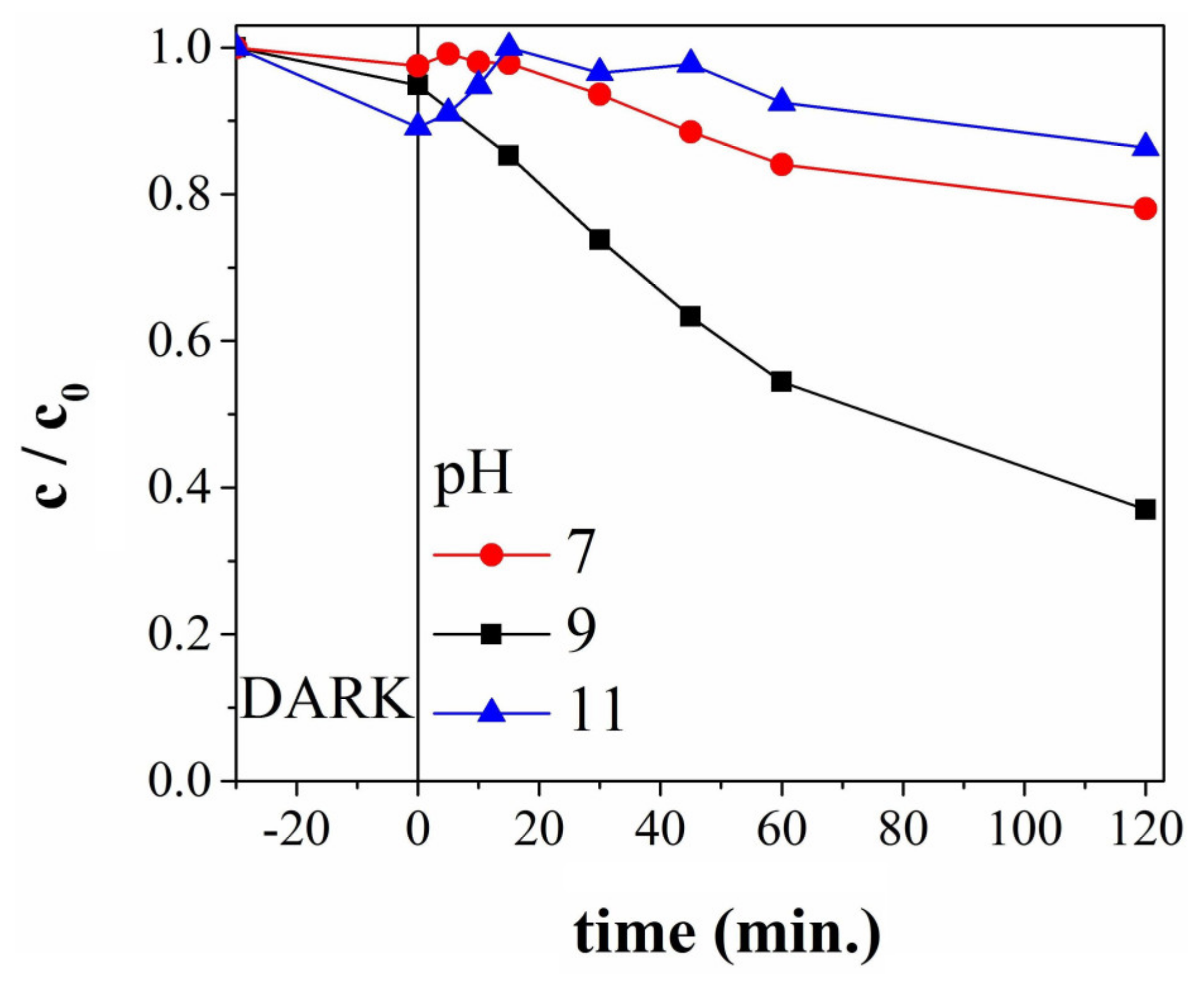
3.4. Photocatalytic Degradation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghandehariun, S.; Yekta, M.A.; Naterer, G.F. Review of Low Temperature Water Splitting Cycles for Thermochemical Hydrogen Production. Int. J. Hydrogen Energy 2024, 96, 1310–1327. [Google Scholar] [CrossRef]
- Sabir, A.S.; Pervaiz, E.; Khosa, R.; Sohail, U. An Inclusive Review and Perspective on Cu-Based Materials for Electrochemical Water Splitting. RSC Adv. 2023, 13, 4963–4993. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.M.; Noor, S.; Parveen, W. Advances in Water Splitting and Lithium-Ion Batteries: Pioneering Sustainable Energy Storage and Conversion Technologies. Front. Energy Res. 2025, 12, 1465349. [Google Scholar] [CrossRef]
- Balcu, I.; Ciucanu, I.; MacArie, C.; Taranu, B.; Ciupageanu, D.-A.; Lazaroiu, G.; Dumbrava, V. Decarbonization of Low Power Applications through Methanation Facilities Integration. In Proceedings of the 2019 IEEE PES Innovative Smart Grid Technologies Europe (ISGT-Europe), Bucharest, Romania, 29 September–2 October 2019; pp. 416–420. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, M.K.; Miotello, A.; Patel, N. Metal Boride-Based Catalysts for Electrochemical Water-Splitting: A Review. Adv. Funct. Mater. 2020, 30, 1906481. [Google Scholar] [CrossRef]
- Hossain Bhuiyan, M.M.; Siddique, Z. Hydrogen as an Alternative Fuel: A Comprehensive Review of Challenges and Opportunities in Production, Storage, and Transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A Comprehensive Review on the Electrochemical Parameters and Recent Material Development of Electrochemical Water Splitting Electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Hamzat, A.K.; Whidborne, J.; Kuang, B.; Jenkins, K.W. Integration of Renewable Energy Sources in Tandem with Electrolysis: A Technology Review for Green Hydrogen Production. Int. J. Hydrogen Energy 2024, 107, 218–240. [Google Scholar] [CrossRef]
- Jain, R.; Raj, R.; Ram, M.; Rani, A.; Dastider, S.G.; Dhayal, R.S.; Haldar, K.K. TiMn2 Based Bimetallic Nano Alloy Electrocatalyst for Improve Hydrogen Evolution Reaction. Discov. Electrochem. 2024, 1, 11. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A Review on Noble-Metal-Free Bifunctional Heterogeneous Catalysts for Overall Electrochemical Water Splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef]
- Aghamohammadi, P.; Hüner, B.; Altıncı, O.C.; Akgul, E.T.; Teymur, B.; Simsek, U.B.; Demir, M. Recent Advances in the Electrocatalytic Applications (HER, OER, ORR, Water Splitting) of Transition Metal Borides (MBenes) Materials. Int. J. Hydrogen Energy 2024, 87, 179–198. [Google Scholar] [CrossRef]
- Abdelraouf, A.A.; Abdelrahim, A.M.; Abd El-Moghny, M.G.; El-Deab, M.S. Interface Engineering: Enhancing the Electrocatalytic Activity of Heterostructure NiFe-Based Alloy over Valorized Carbon Waste towards Water Splitting. Int. J. Hydrogen Energy 2025, 101, 556–567. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, J.; Liao, J.; Peng, H.; Wang, Z.; Wang, Y.; Wang, W.; Luo, M.; Li, S.; Li, W. Transition Metal Single-Atom Catalysts for Water Splitting: Unravelling Coordination Strategies and Catalytic Mechanisms for Sustainable Hydrogen Generation. Next Mater. 2025, 6, 100491. [Google Scholar] [CrossRef]
- Kang, Y.; Hui, J.; Wang, C.; Gao, X.; Zhao, Y.; Qiao, Z.; Ding, C. Modulation of Electrospun Nanomaterials for Boosting Electrocatalytic Water Splitting. J. Electroanal. Chem. 2025, 979, 118941. [Google Scholar] [CrossRef]
- Mehmood, R.A.; Aslam, A.A.; Iqbal, M.J.; Sajid, A.H.; Hamza, A.; Tahira, H.F.; Islam, I.U.; Yabalak, E. A Review on Hydrogen Production Using Decorated Metal-Organic Frameworks by Electrocatalytic and Photocatalytic Water Splitting. Fuel 2025, 387, 134416. [Google Scholar] [CrossRef]
- Janak; Jaryal, R.; Sakshi; Kumar, R.; Khullar, S. Nickel (II) and Cobalt (II) Based 2D Mixed Metal-Metal Organic Frameworks (MM-MOFs) for Electrocatalytic Water Splitting Reactions. Catal. Today 2025, 446, 115117. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Sun, X.; Sun, Y.; Yang, M.; Liu, B.; Yang, D.; Li, H.; Liu, Y. Recent Advances in Functionalizing ZIFs and Their Derived Carbon Materials towards Electrocatalytic Water Splitting. Nano Energy 2025, 136, 110727. [Google Scholar] [CrossRef]
- Liu, S.; Wei, Y.; Wang, M.; Shen, Y. The Future of Alkaline Water Splitting from the Perspective of Electrocatalysts-Seizing Today’s Opportunities. Coord. Chem. Rev. 2025, 522, 216190. [Google Scholar] [CrossRef]
- Tran, D.T.; Tran, P.K.L.; Malhotra, D.; Nguyen, T.H.; Nguyen, T.T.A.; Duong, N.T.A.; Kim, N.H.; Lee, J.H. Current Status of Developed Electrocatalysts for Water Splitting Technologies: From Experimental to Industrial Perspective. Nano Converg. 2025, 12, 9. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Fagadar-Cosma, E. The pH Influence on the Water-Splitting Electrocatalytic Activity of Graphite Electrodes Modified with Symmetrically Substituted Metalloporphyrins. Nanomaterials 2022, 12, 3788. [Google Scholar] [CrossRef]
- Taranu, B.O.; Fagadar-Cosma, E. Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium. Processes 2022, 10, 611. [Google Scholar] [CrossRef]
- Poienar, M.; Svera, P.; Taranu, B.-O.; Ianasi, C.; Sfirloaga, P.; Buse, G.; Veber, P.; Vlazan, P. Electrochemical Investigation of the OER Activity for Nickel Phosphite-Based Compositions and Its Morphology-Dependent Fluorescence Properties. Crystals 2022, 12, 1803. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, A.K. Design Implication of Alkaline Water Electrolyzer for Green Hydrogen Generation towards Efficiency, Sustainability, and Economic Viability. Int. J. Hydrogen Energy 2025, 100, 201–213. [Google Scholar] [CrossRef]
- Aguirre, O.A.; Ocampo-Martinez, C.; Camacho, O. Control Strategies for Alkaline Water Electrolyzers: A Survey. Int. J. Hydrogen Energy 2024, 86, 1195–1213. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Chen, G.; Shao, Z. Perovskite Oxides as Electrocatalysts for Water Electrolysis: From Crystalline to Amorphous. Carbon Energy 2024, 6, e595. [Google Scholar] [CrossRef]
- Lai, S.; Li, K.; Lv, X.; Ye, H.; Wang, J.; Dong, F.; Lin, Z. Molybdenum-Tailored Perovskite Oxides for Efficient Electrocatalytic Hydrogen Evolution via B-Site Engineering in Alkaline Media. J. Power Sources 2025, 630, 236089. [Google Scholar] [CrossRef]
- Levy, M.R. Crystal Structure and Defect Property Predictions in Ceramic Materials. Ph.D. Thesis, Imperial College London, London, UK, 2005. [Google Scholar]
- Kim, D.; Oh, L.S.; Park, J.H.; Kim, H.J.; Lee, S.; Lim, E. Perovskite-Based Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media: A Mini Review. Front. Chem. 2022, 10, 1024865. [Google Scholar] [CrossRef]
- Wang, T.; Gong, F.; Ma, X.; Pan, S.; Wei, X.K.; Kuo, C.; Yoshida, S.; Ku, Y.C.; Wang, S.; Yang, Z.; et al. Large Enhancement of Ferroelectric Properties of Perovskite Oxides via Nitrogen Incorporation. Sci. Adv. 2025, 11, eads8830. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Transition Metal Oxides with Perovskite and Spinel Structures for Electrochemical Energy Production Applications. Environ. Res. 2022, 214, 113731. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhang, K.; Xu, J.; Wu, Q.; Xie, Z.; An, W.; Liang, X.; Zou, X. Electrocatalytic Water Splitting over Perovskite Oxide Catalysts. Chin. J. Catal. 2023, 50, 109–125. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Taranu, B.O.; Poienar, M.; Vlazan, P. Addressing Electrocatalytic Activity of Metal-Substituted Lanthanum Manganite for the Hydrogen Evolution Reaction. Surf. Interfaces 2023, 39, 102881. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Vlazan, P.; Svera (m. Ianasi), P.; Poienar, M.; Sfirloaga, P. New Functional Hybrid Materials Based on Clay Minerals for Enhanced Electrocatalytic Activity. J. Alloys Compd. 2021, 892, 162239. [Google Scholar] [CrossRef]
- Lakshmanan, K.; Govindasamy, P.; Govindasamy, P.; Marappan, S.; Jintae, L. Facile Synthesis of Perovskite-Type CeCuO3 Nanoparticles as a Robust Bifunctional Electrocatalyst for Highly Stable Overall Water-Splitting. J. Alloys Compd. 2025, 1014, 178635. [Google Scholar] [CrossRef]
- Sun, K.; Li, Z.; Cao, Y.; Wang, F.; Qyyum, M.A.; Han, N. Recent Advancements in Perovskite Electrocatalysts for Clean Energy-related Applications: Hydrogen Production, Oxygen Electrocatalysis, and Nitrogen Reduction. Int. J. Hydrogen Energy 2024, 52, 1104–1126. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef]
- Ji, X.; Yang, F.; Du, Y.; Li, J.; Li, J.; Hu, Q. Highly Active and Durable La0.6Ca0.4(CrMnFeCo2Ni)O3 High Entropy Perovskite Oxide as Electrocatalyst for Oxygen Evolution Reaction in Alkaline Media. J. Mater. Sci. Technol. 2024, 168, 71–78. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, J.J.; Meng, Y.; Lu, F.; Ji, M.; Zhu, C.; Xu, J.; Sun, J. Review on Intrinsic Electrocatalytic Activity of Transition Metal Nitrides on HER. Energy Mater. Adv. 2022, 2022, 0006. [Google Scholar] [CrossRef]
- Huang, S.Y.; Le, P.A.; Nguyen, V.T.; Lu, Y.C.; Sung, C.W.; Cheng, H.W.; Hsiao, C.Y.; Dang, V.D.; Chiu, P.W.; Wei, K.H. Surface Plasma–Induced Tunable Nitrogen Doping through Precursors Provides 1T-2H MoSe2/Graphene Sheet Composites as Electrocatalysts for the Hydrogen Evolution Reaction. Electrochim. Acta 2022, 426, 140767. [Google Scholar] [CrossRef]
- Masri, M.; Girisha, K.B.; Hezam, A.; Alkanad, K.; Prashantha, K.; Manjunath, S.H.; Udayabhanu; Masri, F.; Qahtan, T.F.; Byrappa, K. Metal Halide Perovskite-Based Photocatalysts for Organic Pollutants Degradation: Advances, Challenges, and Future Directions. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133387. [Google Scholar] [CrossRef]
- Merkulov, D.S.; Vlazan, P.; Poienar, M.; Bognár, S.; Ianasi, C.; Sfirloaga, P. Sustainable Removal of 17α-Ethynylestradiol from Aqueous Environment Using Rare Earth Doped Lanthanum Manganite Nanomaterials. Catal. Today 2023, 424, 113746. [Google Scholar] [CrossRef]
- Yahya, N.A.; Nasir, A.M.; Daub, N.A.; Aziz, F.; Aizat, A.; Jaafar, J.; Lau, W.J.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F.; et al. Visible light–driven perovskite-based photocatalyst for wastewater treatment. In Handbook of Smart Photocatalytic Materials; Hussain, C.M., Mishra, A.K., Eds.; Elsevier eBooks: Amsterdam, The Netherlands, 2020; pp. 265–302. ISBN 978-0-12-819051-7. [Google Scholar]
- Li, L.; Zhou, J.; Liu, D.; Liu, X.; Sun, Y.; Xiao, S. Performance and Mechanism of Co-Doped Ce–Mn Perovskite for Degradation of Tetracycline via Heterogeneous Photocatalysis Coupled PMS Oxidation. Mater. Sci. Semicond. Process. 2023, 154, 107229. [Google Scholar] [CrossRef]
- Kanmaz, N.; Buğdaycı, M. Promoting Photo-Fenton Catalytic Performance of Novel NiZrO3-Type Perovskite: Optimization with Response Surface Methodology. J. Mol. Struct. 2024, 1295, 136718. [Google Scholar] [CrossRef]
- Tashkandi, N.Y.; Shawky, A. Enhanced Visible-Light Photocatalytic Remediation of Atrazine over CuAl2O4-Modified BaSnO3 Nanoplatelets Synthesized by Sol-Gel Route. J. Alloys Compd. 2023, 968, 171826. [Google Scholar] [CrossRef]
- Zhu, F.; Ge, J.; Gao, Y.; Li, S.; Chen, Y.; Tu, J.; Wang, M.; Jiao, S. Molten Salt Electro-preparation of Graphitic Carbons. Exploration 2023, 3, 20210186. [Google Scholar] [CrossRef]
- Chang, J.; Lv, Q.; Li, G.; Ge, J.; Liu, C.; Xing, W. Core-Shell Structured Ni12P5/Ni3(PO4)2 Hollow Spheres as Difunctional and Efficient Electrocatalysts for Overall Water Electrolysis. Appl. Catal. B Environ. 2017, 204, 486–496. [Google Scholar] [CrossRef]
- Fratilescu, I.; Lascu, A.; Taranu, B.O.; Epuran, C.; Birdeanu, M.; Macsim, A.-M.; Tanasa, E.; Vasile, E.; Fagadar-Cosma, E. One A3B Porphyrin Structure—Three Successful Applications. Nanomaterials 2022, 12, 1930. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Ivanovici, M.G.; Svera, P.; Vlazan, P.; Sfirloaga, P.; Poienar, M. Ni11□(HPO3)8(OH)6 Multifunctional Materials: Electrodes for Oxygen Evolution Reaction and Potential Visible-Light Active Photocatalysts. J. Alloys Compd. 2020, 848, 156595. [Google Scholar] [CrossRef]
- Abdul, M.; Zhang, M.; Ma, T.; Alotaibi, N.H.; Mohammad, S.; Luo, Y.S. Facile Synthesis of Co3Te4-Fe3C for Efficient Overall Water-Splitting in an Alkaline Medium. Nanoscale Adv. 2024, 7, 433–447. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Levin, M.M.; Ivanov, A.S.; Zakharov, M.A.; Kozhatkin, I.D.; Maslakov, K.I.; Singh, P.K.; Savilov, S.V. Electroactive Ferrocene-Based Ionic Liquids: Transport and Electrochemical Properties of Their Acetonitrile Solutions. Electrochim. Acta 2025, 512, 145443. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Orha, C.; Pop, A. Enhanced Electrochemical Response of Diclofenac at a Fullerene–Carbon Nanofiber Paste Electrode. Sensors 2019, 19, 1332. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion Coefficients of Ferri- and Ferrocyanide Ions in Aqueous Media, Using Twin-Electrode Thin-Layer Electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio Structure Determination of LiSbWO6 by X-ray Powder Diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. FULLPROF: A Program for Rietveld Refinement and Pattern Matching Analysis. In Proceedings of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Toulouse, France, 16–19 July 1990; p. 127. [Google Scholar]
- Khan, N.A.; Rahman, G.; Chae, S.Y.; Shah, A.U.H.A.; Joo, O.S.; Mian, S.A.; Hussain, A. Boosting Electrocatalytic Hydrogen Generation from Water Splitting with Heterostructured MoS2/NiFe2O4 Composite in Alkaline Media. Int. J. Hydrogen Energy 2024, 69, 261–271. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, S.; Cheng, L.; Zhou, L.; Cui, H.; Liu, M.; Wen, M.; Wang, C.; Wang, W.; Li, S.; et al. Mass Synthesis of Pt/C Catalysts with High Pt Loading for Low-Overpotential Hydrogen Evolution. Int. J. Hydrogen Energy 2024, 58, 268–278. [Google Scholar] [CrossRef]
- Zhou, Z.; Zaman, W.Q.; Sun, W.; Cao, L.M.; Tariq, M.; Yang, J. Cultivating Crystal Lattice Distortion in IrO2 via Coupling with MnO2 to Boost the Oxygen Evolution Reaction with High Intrinsic Activity. Chem. Commun. 2018, 54, 4959–4962. [Google Scholar] [CrossRef]
- Achilli, E.; Minelli, S.; Casale, I.; He, X.; Agostini, G.; Spinolo, G.; Ghigna, P.; Minguzzi, A.; Vertova, A. Determining the Proton Diffusion Coefficient in Highly Hydrated Iridium Oxide Films by Energy Dispersive X-Ray Absorption Spectroscopy. Electrochim. Acta 2023, 444, 142017. [Google Scholar] [CrossRef]
- Sebarchievici, I.; Taranu, B.-O.; Birdeanu, M.; Rus, S.F.; Fagadar-Cosma, E. Electrocatalytic Behaviour and Application of Manganese Porphyrin/Gold Nanoparticle- Surface Modified Glassy Carbon Electrodes. Appl. Surf. Sci. 2016, 390, 131–140. [Google Scholar] [CrossRef]
- Keeley, G.P.; Lyons, M.E.G. The Effects of Thin Layer Diffusion at Glassy Carbon Electrodes Modified with Porous Films of Single-Walled Carbon Nanotubes. Int. J. Electrochem. Sci. 2009, 4, 794–809. [Google Scholar] [CrossRef]
- Taranu, B.O.; Fagadar-Cosma, E.; Sfirloaga, P.; Poienar, M. Free-Base Porphyrin Aggregates Combined with Nickel Phosphite for Enhanced Alkaline Hydrogen Evolution. Energies 2023, 16, 1212. [Google Scholar] [CrossRef]
- Menezes, P.W.; Panda, C.; Loos, S.; Bunschei-Bruns, F.; Walter, C.; Schwarze, M.; Deng, X.; Dau, H.; Driess, M. A Structurally Versatile Nickel Phosphite Acting as a Robust Bifunctional Electrocatalyst for Overall Water Splitting. Energy Environ. Sci. 2018, 11, 1287–1298. [Google Scholar] [CrossRef]
- Bukalov, S.S.; Zubavichus, Y.V.; Leites, L.A.; Sorokin, A.I.; Kotosonov, A.S. Structural Changes in Industrial Glassy Carbon As a Function of Heat Treatment Temperature According to Raman Spectroscopy and X-Ray Diffraction Data. Nanosyst. Phys. Chem. Math. 2014, 5, 186–191. [Google Scholar]
- Upare, D.P.; Yoon, S.; Lee, C.W. Nano-Structured Porous Carbon Materials for Catalysis and Energy Storage. Korean, J. Chem. Eng. 2011, 28, 731–743. [Google Scholar] [CrossRef]
- Andrianova, N.N.; Borisov, A.M.; Kazakov, V.A.; Makunin, A.V.; Mashkova, E.S.; Ovchinnikov, M.A. Modification of the Nanoglobular Structure of Glassy Carbon by Heat Treatment and Ion Irradiation. J. Surf. Investig. 2019, 13, 802–808. [Google Scholar] [CrossRef]
- Fukumura, H.; Matsui, S.; Harima, H.; Kisoda, K.; Takahashi, T.; Yoshimura, T.; Fujimura, N. Raman Scattering Studies on Multiferroic YMnO3. J. Phys. Condens. Matter 2007, 19, 365239. [Google Scholar] [CrossRef]
- Zhou, G.; Gu, X.; Xie, W.; Gao, T.; Peng, J.; Wu, X.S. Polarized Raman Scattering Studies of Hexagonal YMnO3 Single Crystal. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Bibi, K.; Muqadas, S.; Khan, M.M.; Fatima, R.; Sahar, F.; Abbas Shah, S.I.; Tighezza, A.M.; Khan, M.S. Improved Water Splitting Performance for Green Hydrogen Production Using ZnCo2O4–CoFe2O4 Composite Electrocatalyst. Int. J. Hydrogen Energy 2025, 106, 411–422. [Google Scholar] [CrossRef]
- Sarker, S.; Chaturvedi, P.; Yan, L.; Nakotte, T.; Chen, X.; Richins, S.K.; Das, S.; Peters, J.; Zhou, M.; Smirnov, S.N.; et al. Synergistic Effect of Iron Diselenide Decorated Multi-Walled Carbon Nanotubes for Enhanced Heterogeneous Electron Transfer and Electrochemical Hydrogen Evolution. Electrochim. Acta 2018, 270, 138–146. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Hu, B.C.; Wu, P.; Liang, H.W.; Yu, Z.L.; Lin, Y.; Zheng, Y.R.; Li, Z.; Yu, S.H. Mo2C Nanoparticles Embedded within Bacterial Cellulose-Derived 3D N-Doped Carbon Nanofiber Networks for Efficient Hydrogen Evolution. NPG Asia Mater. 2016, 8, e288. [Google Scholar] [CrossRef]
- Wang, X.; Kolen’Ko, Y.V.; Bao, X.Q.; Kovnir, K.; Liu, L. One-Step Synthesis of Self-Supported Nickel Phosphide Nanosheet Array Cathodes for Efficient Electrocatalytic Hydrogen Generation. Angew. Chem.-Int. Ed. 2015, 54, 8188–8192. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent Progress Made in the Mechanism Comprehension and Design of Electrocatalysts for Alkaline Water Splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Cong, Z.; Shuaiyang, W.; Junfeng, R.; Wanliang, M. Amorphous Catalysts for Electrochemical Water Splitting. China Pet. Process. Petrochem. Technol. 2022, 24, 1–13. [Google Scholar]
- Liu, Z.; Tan, H.; Liu, D.; Liu, X.; Xin, J.; Xie, J.; Zhao, M.; Song, L.; Dai, L.; Liu, H. Promotion of Overall Water Splitting Activity Over a Wide pH Range by Interfacial Electrical Effects of Metallic NiCo-Nitrides Nanoparticle/NiCo2O4 Nanoflake/Graphite Fibers. Adv. Sci. 2019, 6, 1801829. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Q.; Yu, X.; Liu, C.; Li, Y.; Zhang, Y.; Wang, S. Research Progress of Perovskite/Carbon Composites in the Field of Electrocatalysis. Mol. Catal. 2025, 574, 114860. [Google Scholar] [CrossRef]
- Bu, Y.; Jang, H.; Gwon, O.; Kim, S.H.; Joo, S.H.; Nam, G.; Kim, S.; Qin, Y.; Zhong, Q.; Kwak, S.K.; et al. Synergistic Interaction of Perovskite Oxides and N-Doped Graphene in Versatile Electrocatalyst. J. Mater. Chem. A 2019, 7, 2048–2054. [Google Scholar] [CrossRef]
- Sun, H.; Dai, J.; Zhou, W.; Shao, Z. Emerging Strategies for Developing High-Performance Perovskite-Based Materials for Electrochemical Water Splitting. Energy Fuels 2020, 34, 10547–10567. [Google Scholar] [CrossRef]
- Loni, E.; Shokuhfar, A.; Siadati, M.H. Cobalt-Based Electrocatalysts for Water Splitting: An Overview. Catal. Surv. Asia 2021, 25, 114–147. [Google Scholar] [CrossRef]
- Benny, S.; Galeb, W.; Ezhilarasi, S.; Rodney, J.D.; Udayashankar, N.K.; Raja, M.D.; Madhavan, J.; Arulmozhi, S. Engineering of Cobalt Impregnated Sponge like Spinel Nickel Ferrite as an Efficient Electrocatalyst for Sustained Overall Water Splitting. Inorg. Chem. Commun. 2025, 174, 114044. [Google Scholar] [CrossRef]
- Gaso, P.; Jandura, D.; Bulatov, S.; Pudis, D.; Goraus, M. Advancing Atomic Force Microscopy: Design of Innovative IP-Dip Polymer Cantilevers and Their Exemplary Fabrication via 3D Laser Microprinting. Coatings 2024, 14, 841. [Google Scholar] [CrossRef]
- Xun, Y.; Zhang, K.; Jonhson, W.; Ding, J. A Minireview on 3D Printing for Electrochemical Water Splitting Electrodes and Cells. APL Mater. 2023, 11, 061119. [Google Scholar] [CrossRef]
- Despotović, V.; Finčur, N.; Bognar, S.; Merkulov, D.S.; Putnik, P.; Abramović, B.; Panić, S. Characterization and Photocatalytic Performance of Newly Synthesized ZnO Nanoparticles for Environmental Organic Pollutants Removal from Water System. Separations 2023, 10, 258. [Google Scholar] [CrossRef]
- Chemical Book. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB1350377.htm (accessed on 17 February 2025).
- Owens, C.L.; Nash, G.R.; Hadler, K.; Fitzpatrick, R.S.; Anderson, C.G.; Wall, F. Apatite Enrichment by Rare Earth Elements: A Review of the Effects of Surface Properties. Adv. Colloid Interface Sci. 2019, 265, 14–28. [Google Scholar] [CrossRef]
- Ustunel, T.; Ide, Y.; Kaya, S.; Doustkhah, E. Single-Atom Sn-Loaded Exfoliated Layered Titanate Revealing Enhanced Photocatalytic Activity in Hydrogen Generation. ACS Sustain. Chem. Eng. 2023, 11, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Kang, J.; Guo, D.; Zhu, C.; Li, C.; Zhang, X.; Chen, Y. Self-Supported Cobalt Nitride Porous Nanowire Arrays as Bifunctional Electrocatalyst for Overall Water Splitting. Electrochim. Acta 2018, 273, 229–238. [Google Scholar] [CrossRef]
- Xing, Z.; Liu, Q.; Xing, W.; Asiri, A.M.; Sun, X. Interconnected Co-Entrapped, N-Doped Carbon Nanotube Film as Active Hydrogen Evolution Cathode over the Whole pH Range. ChemSusChem 2015, 8, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bhoyate, S.; Kahol, P.K.; Siam, K.; Poudel, T.P.; Mishra, S.R.; Perez, F.; Gupta, A.; Gupta, G.; Gupta, R.K. Highly Efficient and Durable Electrocatalyst Based on Nanowires of Cobalt Sulfide for Overall Water Splitting. ChemNanoMat 2018, 4, 1240–1246. [Google Scholar] [CrossRef]
- Lin, Y.; Pan, Y.; Liu, S.; Sun, K.; Cheng, Y.; Liu, M.; Wang, Z.; Li, X.; Zhang, J. Construction of Multi-Dimensional Core/Shell Ni/NiCoP Nano-Heterojunction for Efficient Electrocatalytic Water Splitting. Appl. Catal. B Environ. 2019, 259, 118039. [Google Scholar] [CrossRef]
- Liu, P.F.; Yang, S.; Zhang, B.; Yang, H.G. Defect-Rich Ultrathin Cobalt-Iron Layered Double Hydroxide for Electrochemical Overall Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 34474–34481. [Google Scholar] [CrossRef]
- Zhai, M.; Wang, F.; Du, H. Transition-Metal Phosphide-Carbon Nanosheet Composites Derived from Two-Dimensional Metal-Organic Frameworks for Highly Efficient Electrocatalytic Water-Splitting. ACS Appl. Mater. Interfaces 2017, 9, 40171–40179. [Google Scholar] [CrossRef]
- Yan, J.; Chen, L.; Liang, X. Co9S8 Nanowires@NiCo LDH Nanosheets Arrays on Nickel Foams towards Efficient Overall Water Splitting. Sci. Bull. 2019, 64, 158–165. [Google Scholar] [CrossRef]
- Li, J.; Kong, X.; Jiang, M.; Lei, X. Hierarchically Structured CoN/Cu3N Nanotube Array Supported on Copper Foam as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Inorg. Chem. Front. 2018, 5, 2906–2913. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0−14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Haiyan, J.; Jing, W.; Diefeng, S.; Zhongzhe, W.; Zhenfeng, P.; Yong, W. In Situ Cobalt-Cobalt Oxide/N-Doped Carbon Hybrids as Superior Bifunctional Electrocatalysts for Hydrogen and Oxygen Evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar]
- Zeng, M.; Liu, Y.; Zhao, F.; Nie, K.; Han, N.; Wang, X.; Huang, W.; Song, X.; Zhong, J.; Li, Y. Metallic Cobalt Nanoparticles Encapsulated in Nitrogen-Enriched Graphene Shells: Its Bifunctional Electrocatalysis and Application in Zinc–Air Batteries. Adv. Funct. Mater. 2016, 26, 4397–4404. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, X.; Zhong, C.; Ma, T.; Deng, Y.; Hu, W.; Han, X. Pyrite-Type CoS2 Nanoparticles Supported on Nitrogen-Doped Graphene for Enhanced Water Splitting. Front. Chem. 2018, 6, 569. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, Q.; Xu, P.; Zhang, D.; Wei, L.; Yuan, D. Three-Dimensional Lily-like CoNi2S4 as an Advanced Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution Reaction. Cuihua Xuebao/Chin. J. Catal. 2018, 39, 1403–1410. [Google Scholar] [CrossRef]
- Meng, T.; Qin, J.; Wang, S.; Zhao, D.; Mao, B.; Cao, M. In Situ Coupling of Co0.85Se and N-Doped Carbon via One-Step Selenization of Metal-Organic Frameworks as a Trifunctional Catalyst for Overall Water Splitting and Zn-Air Batteries. J. Mater. Chem. A 2017, 5, 7001–7014. [Google Scholar] [CrossRef]
- Fang, Z.; Peng, L.; Qian, Y.; Zhang, X.; Xie, Y.; Cha, J.J.; Yu, G. Dual Tuning of Ni-Co-A (A = P, Se, O) Nanosheets by Anion Substitution and Holey Engineering for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 5241–5247. [Google Scholar] [CrossRef]
- Ma, L.; Hu, Y.; Chen, R.; Zhu, G.; Chen, T.; Lv, H.; Wang, Y.; Liang, J.; Liu, H.; Yan, C.; et al. Self-Assembled Ultrathin NiCo2S4 Nanoflakes Grown on Ni Foam as High-Performance Flexible Electrodes for Hydrogen Evolution Reaction in Alkaline Solution. Nano Energy 2016, 24, 139–147. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Soucy, T.L.; Hodges, J.M.; Callejas, J.F.; Mondschein, J.S.; Schaak, R.E. Colloidally-Synthesized Cobalt Molybdenum Nanoparticles as Active and Stable Electrocatalysts for the Hydrogen Evolution Reaction under Alkaline Conditions. J. Mater. Chem. A 2016, 4, 3077–3081. [Google Scholar] [CrossRef]
- Hou, Y.; Lohe, M.R.; Zhang, J.; Liu, S.; Zhuang, X.; Feng, X. Vertically Oriented Cobalt Selenide/NiFe Layered-Double-Hydroxide Nanosheets Supported on Exfoliated Graphene Foil: An Efficient 3D Electrode for Overall Water Splitting. Energy Environ. Sci. 2016, 9, 478–483. [Google Scholar] [CrossRef]
- Liu, D.; Lu, Q.; Luo, Y.; Sun, X.; Asiri, A.M. NiCo2S4 Nanowires Array as an Efficient Bifunctional Electrocatalyst for Full Water Splitting with Superior Activity. Nanoscale 2015, 7, 15122–15126. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhang, B.; Ruan, Y.; Lv, L.; Ji, X.; Xu, K.; Miao, L.; Jiang, J. Hierarchical NiCo2S4@NiFe LDH Heterostructures Supported on Nickel Foam for Enhanced Overall-Water-Splitting Activity. ACS Appl. Mater. Interfaces 2017, 9, 15364–15372. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wang, J.; Wang, Z.; Li, T.; Song, Y.F. Unique NiFe–NiCoO2 Hollow Polyhedron as Bifunctional Electrocatalysts for Water Splitting. J. Energy Chem. 2019, 33, 74–80. [Google Scholar] [CrossRef]

| Electrode Tag | Perovskite Material | Carbon Black (mg) | Nafion Solution 5% (µL) | Ethanol (µL) |
|---|---|---|---|---|
| GC0 | - | - | - | - |
| GCYMO | 5 mg YMO | - | 50 | 450 |
| GCYMO-Sn | 5 mg YMO-Sn | - | 50 | 450 |
| GCYMO-Co | 5 mg YMO-Co | - | 50 | 450 |
| GCCB | - | 5 | 50 | 450 |
| GCCB-YMO | 5 mg YMO | 5 | 50 | 900 |
| GCCB-YMO-Sn | 5 mg YMO-Sn | 5 | 50 | 900 |
| GCCB-YMO-Co | 5 mg YMO-Co | 5 | 50 | 900 |
| Sample | Lattice Parameters (Å) | Space Group | Unit Cell Volume (Å3) | Crystallite Size (nm) | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| YMO | 6.14910 | 6.14910 | 11.375 (2) | P63cm | 372.49840 | 27.3 |
| YMO-Co | 6.151 (2) | 6.151 (2) | 11.336 (5) | P63cm | 371.48790 | 23.07 |
| YMO-Sn | 6.128 (2) | 6.128 (2) | 11.358 (3) | P63cm | 369.36060 | 24.74 |
| Samples | YMO | YMO-Co | YMO-Sn |
|---|---|---|---|
| a (Å) | 6.14517 (6) | 6.14978 (2) | 6.14624 (5) |
| c (Å) | 11.316 (8) | 11.33198 (3) | 11.30616 (6) |
| Volume (Å3) | 370.077 (6) | 371.156 (2) | 369.883 (4) |
| X2 | 3.10 | 3.11 | 2.75 |
| RBragg | 6.86 | 1.69 | 1.88 |
| Rf factor | 5.20 | 1.88 | 2.05 |
| Electrode Tag | EASA (cm2) | Diffusion Coefficient (cm2/s) |
|---|---|---|
| GC0 | 0.123 ± 0.0077 | 1.31 × 10−6 ± 0.161 × 10−6 |
| GCCB | 0.135 ± 0.009 | 1.573 × 10−6 ± 0.214 × 10−6 |
| GCCB-YMO-Co | 0.19 ± 0.0087 | 3.108 × 10−6 ± 0.284 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfirloaga, P.; Bognár, S.; Taranu, B.-O.; Vlazan, P.; Poienar, M.; Šojić Merkulov, D. Co- and Sn-Doped YMnO3 Perovskites for Electrocatalytic Water-Splitting and Photocatalytic Pollutant Degradation. Coatings 2025, 15, 475. https://doi.org/10.3390/coatings15040475
Sfirloaga P, Bognár S, Taranu B-O, Vlazan P, Poienar M, Šojić Merkulov D. Co- and Sn-Doped YMnO3 Perovskites for Electrocatalytic Water-Splitting and Photocatalytic Pollutant Degradation. Coatings. 2025; 15(4):475. https://doi.org/10.3390/coatings15040475
Chicago/Turabian StyleSfirloaga, Paula, Szabolcs Bognár, Bogdan-Ovidiu Taranu, Paulina Vlazan, Maria Poienar, and Daniela Šojić Merkulov. 2025. "Co- and Sn-Doped YMnO3 Perovskites for Electrocatalytic Water-Splitting and Photocatalytic Pollutant Degradation" Coatings 15, no. 4: 475. https://doi.org/10.3390/coatings15040475
APA StyleSfirloaga, P., Bognár, S., Taranu, B.-O., Vlazan, P., Poienar, M., & Šojić Merkulov, D. (2025). Co- and Sn-Doped YMnO3 Perovskites for Electrocatalytic Water-Splitting and Photocatalytic Pollutant Degradation. Coatings, 15(4), 475. https://doi.org/10.3390/coatings15040475








