Study of Gd2O3-Doped La2(Zr0.7Ce0.3)2O7 Thermal Barriers for Coating Ceramic Materials for CMAS Resistance
Abstract
1. Introduction
2. Experimental Procedure
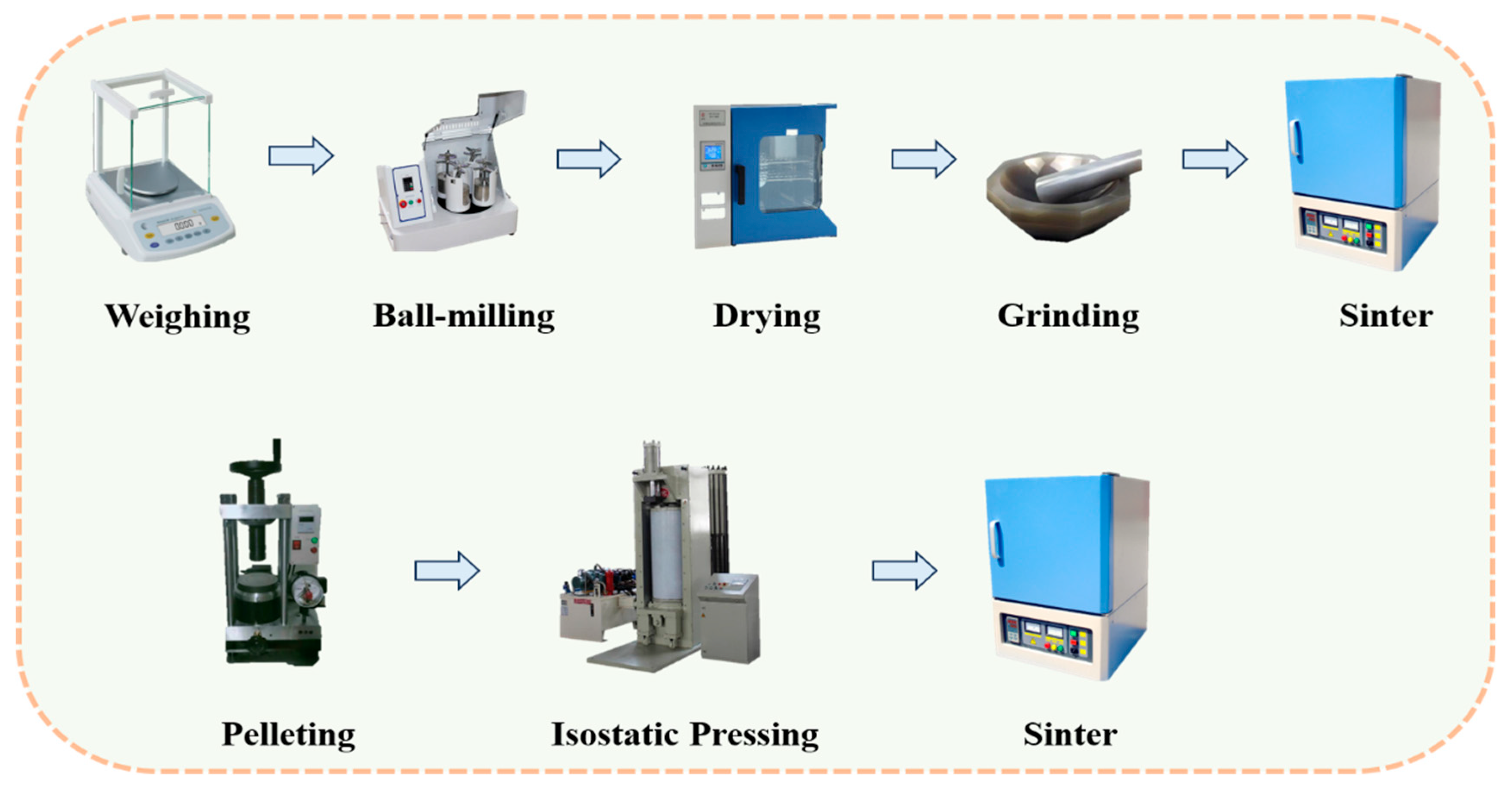
2.1. Sample Preparation
2.2. CMAS Powder Preparation and Ceramic Corrosion
2.3. Characterisation
3. Results and Discussion
3.1. Experimentail Data Characterization
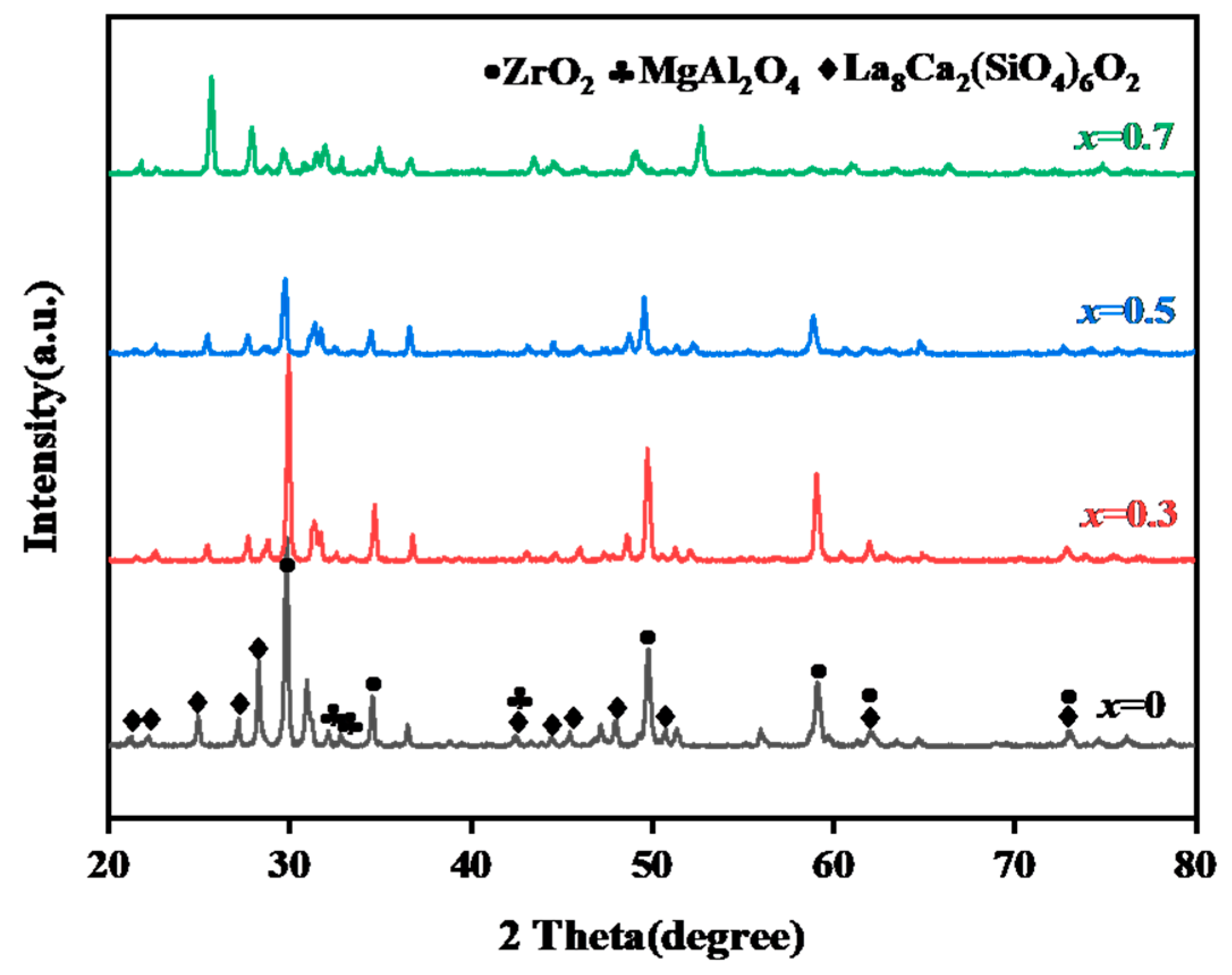
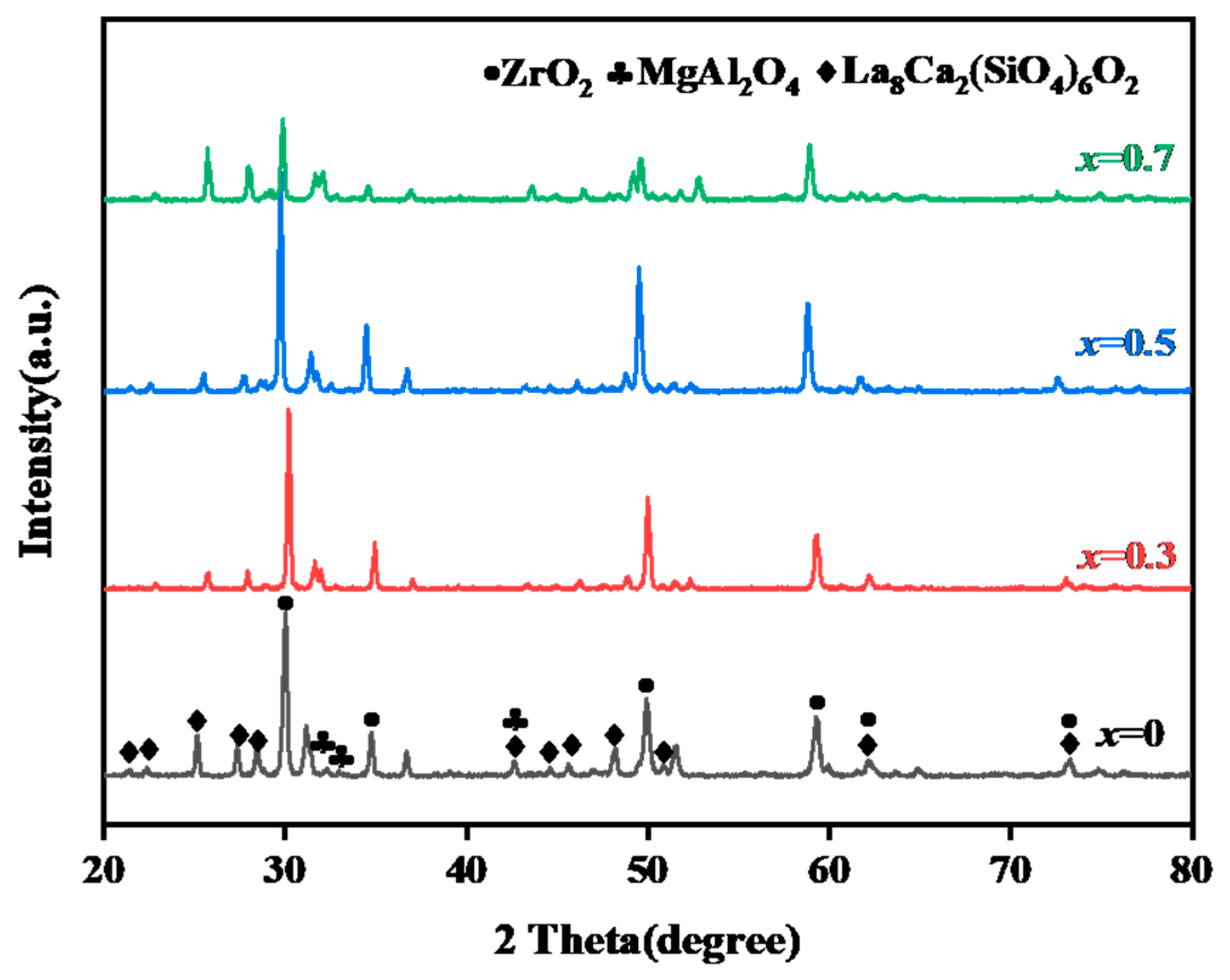
3.1.1. Characterization of Original Sintered Ceramics
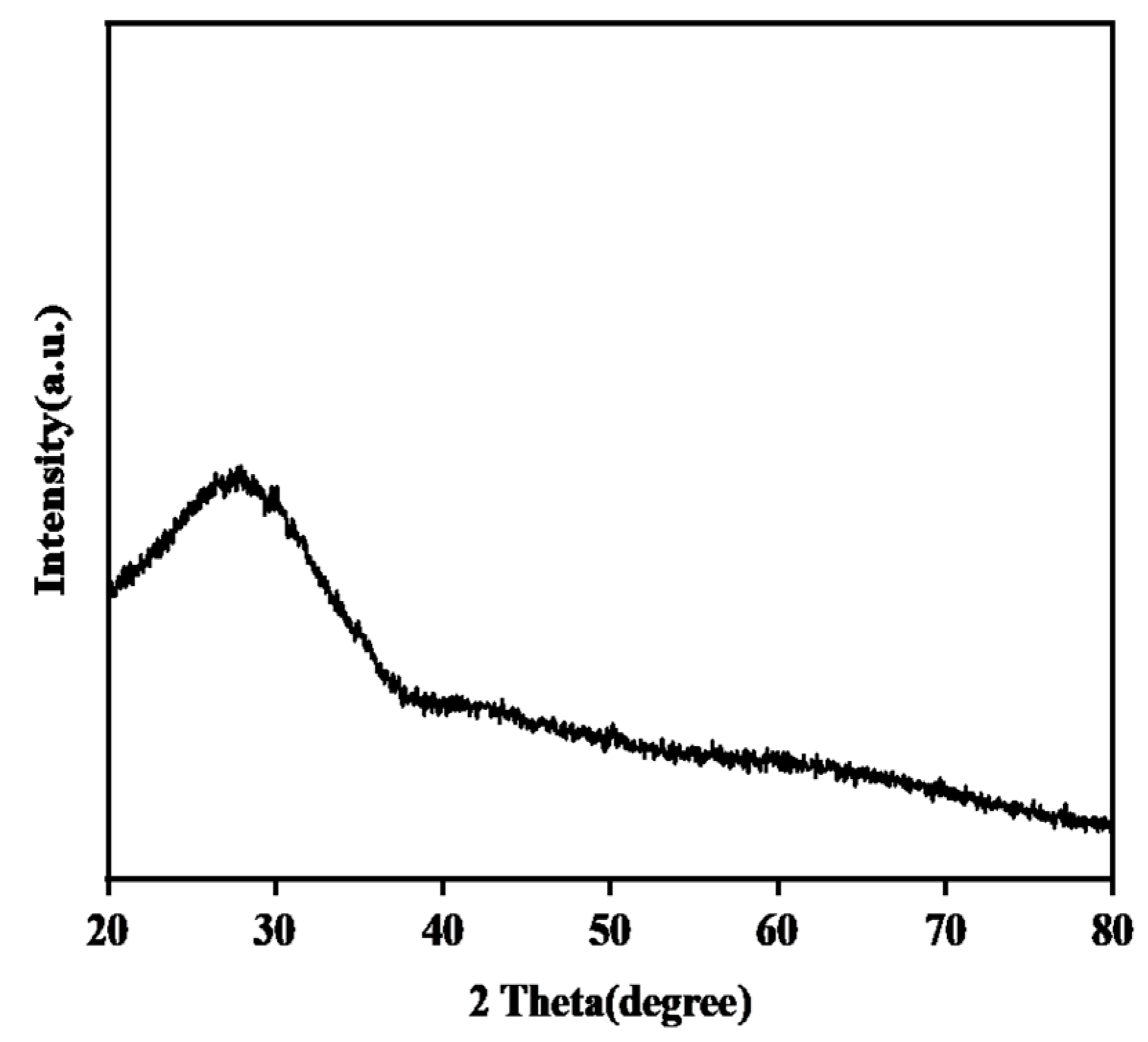
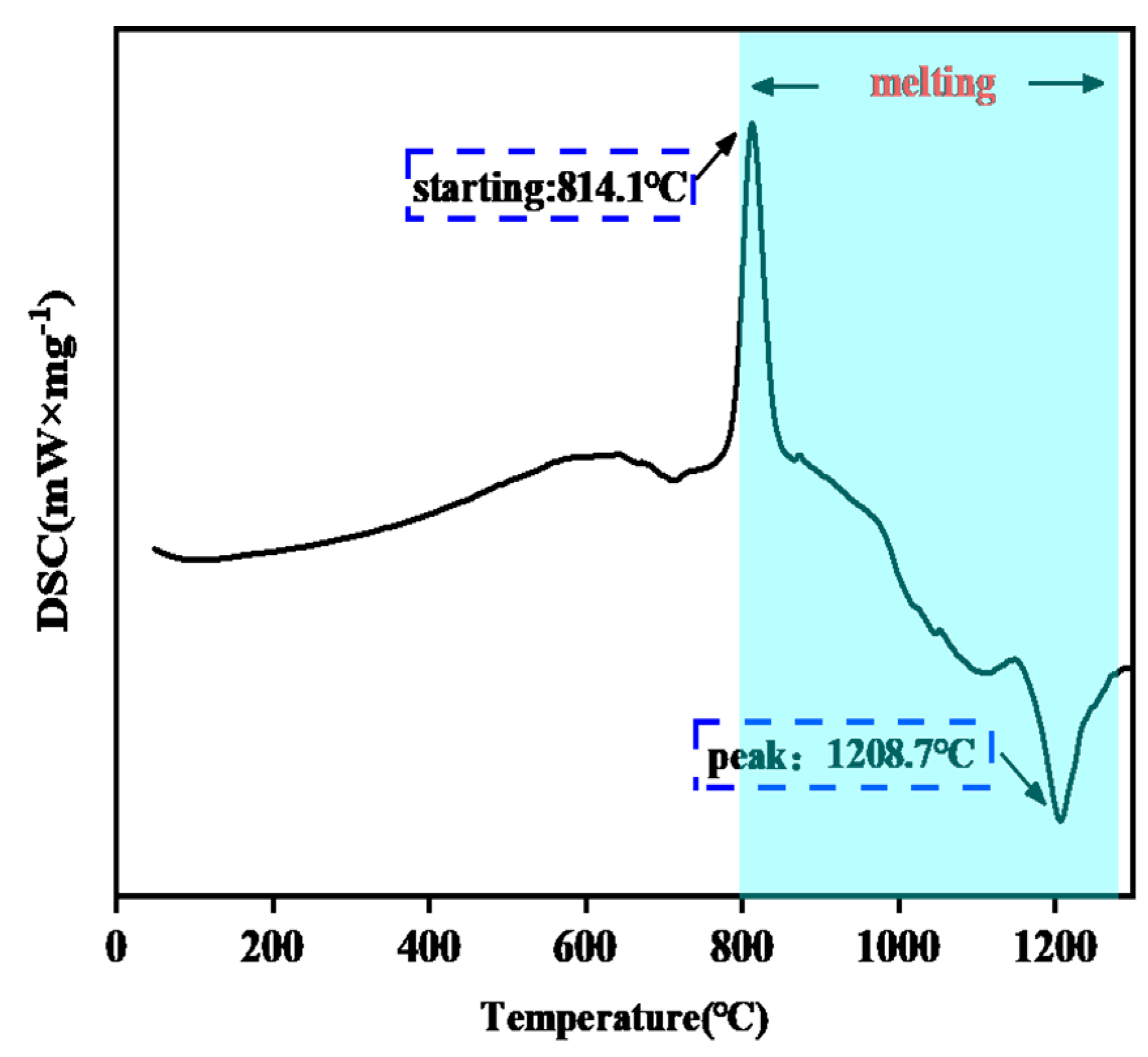
3.1.2. Phase Structure and DSC of the CMAS Powder
3.2. Characterization of Corrosive Ceramics
- (1)
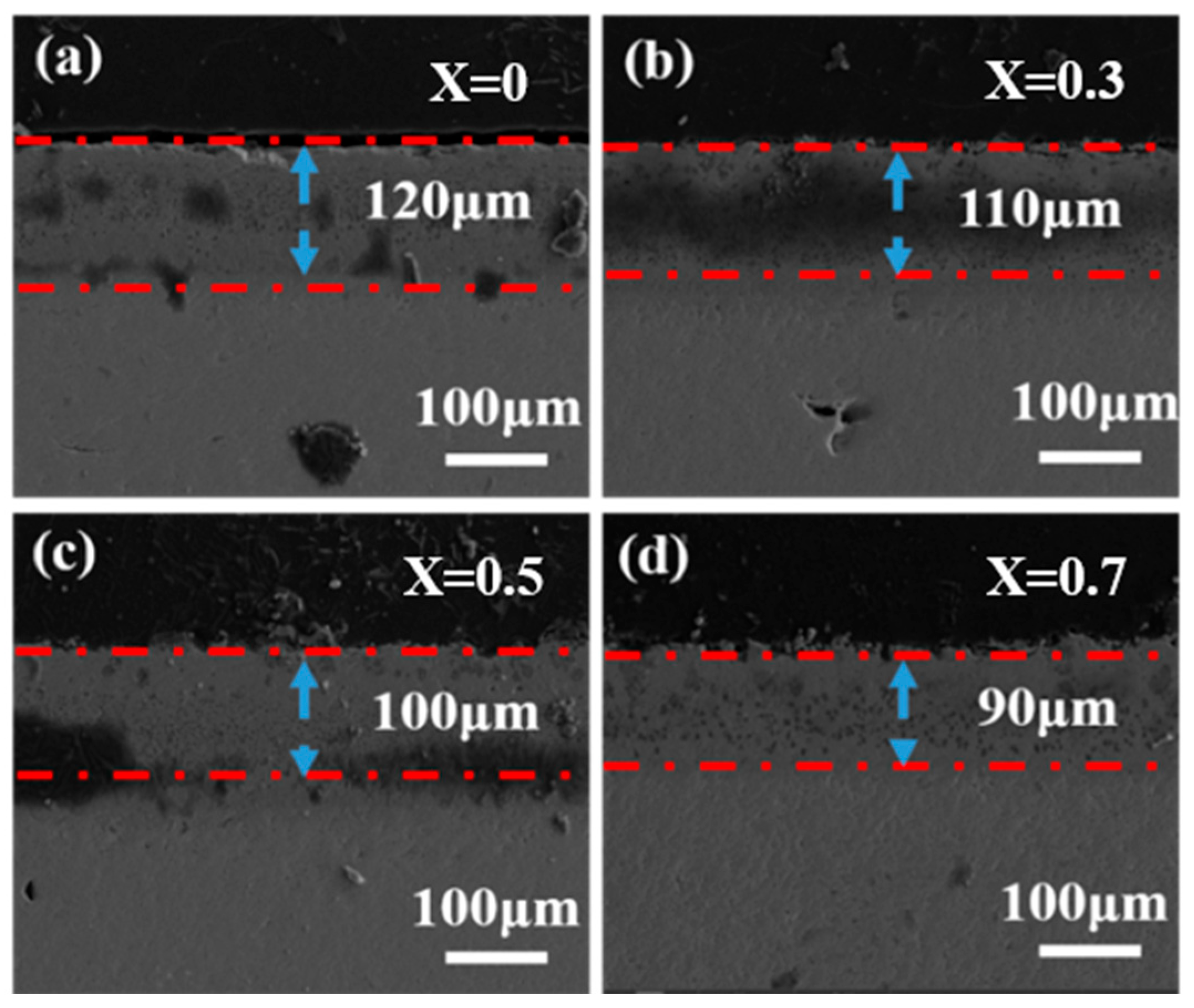
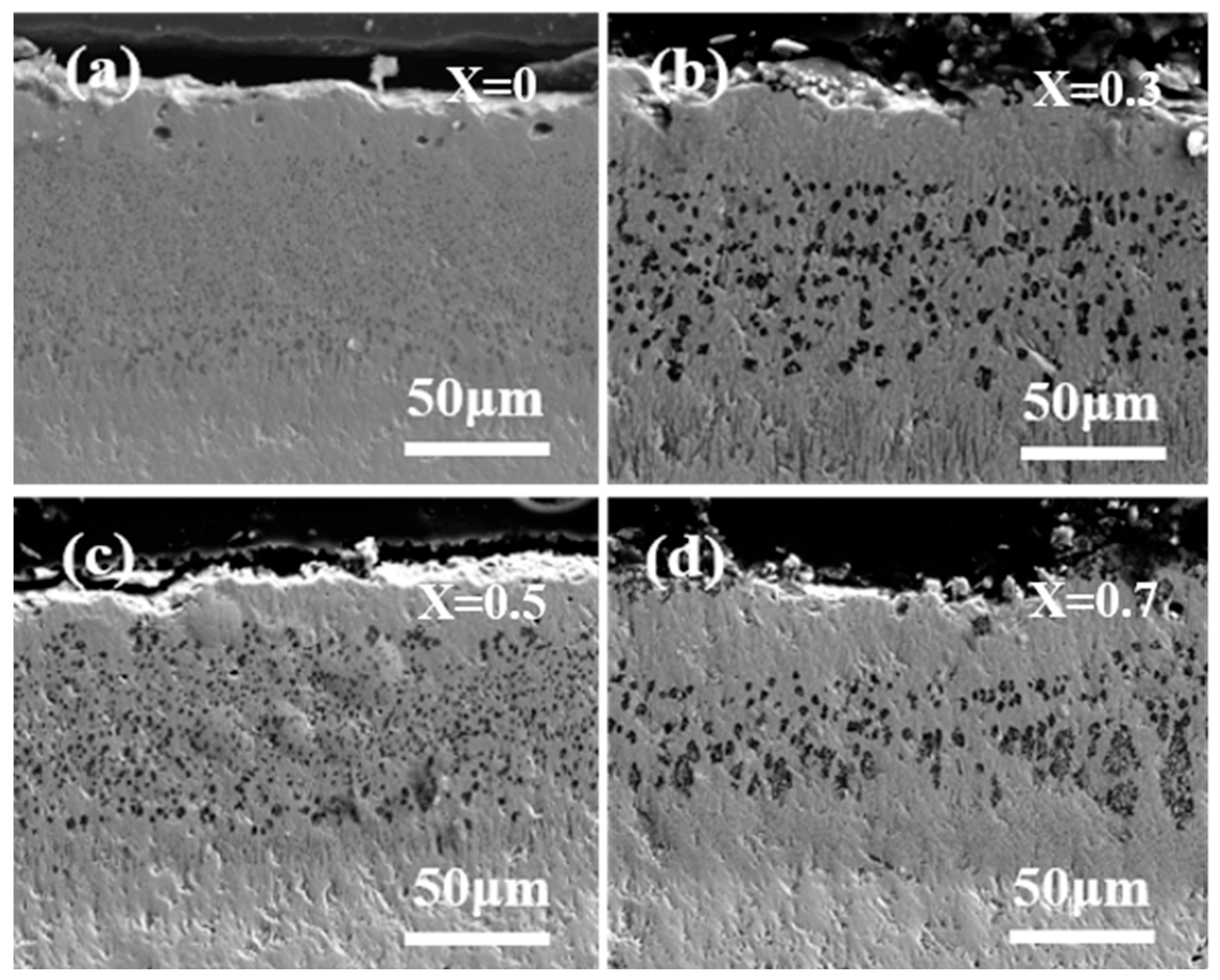
- In the first stage, the molten CMAS spreads out on the specimen surface and permeates continuously into the high-entropy ceramic. The section diagram for 5 h shows that an intermediate reaction layer is initially formed between CMAS and the high-entropy ceramics, and the average thickness of the corrosion at 1250 °C for 5 h is about 100 μm. Combined with the surface topography images at 1250 °C for 5 h and the surface and XRD patterns of the CMAS corrosion samples at different times, white apatite precipitates from the surface of CMAS. This indicates that the ceramics decompose when encountering CMAS, and the rare-earth elements in the ceramics diffuse into the CMAS melt.
- (2)
- In the second stage, with the increase in the reaction time, Ca and Si in the CMAS are continuously consumed, and a large amount of apatite and MgAl2O4, formed partly by MgO and Al2O3, exist at the top of the sample section, with only a small amount of CMAS remaining on the surface. The results show that molten CMAS has essentially penetrated into the ceramic layer, and this stage mainly shows the process of CMAS infiltration and reaction with ceramics to form apatite and precipitate RE(Ca)-ZrO2 with a fluorite structure.
- (3)
- In the third stage, the CMAS vitreous body completely permeates into the ceramic. In the long-term high-temperature environment, the grain size increases in the reaction layer, and some of the CMAS reacts with the apatite to continuously form stable apatite. As shown in the CA-Si-Al ternary phase diagram in the figure, with the continuous reduction of the Ca and Si components in CMAS, the residual CMAS forms a yellow feldspar Ca2Al2SiO7 sediment with a higher melting point, so it cannot continue to penetrate into the ceramic interior.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barwinska, I.; Kopec, M.; Kukla, D.; Senderowski, C.; Kowalewski, Z.L. Thermal Barrier Coatings for High-Temperature Performance of Nickel-Based Superalloys: A Synthetic Review. Coatings 2023, 13, 769. [Google Scholar] [CrossRef]
- Bogdan, M.; Peter, I. A Comprehensive Understanding of Thermal Barrier Coatings (TBCs): Applications, Materials, Coating Design and Failure Mechanisms. Metals 2024, 14, 575. [Google Scholar] [CrossRef]
- Chellaganesh, D.; Adam Khan, M.; Winowlin Jappes, J.T. Thermal Barrier Coatings for High Temperature Applications: A Short Review. Mater. Today 2021, 45, 1529. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Yao, J.; Dong, H. Multi-Scale Structural Design and Advanced Materials for Thermal Barrier Coatings with High Thermal Insulation: A Review. Coatings 2023, 13, 343. [Google Scholar] [CrossRef]
- Ariharan, S.; Parchovianský, M.; Singh, P. Hot Corrosion Behavior of La2Ce2O7-Based Plasma-Sprayed Coating. High Temp. Corros. Mater. 2024, 101, 779–788. [Google Scholar] [CrossRef]
- Bakan, E.; Vaßen, R. Ceramic Top Coats of Plasma-Sprayed Thermal Barrier Coatings: Materials, Processes, and Properties. J. Therm. Spray Technol. 2017, 26, 992. [Google Scholar] [CrossRef]
- Ajay, A.; Raja, V.S.; Sivakumar, G.; Joshi, S.V. Hot Corrosion Behavior of Solution Precursor and Atmospheric Plasma Sprayed Thermal Barrier Coatings. Corros. Sci. 2015, 98, 271. [Google Scholar]
- Huang, Y.; Shen, Y.; Zeng, Y.; Song, X.; Guo, S. EBSD Analysis of Microstructure Changes in YSZ Coatings During Thermal Cycling. Ceram. Int. 2020, 47, 5559. [Google Scholar] [CrossRef]
- Gildersleeve, J.; Sampath, S. Process-Geometry Interplay in the Deposition and Microstructural Evolution of 7YSZ Thermal Barrier Coatings by Air Plasma Spray. J. Therm. Spray Technol. 2020, 29, 560. [Google Scholar]
- Li, F.; Zhou, L.; Liu, J. High-Entropy Pyrochlores with Low Thermal Conductivity for Thermal Barrier Coating Materials. J. Adv. Ceram. 2019, 8, 76–582. [Google Scholar]
- Zhao, H.; Richards, B.T.; Levi, C.G.; Wadley, H.N. Molten silicate reactions with plasma sprayed ytterbium silicate coatings. Surf. Coat. Technol. 2016, 288, 151–162. [Google Scholar] [CrossRef]
- Jarligo, M.; Mack, D.; Mauer, G. Atmospheric Plasma Spraying of High Melting Temperature Complex Perovskites for TBC Application. J. Therm. Spray Technol. 2010, 19, 303–310. [Google Scholar] [CrossRef]
- Zaleski, E.M.; Ensslen, C.; Levi, C.G. Melting and Crystallization of Silicate Systems Relevant to Thermal Barrier Coating Damage. J. Am. Ceram. Soc. 2015, 98, 1642–1649. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Tietz, F.; Stoever, D. New double-ceramic-layer thermal barrier coatings based on zirconiaerare earth composite oxides. J. Eur. Ceram. Soc. 2006, 26, 247–251. [Google Scholar]
- Levi, C.G. Emerging materials and processes for thermal barrier systems. Curr. Opin. Solid State Mater. Sci. 2004, 8, 77–91. [Google Scholar]
- Xu, Z.; He, L.; Zhong, X.; Mu, R.D.; He, S.M.; Cao, X.Q. Thermal barrier coating of lanthanum-zirconium-cerium composite oxide made by electron beamphysical vapor deposition. J. Alloys Comp. 2009, 478, 168–172. [Google Scholar]
- Cao, X.Q.; Li, J.; Zhong, X.; Zhang, J.F.; Zhang, Y.F.; Vassen, R.; Stoever, D. La2(Zr0.7Ce0.3)2O7-a new oxide ceramic material with high sintering resistance. Mater. Lett. 2008, 62, 2667–2669. [Google Scholar]
- Vaßen, R.; Jarligo, M.; Steinke, T.; Mack, D.; Stöver, D. Overview on Advanced Thermal Barrier Coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar]
- Schulz, U.; Braue, W. Degradation of La2Zr2O7 and Other Novel EB-PVD Thermal Barrier Coatings by CMAS (CaO–MgO–Al2O3–SiO2) and Volcanic Ash Deposits. Surf. Coat. Technol. 2013, 235, 165. [Google Scholar]
- Cheng, B.; Wang, Y.; Zhang, X.; An, G.; Chu, Q.; Zhang, X.; He, D.; Zhai, H.; Li, W. Sintering Governing the Cracking Behaviors of Different La2Zr2O7/YSZ Ceramic Layer Combination TBCs at 1150 °C. Surf. Coat. Technol 2021, 428, 127910. [Google Scholar] [CrossRef]
- Wright, A.; Huang, C.; Walock, M.; Ghoshal, A.; Murugan, M. Sand Corrosion, Thermal Expansion, and Ablation of Medium- and High-Entropy Compositionally Complex Fluorite Oxides. J. Am. Ceram. Soc. 2020, 104, 448–462. [Google Scholar]
- Bahamirian, M. Nanostructured Gd2Zr2O7: A Promising Thermal Barrier Coating with High Resistance to CaO–MgO–Al2O3–SiO2 Corrosion. J. Aust. Ceram. Soc. 2023, 59, 165–177. [Google Scholar]
- Tong, Y.P. Study on Preparation, Structure and Catalytic Properties of Pyrochrite Type Rare Earth Zirconates. Ph.D. Thesis, Nanjing University of Science and Technology, Nanjing, China, 2008. [Google Scholar]
- Levi, C.G.; Hutchinson, J.W.; Vidal-Sétif, M.-H.; Johnson, C.A. Environmental Degradation of Thermal-Barrier Coatings by Molten Deposits. MRS Bull. 2012, 37, 932–941. [Google Scholar]
- Zhou, X.; Zou, B.; He, L.; Xu, Z.; Xu, J.; Mu, R.; Cao, X. Hot Corrosion Behaviour of La2(Zr0.7Ce0.3)2O7 Thermal Barrier Coating Ceramics Exposed to Molten Calcium Magnesium Aluminosilicate at Different Temperatures. Corros. Sci. 2015, 100, 566–578. [Google Scholar]
- Liu, Y.; Liu, Y.W.; Zhuang, L.; Yu, H.L.; Chu, Y.H. Composition-driven superior CMAS corrosion resistance of high-entropy rare-earth disilicates. Corros. Sci. 2024, 233, 112108. [Google Scholar]
- Guo, L.; Li, G.; Gan, Z. Effects of surface roughness on CMAS corrosion behavior for thermal barrier coating applications. Adv. Ceram. 2021, 10, 472–481. [Google Scholar]
- Deng, W.Z.; Fergus, J.W. Effect of CMAS composition on hot corrosion behavior of gadolinium zirconate thermal barrier coating materials. Electrochem. Soc. 2017, 164, 526. [Google Scholar]
- Krämer, S.; Yang, J.; Levi, C.G.; Johnson, C.A. Thermochemical interaction of thermal barrier coatings with molten CaO–MgO–Al2O3–SiO2 (CMAS) deposits. J. Am. Ceram. Soc. 2006, 89, 3167–3175. [Google Scholar]







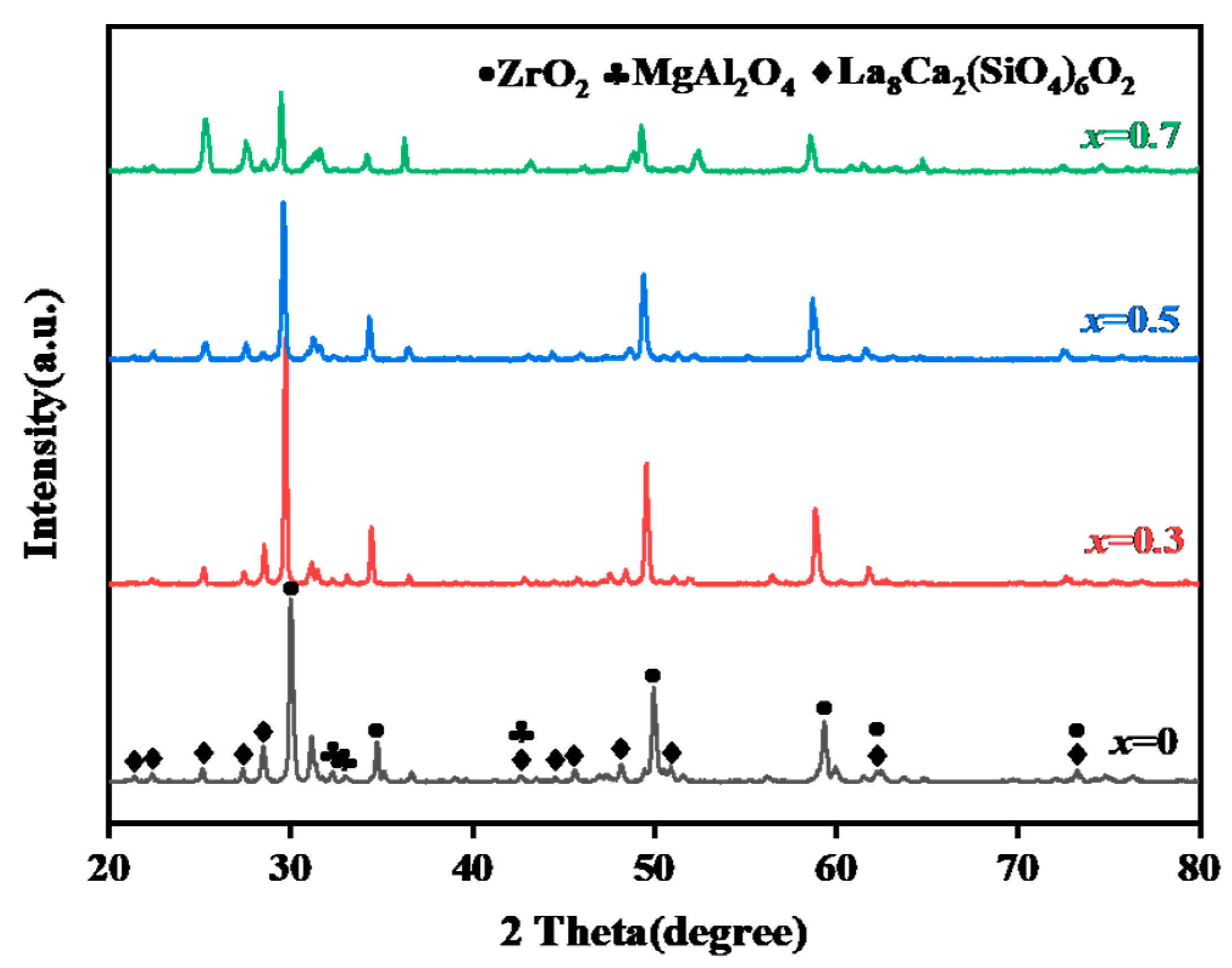
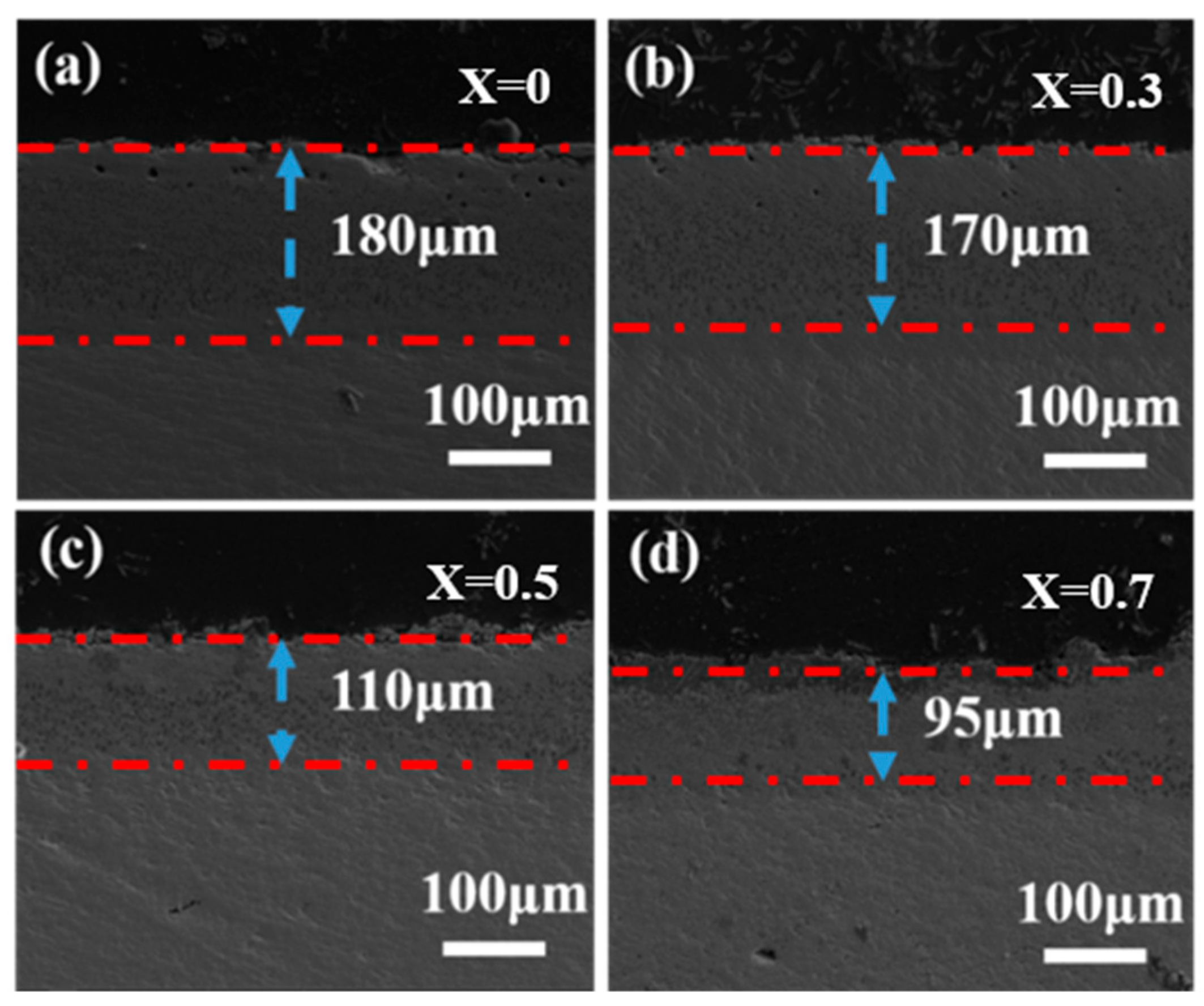
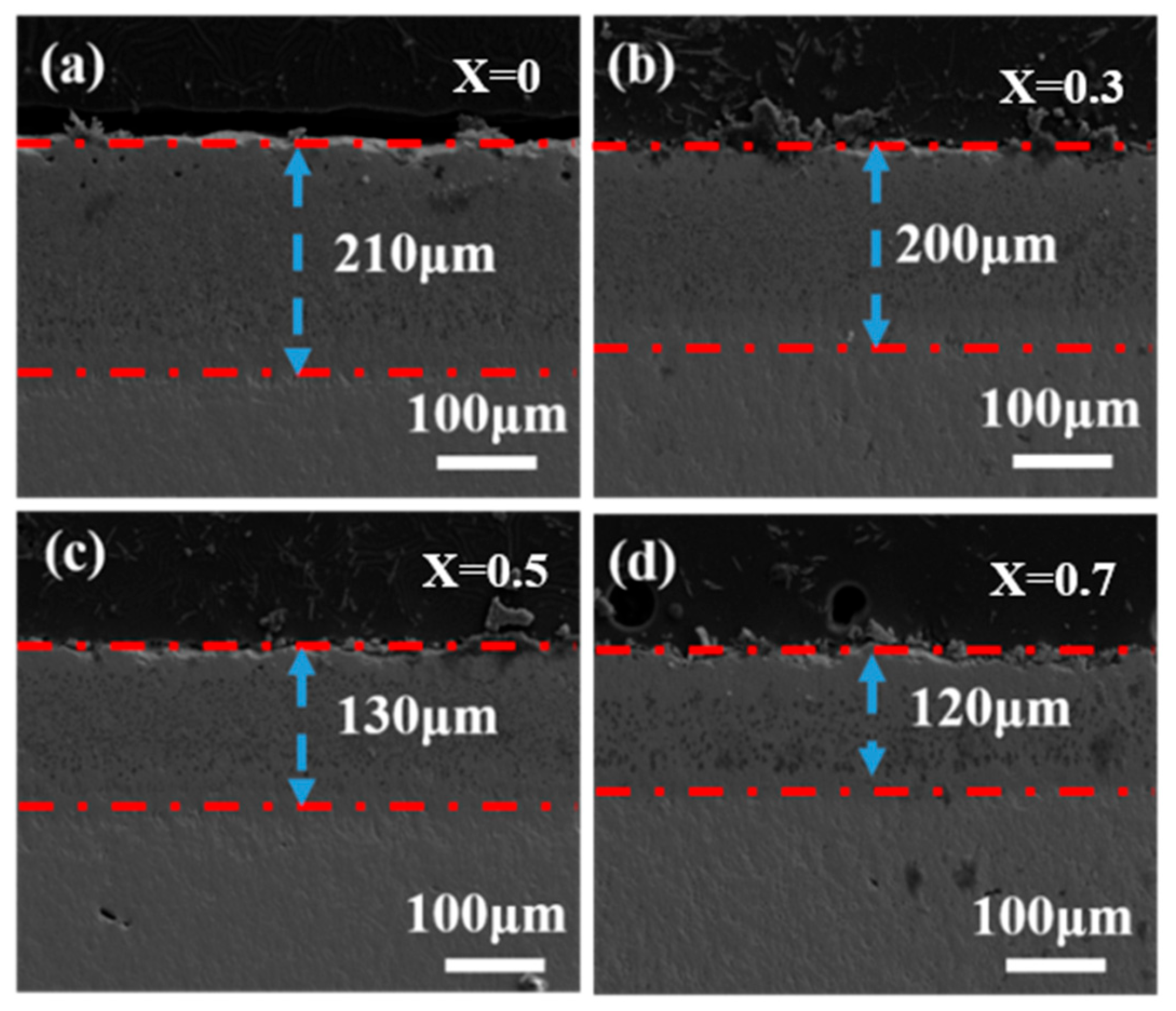

| aǺ | Theoretical Density/g·cm3 | Bulk Density/g·cm3 | Relatie Density/% | |
|---|---|---|---|---|
| x = 0 | 10.8241 | 6.0259 | 5.9883 | 96.19 |
| x = 0.3 | 10.7537 | 6.1727 | 6.0267 | 95.47 |
| x = 0.5 | 10.7209 | 6.2315 | 6.1628 | 96.90 |
| x = 0.7 | 10.7020 | 6.3582 | 6.2015 | 95.92 |
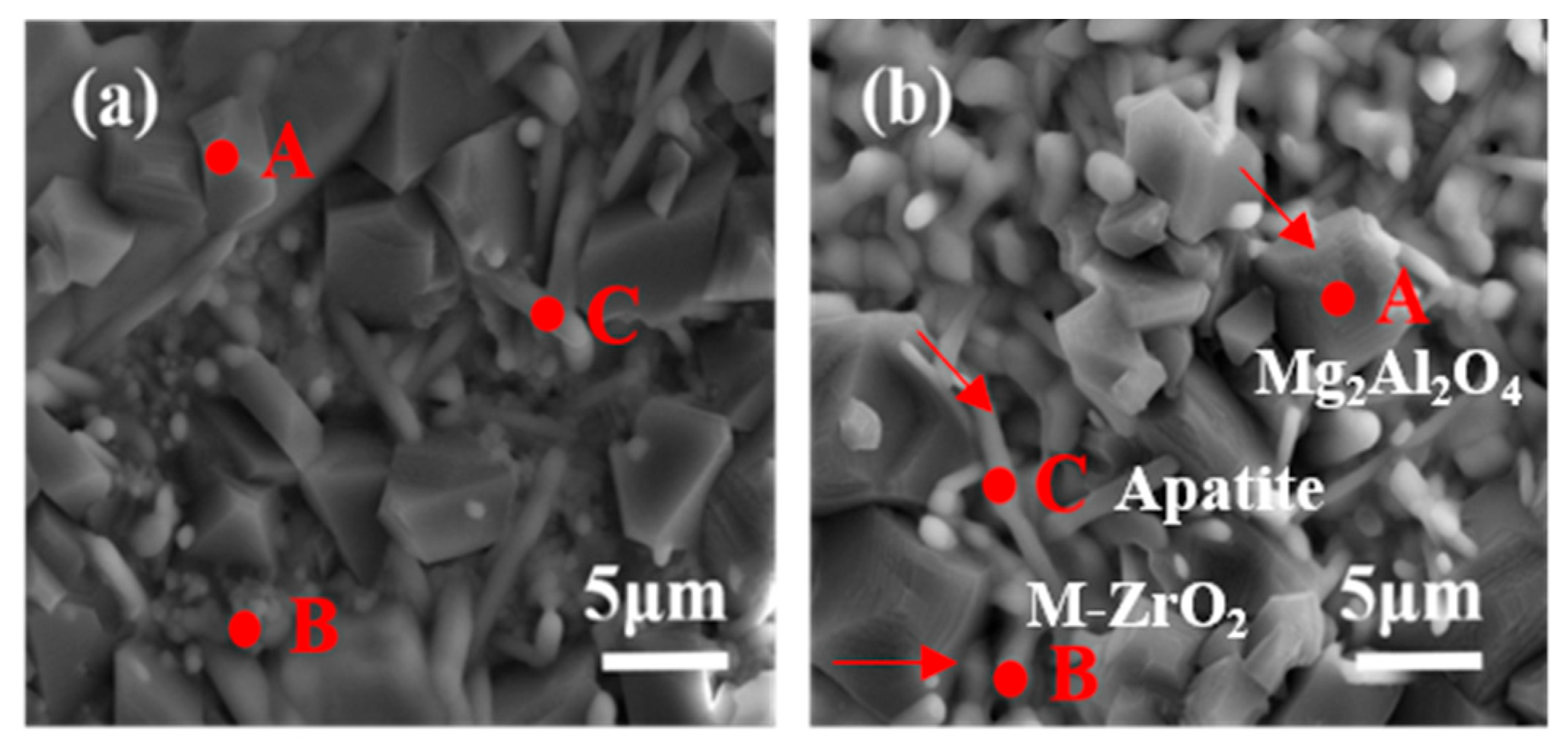
| Position | Component | Zr (at.%) | La (at.%) | Ce (at.%) | Gd (at.%) | |
|---|---|---|---|---|---|---|
| A | La(Zr0.7Ce0.3)2O7 | 0.46 | 1.15 | 0.27 | 0.00 | |
| (La0.7Gd0.3)2(Zr0.7Ce0.3)2O7 | 0.08 | 1.00 | 0.50 | 0.18 | ||
| (La0.5Gd0.5)2(Zr0.7Ce0.3)2O7 | 0.00 | 0.04 | 0.02 | 0.17 | ||
| (La0.3Gd0.7)2(Zr0.7Ce0.3)2O7 | 0.00 | 0.15 | 0.12 | 0.14 | ||
| B | La(Zr0.7Ce0.3)2O7 | 10.58 | 3.09 | 0.97 | 0.00 | |
| (La0.7Gd0.3)2(Zr0.7Ce0.3)2O7 | 11.14 | 1.48 | 2.47 | 1.96 | ||
| (La0.5Gd0.5)2(Zr0.7Ce0.3)2O7 | 13.97 | 1.47 | 3.33 | 3.64 | ||
| (La0.3Gd0.7)2(Zr0.7Ce0.3)2O7 | 6.22 | 1.09 | 1.97 | 4.08 | ||
| C | La(Zr0.7Ce0.3)2O7 | 0.8 | 14.17 | 2.66 | 0.00 | |
| (La0.7Gd0.3)2(Zr0.7Ce0.3)2O7 | 0.00 | 4.55 | 1.41 | 1.76 | ||
| (La0.5Gd0.5)2(Zr0.7Ce0.3)2O7 | 2.66 | 4.7 | 2.01 | 3.97 | ||
| (La0.3Gd0.7)2(Zr0.7Ce0.3)2O7 | 0.90 | 2.78 | 2.01 | 6.30 | ||
| Position | Component | Ca(at.%) | Mg(at.%) | Al(at.%) | Si(at.%) | Results |
| A | La(Zr0.7Ce0.3)2O7 | 0.45 | 11.05 | 19.32 | 1.08 | MgAL2O4 |
| (La0.7Gd0.3)2(Zr0.7Ce0.3)2O7 | 0.61 | 10.11 | 19.18 | 0.61 | ||
| (La0.5Gd0.5)2(Zr0.7Ce0.3)2O7 | 0.16 | 10.47 | 19.59 | 0.05 | ||
| (La0.3Gd0.7)2(Zr0.7Ce0.3)2O7 | 0.08 | 11.84 | 22.68 | 0.17 | ||
| B | La(Zr0.7Ce0.3)2O7 | 1.32 | 3.53 | 5.96 | 2.34 | ZrO2 |
| (La0.7Gd0.3)2(Zr0.7Ce0.3)2O7 | 1.58 | 0.25 | 0.10 | 0.68 | ||
| (La0.5Gd0.5)2(Zr0.7Ce0.3)2O7 | 1.97 | 0.31 | 0.64 | 1.94 | ||
| (La0.3Gd0.7)2(Zr0.7Ce0.3)2O7 | 1.97 | 1.20 | 2.06 | 3.94 | ||
| C | La(Zr0.7Ce0.3)2O7 | 5.02 | 3.17 | 4.61 | 8.60 | RexCay(SiO4)6O2 |
| (La0.7Gd0.3)2(Zr0.7Ce0.3)2O7 | 3.03 | 0.23 | 0.17 | 9.70 | ||
| (La0.5Gd0.5)2(Zr0.7Ce0.3)2O7 | 3.47 | 0.44 | 0.96 | 7.90 | ||
| (La0.3Gd0.7)2(Zr0.7Ce0.3)2O7 | 3.96 | 1.44 | 2.13 | 9.08 | ||
| La2(Zr0.7Ce0.3)2O7 Corroded After 5 h | Element Content | (La0.3Gd0.7)2(Zr0.7Ce0.3)2O7 After Corrosion for 5 h | Element Content |
|---|---|---|---|
| Al2O3 | 39.53% | Al2O3 | 18.66% |
| ZrO2 | 34.29% | ZrO2 | 30.40% |
| SiO2 | 10.43% | SiO2 | 18.24% |
| La2O3 | 7.20% | La2O3 | 3.00% |
| CeO2 | 3.82% | CeO2 | 3.87% |
| CaO | 3.72% | CaO | 8.20% |
| Gd2O3 | 8.57% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Xie, M.; Qu, X.; Song, X.; Zhang, Y.; Mu, R. Study of Gd2O3-Doped La2(Zr0.7Ce0.3)2O7 Thermal Barriers for Coating Ceramic Materials for CMAS Resistance. Coatings 2025, 15, 483. https://doi.org/10.3390/coatings15040483
Song X, Xie M, Qu X, Song X, Zhang Y, Mu R. Study of Gd2O3-Doped La2(Zr0.7Ce0.3)2O7 Thermal Barriers for Coating Ceramic Materials for CMAS Resistance. Coatings. 2025; 15(4):483. https://doi.org/10.3390/coatings15040483
Chicago/Turabian StyleSong, Xiaowei, Min Xie, Xiaofu Qu, Xiwen Song, Yonghe Zhang, and Rende Mu. 2025. "Study of Gd2O3-Doped La2(Zr0.7Ce0.3)2O7 Thermal Barriers for Coating Ceramic Materials for CMAS Resistance" Coatings 15, no. 4: 483. https://doi.org/10.3390/coatings15040483
APA StyleSong, X., Xie, M., Qu, X., Song, X., Zhang, Y., & Mu, R. (2025). Study of Gd2O3-Doped La2(Zr0.7Ce0.3)2O7 Thermal Barriers for Coating Ceramic Materials for CMAS Resistance. Coatings, 15(4), 483. https://doi.org/10.3390/coatings15040483






