Stress-Affected Lithiation Reactions in Elasto-Viscoplastic Si Particles with Hyperelastic Polymer Coatings: A Nonlinear Chemo-Mechanical Finite-Element Study
Abstract
:1. Introduction
2. Chemo-Mechanical Model for a Polymer-Coated Spherical Si Particle
2.1. Model Background
2.2. Governing Equations for a Coated Spherical Si Particle With a Non-Stationary Reaction Front
2.3. Constitutive Relations
2.3.1. Transformed and Untransformed Components of Si Particle
2.3.2. Hyperelastic Polymer Coating
3. Finite Element solution of the Problem
4. Results and Discussion
4.1. Model Parameters
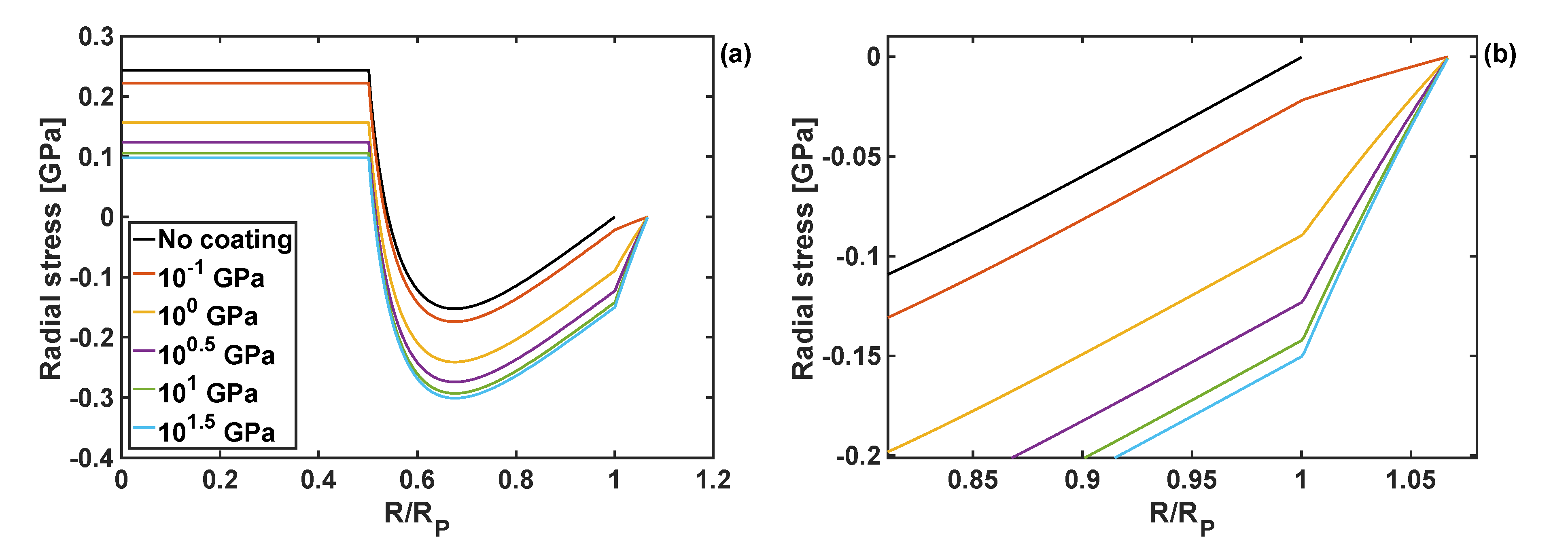
4.2. Effects of Coating Moduli on Stresses During Lithiation Process
4.3. Effect of Coating Moduli on Reaction Front Velocity
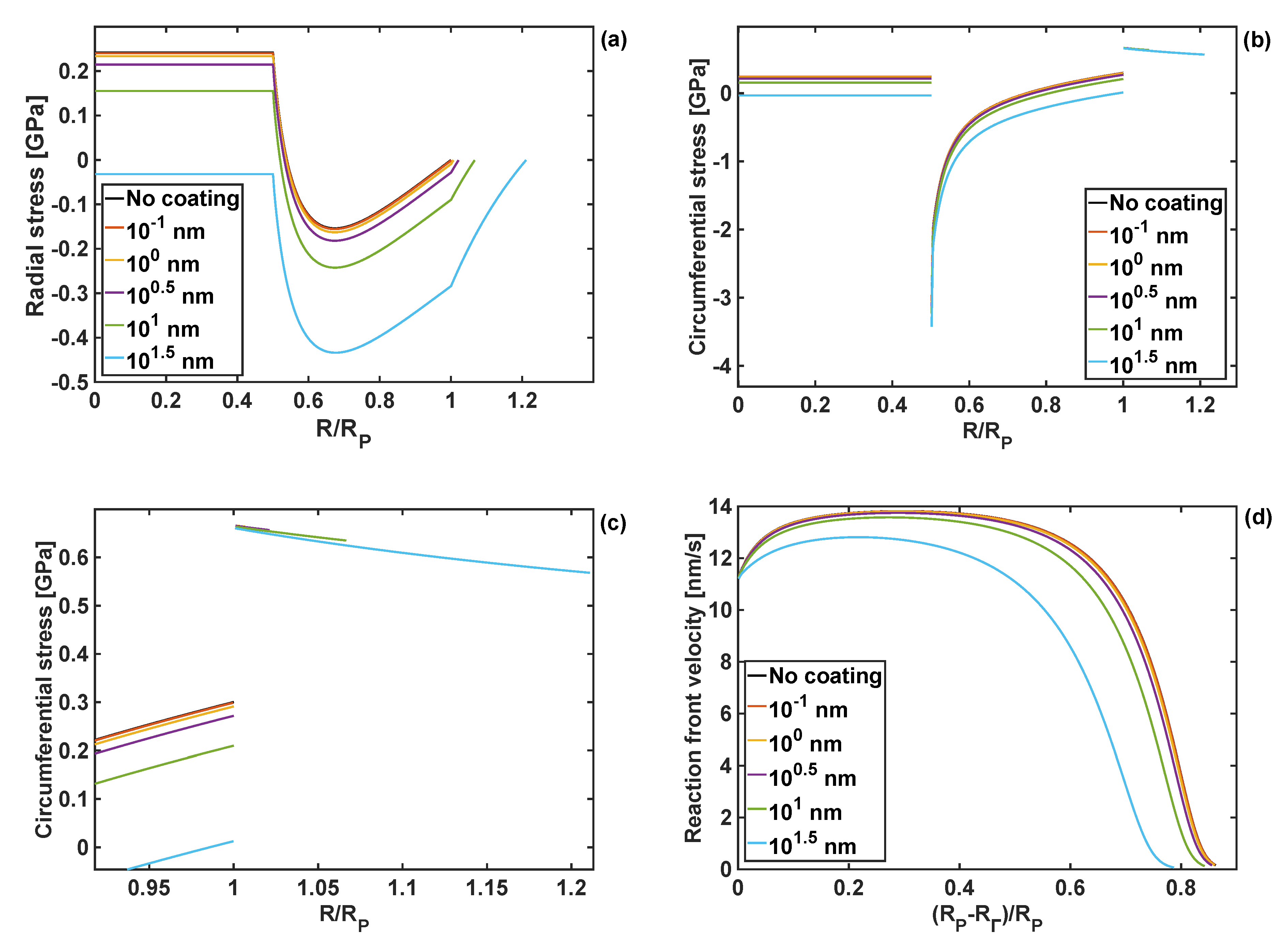
4.4. Effect of Coating Thickness on Stresses and Reaction Front Velocity
4.5. Sensitivity Analysis of Reaction Front Velocity to Coating Properties
4.6. Combined Effects of Coating Parameters and Chemical Energy
4.7. Effect of Particle Constraints (Non-Stress-Free Boundary Conditions)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Parameter | Value | Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|---|---|
| [] | 28.5 | [] | 2.285 | [] | 28.0855 | [None] | 1/15 |
| [] | 0.5 | [None] | 24 | [] | 293.15 | [] | 5 |
| [] | [] | 2000 | [None] | 41/3 | [] | 80 | |
| [] | 2 | [] | 0.053 | [None] | 4/15 | [None] | 0.22 |
| [] | 1 | [] | 86 | [None] | 1 | [] | 41 |
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Ma, D.; Cao, Z.; Hu, A. Si-based anode materials for Li-ion batteries: A mini review. Nano-Micro Lett. 2014, 6, 347–358. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. 25th anniversary article: Understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef]
- Endo, M.; Kim, C.; Nishimura, K.; Fujino, T.; Miyashita, K. Recent developments of carbon materials for Li ion batteries. Carbon 2000, 38, 183–197. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chang, C.C.; Duh, J.G. Silicon nitride coated silicon thin film on three dimensions current collector for lithium ion battery anode. J. Power Sources 2016, 325, 64–70. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Lesh, G.C.; Huggins, R.A. All-solid lithium electrodes with mixed-conductor matrix. J. Electrochem. Soc. 1981, 128, 725–729. [Google Scholar] [CrossRef]
- Beaulieu, L.Y.; Eberman, K.W.; Turner, R.L.; Krause, L.J.; Dahn, J.R. Colossal reversible volume changes in lithium alloys. Electrochem. Solid-State Lett. 2001, 4, A137–A140. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Van Havenbergh, K.; Turner, S.; Marx, N.; Van Tendeloo, G. The mechanical behavior during (De)lithiation of coated silicon nanoparticles as anode material for lithium-ion batteries studied by in situ transmission electron microscopy. Energy Technol. 2016, 4, 1005–1012. [Google Scholar] [CrossRef]
- McDowell, M.T.; Ryu, I.; Lee, S.W.; Wang, C.; Nix, W.D.; Cui, Y. Studying the kinetics of crystalline silicon nanoparticle lithiation with in situ transmission electron microscopy. Adv. Mater. 2012, 24, 6034–6041. [Google Scholar] [CrossRef]
- Zhang, W.J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Yao, Y.; McDowell, M.T.; Ryu, I.; Wu, H.; Liu, N.; Hu, L.; Nix, W.D.; Cui, Y. Interconnected silicon hollow nanospheres for lithium-ion battery anodes with long cycle life. Nano Lett. 2011, 11, 2949–2954. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Yen, Y.C.; Chao, S.C.; Wu, H.C.; Wu, N.L. Study on solid-electrolyte-interphase of Si and C-coated Si electrodes in lithium cells. J. Electrochem. Soc. 2009, 156, A95–A102. [Google Scholar] [CrossRef]
- Liu, B.; Soares, P.; Checkles, C.; Zhao, Y.; Yu, G. Three-dimensional hierarchical ternary nanostructures for high-performance Li-ion battery anodes. Nano Letters 2013, 13, 3414–3419. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Zhao, P.; Yang, H.; Liu, B.; Zhang, J.G.; Cui, Y.; Yu, G.; Zhang, S.; Wang, C.M. Surface coating constraint induced self-discharging of silicon nanoparticles as anodes for lithium ion batteries. Nano Lett. 2015, 15, 7016–7022. [Google Scholar] [CrossRef]
- Gao, P.; Fu, J.; Yang, J.; Lv, R.; Wang, J.; Nuli, Y.; Tang, X. Microporous carbon coated silicon core/shell nanocomposite via in situ polymerization for advanced Li-ion battery anode material. Phys. Chem. Chem. Phys. 2009, 11, 11101–11105. [Google Scholar] [CrossRef]
- Levitas, V.I.; Attariani, H. Anisotropic compositional expansion in elastoplastic materials and corresponding chemical potential: Large-strain formulation and application to amorphous lithiated silicon. J. Mech. Phys. Solids 2014, 69, 84–111. [Google Scholar] [CrossRef]
- Loeffel, K.; Anand, L. A chemo-thermo-mechanically coupled theory for elastic–viscoplastic deformation, diffusion, and volumetric swelling due to a chemical reaction. Int. J. Plast. 2011, 27, 1409–1431. [Google Scholar] [CrossRef]
- Jia, Z.; Li, T. Stress-modulated driving force for lithiation reaction in hollow nano-anodes. J. Power Sources 2015, 275, 866–876. [Google Scholar] [CrossRef]
- Freidin, A.B.; Vilchevskaya, E.N.; Korolev, I.K. Stress-assist chemical reactions front propagation in deformable solids. Int. J. Eng. Sci. 2014, 83, 57–75. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Piper, D.M.; Gu, M.; Travis, J.J.; George, S.M.; Lee, S.H.; Genc, A.; Pullan, L.; Liu, J.; Mao, S.X.; et al. In situ transmission electron microscopy probing of native oxide and artificial layers on silicon nanoparticles for lithium ion batteries. ACS Nano 2014, 8, 11816–11823. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Cao, K.; Tang, J.; Wang, H.; Zhou, L.; Yao, H. Mechanics-based optimization of yolk-shell carbon-coated silicon nanoparticle as electrode materials for high-capacity lithium ion battery. Compos. Commun. 2016, 1, 1–5. [Google Scholar] [CrossRef]
- Wang, J.W.; He, Y.; Fan, F.; Liu, X.H.; Xia, S.; Liu, Y.; Harris, C.; Li, H.; Huang, J.; Mao, S.; et al. Two-phase electrochemical lithiation in amorphous silicon. Nano Lett. 2013, 13, 709–715. [Google Scholar] [CrossRef]
- Liu, X.H.; Wang, J.W.; Huang, S.; Fan, F.; Huang, X.; Liu, Y.; Krylyuk, S.; Yoo, J.; Dayeh, A.V.; Mao, S.X.; et al. In situ atomic-scale imaging of electrochemical lithiation in silicon. Nat. Nanotechnol. 2012, 7, 749–756. [Google Scholar] [CrossRef]
- Li, W.; Cao, K.; Wang, H.; Liu, J.; Zhou, L.; Yao, H. Carbon coating may expedite the fracture of carbon-coated silicon core–shell nanoparticles during lithiation. Nanoscale 2016, 8, 5254–5259. [Google Scholar] [CrossRef]
- Poluektov, M.; Freidin, A.B.; Figiel, Ł. Modelling stress-affected chemical reactions in non-linear viscoelastic solids with application to lithiation reaction in spherical Si particles. Int. J. Eng. Sci. 2018, 128, 44–62. [Google Scholar] [CrossRef]
- Bower, A.F.; Guduru, P.R.; Chason, E. Analytical solutions for composition and stress in spherical elastic–plastic lithium-ion electrode particles containing a propagating phase boundary. Int. J. Solids Struct. 2015, 69, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Freidin, A.B. On the chemical affinity tensor for chemical reactions in deformable materials. Mech. Solids 2015, 50, 260–285. [Google Scholar] [CrossRef]
- Freidin, A.B. Chemical affinity tensor and stress-assist chemical reactions front propagation in solids. In Proceedings of the ASME 2013 International Mechanical Engineering Congress and Exposition, San Diego, CA, USA, 15–21 November 2013; The American Society of Mechanical Engineers: New York, NY, USA, 2013. IMECE2013-64957. [Google Scholar]
- Liu, N.; Wu, H.; McDowell, M.T.; Yao, Y.; Wang, C.; Cui, Y. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 2012, 12, 3315–3321. [Google Scholar] [CrossRef]
- Frank, P.M. Introduction to System Sensitivity Theory; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Figiel, Ł.; Kamiński, M. Numerical probabilistic approach to sensitivity analysis in a fatigue delamination problem of a two layer composite. Appl. Math. Comput. 2009, 209, 75–90. [Google Scholar] [CrossRef]












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippmann, P.; Poluektov, M.; Figiel, Ł. Stress-Affected Lithiation Reactions in Elasto-Viscoplastic Si Particles with Hyperelastic Polymer Coatings: A Nonlinear Chemo-Mechanical Finite-Element Study. Coatings 2018, 8, 455. https://doi.org/10.3390/coatings8120455
Lippmann P, Poluektov M, Figiel Ł. Stress-Affected Lithiation Reactions in Elasto-Viscoplastic Si Particles with Hyperelastic Polymer Coatings: A Nonlinear Chemo-Mechanical Finite-Element Study. Coatings. 2018; 8(12):455. https://doi.org/10.3390/coatings8120455
Chicago/Turabian StyleLippmann, Philip, Michael Poluektov, and Łukasz Figiel. 2018. "Stress-Affected Lithiation Reactions in Elasto-Viscoplastic Si Particles with Hyperelastic Polymer Coatings: A Nonlinear Chemo-Mechanical Finite-Element Study" Coatings 8, no. 12: 455. https://doi.org/10.3390/coatings8120455
APA StyleLippmann, P., Poluektov, M., & Figiel, Ł. (2018). Stress-Affected Lithiation Reactions in Elasto-Viscoplastic Si Particles with Hyperelastic Polymer Coatings: A Nonlinear Chemo-Mechanical Finite-Element Study. Coatings, 8(12), 455. https://doi.org/10.3390/coatings8120455






