Application of AC-DC-AC Accelerated Aging to Assess the Galvanic Corrosion Risk of Mild Steel Coated with Graphene-Embedded Epoxy Coatings
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials and Methods
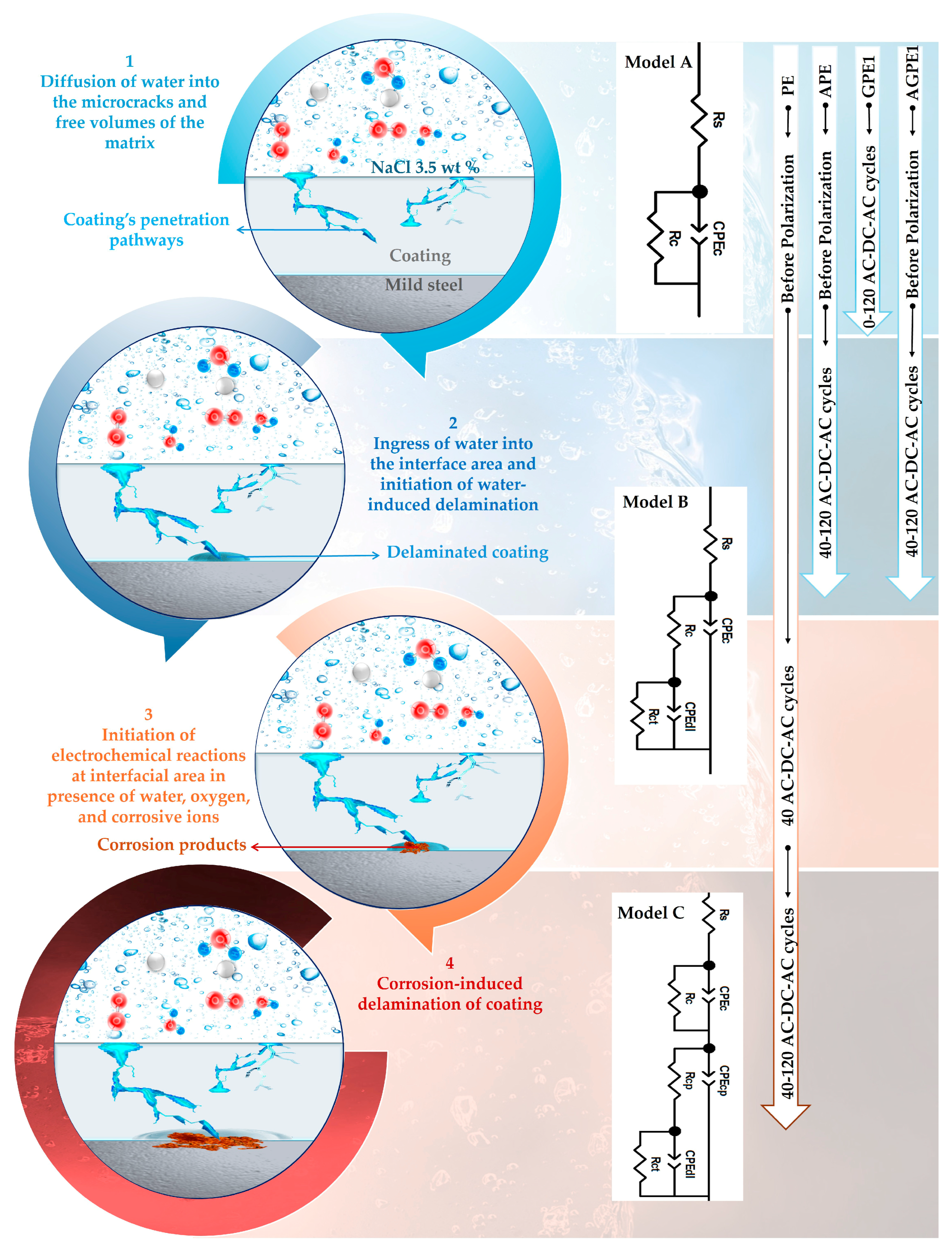
2.2. Corrosion-Induced Delamination
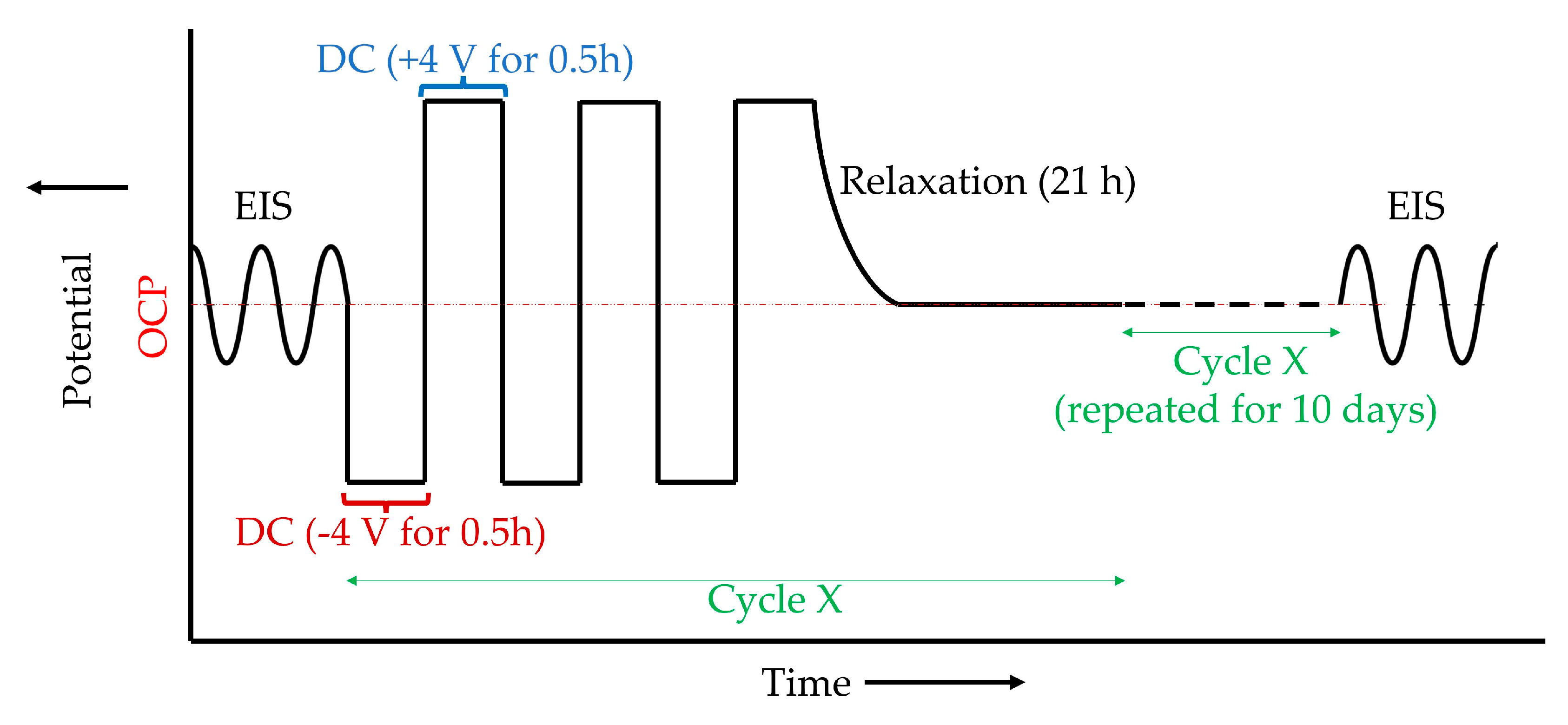
2.3. AC-DC-AC Aging Test
2.4. Cathodic Disbondment Test
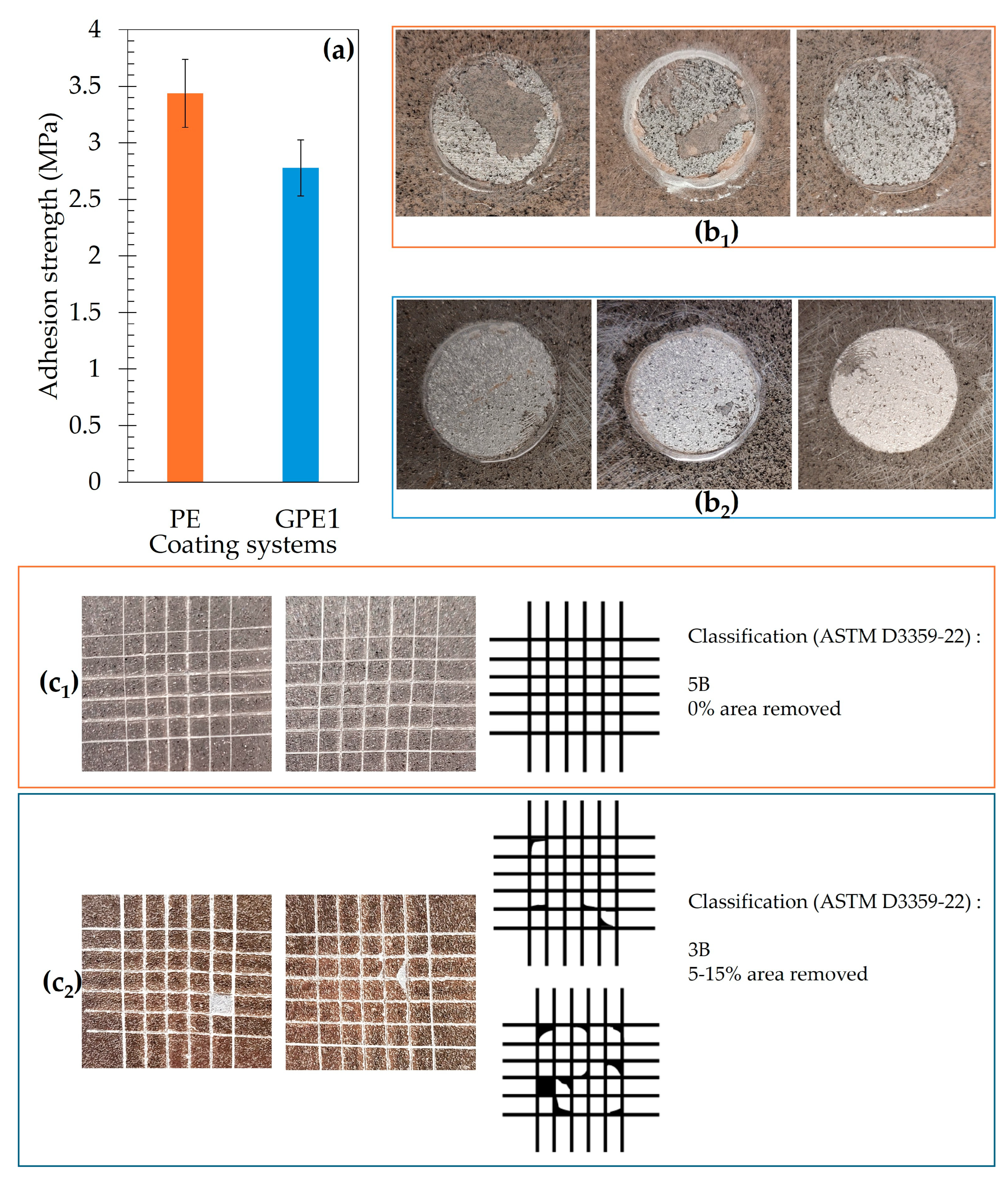
2.5. Adhesion Strength Evaluation
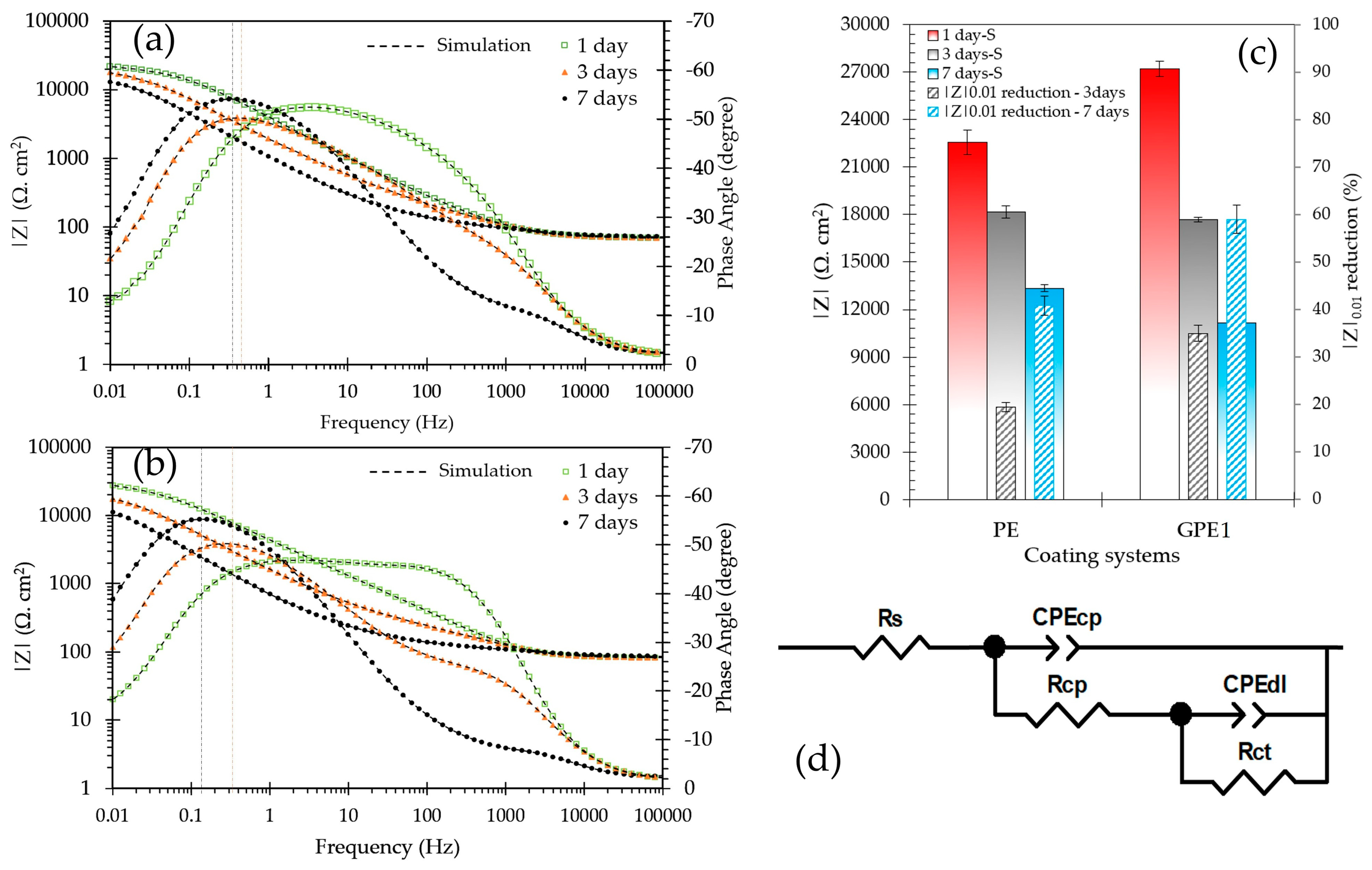
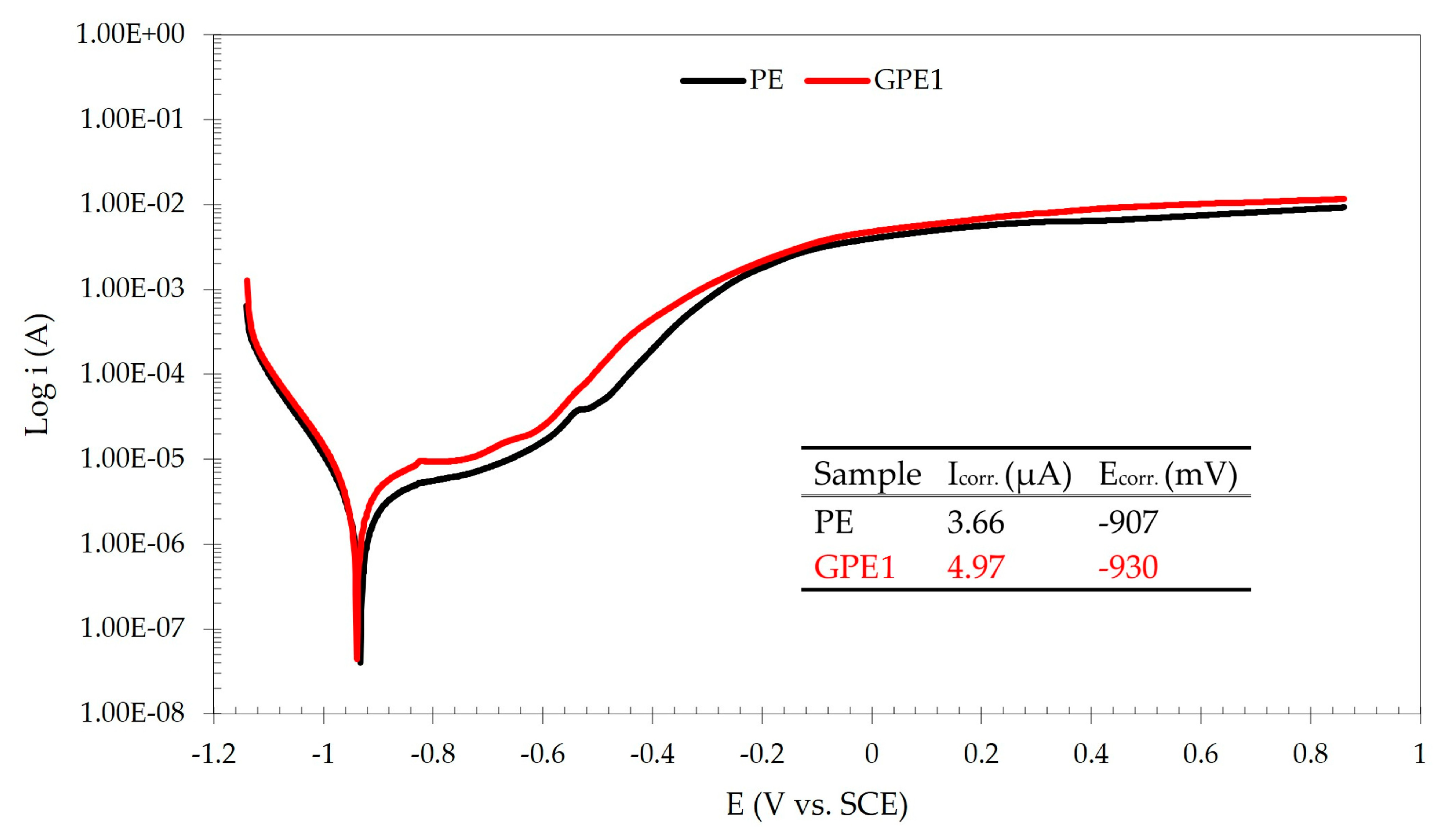
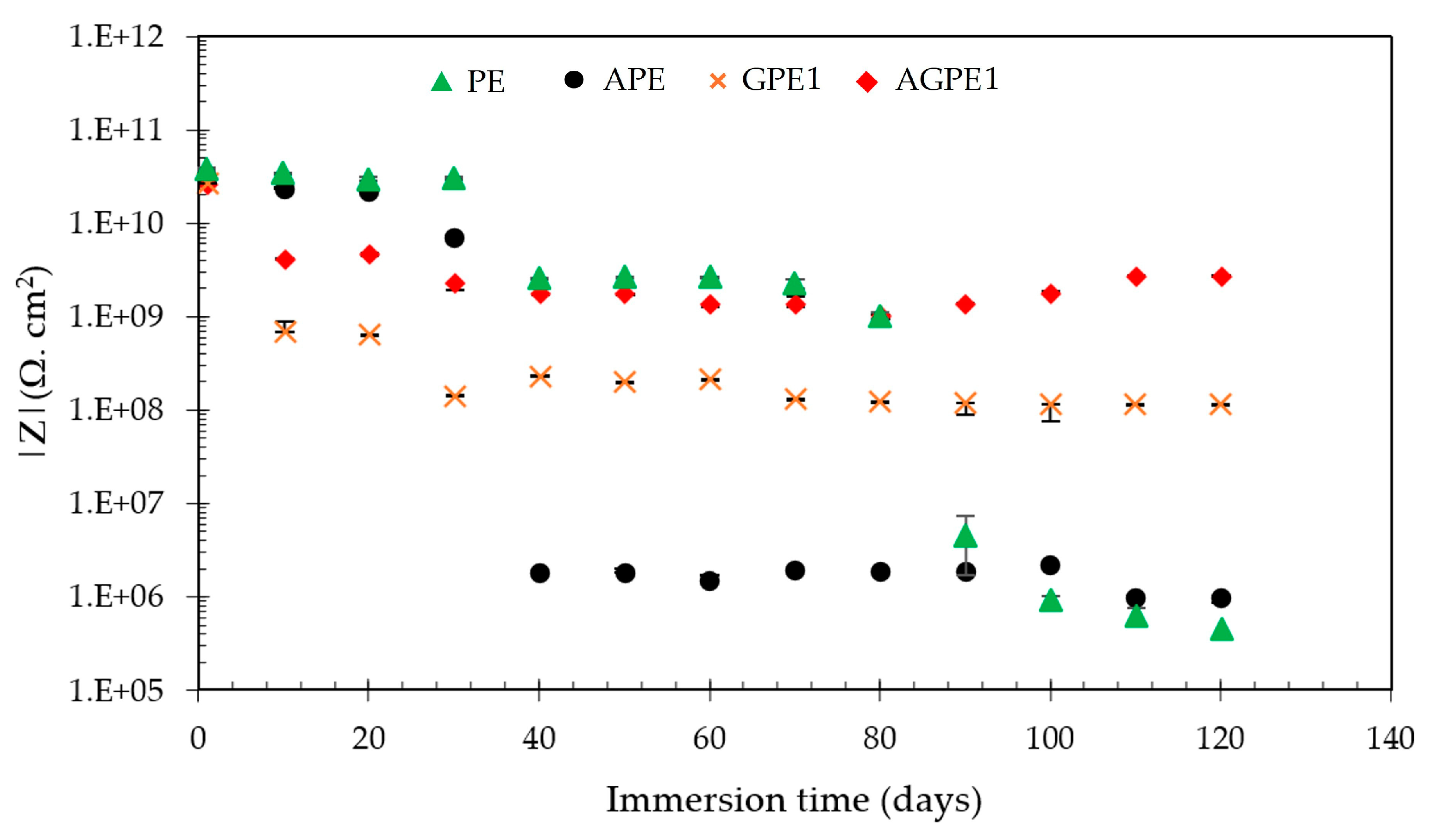
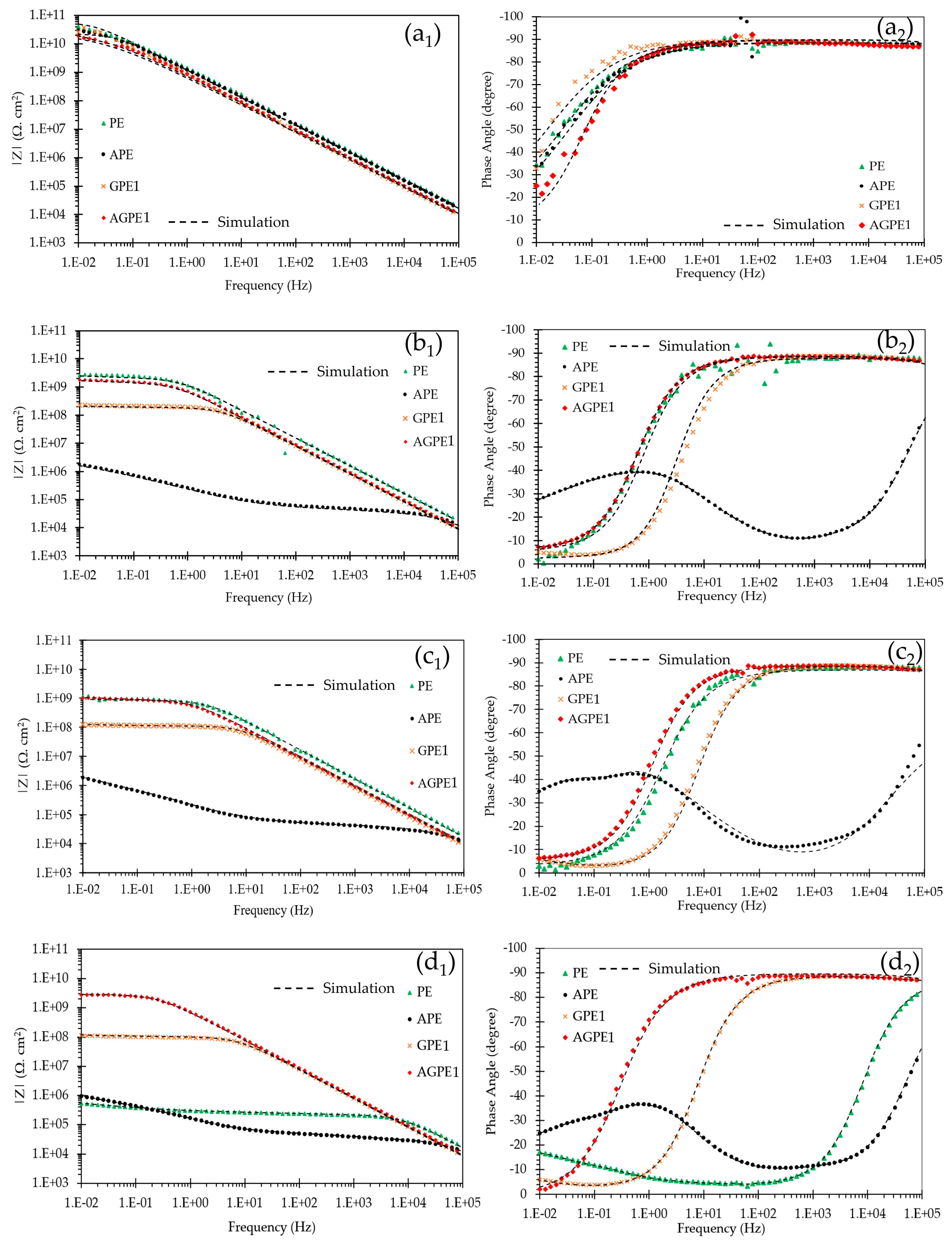
3. Results and Discussion
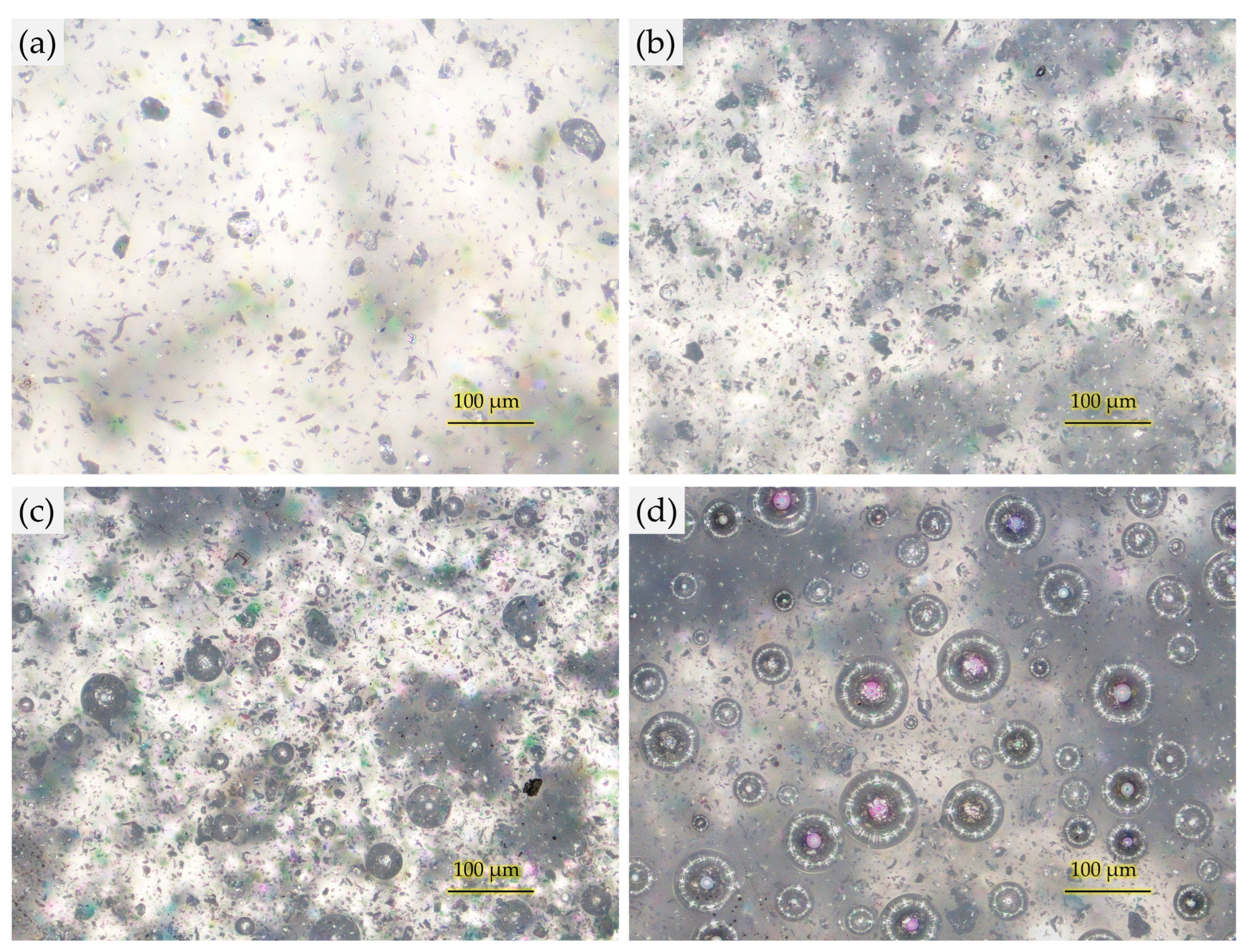
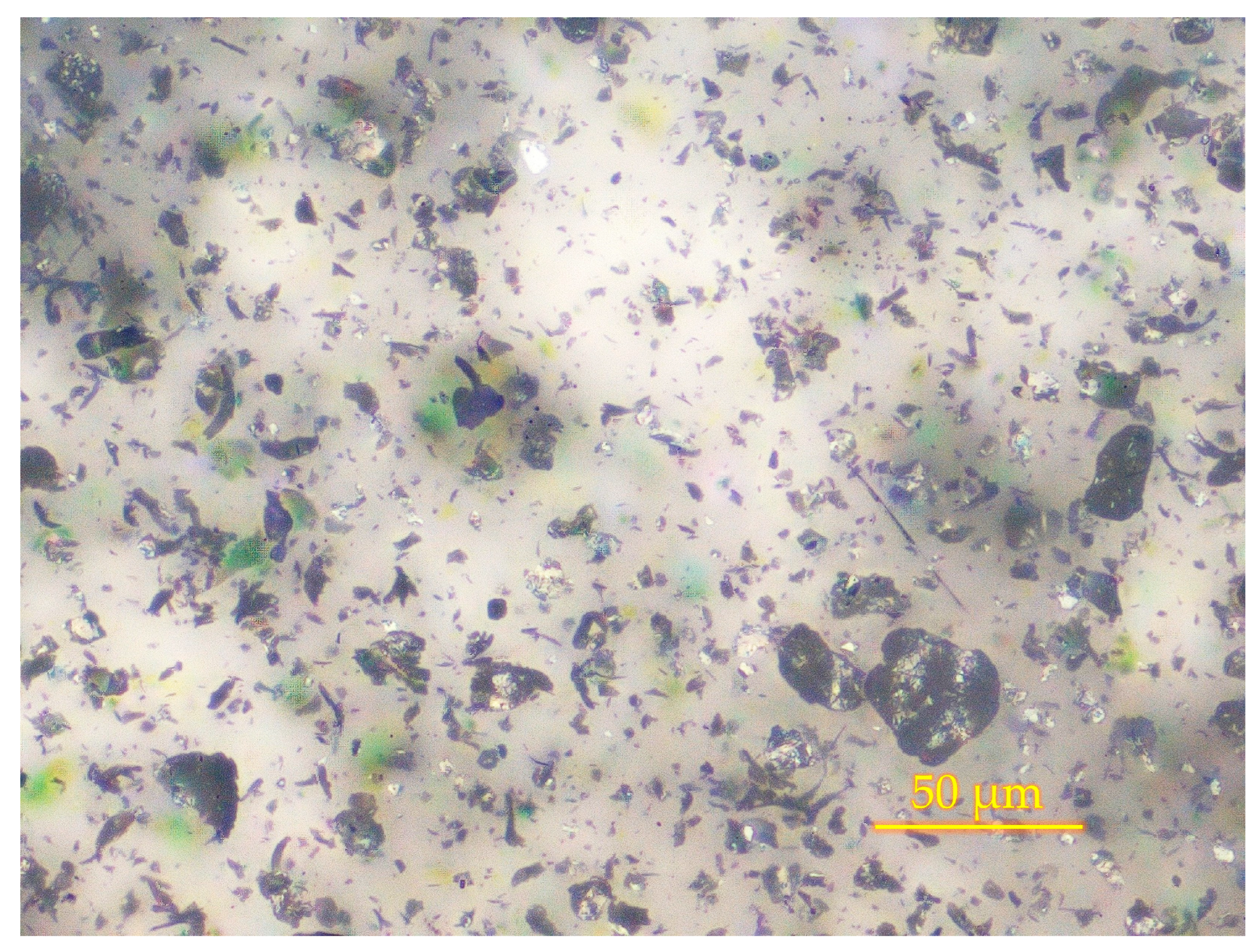
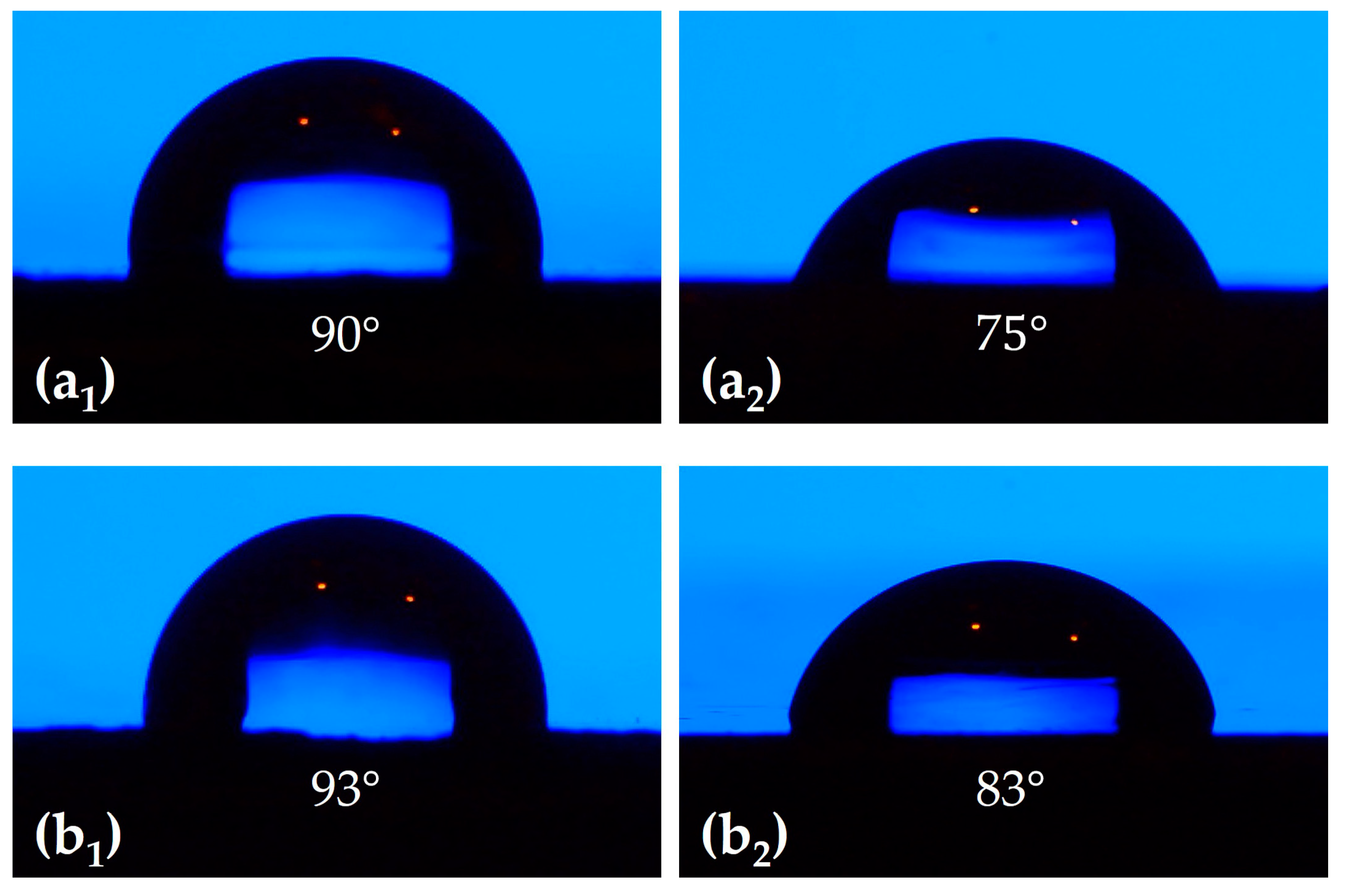
3.1. Optimization of Graphene Platelets Concentration for Coating Formulation
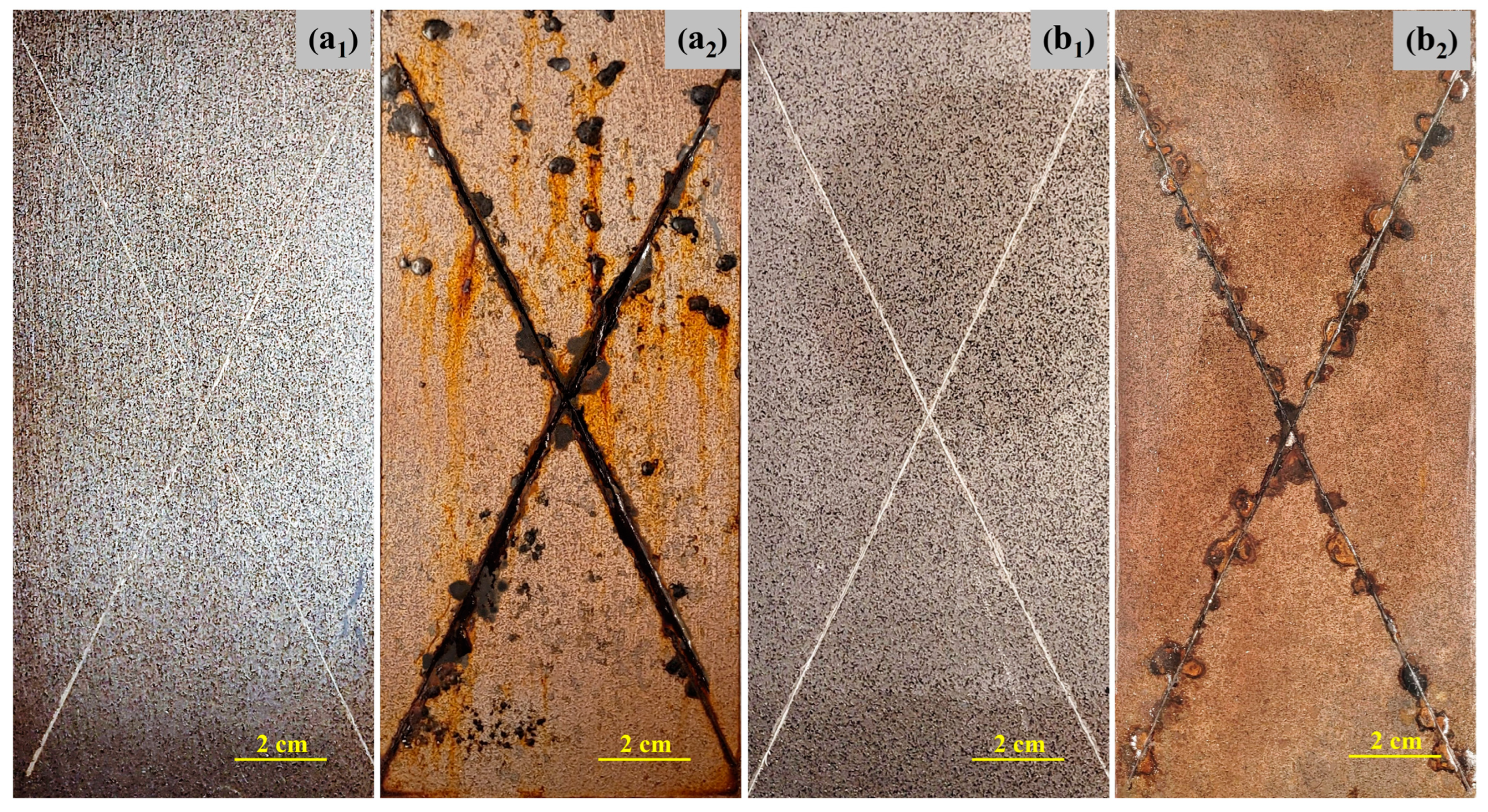
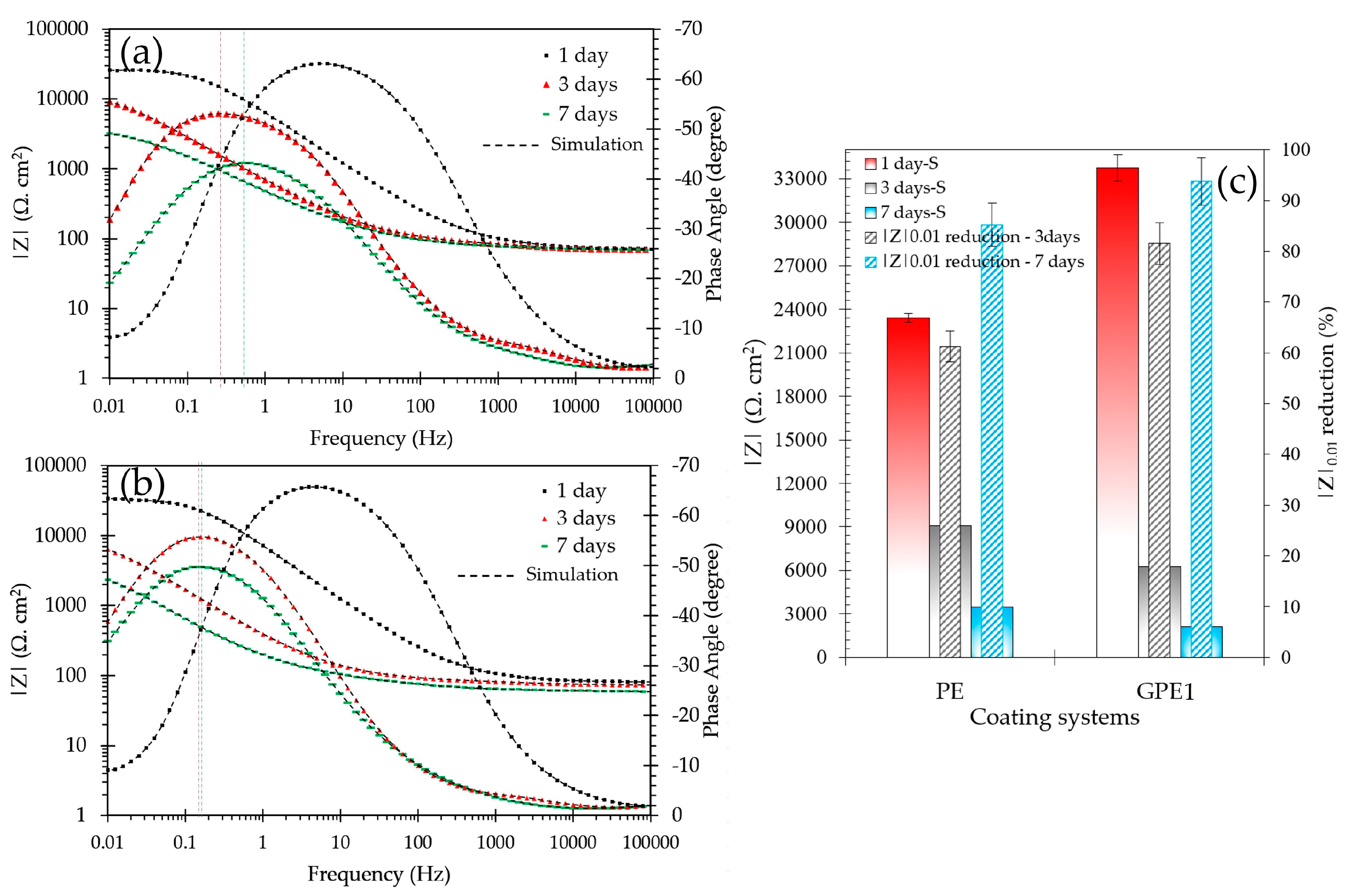
3.2. Corrosion-Induced Delamination of the Coatings
3.3. Accelerated Aging of Coatings by AC/DC/AC Technique
3.4. Cathodic Disbondment
3.5. Interfacial Adhesion Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, X.; Song, S.; Shi, Y.; Cai, M.; Huang, Y.; Zhang, B.; Zhu, M. Mechanochemical Stable Superhydrophobic Coating toward Lasting Corrosion Protection. Prog. Org. Coat. 2023, 178, 107478. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Wu, J.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Yuan, Y.; Xie, Z.; Pan, F. “Smart” Micro/Nano Container-Based Self-Healing Coatings on Magnesium Alloys: A Review. J. Magnes. Alloys 2023, 11, 2230–2259. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Pan, F. Self-Healing Coatings. In Advances in Corrosion Control of Magnesium and Its Alloys; CRC Press: Boca Raton, FL, USA, 2024; pp. 375–398. [Google Scholar]
- Sabet-Bokati, K.; Plucknett, K. Water-Induced Failure in Polymer Coatings: Mechanisms, Impacts and Mitigation Strategies—A Comprehensive Review. Polym. Degrad. Stab. 2024, 230, 111058. [Google Scholar] [CrossRef]
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Yan, M.; Lan, Y.X.; Yeh, J.-M. Application of Graphene and Its Derivatives in Anticorrosion Coatings: Research Advances and Future Perspectives. J. Taiwan. Inst. Chem. Eng. 2024, 154, 105130. [Google Scholar] [CrossRef]
- Sabet-Bokati, Z.; Sabet-Bokati, K.; Russell, Z.; Morshed-Behbahani, K.; Ouanani, S. Anticorrosion Shape Memory-Assisted Self-Healing Coatings: A Review. Prog. Org. Coat. 2024, 188, 108193. [Google Scholar] [CrossRef]
- Udoh, I.I.; Ekerenam, O.O.; Daniel, E.F.; Ikeuba, A.I.; Njoku, D.I.; Kolawole, S.K.; Etim, I.-I.N.; Emori, W.; Njoku, C.N.; Etim, I.P. Developments in Anticorrosive Organic Coatings Modulated by Nano/Microcontainers with Porous Matrices. Adv. Colloid Interface Sci. 2024, 330, 103209. [Google Scholar] [CrossRef]
- Mohammadi, I.; Shahrabi, T.; Mahdavian, M.; Izadi, M. Construction of an Epoxy Coating with Excellent Protection Performance on the AA 2024-T3 Using Ion-Exchange Materials Loaded with Eco-Friendly Corrosion Inhibitors. Prog. Org. Coat. 2022, 166, 106786. [Google Scholar] [CrossRef]
- Kopsidas, S.; Olowojoba, G.B.; Kinloch, A.J.; Taylor, A.C. Examining the Effect of Graphene Nanoplatelets on the Corrosion Resistance of Epoxy Coatings. Int. J. Adhes. Adhes. 2021, 104, 102723. [Google Scholar] [CrossRef]
- Sun, W.; Wu, T.; Wang, L.; Yang, Z.; Zhu, T.; Dong, C.; Liu, G. The Role of Graphene Loading on the Corrosion-Promotion Activity of Graphene/Epoxy Nanocomposite Coatings. Compos. B Eng. 2019, 173, 106916. [Google Scholar] [CrossRef]
- Sun, W.; Yang, Y.; Yang, Z.; Wang, L.; Wang, J.; Xu, D.; Liu, G. Review on the Corrosion-Promotion Activity of Graphene and Its Inhibition. J. Mater. Sci. Technol. 2021, 91, 278–306. [Google Scholar] [CrossRef]
- Ren, S.; Cui, M.; Liu, C.; Wang, L. A Comprehensive Review on Ultrathin, Multi-Functionalized, and Smart Graphene and Graphene-Based Composite Protective Coatings. Corros. Sci. 2023, 212, 110939. [Google Scholar] [CrossRef]
- Glover, C.F.; Richards, C.; Baker, J.; Williams, G.; McMurray, H.N. In-Coating Graphene Nano-Platelets for Environmentally-Friendly Corrosion Protection of Iron. Corros. Sci. 2017, 114, 169–172. [Google Scholar] [CrossRef]
- Wang, X.; Tang, F.; Qi, X.; Lin, Z. Mechanical, Electrochemical, and Durability Behavior of Graphene Nano-Platelet Loaded Epoxy-Resin Composite Coatings. Compos. B Eng. 2019, 176, 107103. [Google Scholar] [CrossRef]
- Um, J.G.; Jun, Y.-S.; Alhumade, H.; Krithivasan, H.; Lui, G.; Yu, A. Investigation of the Size Effect of Graphene Nano-Platelets (GnPs) on the Anti-Corrosion Performance of Polyurethane/GnP Composites. RSC Adv. 2018, 8, 17091–17100. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, H.; Mao, F.; Li, J. Electrochemical Investigation of Graphene on the Corrosion of Scratched Polyurea Based Organic Coating. Mater. Res. Express 2019, 6, 125619. [Google Scholar] [CrossRef]
- Pierleoni, D.; Xia, Z.Y.; Christian, M.; Ligi, S.; Minelli, M.; Morandi, V.; Doghieri, F.; Palermo, V. Graphene-Based Coatings on Polymer Films for Gas Barrier Applications. Carbon 2016, 96, 503–512. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A Review of the Water Barrier Properties of Polymer/Clay and Polymer/Graphene Nanocomposites. J. Memb. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef]
- Abakah, R.R.; Huang, F.; Hu, Q.; Wang, Y.; Liu, J. Comparative Study of Corrosion Properties of Different Graphene Nanoplate/Epoxy Composite Coatings for Enhanced Surface Barrier Protection. Coatings 2021, 11, 285. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A Comprehensive Review on Graphene-Based Anti-Corrosive Coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y. CeO2 Grafted Polydopamine-Wrapped Graphene to Enhance Corrosion Resistance of Coated Steel. Prog. Org. Coat. 2022, 164, 106698. [Google Scholar] [CrossRef]
- Glover, C.F.; Richards, C.A.J.; Williams, G.; McMurray, H.N. Evaluation of Multi-Layered Graphene Nano-Platelet Composite Coatings for Corrosion Control Part II–Cathodic Delamination Kinetics. Corros. Sci. 2018, 136, 304–310. [Google Scholar] [CrossRef]
- Mahdavi, F.; Tan, M.Y.J.; Forsyth, M. Electrochemical Impedance Spectroscopy as a Tool to Measure Cathodic Disbondment on Coated Steel Surfaces: Capabilities and Limitations. Prog. Org. Coat. 2015, 88, 23–31. [Google Scholar] [CrossRef]
- Schmidt, R.G.; Bell, J.P. Epoxy Adhesion to Metals. In Advances in Polymer Science; Epoxy Resins and Composites II; Springer: Berlin/Heidelberg, Germany, 2005; pp. 33–71. [Google Scholar]
- Garcia, S.J.; Suay, J. A Comparative Study between the Results of Different Electrochemical Techniques (EIS and AC/DC/AC): Application to the Optimisation of the Cataphoretic and Curing Parameters of a Primer for the Automotive Industry. Prog. Org. Coat. 2007, 59, 251–258. [Google Scholar] [CrossRef]
- Zheng, D.; Gui, Q.; Xu, Y.; Song, G.-L. Modified AC-DC-AC Method for Evaluation of Corrosion Damage of Acrylic Varnish Paint Coating/Q215 Steel System. Prog. Org. Coat. 2021, 159, 106401. [Google Scholar] [CrossRef]
- Da Silva Lopes, T.; Lopes, T.; Martins, D.; Carneiro, C.; Machado, J.; Mendes, A. Accelerated Aging of Anticorrosive Coatings: Two-Stage Approach to the AC/DC/AC Electrochemical Method. Prog. Org. Coat. 2020, 138, 105365. [Google Scholar] [CrossRef]
- Berry, V. Impermeability of Graphene and Its Applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Chang, K.-C.; Hsu, M.-H.; Lu, H.-I.; Lai, M.-C.; Liu, P.-J.; Hsu, C.-H.; Ji, W.-F.; Chuang, T.-L.; Wei, Y.; Yeh, J.-M. Room-Temperature Cured Hydrophobic Epoxy/Graphene Composites as Corrosion Inhibitor for Cold-Rolled Steel. Carbon 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Schiller, T.; Medhekar, N.; Birbilis, N. Exploring Graphene as a Corrosion Protection Barrier. Corros. Sci. 2012, 56, 1–4. [Google Scholar] [CrossRef]
- Watson, T.M.; Coleman, A.J.; Williams, G.; McMurray, H.N. The Effect of Oxygen Partial Pressure on the Filiform Corrosion of Organic Coated Iron. Corros. Sci. 2014, 89, 46–58. [Google Scholar] [CrossRef]
- Cristoforetti, A.; Rossi, S.; Deflorian, F.; Fedel, M. An Electrochemical Study on the Mechanism of Filiform Corrosion on Acrylic-Coated Carbon Steel. Prog. Org. Coat. 2023, 179, 107525. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Wang, Y.; Guo, X.; Wu, S.; Fu, D.; Zhang, D. Enhanced Active Corrosion Protection Coatings for Aluminum Alloys with Two Corrosion Inhibitors Co-Incorporated in Nanocontainers. Corros. Sci. 2022, 208, 110663. [Google Scholar] [CrossRef]
- Meeusen, M.; Zardet, L.; Homborg, A.M.; Lekka, M.; Andreatta, F.; Fedrizzi, L.; Boelen, B.; Mol, J.M.C.; Terryn, H. The Effect of Time Evolution and Timing of the Electrochemical Data Recording of Corrosion Inhibitor Protection of Hot-Dip Galvanized Steel. Corros. Sci. 2020, 173, 108780. [Google Scholar] [CrossRef]
- Xu, Y.; Song, G.-L.; Zheng, D.; Feng, Z. The Corrosion Damage of an Organic Coating Accelerated by Different AC-DC-AC Tests. Eng. Fail. Anal. 2021, 126, 105461. [Google Scholar] [CrossRef]
- Shirangi, M.H.; Michel, B. Mechanism of Moisture Diffusion, Hygroscopic Swelling, and Adhesion Degradation in Epoxy Molding Compounds. In Moisture Sensitivity of Plastic Packages of IC Devices; Springer: Boston, MA, USA, 2010; pp. 29–69. [Google Scholar]
- Grujicic, M.; Sellappan, V.; Omar, M.A.; Seyr, N.; Obieglo, A.; Erdmann, M.; Holzleitner, J. An Overview of the Polymer-to-Metal Direct-Adhesion Hybrid Technologies for Load-Bearing Automotive Components. J. Mater. Process Technol. 2008, 197, 363–373. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of Polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, H.; Wang, L.; Li, X. Comparison of Barrier Properties for a Superhydrophobic Epoxy Coating under Different Simulated Corrosion Environments. Corros. Sci. 2016, 103, 230–241. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Water Uptake in Protective Organic Coatings and Its Reflection in Measured Coating Impedance. Prog. Org. Coat. 2018, 124, 296–302. [Google Scholar] [CrossRef]
- Eayal Awwad, K.Y.; Yousif, B.F.; Fallahnezhad, K.; Saleh, K.; Zeng, X. Influence of Graphene Nanoplatelets on Mechanical Properties and Adhesive Wear Performance of Epoxy-Based Composites. Friction 2021, 9, 856–875. [Google Scholar] [CrossRef]
- Yuan, X.; Yue, Z.F.; Chen, X.; Wen, S.F.; Li, L.; Feng, T. EIS Study of Effective Capacitance and Water Uptake Behaviors of Silicone-Epoxy Hybrid Coatings on Mild Steel. Prog. Org. Coat. 2015, 86, 41–48. [Google Scholar] [CrossRef]
- Mallarino, S.; Renaud, A.; Trinh, D.; Touzain, S. The Role of Internal Stresses, Temperature, and Water on the Swelling of Pigmented Epoxy Systems during Hygrothermal Aging. J. Appl. Polym. Sci. 2022, 139, e53162. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Lv, Y.; Zuo, Y. Study on Corrosion Electrochemical Behavior of Several Different Coating Systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S.; Deflorian, F.; Dirè, S.; Ceccato, R.; Guo, X.; Frankel, G.S. Effects of Graphene-Based Fillers on Cathodic Delamination and Abrasion Resistance of Cataphoretic Organic Coatings. Coatings 2020, 10, 602. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, H.; Zhong, W.H. Wetting Characteristics of Epoxy Resins Modified by Graphitic Nanofibers with Different Functional Groups. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 196–202. [Google Scholar] [CrossRef]

| Sample | Resin + Pigment | Curing Agent | |
|---|---|---|---|
| Epoxy (g) | Graphene Platelets (g) | (g) | |
| PE | 100 | 0 | 74.5 |
| GPE0.5 | 100 | 0.5 | 74.5 |
| GPE1 | 100 | 1 | 74.5 |
| GPE2 | 100 | 2 | 74.5 |
| GPE4 | 100 | 4 | 74.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabet-Bokati, K.; Plucknett, K.P. Application of AC-DC-AC Accelerated Aging to Assess the Galvanic Corrosion Risk of Mild Steel Coated with Graphene-Embedded Epoxy Coatings. Coatings 2025, 15, 501. https://doi.org/10.3390/coatings15050501
Sabet-Bokati K, Plucknett KP. Application of AC-DC-AC Accelerated Aging to Assess the Galvanic Corrosion Risk of Mild Steel Coated with Graphene-Embedded Epoxy Coatings. Coatings. 2025; 15(5):501. https://doi.org/10.3390/coatings15050501
Chicago/Turabian StyleSabet-Bokati, Kazem, and Kevin Paul Plucknett. 2025. "Application of AC-DC-AC Accelerated Aging to Assess the Galvanic Corrosion Risk of Mild Steel Coated with Graphene-Embedded Epoxy Coatings" Coatings 15, no. 5: 501. https://doi.org/10.3390/coatings15050501
APA StyleSabet-Bokati, K., & Plucknett, K. P. (2025). Application of AC-DC-AC Accelerated Aging to Assess the Galvanic Corrosion Risk of Mild Steel Coated with Graphene-Embedded Epoxy Coatings. Coatings, 15(5), 501. https://doi.org/10.3390/coatings15050501






