Progress in Surface Engineering Techniques for Magnesium–Lithium-Based Alloys
Abstract
:1. Introduction
2. Different Surface Engineering of Mg–Li Alloys
2.1. Chemical Conversion
2.2. Electroless Plating
2.3. Anodic Oxidation
2.4. Coating Technology
2.5. Other Surface Modification Techniques
3. Innovation and Further Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.J.; Luan, J.Y.; Xu, D.K.; Sun, J.; Li, C.Q.; Han, E.H. Research progress on the corrosion behavior of magnesiumlithium-based alloys: A review. Acta Metall. Sin.-Engl. Lett. 2019, 32, 1–9. [Google Scholar] [CrossRef]
- Peng, X.; Xu, S.H.; Ding, D.H.; Liao, G.L.; Wu, G.H.; Liu, W.C.; Ding, W.J. Microstructural evolution, mechanical properties and corrosion behavior of as-cast Mg-5Li-3Al-2Zn alloy with different Sn and Y addition. J. Mater. Sci. Technol. 2021, 72, 16–22. [Google Scholar] [CrossRef]
- Cain, T.W.; Labukas, J.P. The development of β phase Mg-Li alloys for ultralight corrosion resistant applications. NPJ Mater. Degrad. 2020, 4, 1–10. [Google Scholar] [CrossRef]
- Tang, H.; Yan, Y.D.; Zhang, M.L.; Li, X.; Han, W.; Xue, Y.; Zhang, Z.J.; He, H. Fabrication of Mg-Pr and Mg-Li-Pr alloys by electrochemical co-reduction from their molten chlorides. Electrochim. Acta 2013, 107, 209–215. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards developing mg alloys with simultaneously improved strength and corrosion resistance via re alloying. J. Magnes. Alloys 2021, 9, 41–56. [Google Scholar] [CrossRef]
- Ouyang, Y.B.; Guo, E.Y.; Chen, X.B.; Kang, H.J.; Chen, Z.N.; Wang, T.M. Recent progress in protective coatings against corrosion upon magnesium–lithium alloys: A critical review. J. Magnes. Alloys 2024, 12, 3967–3995. [Google Scholar] [CrossRef]
- Kojima, Y. Platform science and technology for advanced magnesium alloys. Mater. Sci. Forum. 2000, 350–351, 3–18. [Google Scholar] [CrossRef]
- Friedrich, H.; Schumann, S. Research for a “new age of magnesium” in the automotive industry. J. Mater. Process. Technol. 2001, 117, 276–281. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Han, E.H. Corrosion characterization of Mg-8Li alloy in NaCl solution. Corros. Sci. 2009, 51, 1087–1094. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, X.; Zhang, M.; Gao, L.; Wu, R. Electrochemical characterization of the corrosion of a Mg–Li alloy. Mater. Lett. 2008, 62, 2177–2180. [Google Scholar] [CrossRef]
- Peng, X.; Liu, W.C.; Wu, G.H. Strengthening-toughening methods and mechanisms of Mg–Li alloy: A review. Rare Met. 2022, 41, 1176–1188. [Google Scholar] [CrossRef]
- Liu, Z.; Nie, J.F.; Zhao, Y.H. Effect of deformation processing on microstructure evolution and mechanical properties of Mg–Li alloys: A review. Trans. Nonferrous Met. Soc. China 2024, 34, 1–25. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, B.; Wu, R.; Zhou, Y.; Wu, Q. Nanocrystalline strengthened Mg-Li alloy with a BCC structure prepared via heat treatment and rolling. Mater. Lett. 2022, 312, 131680. [Google Scholar] [CrossRef]
- Ma, Y.; Li, N.; Li, D.; Zhang, M.; Huang, X. Characteristics and corrosion studies of vanadate conversion coating formed on Mg–14 wt% Li–1 wt% Al–0.1 wt% Ce alloy. Appl. Surf. Sci. 2012, 261, 59–67. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Yu, X.; Zhang, M.; Huang, X. Lanthanum-based conversion coating on Mg-8Li alloy. Appl. Surf. Sci. 2008, 255, 2338–2341. [Google Scholar] [CrossRef]
- Zeng, R.C.; Sun, X.X.; Song, Y.W.; Zhang, F.; Li, S.Q.; Cui, H.Z.; Han, E.H. Influence of solution temperature on corrosion resistance of Zn-Ca phosphate conversion coating on biomedical Mg-Li-Ca alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 3293–3299. [Google Scholar] [CrossRef]
- Luo, H.J.; Song, B.N.; Liu, Y.H.; Yao, G.C. Electroless Ni-P plating on Mg-Li alloy by two-step method. Trans. Nonferrous Met. Soc. China 2011, 21, 2225–2230. [Google Scholar] [CrossRef]
- Yin, T.; Wu, R.; Leng, Z.; Du, G.; Guo, X.; Zhang, M.; Zhang, J. The process of electroplating with Cu on the surface of Mg-Li alloy. Surf. Coat. Technol. 2013, 225, 119–125. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Zheng, Y.; Jiang, W.; Zhang, M. Electroless Ni-P plating with molybdate pretreatment on Mg-8Li alloy. J. Alloys Compd. 2009, 467, 562–566. [Google Scholar] [CrossRef]
- Wang, L.; Wu, T.; Cao, D.; Yin, J.; Wang, G.; Zhang, M. Self-growth of micro-and nano-structured Mg(OH)2 on electrochemically anodised Mg–Li alloy surface. J. Exp. Nanosci. 2015, 10, 56–65. [Google Scholar] [CrossRef]
- Sharma, A.K.; Rani, R.U.; Bhojaraj, H.; Narayanamurthy, H. Galvanic black anodizing on Mg-Li alloys. J. Appl. Electrochem. 1993, 23, 500–507. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, C.M.; Chen, W.K.; Liu, Y.M.; Ger, M.D. Microstructure and corrosion resistance of anodized Mg-9 mass% Li-1 mass% Zn alloy. Mater. Trans. 2008, 49, 1355–1358. [Google Scholar] [CrossRef]
- Ji, P.; Long, R.; Hou, L.; Wu, R.; Zhang, J.; Zhang, M. Study on hydrophobicity and wettability transition of Ni-Cu-SiC coating on Mg-Li alloy. Surf. Coat. Technol. 2018, 350, 428–435. [Google Scholar] [CrossRef]
- Yamauchi, N.; Ueda, N.; Okamoto, A.; Sone, T.; Tsujikawa, M.; Oki, S.D.L.C. DLC coating on Mg-Li alloy. Surf. Coat. Technol. 2007, 201, 4913–4918. [Google Scholar] [CrossRef]
- Yao, Z.; Ju, P.; Xia, Q.; Wang, J.; Su, P.; Wei, H.; Li, D.; Jiang, Z. Preparation of thermal control coatings on Mg–Li alloys by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 307, 1236–1240. [Google Scholar] [CrossRef]
- Ma, Y.; Li, N.; Li, D.; Zhang, M.; Huang, X. A two-step surface treatment, combining fluoride pretreatment and anodic electrophoresis deposition of waterborne acrylic resin, for Mg–Li–Al–Ce alloy. Mater. Lett. 2013, 90, 11–13. [Google Scholar] [CrossRef]
- Li, C.; Liang, D.; Lin, Y.; Dong, Y.; Shi, B.; Yan, C.; Zhang, Z. Effect of Li content on the surface film formed on the binary Mg-Li alloys in NaCl solution. Met. Mater. Int. 2024, 30, 127–142. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, X.C.; Sun, J.; Yao, Q.T.; Du, X.D.; Wu, Y.C. Nitriding behaviour of Mg-Li alloy with surface mechanical nano-alloying pretreatment. Surf. Eng. 2021, 37, 1075–1083. [Google Scholar] [CrossRef]
- Zou, Y.; Shen, R.T.; Lu, Z.T.; Zhou, Y.; Li, Y. Enhanced low-cycle fatigue behavior LZ91 Mg–Li alloy with ultrasonic nanocrystal surface modification. Fatigue Fract. Eng. Mater. Struct. 2023, 46, 2485–2495. [Google Scholar] [CrossRef]
- Mozetič, M. Surface modification to improve properties of materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef]
- Prakash, S.; Karacor, M.B.; Banerjee, S. Surface modification in microsystems and nanosystems. Surf. Sci. Rep. 2009, 64, 233–254. [Google Scholar] [CrossRef]
- Montealegre, M.A.; Castro, G.; Rey, P.; Arias, J.L.; Vázquez, P.; González, M. Surface treatments by laser technology. Contemp. Mater. 2010, 1, 19–30. [Google Scholar] [CrossRef]
- Wagner, H.E.; Brandenburg, R.; Kozlov, K.V.; Sonnenfeld, A.; Michel, P.; Behnke, J.F. The barrier discharge: Basic properties and applications to surface treatment. Vacuum 2003, 71, 417–436. [Google Scholar] [CrossRef]
- Sun, Y.H.; Wang, R.C.; Peng, C.Q.; Yan, F.; Ming, Y. Corrosion behavior and surface treatment of superlight Mg–Li alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 1455–1475. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. J. Energy Chem. 2020, 43, 220–235. [Google Scholar] [CrossRef]
- Rickerby, D.S.; Bull, S.J. Engineering with surface coatings: The role of coating microstructure. Surf. Coat. Technol. 1989, 39, 315–328. [Google Scholar] [CrossRef]
- Ma, X.C.; Jin, S.Y.; Wu, R.Z.; Wang, J.X.; Wang, G.X.; Krit, B.; Betsofen, S. Corrosion behavior of Mg–Li alloys: A review. Trans. Nonferrous Met. Soc. China 2021, 31, 3228–3254. [Google Scholar] [CrossRef]
- Chang, T.C.; Wang, J.Y.; Chu, C.L.; Lee, S. Mechanical properties and microstructures of various Mg–Li alloys. Mater. Lett. 2006, 60, 3272–3276. [Google Scholar] [CrossRef]
- Li, C.Q.; Xu, D.K.; Chen, X.B.; Wang, B.J.; Wu, R.Z.; Han, E.H.; Birbilis, N. Composition and microstructure dependent corrosion behaviour of Mg-Li alloys. Electrochim. Acta 2018, 260, 55–64. [Google Scholar] [CrossRef]
- Cheng, D.; Negreiros, F.R.; Aprà, E.; Fortunelli, A. Computational approaches to the chemical conversion of carbon dioxide. ChemSusChem 2013, 6, 944–965. [Google Scholar] [CrossRef]
- Jiang, C.C.; Cao, Y.K.; Xiao, G.Y.; Zhu, R.F.; Lu, Y.P. A review on the application of inorganic nanoparticles in chemical surface coatings on metallic substrates. RSC Adv. 2017, 7, 7531–7539. [Google Scholar] [CrossRef]
- Zhang, C.H.; Li, R.M.; Gao, L.L.; Zhang, M.L. Corrosion protection of Mg–11Li–3Al–0·5RE alloy using hybrid epoxy/silica conversion coatings. Corros. Eng. Sci. Technol. 2013, 48, 276–281. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.H. A novel phosphate conversion film on Mg-8.8 Li alloy. Surf. Coat. Technol. 2009, 203, 1107–1113. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of corrosion-resistant conversion coatings for magnesium and its alloys. Corrosion 2011, 67, 35005. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Dong, G.; Gong, F.; Zhang, M. Rare earth conversion coating on Mg-8.5 Li alloys. J. Alloys Compd. 2009, 487, 64–68. [Google Scholar] [CrossRef]
- Shi, W.; Song, Z.; Wang, J.; Li, Q.; An, Q. Phytic acid conversion film interfacial engineering for stabilizing zinc metal anode. Chem. Eng. J. 2022, 446, 137295. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, C.; Zhang, M.; Huang, X.; Jiang, X. Phytic acid conversion coating on Mg-Li alloy. J. Alloys Compd. 2009, 485, 789–793. [Google Scholar] [CrossRef]
- Zhou, W.; Shan, D.; Han, E.H.; Ke, W. Structure and formation mechanism of phosphate conversion coating on die-cast AZ91D magnesium alloy. Corros. Sci. 2008, 50, 329–337. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.H. Formation mechanism of phosphate conversion film on Mg-8.8 Li alloy. Corros. Sci. 2009, 51, 62–69. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, M.; Li, J.; Yu, X.; Niu, Z. Stannate conversion coatings on Mg-8Li alloy. J. Alloys Compd. 2009, 471, 197–200. [Google Scholar] [CrossRef]
- Hung, S.M.; Lin, H.; Chen, H.W.; Chen, S.Y.; Lin, C.S. Corrosion resistance and electrical contact resistance of a thin permanganate conversion coating on dual-phase LZ91 Mg-Li alloy. J. Mater. Res. Technol. 2021, 11, 1953–1968. [Google Scholar] [CrossRef]
- Saji, V.S. Review of rare-earth-based conversion coatings for magnesium and its alloys. J. Mater. Res. Technol. 2019, 8, 5012–5035. [Google Scholar] [CrossRef]
- Song, D.; Jing, X.; Wang, J.; Lu, S.; Yang, P.; Wang, Y.; Zhang, M. Microwave-assisted synthesis of lanthanum conversion coating on Mg-Li alloy and its corrosion resistance. Corros. Sci. 2011, 53, 3651–3656. [Google Scholar] [CrossRef]
- Xu, F.F.; Zhao, Y.; Xu, Y. Preparation and corrosion resistance of rare earth-silane composite conversion coatings on magnesium-lithium alloy surface. Rare Met. 2023, 42, 1011–1017. [Google Scholar] [CrossRef]
- Mazur, K.; Stefańska, A.; Hebda, M. Analysis of chemical nickel-plating process. Mater. Sci. 2018, 54, 387–394. [Google Scholar] [CrossRef]
- Bremner, J.G.M. Nickel plating by chemical reduction. Nature 1948, 162, 183–184. [Google Scholar] [CrossRef]
- Huang, Z.; Nguyen, T.T.; Zhou, Y.; Qi, G. A low temperature electroless nickel plating chemistry. Surf. Coat. Technol. 2019, 372, 160–165. [Google Scholar] [CrossRef]
- Pei, S.F.; Li, S.Q.; Zhong, L.; Cui, K.F.; Yang, J.; Yang, Z.G. Analysis of the causes of differences between the upper and lower surfaces of electroless Ni-P coating on LZ91 magnesium-lithium alloy. Coatings 2022, 12, 1157. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Z.; Liu, S.; Chen, D.; Wang, G.; Wang, Y.; Zhang, M.; Chen, Y. Ultrasonic-assisted electroless Ni-P plating on dual phase Mg-Li alloy. J. Electrochem. Soc. 2014, 162, C64. [Google Scholar] [CrossRef]
- Yue, A.; Cao, Y.; Zhang, Y.; Zhou, S. Study of electroless-deposited Zn on the surface of Mg-Li Alloy. Materials 2023, 16, 5511. [Google Scholar] [CrossRef] [PubMed]
- Paz Martínez-Viademonte, M.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing treatments for magnesium alloys and their effect on corrosion resistance in various environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Zhu, X.; Song, Y.; Yu, D.; Zhang, C.; Yao, W. A novel nanostructure fabricated by an improved two-step anodizing technology. Electrochem. Commun. 2013, 29, 71–74. [Google Scholar] [CrossRef]
- Sharma, A.K.; Rani, R.U.; Malek, A.; Acharya, K.S.N.; Muddu, M.; Kumar, S. Black anodizing of a magnesium-lithium alloy. Met. Finish. 1996, 94, 16–27. [Google Scholar] [CrossRef]
- Li, J.F.; Zheng, Z.Q.; Li, S.C.; Ren, W.D.; Zhang, Z. Preparation and galvanic anodizing of a Mg-Li alloy. Mater. Sci. Eng. A 2006, 433, 233–240. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F.S. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloys Compd. 2023, 948, 169773. [Google Scholar] [CrossRef]
- Dou, J.; Chen, Y.; Yu, H.; Chen, C. Research status of magnesium alloys by micro-arc oxidation: A review. Surf. Eng. 2017, 33, 731–738. [Google Scholar] [CrossRef]
- Jin, S.; Ma, X.; Wu, R.; Wang, G.; Zhang, J.; Krit, B.; Betsofen, S.; Liu, B. Advances in micro-arc oxidation coatings on Mg-Li alloys. Appl. Surf. Sci. Adv. 2022, 8, 100219. [Google Scholar] [CrossRef]
- Shi, L.; Xu, Y.; Li, K.; Yao, Z.; Wu, S. Effect of additives on structure and corrosion resistance of ceramic coatings on Mg-Li alloy by micro-arc oxidation. Curr. Appl. Phys. 2010, 10, 719–723. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Zhang, Y. Effect of graphene on micro-structure and properties of MAO coating prepared on Mg-Li alloy. Int. J. Electrochem. Sci. 2017, 12, 6081–6091. [Google Scholar] [CrossRef]
- Song, L.; Kou, Y.; Song, Y.; Shan, D.; Zhu, G.; Han, E.H. Fabrication and characterization of micro-arc oxidation (MAO) coatings on Mg-Li alloy in alkaline polyphosphate electrolytes without and with the addition of K2TiF6. Mater. Corros. 2011, 62, 1124–1132. [Google Scholar] [CrossRef]
- Qian, B.Y.; Miao, W.; Qiu, M.; Gao, F.; Hu, D.H.; Sun, J.F.; Wu, R.Z.; Betsofen Sergey, B.K. Influence of voltage on the corrosion and wear resistance of micro-arc oxidation coating on Mg-8Li-2Ca alloy. Acta Metall. Sin.-Engl. Lett. 2019, 32, 194–204. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Gao, F.Y.; Peng, H.Y.; Jiang, H.W.; Yin, L.C.; Wang, D.; Huang, H.L. Deposition of titanium nitride film on Mg-Li alloys by Dc reactive magnetron sputtering. Adv. Mater. Res. 2011, 204, 1685–1690. [Google Scholar] [CrossRef]
- Wang, P.C.; Shih, Y.T.; Lin, M.C.; Lin, H.C.; Chen, M.J.; Lin, K.M. A study of atomic layer deposited LiAlxOy films on Mg-Li alloys. Thin Solid Films. 2010, 518, 7501–7504. [Google Scholar] [CrossRef]
- Li, S.Q.; Zhang, L.; Liu, T.T.; Zhang, Y.W.; Guo, C.; Wang, Y.; Du, F.H. A dendrite-free lithium-metal anode enabled by designed ultrathin MgF2 nanosheets encapsulated inside nitrogen-doped graphene-like hollow nanospheres. Adv. Mater. 2022, 34, 2201801. [Google Scholar] [CrossRef]
- Yang, X.; Tian, B.; Jian, M.; Wu, M.; Li, W.; Jiang, J.; Guo, Z.; Yang, L. Molecular dynamics simulation of uniaxial stretching for silicon nitride deposited by PECVD. Appl. Surf. Sci. 2025, 682, 161696. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Adachi, S.I.; Abe, Y.; Oki, S.; Nakata, K.; Kamita, M. Corrosion protection of Mg-Li alloy by plasma thermal spraying of aluminum. Plasma Process. Polym. 2007, 4, S593–S596. [Google Scholar] [CrossRef]
- Oki, S.; Tsujikawa, M.; Morishige, T.; Kamita, M. Thin protective aluminum layer on Mg-Li alloy by plasma spraying and cold rolling. Plasma Process. Polym. 2009, 6, S954–S957. [Google Scholar] [CrossRef]
- Bao, Y.; Fu, B.; Jiao, Y.; Dong, T.; Li, J.; Li, G. Study of wear and corrosion resistance of cold sprayed TC4 coating on the surface of Mg-Li alloy. Coatings 2023, 13, 988. [Google Scholar] [CrossRef]
- Feng, K.; Wang, S.; Zhang, K.; Huo, L.; Zhou, H. Microstructure and properties of cold-sprayed Al-x% Al2O3 composite coatings on LA43M Mg-Li alloy. J. Therm. Spray Technol. 2024, 33, 308–320. [Google Scholar] [CrossRef]
- Wan, S.M.; Cui, X.F.; Jin, Q.W.; Ma, J.J.; Wen, X.; Su, W.N.; Zhang, X.R.; Jin, G.; Tian, H.L. Microstructure and properties of cold sprayed aluminum bronze coating on MBLS10A-200 magnesium-lithium alloy. Mater. Chem. Phys. 2022, 281, 125832. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Recent progresses of superhydrophobic coatings in different application fields: An overview. Coatings 2021, 11, 116. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic coatings from ecofriendly materials and processes: A review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, Z.; Guo, E.; Qiu, R.; Wang, X.; Kang, H.; Wang, T. Bioinspired superhydrophobic surface via one-step electrodeposition and its corrosion inhibition for Mg-Li alloy. Colloids Surf. A 2022, 648, 129145. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, Y. Preparation and characterization of superhydrophobic composite coatings on a magnesium-lithium alloy. RSC Adv. 2016, 6, 90587–90596. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, J. Fabrication of biodegradable superhydrophobic Zn-Fe coating on ultra-light Mg-Li alloy. Surf. Coat. Technol. 2024, 486, 130677. [Google Scholar] [CrossRef]
- He, H.; Zhou, S.; Du, J.; Yang, H.; Chen, D. Anti-icing and corrosion resistance of superhydrophobic coatings by precision machining and one-step electrodeposition on Mg-Li alloy. Colloids Surf. A 2024, 685, 133294. [Google Scholar] [CrossRef]
- He, H.; Du, J.; Sang, J.; Hirahara, H.; Aisawa, S.; Chen, D. Superhydrophobic coatings by electrodeposition on Mg-Li alloys: Attempt of armor-like Ni patterns to improve the robustness. Mater. Chem. Phys. 2023, 304, 127902. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, Y.; Jing, X. Composite coatings prepared by combined plasma electrolytic oxidation and chemical conversion routes on magnesium-lithium alloy. J. Alloys Compd. 2017, 706, 419–429. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Lu, Z.; Zhang, Y.; Zou, Y. Effects of ultrasonic nanocrystal surface modification on mechanical and corrosion behavior of LZ91 Mg-Li alloy. Mater. Trans. 2020, 61, 1258–1264. [Google Scholar] [CrossRef]
- Zhang, J.M.; Lian, D.D.; Hou, A.R.; Wang, Z.H.; Zhang, M.C.; Wu, J.W.; Wang, C.C. Comparative study on microstructure, corrosion morphology, and friction wear properties of layered double hydroxide/steam coating composite coatings on Mg–Li alloy. Adv. Eng. Mater. 2024, 26, 2400058. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, Y.; Wang, H.; Meng, L.; Liu, J.; Zhang, Z. Surface nanocrystallization of Mg-3 wt.% Li-6 wt.% Al alloy by surface mechanical attrition treatment. Mater. Charact. 2016, 120, 124–128. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, S.; Wang, Q.; Li, Y. A comparative study on mechanical and corrosion behaviours of α/(α + β) Mg-Li alloys subjected to ultrasonic nanocrystal surface modification. Metals 2022, 12, 681. [Google Scholar] [CrossRef]
- Li, Z.; Jing, X.; Yuan, Y.; Zhang, M. Composite coatings on a Mg-Li alloy prepared by combined plasma electrolytic oxidation and sol-gel techniques. Corros. Sci. 2012, 63, 358–366. [Google Scholar] [CrossRef]
- Li, D.; Cui, X.; Wen, X.; Li, Y.; Liu, E.; Jin, G.; Zheng, W. Improved electrochemical behavior of Mg-Li alloys by superhydrophobic layered double hydroxides/Ni-based composite coatings. J. Alloys Compd. 2023, 947, 169666. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhou, Z.; Guo, E.; Qiu, R.; Chen, Z.; Kang, H.; Wang, T. Boosting corrosion resistance of Mg-Li alloy: Implanting bioinspired superhydrophobic surfaces into MAO matrix for enhanced protection. Ceram. Int. 2024, 50, 48425–48439. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhang, F.; Song, L.; Zeng, R.C.; Li, S.Q.; Han, E.H. Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg-Li-Ca alloy. J. Alloys Compd. 2017, 728, 815–826. [Google Scholar] [CrossRef]
- Yu, C.; Cui, L.Y.; Zhou, Y.F.; Han, Z.Z.; Chen, X.B.; Zeng, R.C.; Zou, Y.H.; Li, S.Q.; Zhang, F.; Han, E.H.; et al. Self-degradation of micro-arc oxidation/chitosan composite coating on Mg-4Li-1Ca alloy. Surf. Coat. Technol. 2018, 344, 1–11. [Google Scholar] [CrossRef]


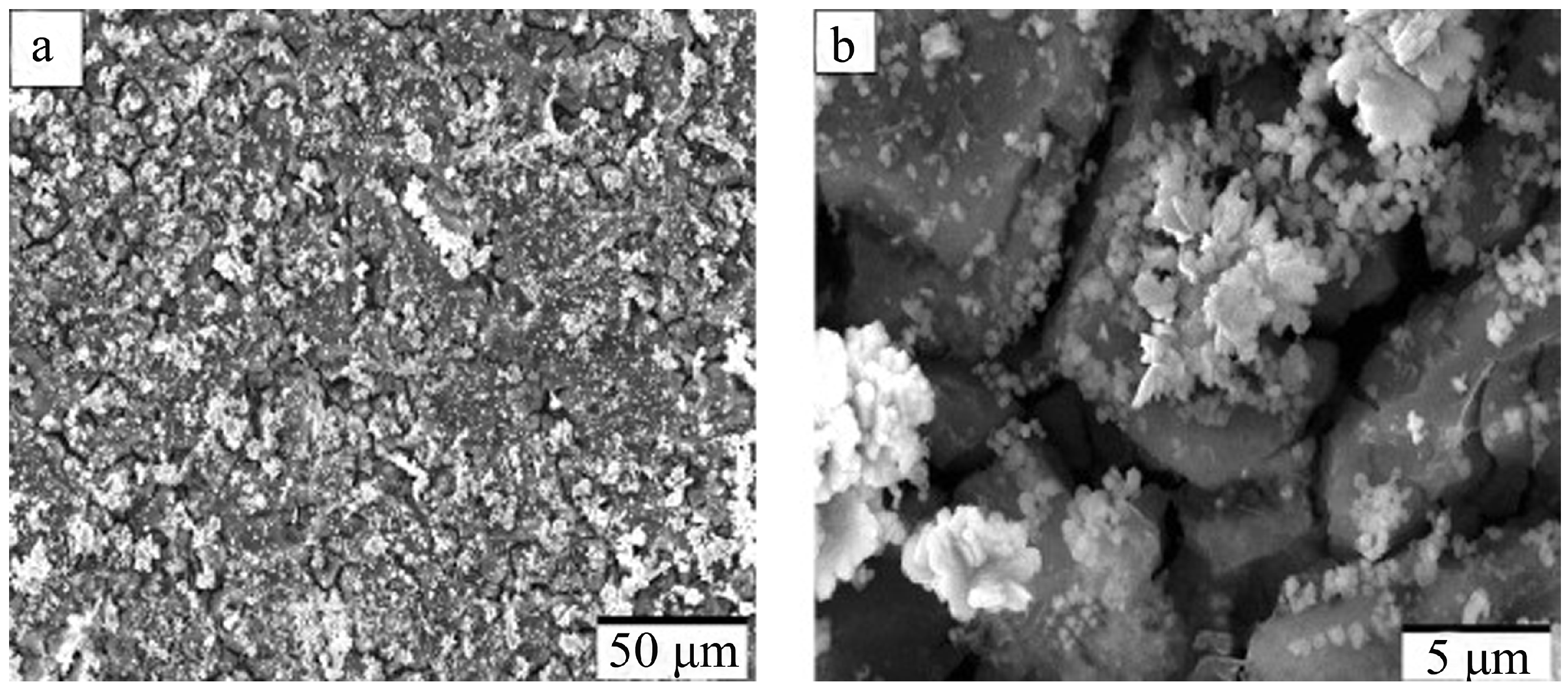

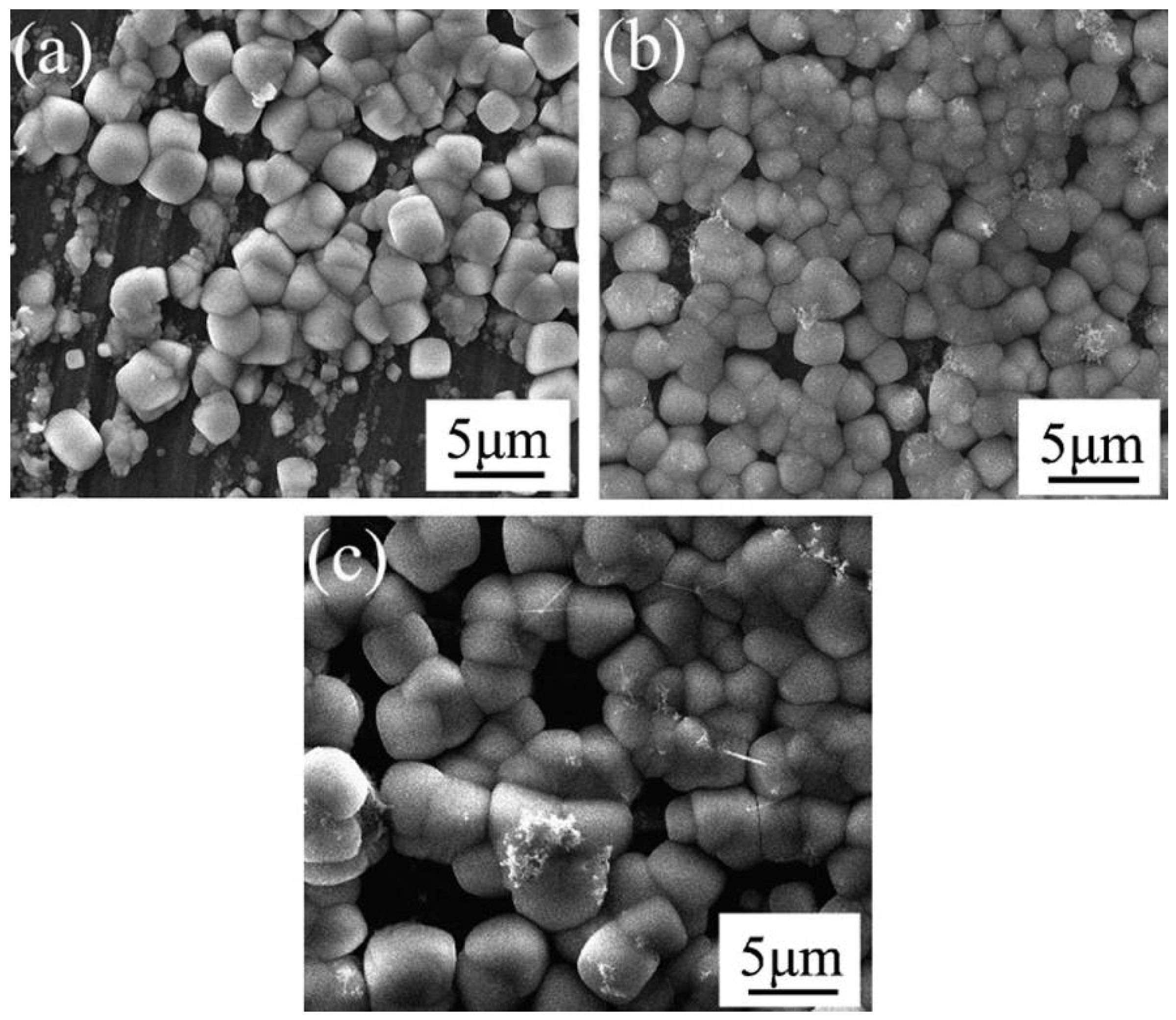
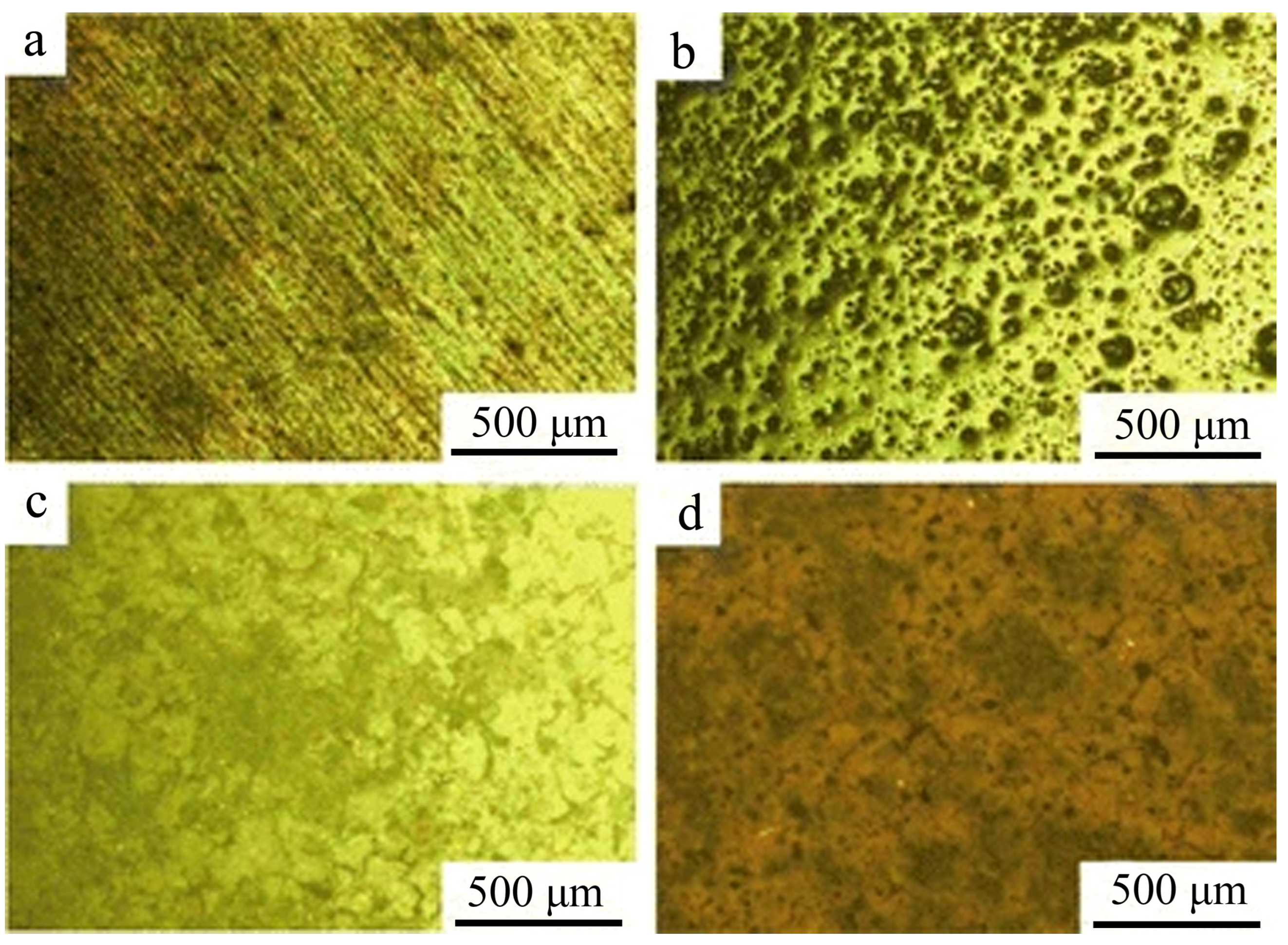
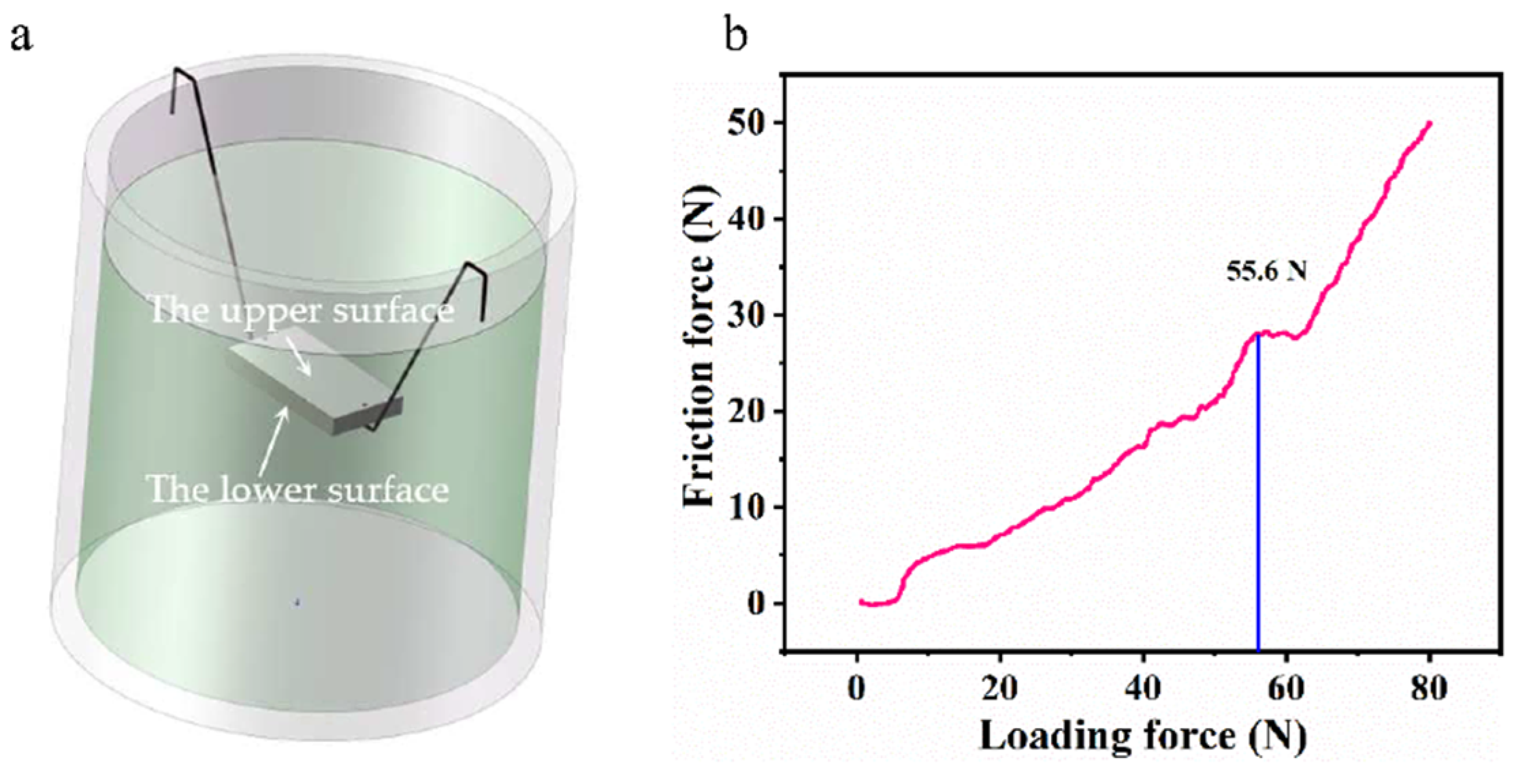
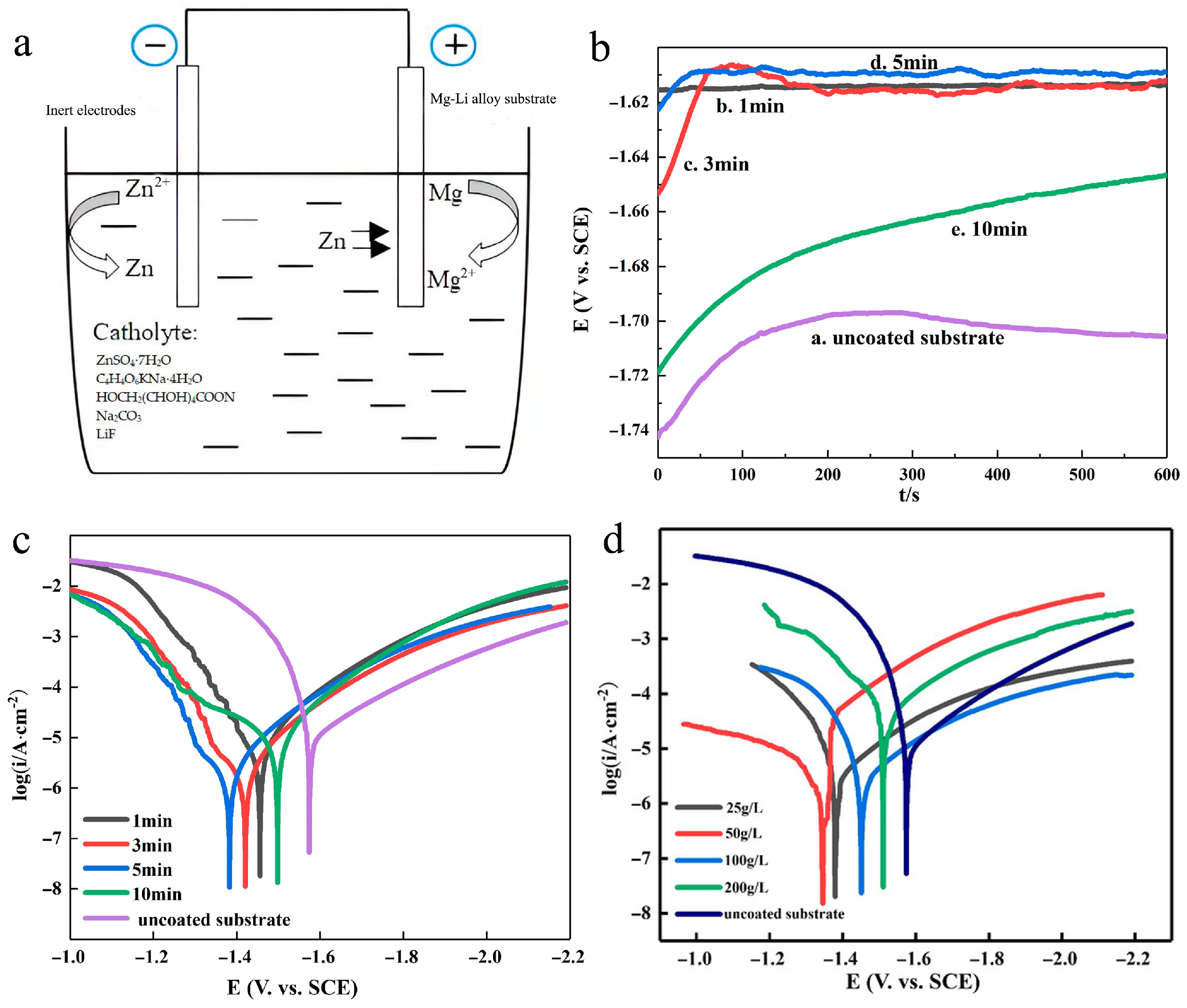

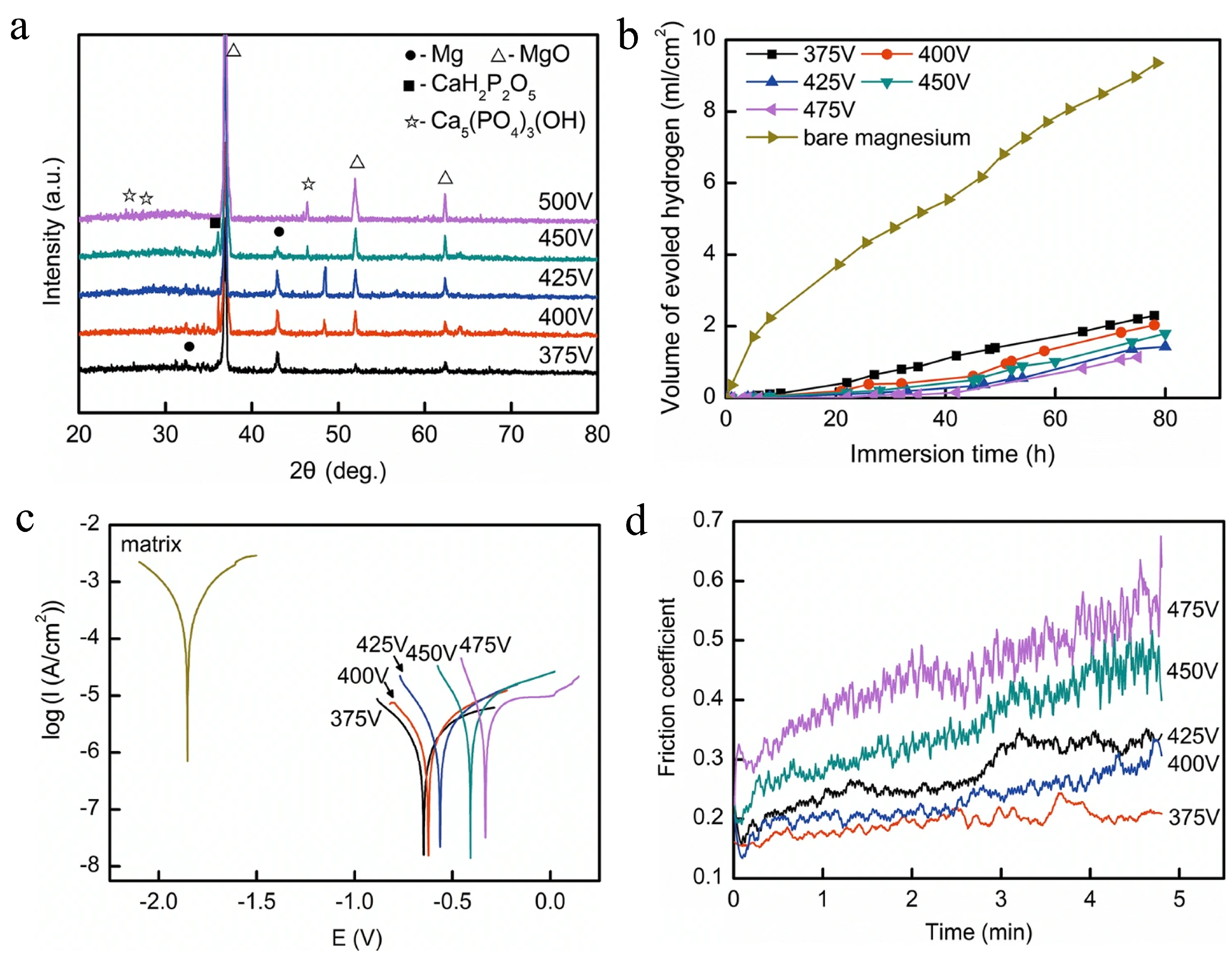
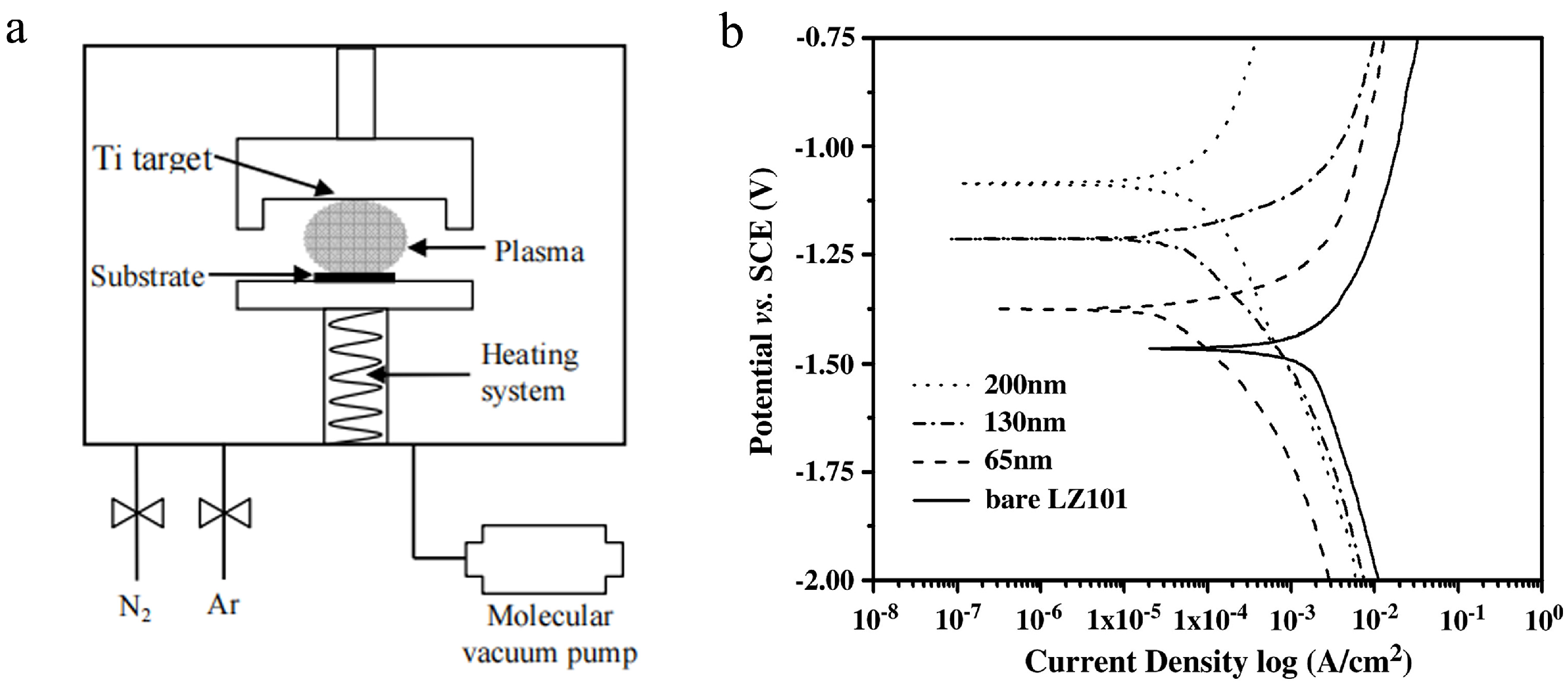
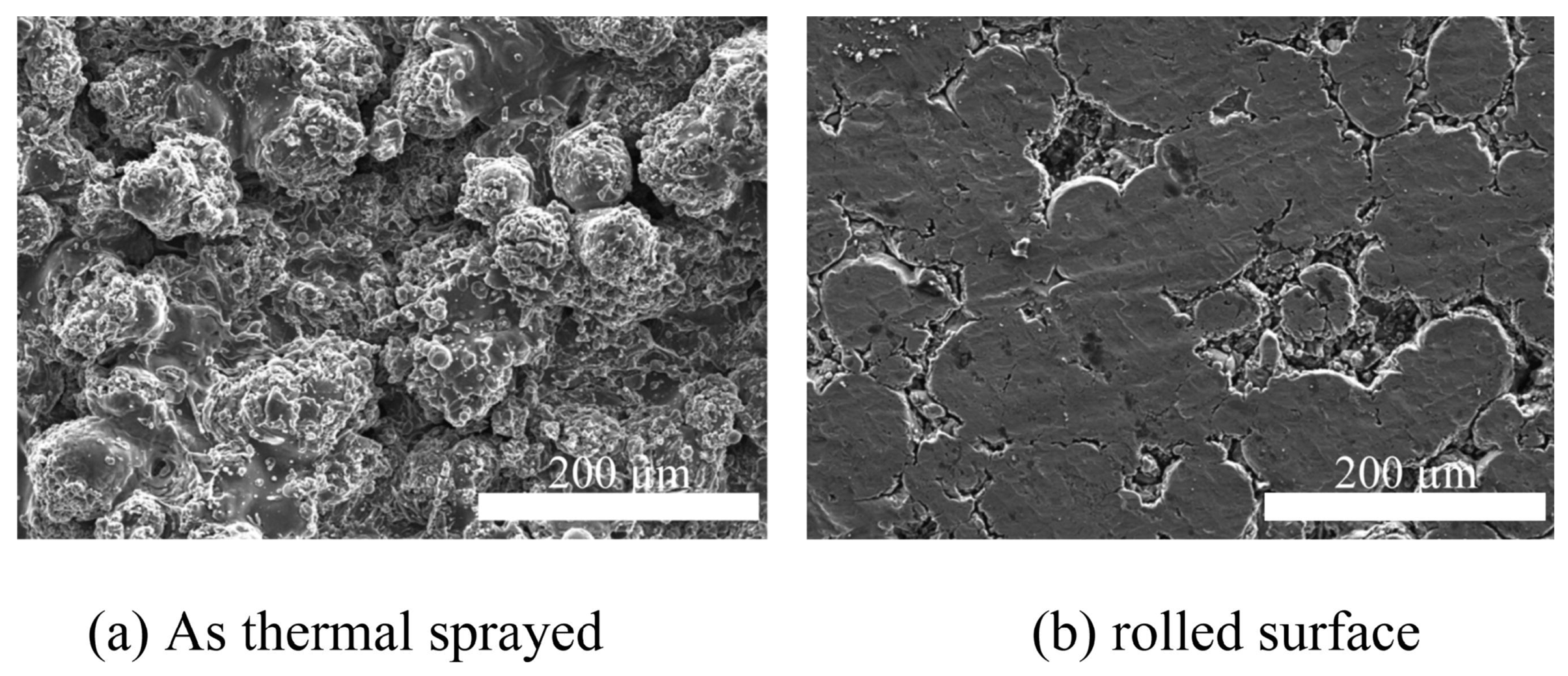
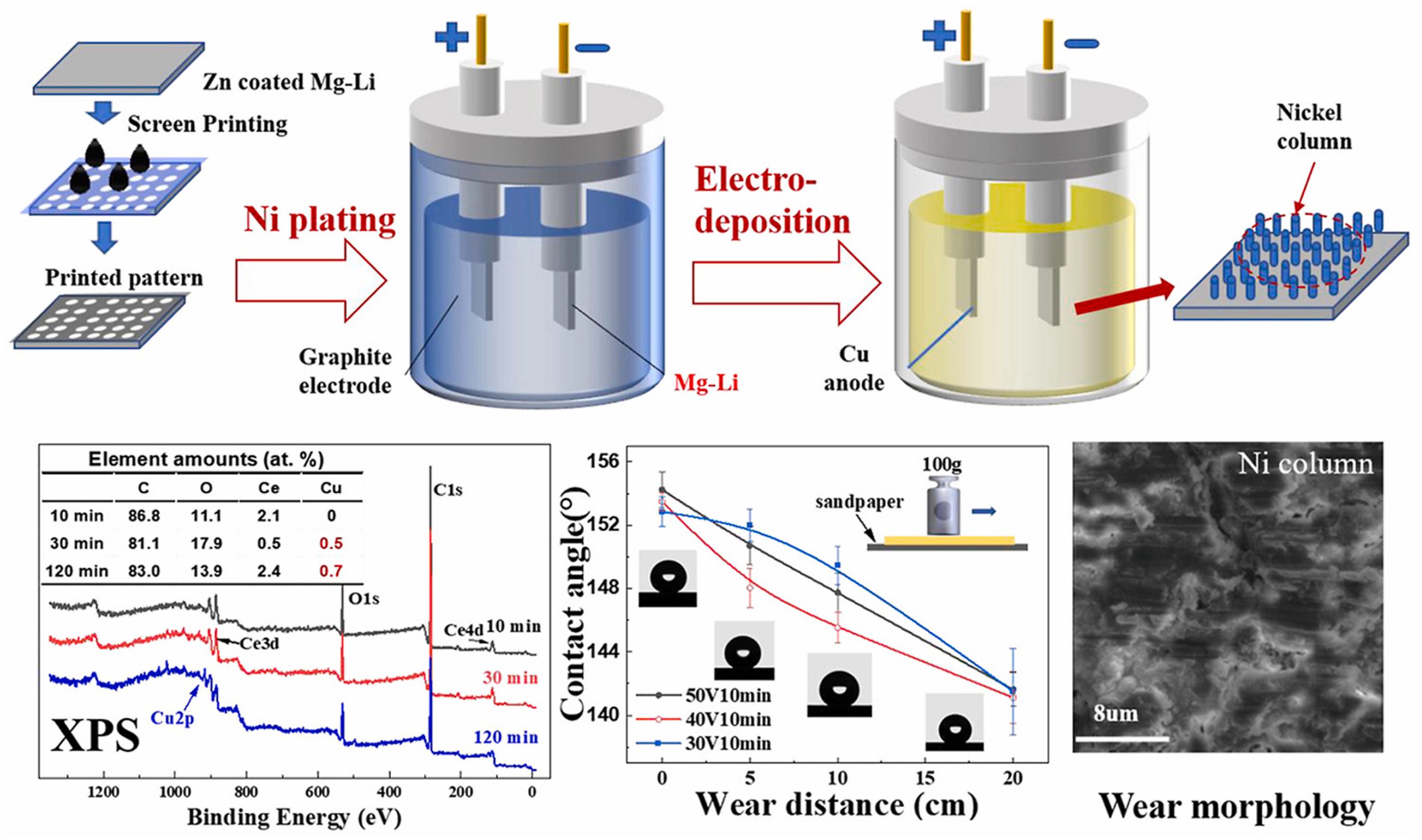

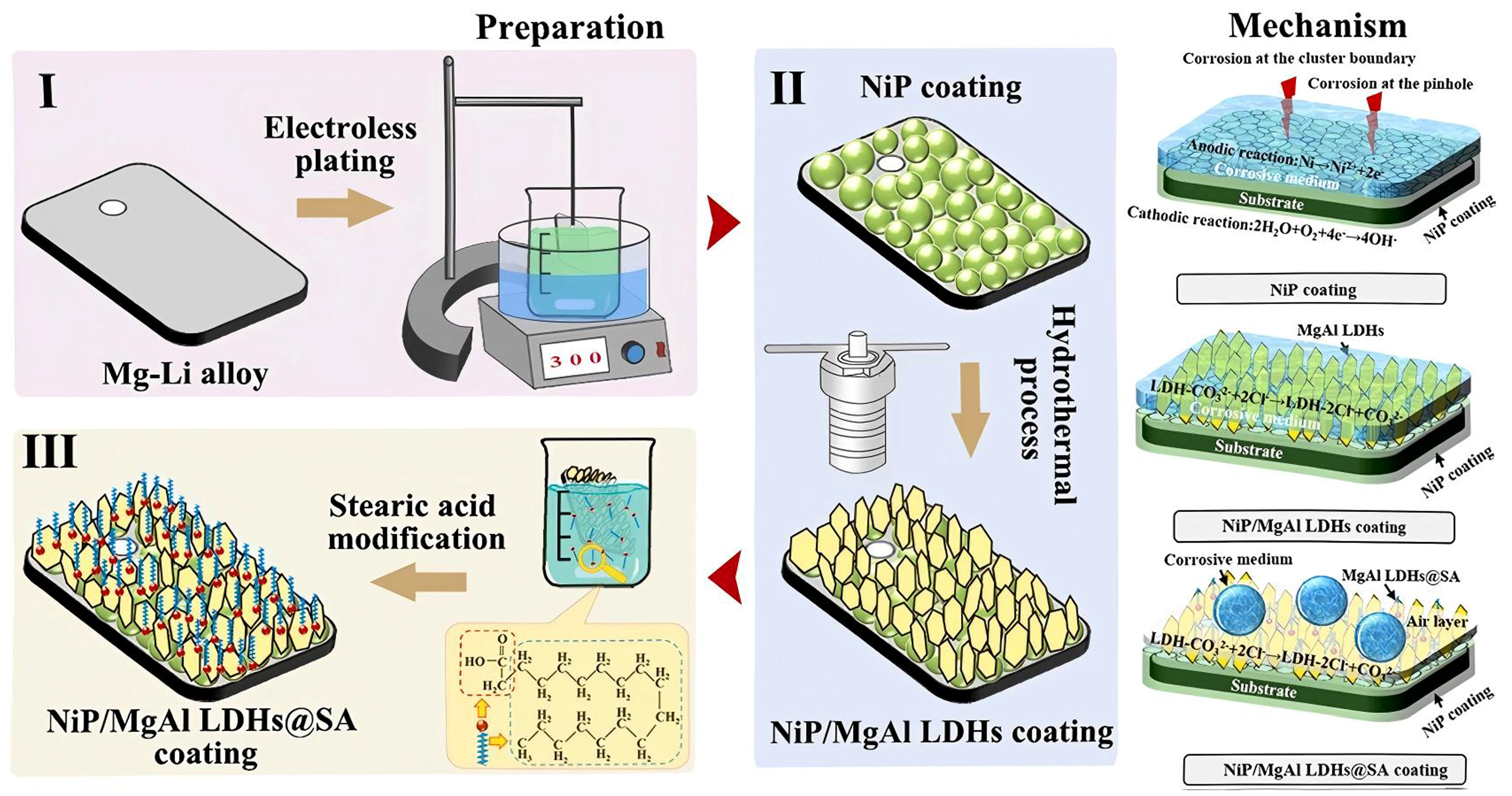
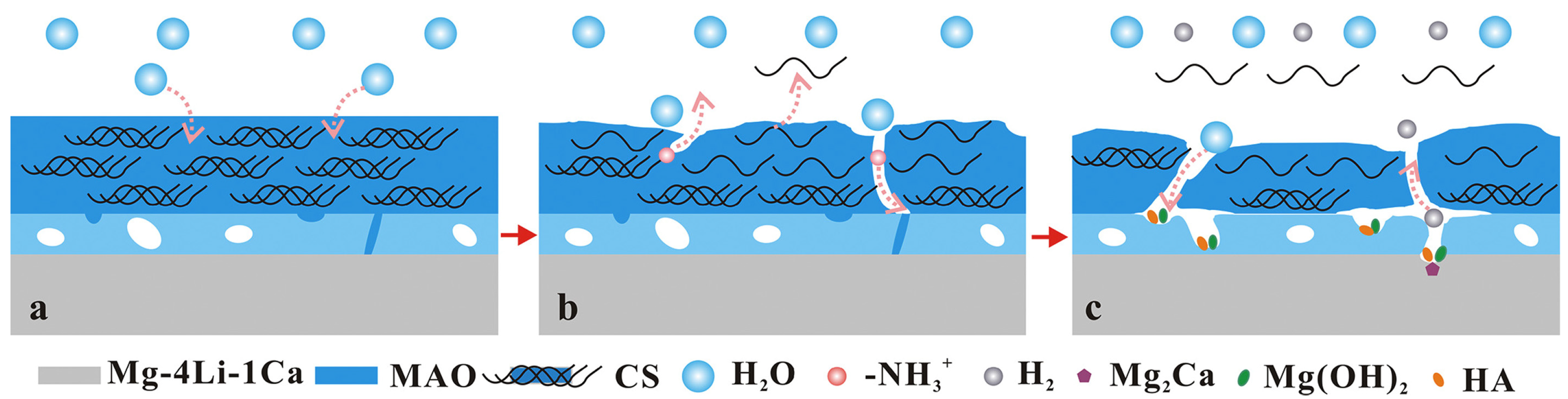
| Anodizing Condition | Ecorr (VSCE) | I0 (A/cm2) | RP (Ω cm2) |
|---|---|---|---|
| Mg–Li alloy base | −1.547 | 0.00124 | 21.1 |
| pH 2.5, room temperature | −1.356 | 7.915 × 10−6 | 3296 |
| pH 3.5, room temperature | −1.367 | 1.76 × 10−5 | 1483 |
| pH 4.5, room temperature | −1.395 | 3.45 × 10−6 | 7551 |
| pH 5.5, room temperature | −1.481 | 6.21 × 10−6 | 4200 |
| pH 4.5, 40 °C | −1.417 | 2.95 × 10−5 | 885.5 |
| pH 4.5, 50 °C | −1.091 | 0.00011 | 236.5 |
| Methods | Types | Advantages | Limitations | Refs. |
|---|---|---|---|---|
| Chemical Conversion | Phytic acid |
|
| [47,48] |
| Phosphate conversion |
|
| [49,50] | |
| Stannate conversion |
|
| [51,52,53,54] | |
| Rare earth-based conversion coatings |
|
| [46,55] | |
| Electroless Plating | Ni–P |
|
| [59,60] |
| Zinc |
|
| [61] | |
| Anodic Oxidation | Ordinary anodizing |
|
| [66] |
| Micro-arc oxidation |
|
| [67,68,69,70,71,72,73,74] | |
| Coating Technologies | Vapor deposition |
|
| [75,76,77,78] |
| Thermal spraying |
|
| [79,80,81,82,83] | |
| Superhydrophobic coatings |
|
| [84,85,86,87,88,89,90,91] | |
| Other Surface Modification Techniques | SMAT |
|
| [95] |
| UNSM |
|
| [29,96] | |
| superhydrophobic composite coating |
|
| [98] | |
| MAO/CS composite coatings |
|
| [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Hao, Z.; Feng, Y.; Shang, Y.; Tong, Y.; Lu, F.; Xu, L.; Chen, X.; Li, S.; Peng, J. Progress in Surface Engineering Techniques for Magnesium–Lithium-Based Alloys. Coatings 2025, 15, 502. https://doi.org/10.3390/coatings15050502
Li N, Hao Z, Feng Y, Shang Y, Tong Y, Lu F, Xu L, Chen X, Li S, Peng J. Progress in Surface Engineering Techniques for Magnesium–Lithium-Based Alloys. Coatings. 2025; 15(5):502. https://doi.org/10.3390/coatings15050502
Chicago/Turabian StyleLi, Ningning, Zhenjie Hao, Yaya Feng, Yan Shang, Yuping Tong, Fan Lu, Lei Xu, Xi Chen, Shuai Li, and Jin Peng. 2025. "Progress in Surface Engineering Techniques for Magnesium–Lithium-Based Alloys" Coatings 15, no. 5: 502. https://doi.org/10.3390/coatings15050502
APA StyleLi, N., Hao, Z., Feng, Y., Shang, Y., Tong, Y., Lu, F., Xu, L., Chen, X., Li, S., & Peng, J. (2025). Progress in Surface Engineering Techniques for Magnesium–Lithium-Based Alloys. Coatings, 15(5), 502. https://doi.org/10.3390/coatings15050502






