Improved Corrosion Resistance of 5XXX Aluminum Alloy by Homogenization Heat Treatment
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adam, H. Carbon fiber in automotive applications. Mater. Des. 1997, 18, 349–355. [Google Scholar] [CrossRef]
- Erica, R.-F.; Frank, R.-F.; Richard, R.; Randolph, E.-K. Strategic materials selection in the automobile body: Economic opportunities for polymer composite design. Compos. Sci. Technol. 2008, 68, 1989–2002. [Google Scholar]
- Jürgen, H. Aluminum in innovative light-weight car design. Mater. Trans. 2011, 52, 818–824. [Google Scholar]
- Miller, W.-S.; Zhuang, L.; Bottema, J.; Wittebrood, A.-J.; Smet, P.-D.; Haszler, A.; Vieregge, A. Recent development in aluminum alloys for the automotive industry. Mater. Sci. Eng. 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Jones, R.-H.; Baer, D.-R.; Danielson, M.-J.; Vetrano, J.-S. Role of Mg in the stress corrosion cracking of an Al-Mg alloy. Metall. Mater. Trans. A 2001, 32, 1699–1711. [Google Scholar] [CrossRef]
- Kim, J.-M.; Seong, K.-D.; Jun, J.H.; Kim, K.-T.; Jung, W.-J. Variation of fluidity and mechanical properties of Al-Mg alloys with the addition of Si, Mn and Zn. J. Korean Foundry Soc. 2004, 24, 138–144. [Google Scholar]
- Searles, J.-L.; Gouma, P.-I.; Buchheit, R.-G. Stress corrosion cracking of sensitized AA5083. Metall. Mater. Trans. A 2001, 32, 2859–2867. [Google Scholar] [CrossRef]
- Romhanji, E.; Popović, M.; Glišić, D.; Stefanović, M.; Milovanović, M. On the Al-Mg Alloy sheets for automotive application: Problems and solutions. Metalurgija 2004, 10, 205–216. [Google Scholar]
- Braun, R. Environmentally assisted cracking of aluminum alloys in chloride solutions. In Proceedings of the ICAA-6: 6th International Conference on Aluminium Alloys, Toyohashi, Japan, 5–10 July 1998; pp. 153–164. [Google Scholar]
- Meng, C.; Zhang, D.; Hua, C.; Zhuang, L.; Zhang, J. Mechanical properties, intergranular corrosion behavior and microstructure of Zn modified Al–Mg alloys. J. Alloy. Compd. 2014, 617, 925–932. [Google Scholar] [CrossRef]
- Cho, J.-I.; Kim, C.-W. Effects of Mg and Si on microstructure and mechanical properties of Al-Mg die casting alloy. J. Korean Foundry Soc. 2012, 32, 219–224. [Google Scholar] [CrossRef]
- Davis, J.-R. Fabrication and Finishing of Aluminum Alloys. In Aluminum and Aluminum Alloys; Davis, J.-R., Ed.; ASM International: Novelty, OH, USA, 1991; pp. 351–416. [Google Scholar]
- Yun, H.-S.; Kim, J.-M.; Park, J.-S.; Kim, K.-T. Properties and casting characteristics of Al-Zn-Fe-Si alloys. J. Korean Foundry Soc. 2013, 33, 8–12. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, D.-H.; Nam, S.-W. Effect of Mn addition and homogenization heat treatment on the tensile properties in Al-Mg-Si alloy system. J. Korean Inst. Met. Mater. 1997, 35, 281–287. [Google Scholar]
- Popović, M.; Romhanji, E. Stress corrosion cracking susceptibility of Al–Mg alloy sheet with high Mg content. J. Mater. Proc. Technol. 2002, 125, 275–280. [Google Scholar] [CrossRef]
- Cerri, E.; Evangelista, E. Metallography of Aluminum alloys. In TALAT Lecture 1202: Metallography of Aluminum Alloys; Core-Materials: Liverpool, UK, 2009; pp. 1–20. [Google Scholar]
- Jiang, H.-C.; Ye, L.-Y.; Zhang, X.-M.; Gu, G.; Zhang, P.; Wu, Y.-L. Intermetallic phase evolution of 5059 aluminum alloy during homogenization. Trans. Nonferrous Met. Soc. China 2013, 23, 3553–3560. [Google Scholar] [CrossRef]
- ASTM G102 Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM: West Conshohocken, PA, USA, 1994; pp. 416–422.
- Li, H.-Y.; Su, X.-J.; Yin, H.; Huang, D.-S. Microstructural evolution during homogenization of Al-Cu-Li-Mn-Zr-Ti alloy. Nonferrous Met. Soc. China 2013, 23, 2543–2550. [Google Scholar] [CrossRef]
- Auld, J.-H.; Williams, B.-E. X-ray powder data of Tphases composed of aluminium and magnesium with silver, copper or zinc. Acta Cryst. 1966, 21, 830–831. [Google Scholar] [CrossRef]
- Du, Y.; Chang, Y.-A.; Huang, B.; Gong, W.; Jin, Z.; Xu, H.; Yuan, Z.; Liu, Y.; He, Y.; Xie, F.-Y. Diffusion coefficients of some solutes in fcc and liquid Al: Critical evaluation and correlation. Mater. Sci. Eng. A 2003, 363, 140–151. [Google Scholar] [CrossRef]
- Tan, L.; Allen, T.-R. Effect of thermomechanical treatment on the corrosion of AA5083. Corros. Sci. 2010, 52, 548–554. [Google Scholar] [CrossRef]
- Yan, J.; Andrea, M.-H. Study of β precipitation and layer structure formation in Al 5083: The role of dispersoids and grain boundaries. J. Alloy. Compd. 2017, 703, 242–250. [Google Scholar] [CrossRef]
- Sukiman, N.-L.; Gupta, R.-K.; Buchheit, R.-G.; Birbilis, N. Influence of microalloying additions on Al-Mg alloy. Part 1: Corrosion and electrochemical response. Corros. Eng. Sci. Technol. 2014, 49, 254–262. [Google Scholar] [CrossRef]
- Jones, D.-A. Principles and Prevention of Corrosion; Macmillan Publishing Co.: London, UK, 1992. [Google Scholar]
- Zeng, F.-L.; Wei, Z.-L.; Li, J.-F.; Li, C.-X.; Tan, X.; Zhang, Z.; Zheng, Z.-Q. Corrosion mechanism associated with Mg2Si and Si particles in Al−Mg−Si alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]





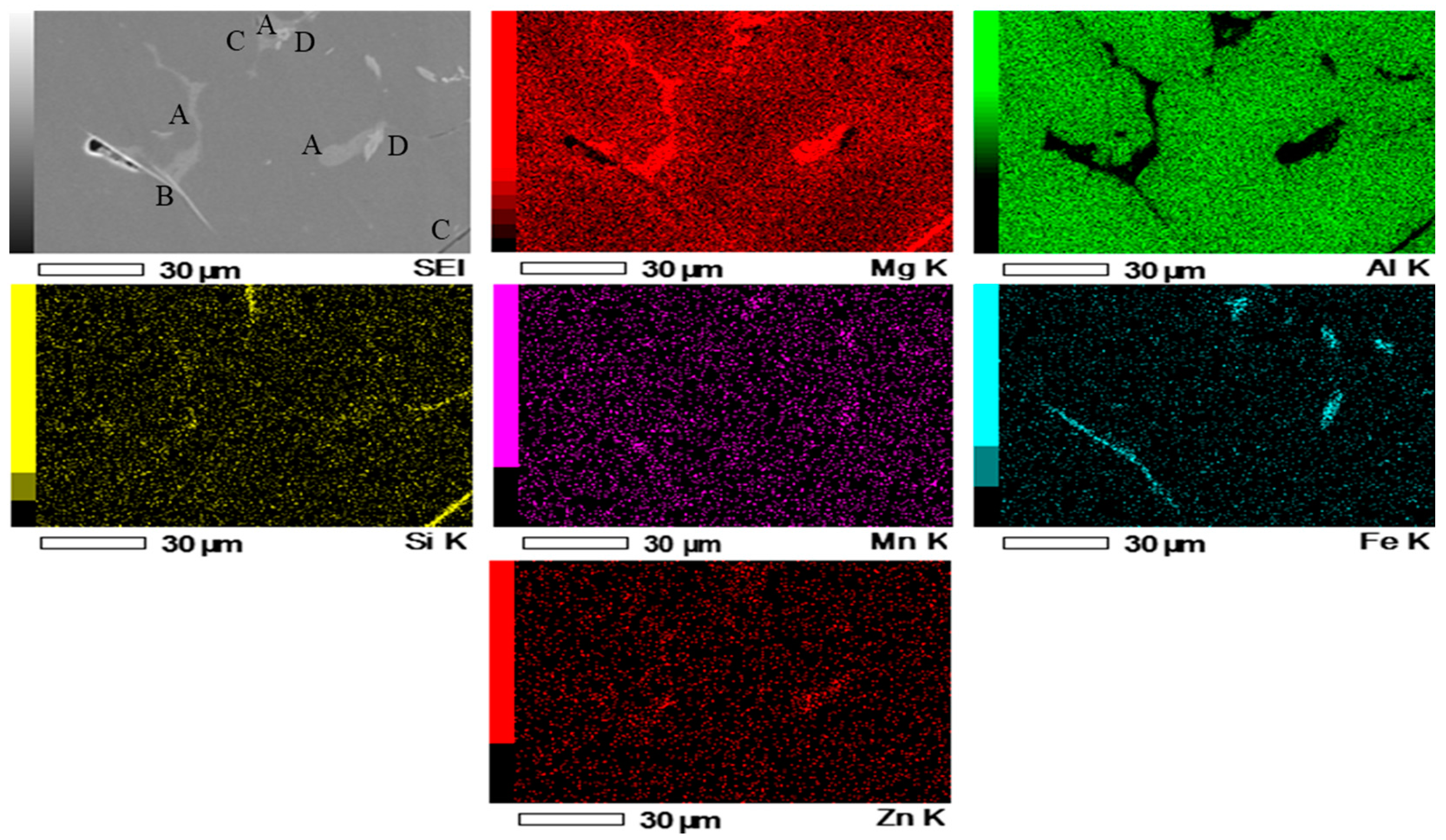

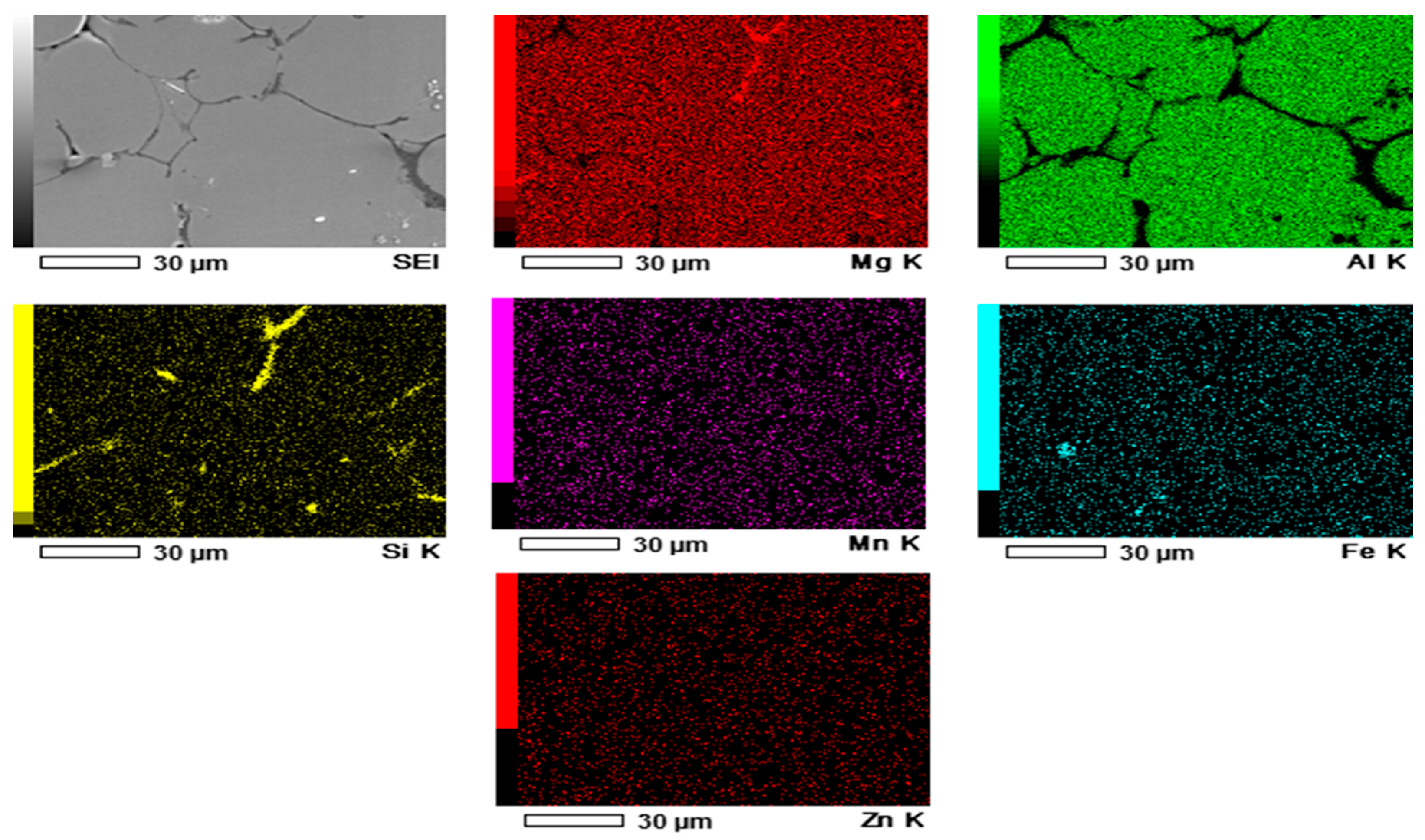

| Mg | Zn | Si | Mn | Fe | Cu | Ti | B | Al |
|---|---|---|---|---|---|---|---|---|
| 6.5 | 1.5 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | Balanced |
| Parameter | Vaule |
|---|---|
| Start Potential (V) | −1.3 |
| Final Potential (V) | −0.2 |
| Scanning Speed (mV/s) | 0.5 |
| Time (s) | 2200 |
| Temperature (°C) | 25 |
| Standard Electrode | Saturated calomel electrode |
| Counter Electrode | Graphite electrode |
| Electrolyte | 3.5 wt % NaCl |
| Specimen Density | 2.6 g/cm3 |
| Phase | Composition (wt %) | ||||||
|---|---|---|---|---|---|---|---|
| Al | Mg | Zn | Fe | Mn | Si | Cu | |
| A | 51.54 | 37.64 | 7.73 | – | – | – | 3.09 |
| B | 79.81 | 8.82 | – | 4.34 | 7.03 | – | – |
| C | 87.67 | 8.37 | – | – | – | 3.96 | – |
| D | 69.73 | 10.66 | – | 2.43 | 17.18 | – | – |
| Phase | Composition (wt %) | ||||||
|---|---|---|---|---|---|---|---|
| Al | Mg | Zn | Fe | Mn | Si | Cu | |
| (a) | 90.39 | 8.22 | – | – | – | 1.39 | – |
| (b) | 90.60 | 8.06 | – | – | – | 1.34 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, I.-K.; Cho, S.-H.; Kim, S.-J.; Jo, Y.-S.; Kim, S.-H. Improved Corrosion Resistance of 5XXX Aluminum Alloy by Homogenization Heat Treatment. Coatings 2018, 8, 39. https://doi.org/10.3390/coatings8010039
Choi I-K, Cho S-H, Kim S-J, Jo Y-S, Kim S-H. Improved Corrosion Resistance of 5XXX Aluminum Alloy by Homogenization Heat Treatment. Coatings. 2018; 8(1):39. https://doi.org/10.3390/coatings8010039
Chicago/Turabian StyleChoi, In-Kyu, Soo-Ho Cho, Sung-Joon Kim, Yoo-Shin Jo, and Sang-Ho Kim. 2018. "Improved Corrosion Resistance of 5XXX Aluminum Alloy by Homogenization Heat Treatment" Coatings 8, no. 1: 39. https://doi.org/10.3390/coatings8010039
APA StyleChoi, I.-K., Cho, S.-H., Kim, S.-J., Jo, Y.-S., & Kim, S.-H. (2018). Improved Corrosion Resistance of 5XXX Aluminum Alloy by Homogenization Heat Treatment. Coatings, 8(1), 39. https://doi.org/10.3390/coatings8010039




