Unmodified Hemp Biowaste as a Sustainable Biosorbent for Congo Red and Remazol Brilliant Blue R
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosorbent Preparation
2.2. Biosorbent Characterization
2.3. Sorption Experiments
- Ci— initial concentration (mg/L)
- C—residual dye concentration in solution (mg/L)
- V—solution volume (L)
- m—biosorbent mass (g)
2.4. Theoretical Analysis of Sorption
3. Results and Discussion
3.1. Elemental Analysis
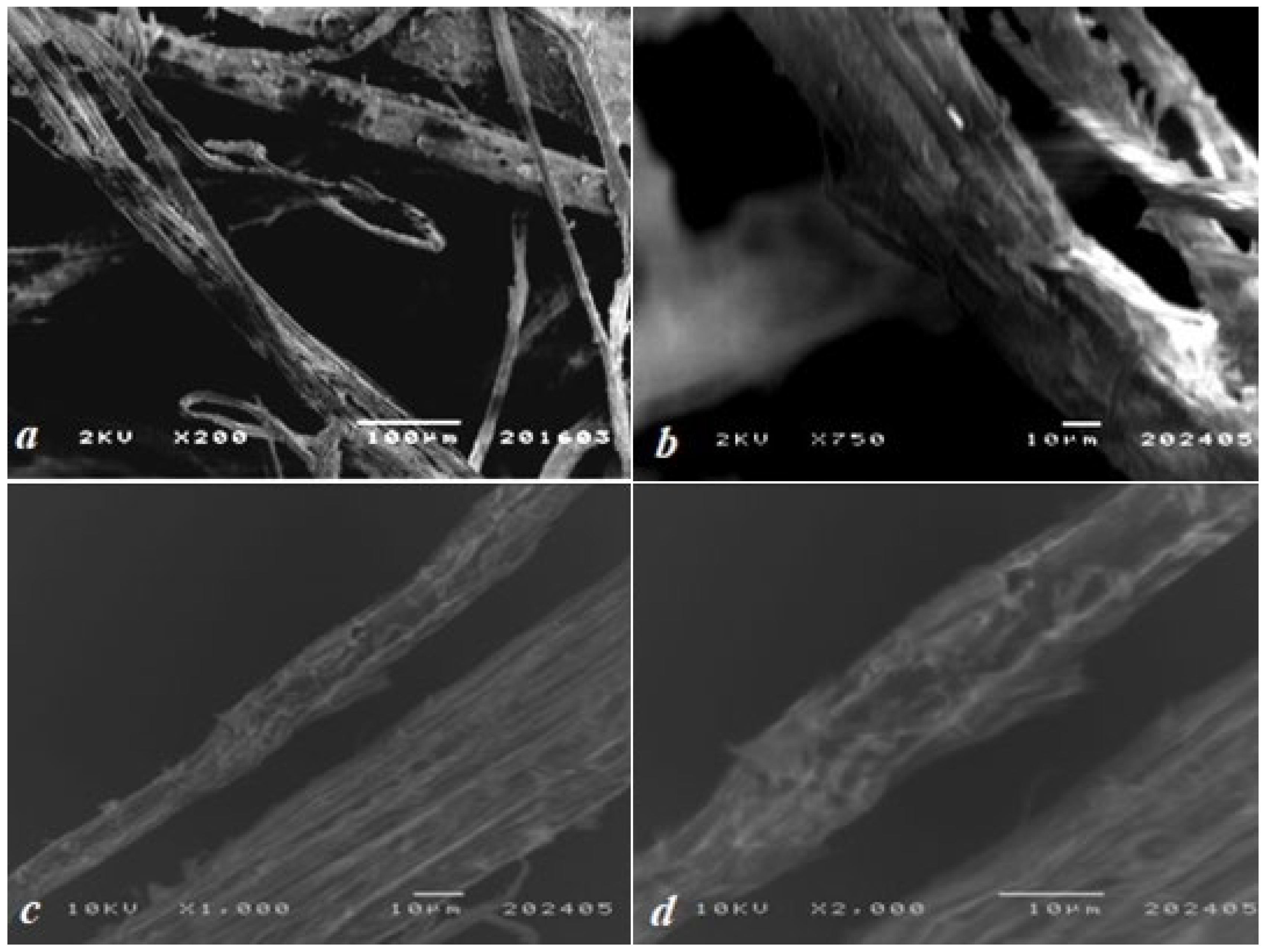
3.2. SEM Analysis
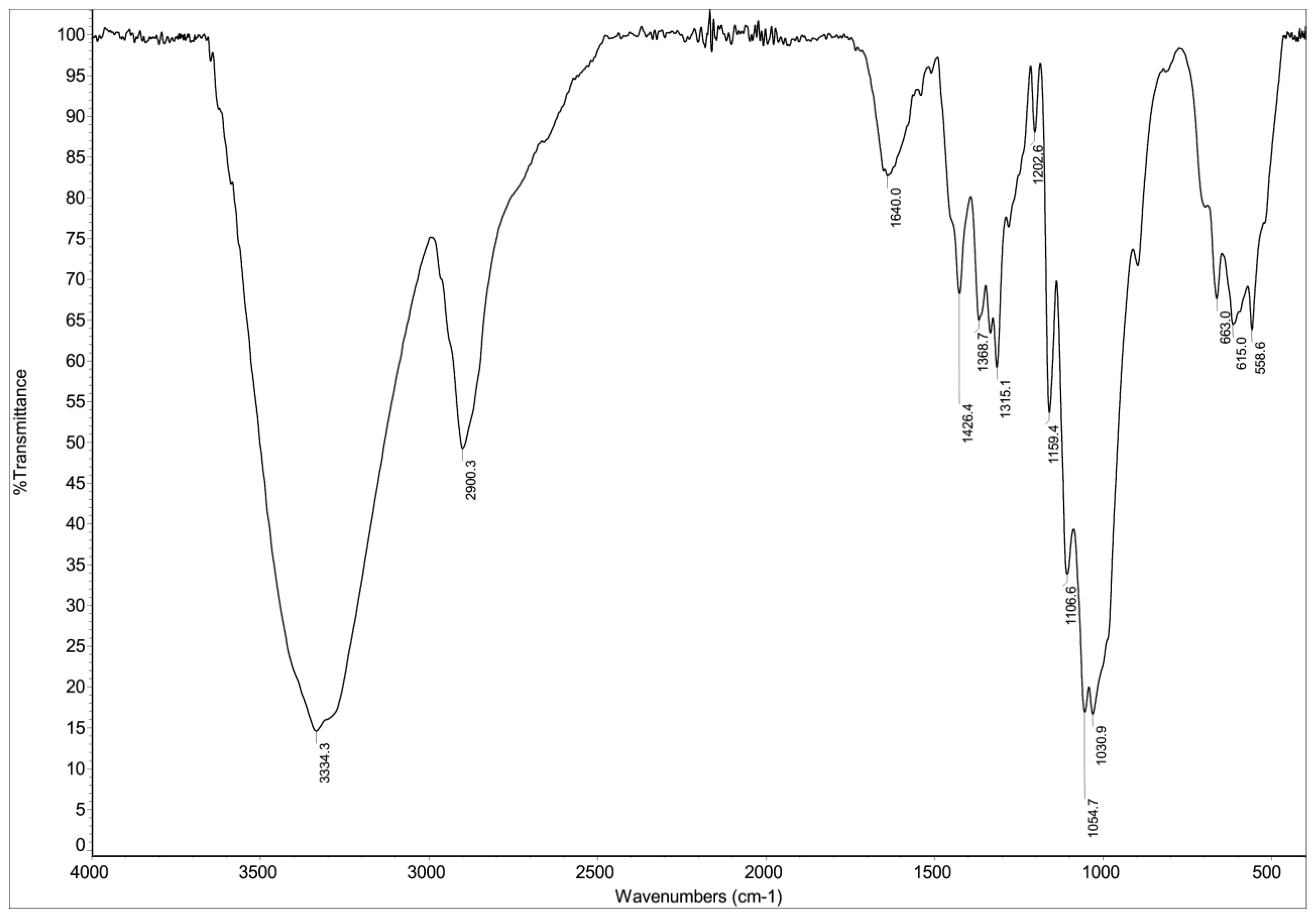
3.3. FTIR Analysis
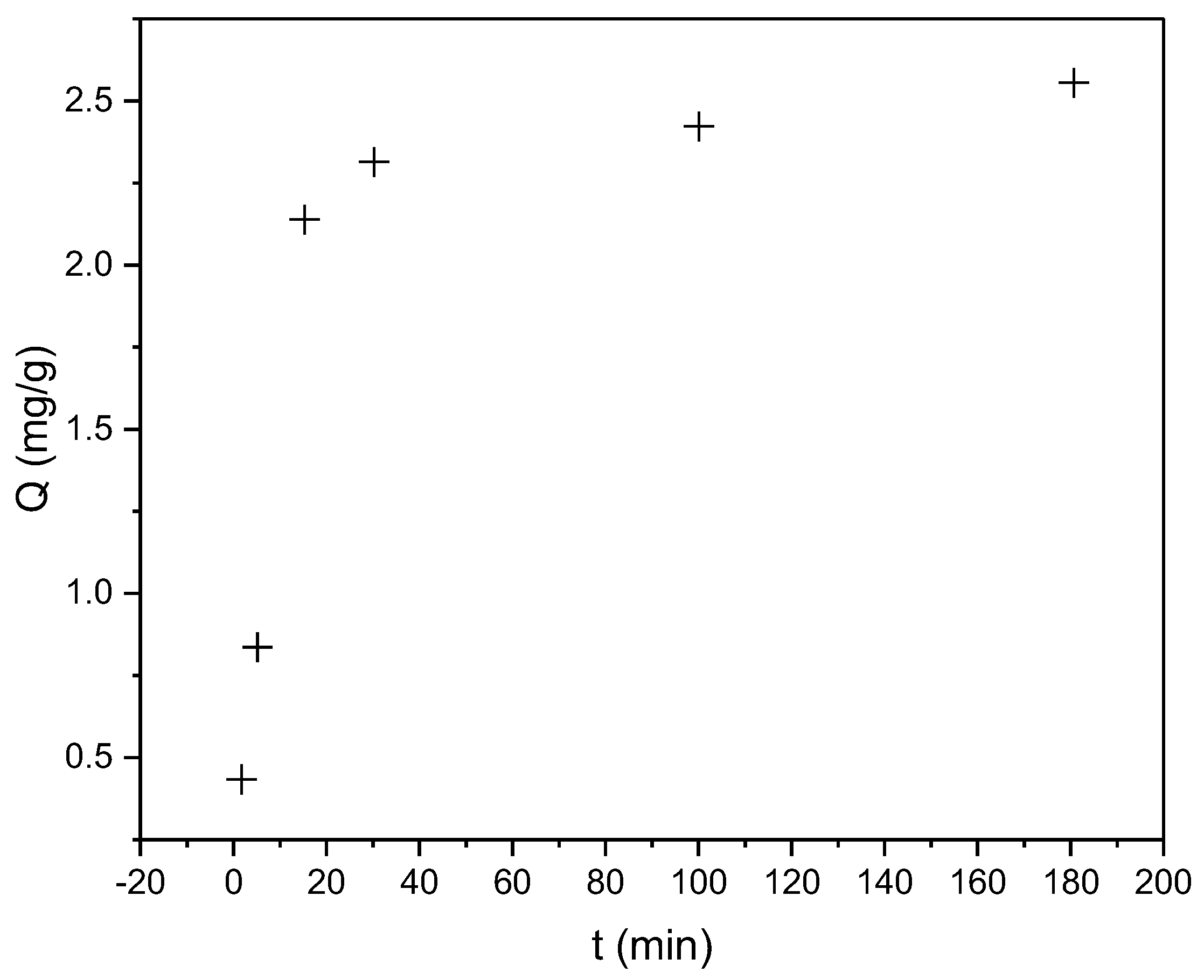
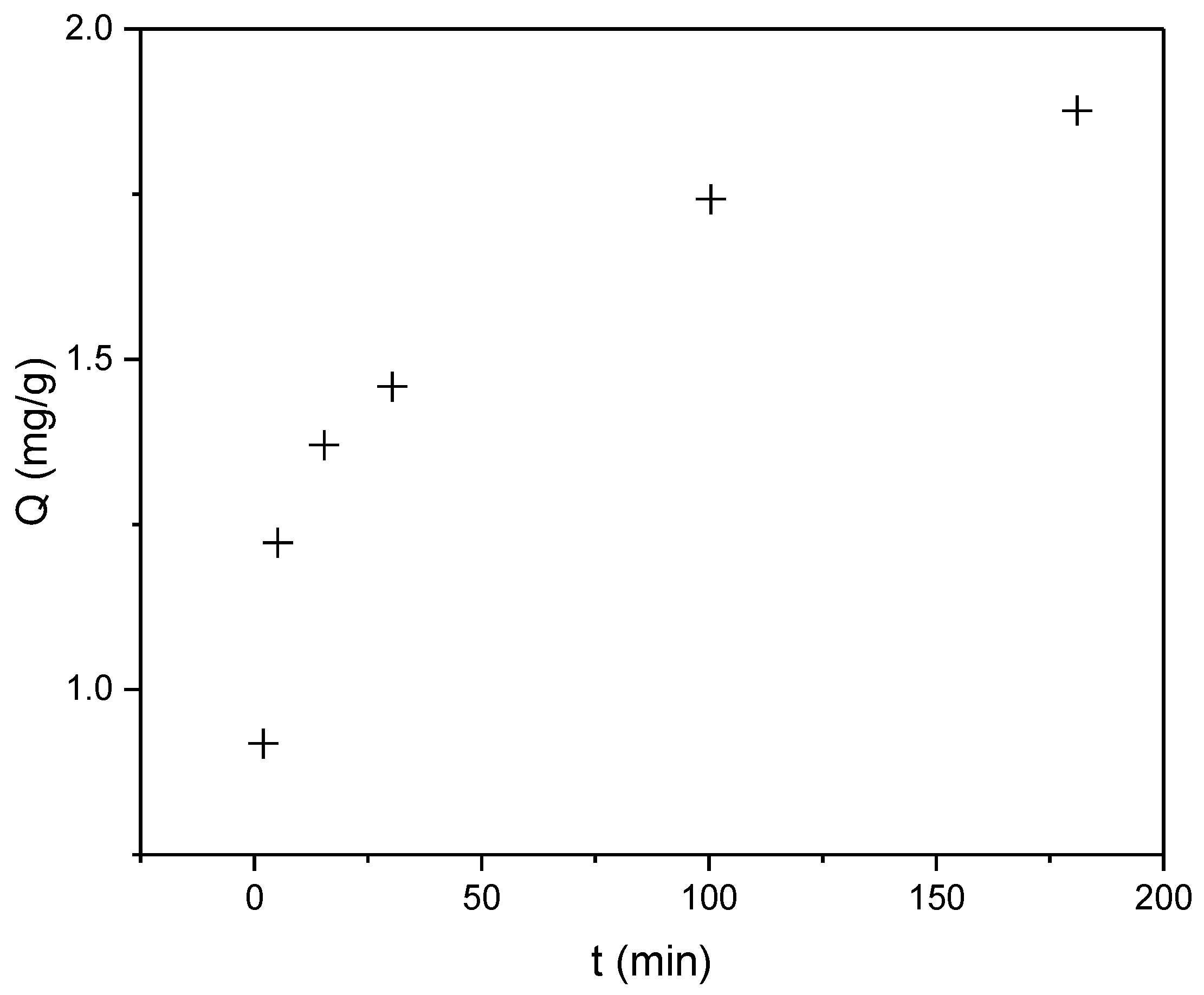
3.4. Sorption Experiments
3.4.1. Kinetic Analysis
3.4.2. Adsorption Isotherm Analysis
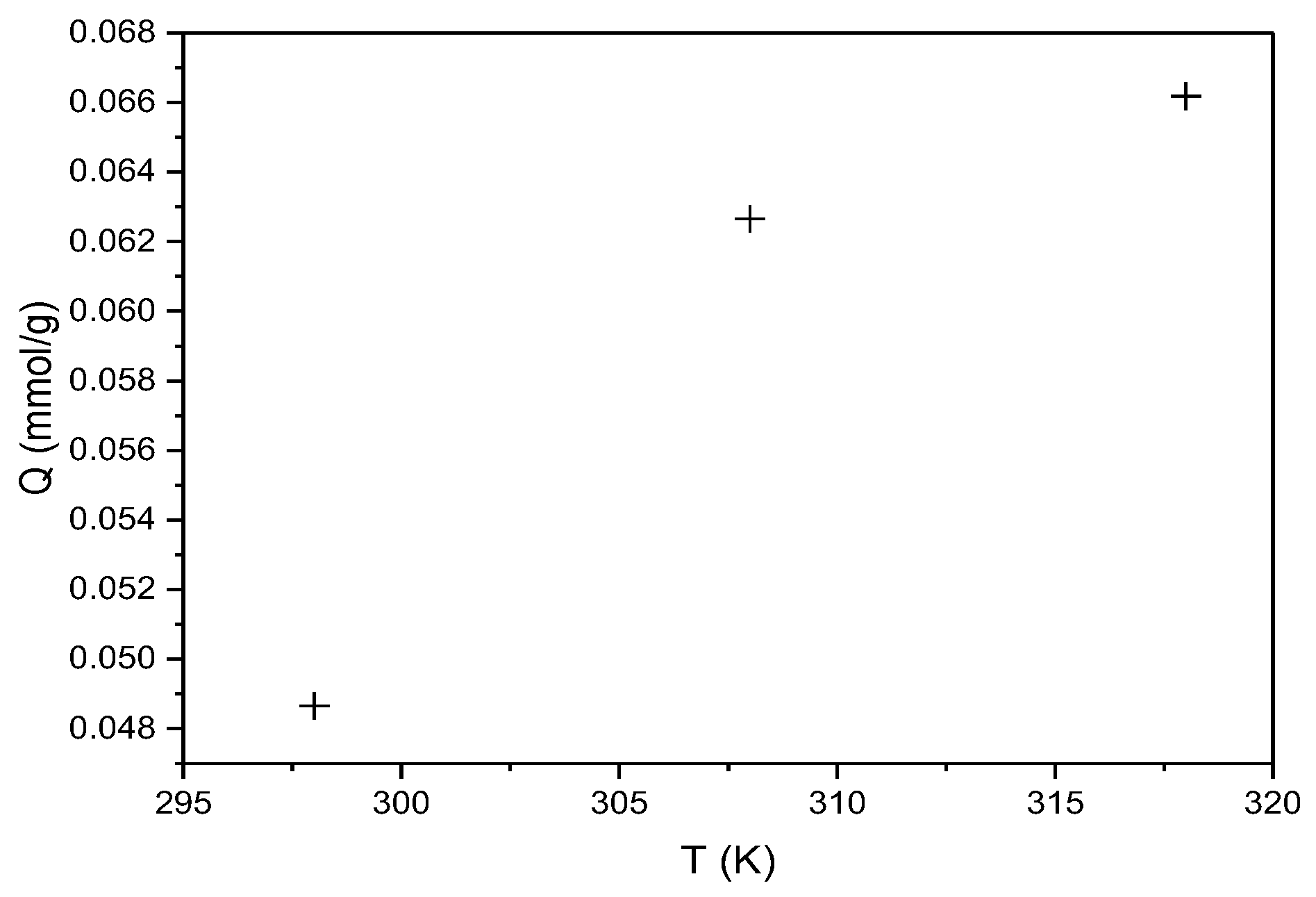
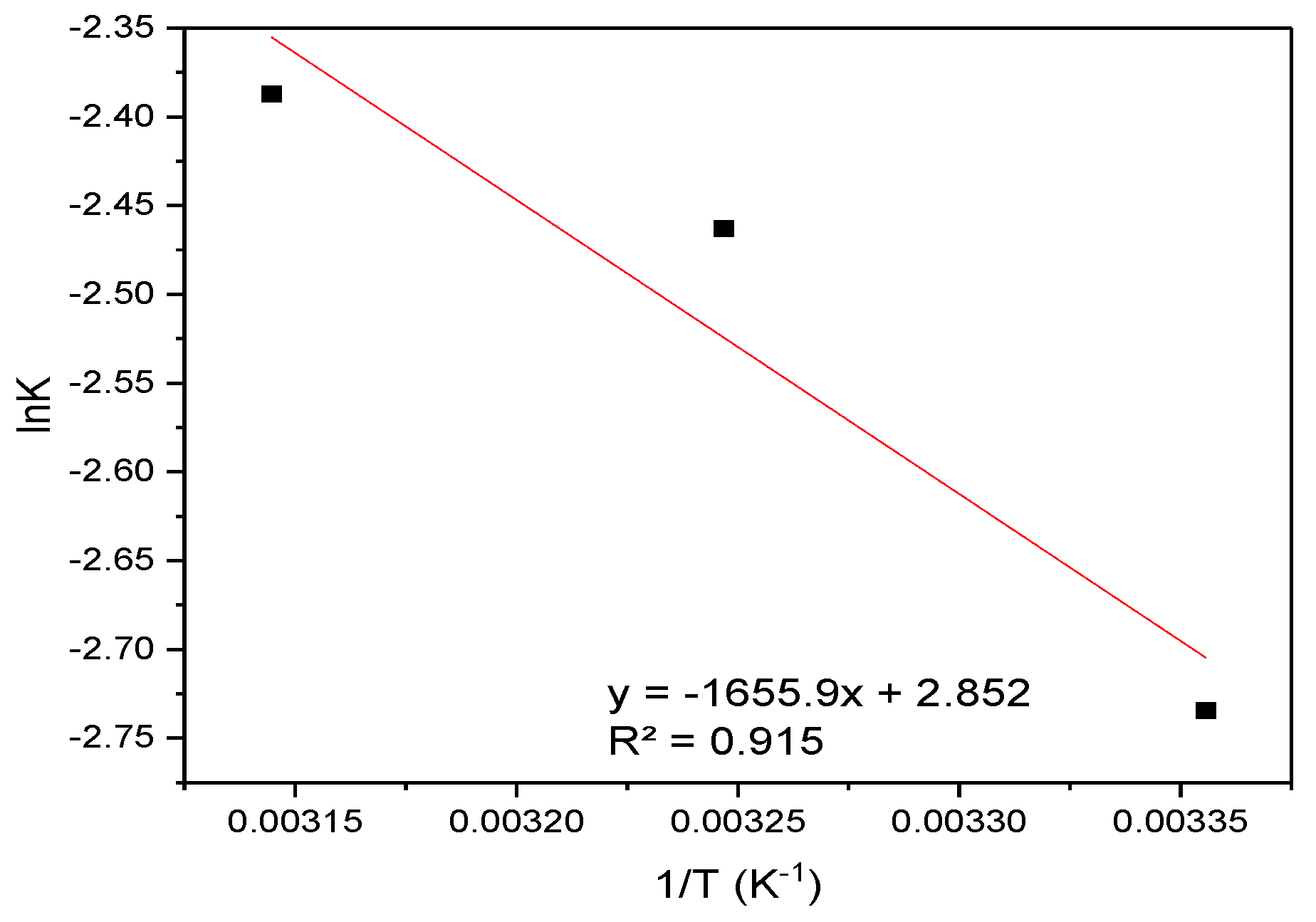
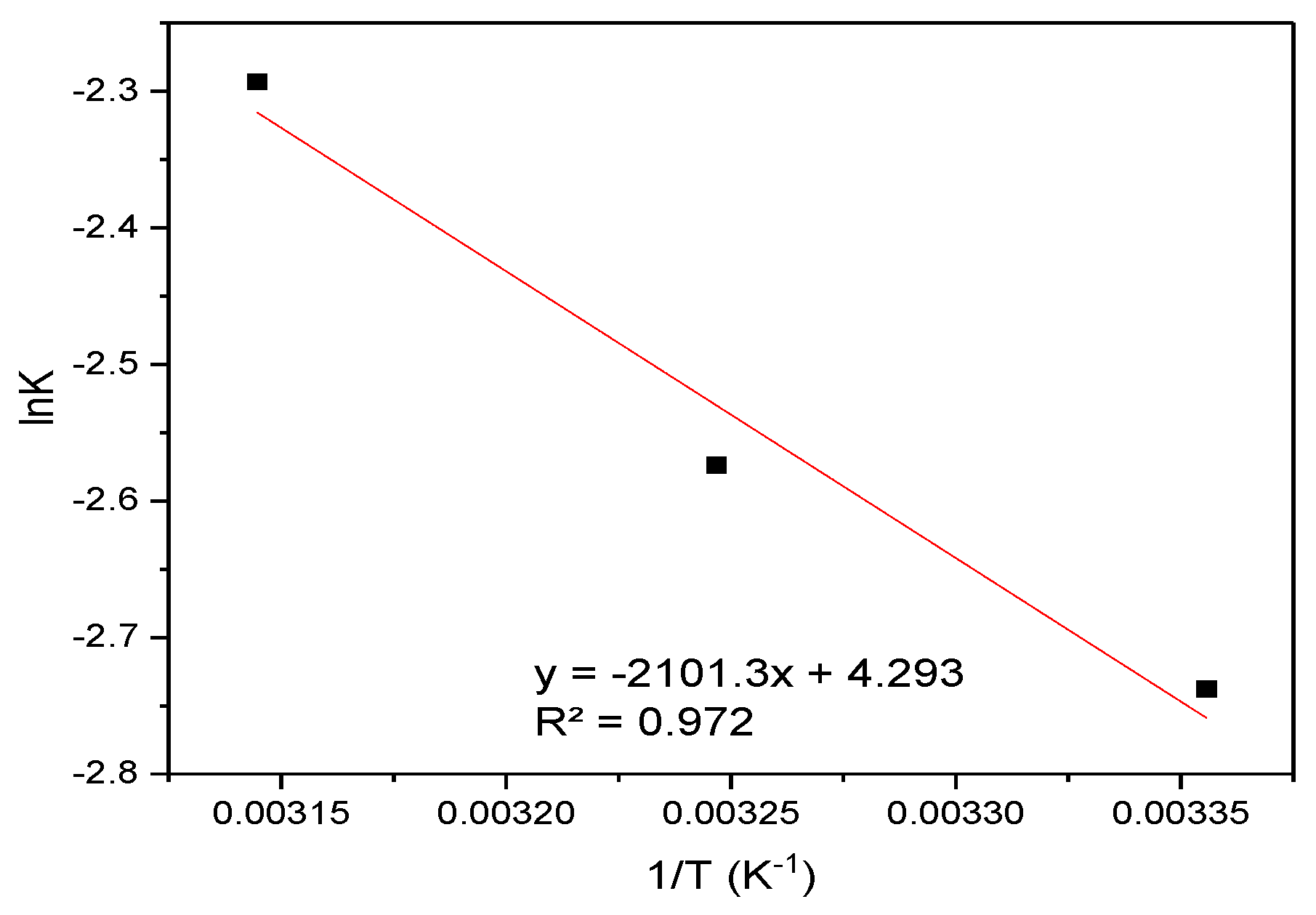
3.4.3. Thermodynamic Analysis
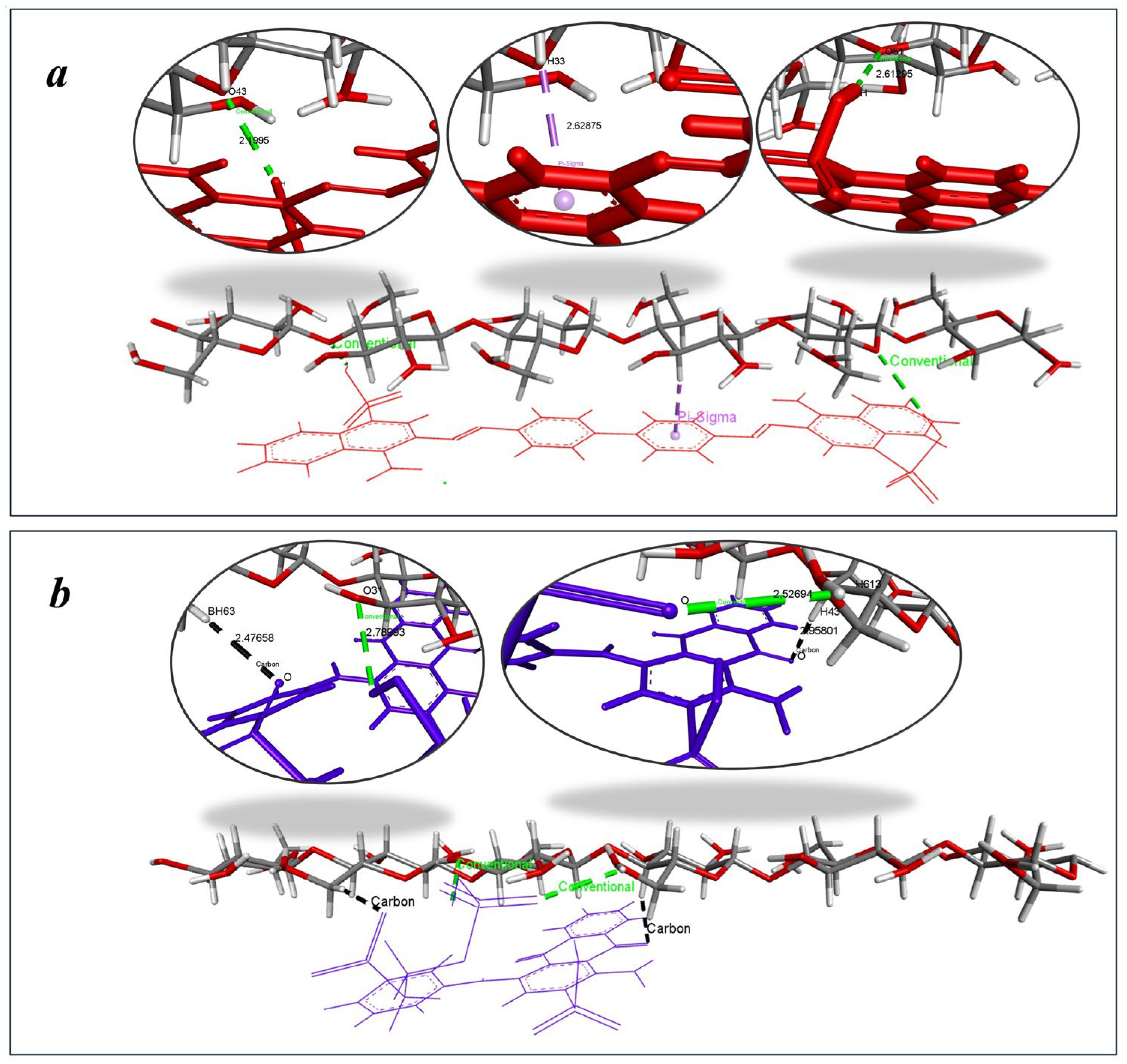
- Molecular modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Chandanshive, V.V.; Kadam, S.; Rane, N.; Jeon, B.-H.; Jadhav, J.; Govindwar, S. In situ textile wastewater treatment in high rate transpiration system furrows planted with aquatic macrophytes and floating phytobeds. Chemosphere 2020, 252, 126513. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Abe, F.R.; Soares, A.M.; de Oliveira, D.P.; Gravato, C.A. Toxicity of dyes to zebrafish at the biochemical level: Cellular energy allocation and neurotoxicity. Environ. Pollut. 2018, 235, 255–262. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Wastewater treatment: An overview. In Green Adsorbents for Pollutant Removal; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Nidheesh, P.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of synthetic dyes—A review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Khan, R.; Bhawana, P.; Fulekar, M.H. Microbial decolorization and degradation of synthetic dyes: A review. Rev. Environ. Sci. Bio/Technol. 2013, 12, 75–97. [Google Scholar] [CrossRef]
- Srinivasan, S.; Sadasivam, S.K. Biodegradation of textile azo dyes by textile effluent non-adapted and adapted Aeromonas hydrophila. Environ. Res. 2021, 194, 110643. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Kostoglou, M. Green adsorbents for wastewaters: A critical review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2013, 160, 3–14. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Nasuha, N.; Hameed, B. Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem. Eng. J. 2011, 166, 783–786. [Google Scholar] [CrossRef]
- Momčilović, M.Z.; Vučić, M.R.; Meseldžija, S.; Velinov, N.; Suručić, L.; Bojić, A.L. Batch sorption dynamics and equilibrium for the capture of Ni(II) onto activated carbon developed from yellow dock (Rumex crispus). Biomass Conv. Bioref. 2024, 1–10. [Google Scholar] [CrossRef]
- Khelifa, N.; Aithamoudi, S.; Laoufi, N.A. Preparation and characterization of biosorbent of shrimp co products-based and its potential application in the removal of an anionic dye. Desalination Water Treat. 2022, 279, 195–202. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Agricultural peels for dye adsorption: A review of recent literature. J. Mol. Liq. 2014, 200, 381–389. [Google Scholar] [CrossRef]
- Liu, J.; Beckerman, J. Application of sustainable biosorbents from hemp for remediation of copper(II)-containing wastewater. J. Environ. Chem. Eng. 2022, 10, 107494. [Google Scholar] [CrossRef]
- Mongioví, C.; Morin-Crini, N.; Lacalamita, D.; Bradu, C.; Raschetti, M.; Placet, V.; Ribeiro, A.R.L.; Ivanovska, A.; Kostić, M.; Crini, G. Biosorbents from plant fibers of hemp and flax for metal removal: Comparison of their biosorption properties. Molecules 2021, 26, 4199. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, G.; Napolitano, F.; Gorrasi, G. Modified hemp fibers as a novel and green adsorbent for organic dye adsorption: Adsorption, kinetic studies and modeling. Euro-Mediterr. J. Environ. Integr. 2024, 9, 591–604. [Google Scholar] [CrossRef]
- Al-Rashed, S.M.; Al-Gaid, A.A. Kinetic and thermodynamic studies on the adsorption behavior of Rhodamine B dye on Duolite C-20 resin. J. Saudi Chem. Soc. 2012, 16, 209–215. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Kumar, V.B.; Luong, J.H.T.; Gedanken, A. Kinetics, isotherm, and thermodynamic studies of methylene blue adsorption on polyaniline and polypyrrole macro-nanoparticles synthesized by C-dot-initiated polymerization. ACS Omega 2018, 3, 7196–7203. [Google Scholar] [CrossRef]
- Aljeddani, G.S.; Alghanmi, R.M.; Hamouda, R.A. Study on the isotherms, kinetics, and thermodynamics of adsorption of crystal violet dye using Ag-NPs-loaded cellulose de-rived from peanut-husk agro-waste. Polymers 2023, 15, 4394. [Google Scholar] [CrossRef]
- Suručić, L.T.; Nastasović, A.B.; Onjia, A.E.; Janjić, G.V.; Rakić, A.A. Design of an amino-functionalized chelating macroporous copolymer poly(GMA-co-EGDMA) for the sorption of Cu(II) ions. J. Serb. Chem. Soc. 2019, 84, 1391–1404. [Google Scholar] [CrossRef]
- Suručić, L.T.; Janjić, G.V.; Rakić, A.A.; Nastasović, A.B.; Popović, A.R.; Milčić, M.K.; Onjia, A.E. Theoretical modeling of sorption of metal ions on amino-functionalized macroporous copolymer in aqueous solution. J. Mol. Model. 2019, 25, 177. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die Adsorption in Lösungen [On adsorption in solutions]. Z. Phys. Chemie 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.I. Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and the interaction between adsorbed molecules. Zhurnal Fiz. Khimii 1941, 15, 296–332. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Database. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 16 February 2025).
- Land, H.; Humble, M.S. YASARA: A tool to obtain structural guidance in biocatalytic investigations. Methods Mol. Biol. 2018, 1685, 43–67. [Google Scholar] [CrossRef] [PubMed]
- CERMAV. PolySac3DB. 2023. Available online: https://polysac3db.cermav.cnrs.fr (accessed on 16 February 2025).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- van der Werf, H.M.G.; Harsveld van der Veen, J.E.; Bouma, A.T.M.; ten Cate, M. Quality of hemp (Cannabis sativa L.) stems as a raw material for paper. Ind. Crop. Prod. 1994, 2, 219–227. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- Kavitha, D.; Namasivayam, C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour. Technol. 2007, 98, 14–21. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Kinetics, isotherm and thermodynamic studies for efficient adsorption of Congo red dye from aqueous solution onto novel cyanoguanidine-modified chitosan adsorbent. Polymers 2021, 13, 4446. [Google Scholar] [CrossRef]
- Farias, K.C.S.; Guimarães, R.C.A.; Oliveira, K.R.W.; Nazário, C.E.D.; Ferencz, J.A.P.; Wender, H. Banana peel powder biosorbent for removal of hazardous organic pollutants from wastewater. Toxics 2023, 11, 664. [Google Scholar] [CrossRef]
- Zhang, Z.; Moghaddam, L.; O’hara, I.M.; Doherty, W.O. Congo Red adsorption by ball-milled sugarcane bagasse. Chem. Eng. J. 2011, 178, 122–128. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.; Lee, D. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Rahmat, N.A.; Ali, A.A.; Salmiati; Hussain, N.; Muhamad, M.S.; Kristanti, R.A.; Hadibarata, T. Removal of Remazol Brilliant Blue R from Aqueous Solution by Adsorption Using Pineapple Leaf Powder and Lime Peel Powder. Water Air Soil Pollut. 2016, 227, 105. [Google Scholar] [CrossRef]



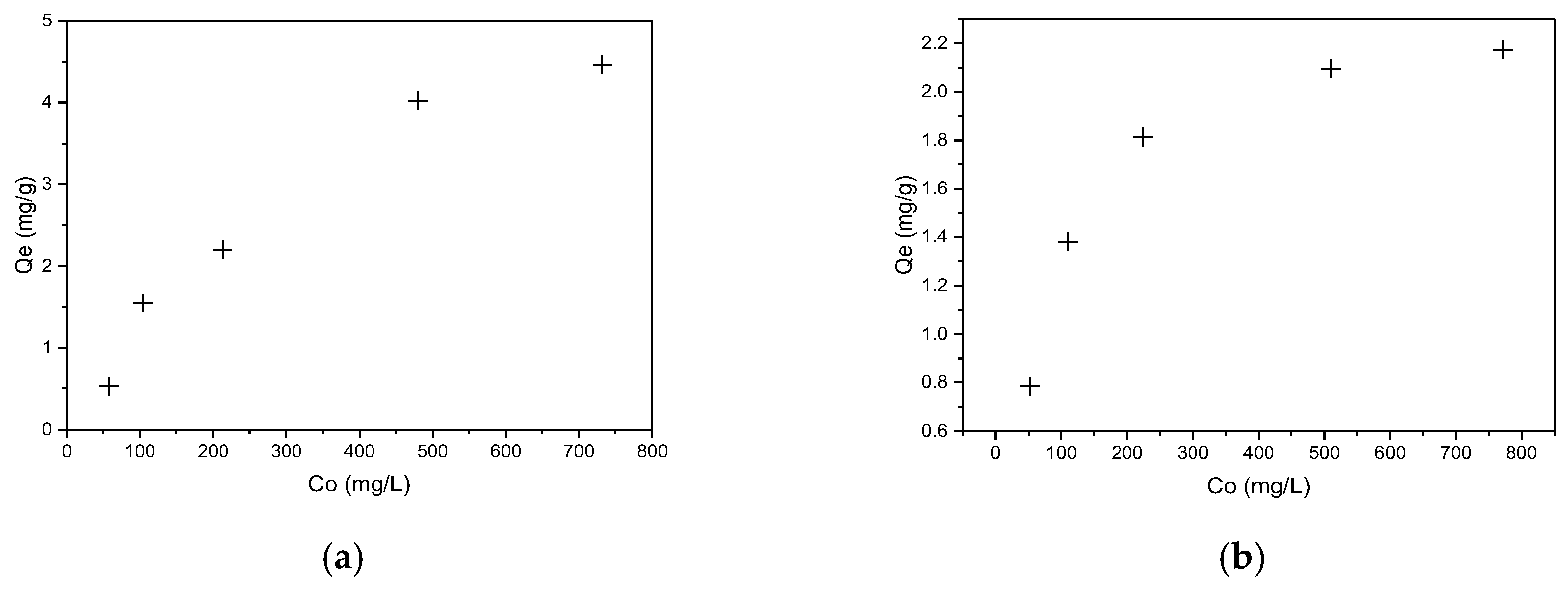
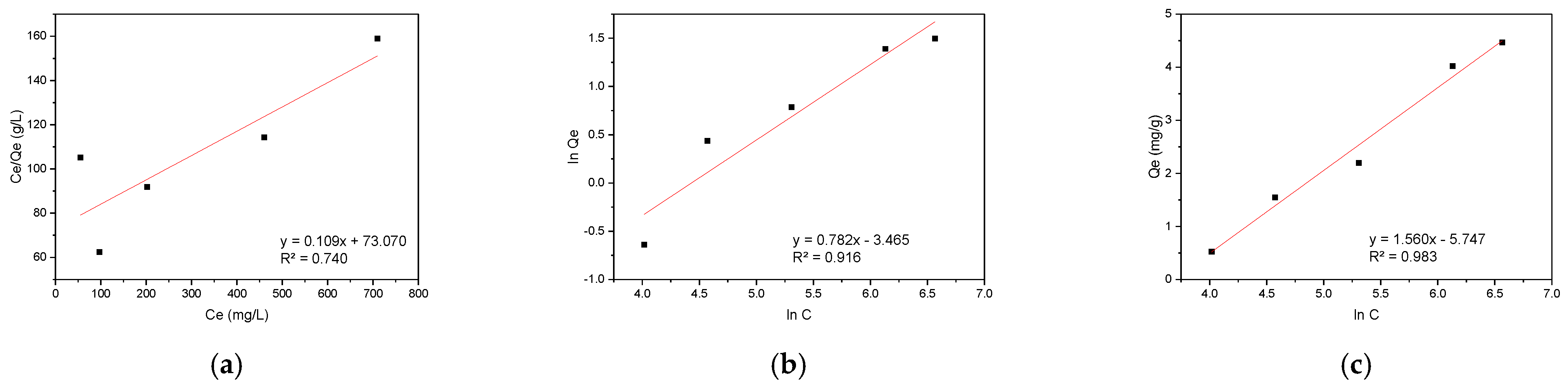
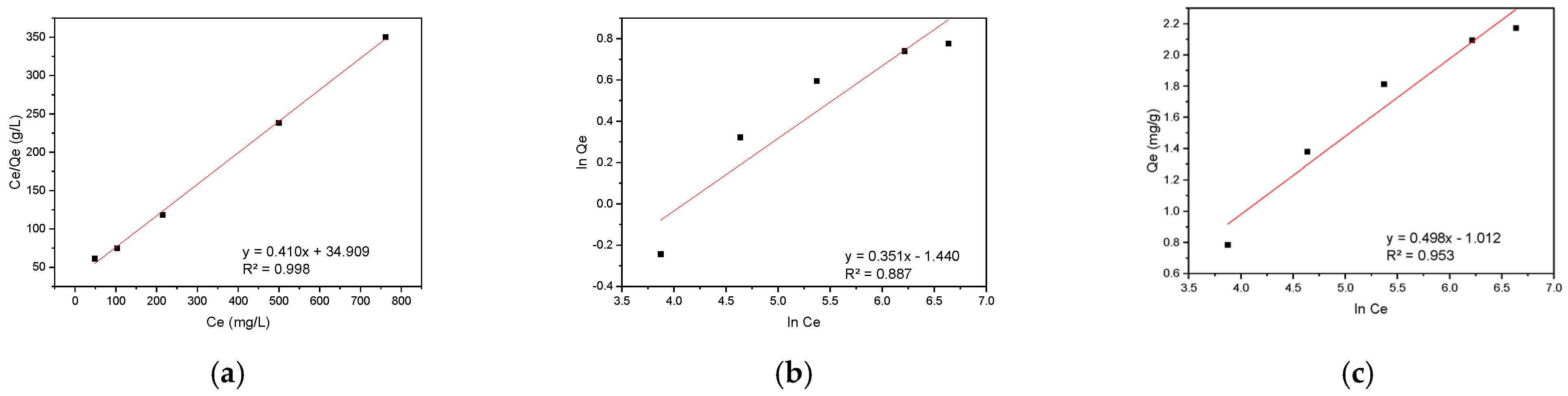

| Models | Parameters | CR | RBBR |
|---|---|---|---|
| Langmuir | Qmax (mg·g−1) | 4.47 | 2.44 |
| KL (dm−3·mg−1) | 0.0015 | 0.0117 | |
| R2 | 0.740 | 0.998 | |
| Freundlich | n | 1.28 | 2.85 |
| KF (mg·g−1)/(mg·dm−3)1/n | 0.00034 | 0.0363 | |
| R2 | 0.916 | 0.887 | |
| Tempkin | AT (dm3·mg−1) | 0.025 | 0.131 |
| bT (kJ·mg−1) | 1.588 | 4.986 | |
| R2 | 0.983 | 0.953 | |
| RL | 0.77–0.93 | 0.30–0.63 | |
| Biosorbent Material | Dye | Qmax (mg/g) | Best Fit Model | Reference |
|---|---|---|---|---|
| Hemp | CR | 4.47 | Temkin (R2 = 0.983) | (Current Study) |
| Hemp | RBBR | 2.44 | Langmuir (R2 = 0.998) | (Current Study) |
| Sugarcane bagasse | CR | 9.8 | Freundlich (R2 = 0.9971) | [42] |
| Banana peel | CR | 18.2 | Langmuir (R2 = 0.9971) | [43] |
| Lime peel powder | RBBR | 7.29 | Langmuir (R2 = 0.999) | [44] |
| Pineapple leaf powder | RBBR | 7.48 | Langmuir (R2 = 0.9945) | [44] |
| T(K) | ΔG kJ(mol)−1 | ΔS | TΔS | ΔH kJ(mol)−1 |
|---|---|---|---|---|
| 298 | −2.50 | 2.85 | 849.90 | −1.65 |
| 308 | −2.53 | 878.42 | ||
| 318 | −2.56 | 906.94 |
| T(K) | ΔG kJ (mol)−1 | ΔS | TΔS | ΔH kJ (mol)−1 |
|---|---|---|---|---|
| 298 | −3.38 | 4.29 | 1278.42 | −2.1 |
| 308 | −3.42 | 1321.32 | ||
| 318 | −3.46 | 1364.22 |
| Dye | Binding Energy (kcal/mol) | Dominant Interactions | Exp. Sorption Capacity, Qmax (mg/g) |
|---|---|---|---|
| CR | −7.5 |
| 4.47 |
| RBBR | −5.4 |
| 2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suručić, L.; Andrić, D.; Jevtić, I.; Momčilović, M.; Suručić, R.; Penjišević, J. Unmodified Hemp Biowaste as a Sustainable Biosorbent for Congo Red and Remazol Brilliant Blue R. Coatings 2025, 15, 519. https://doi.org/10.3390/coatings15050519
Suručić L, Andrić D, Jevtić I, Momčilović M, Suručić R, Penjišević J. Unmodified Hemp Biowaste as a Sustainable Biosorbent for Congo Red and Remazol Brilliant Blue R. Coatings. 2025; 15(5):519. https://doi.org/10.3390/coatings15050519
Chicago/Turabian StyleSuručić, Ljiljana, Deana Andrić, Ivana Jevtić, Milan Momčilović, Relja Suručić, and Jelena Penjišević. 2025. "Unmodified Hemp Biowaste as a Sustainable Biosorbent for Congo Red and Remazol Brilliant Blue R" Coatings 15, no. 5: 519. https://doi.org/10.3390/coatings15050519
APA StyleSuručić, L., Andrić, D., Jevtić, I., Momčilović, M., Suručić, R., & Penjišević, J. (2025). Unmodified Hemp Biowaste as a Sustainable Biosorbent for Congo Red and Remazol Brilliant Blue R. Coatings, 15(5), 519. https://doi.org/10.3390/coatings15050519








