The Influence of Oxygen Flow Ratio on the Optoelectronic Properties of p-Type Ni1?xO Films Deposited by Ion Beam Assisted Sputtering
Abstract
:1. Introduction
2. Materials and Methods
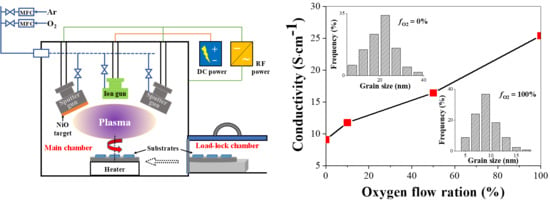
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Frenzel, H.; Lajn, A.; Grundmann, M. One decade of fully transparent oxide thin-film transistors: Fabrication, performance and stability. Phys. Status Solidi R 2013, 7, 605–615. [Google Scholar] [CrossRef]
- Sun, H.; Liao, M.H.; Chen, S.C.; Li, Z.Y.; Lin, P.J.; Song, S.M. Synthesis and characterization of n-type NiO:Al thin films for fabrication of p-n NiO homojunctions. J. Phys. D 2018, 51, 105109. [Google Scholar] [CrossRef]
- Szyszka, B.; Dewald, W.; Gurram, S.K.; Pflug, A.; Schulz, C.; Siemers, M.; Sittinger, V.; Ulrich, S. Recent developments in the field of transparent conductive oxide films for spectral selective coatings, electronics and photovoltaics. Curr. Appl. Phys. 2012, 12, S2–S11. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D. Synergetic effect in the multifunctional composite film of graphene-TiO2 with transparent conductive, photocatalytic and strain sensing properties. J. Alloys Compd. 2017, 698, 60–67. [Google Scholar] [CrossRef]
- He, L.X.; Tjong, S.C. Nanostructured transparent conductive films: Fabrication, characterization and applications. Mater. Sci. Eng. R 2016, 109, 1–101. [Google Scholar] [CrossRef]
- Wu, F.Y.; Tong, X.R.; Zhao, Z.; Gao, J.B.; Zhou, Y.W.; Kelly, P. Oxygen-controlled structures and properties of transparent conductive SnO2:F films. J. Alloys Compd. 2017, 695, 765–770. [Google Scholar] [CrossRef]
- Besleaga, C.; Stan, G.E.; Pintilie, I.; Barquinha, P.; Fortunato, E.; Martins, R. Transparent field-effect transistors based on AlN-gate dielectric and IGZO-channel semiconductor. Appl. Surf. Sci. 2016, 379, 270–276. [Google Scholar] [CrossRef]
- Mickan, M.; Helmersson, U.; Rinnert, H.; Ghanbaja, J.; Muller, D.; Horwat, D. Room temperature deposition of homogeneous, highly transparent and conductive Al-doped ZnO films by reactive high power impulse magnetron sputtering. Sol. Energy Mater. Sol. Cells 2016, 157, 742–749. [Google Scholar] [CrossRef]
- Wang, G.H.; Shi, C.Y.; Zhao, L.; Diao, H.W.; Wang, W.J. Transparent conductive Hf-doped In2O3 thin films by RF sputtering technique at low temperature annealing. Appl. Surf. Sci. 2017, 399, 716–720. [Google Scholar] [CrossRef]
- Al-Jawhari, H.A. A review of recent advances in transparent p-type Cu2O-based thin film transistors. Mater. Sci. Semicond. Process. 2015, 40, 241–252. [Google Scholar] [CrossRef]
- Sun, H.; Arab Pour Yazdi, M.; Chen, S.C.; Wen, C.K.; Sanchette, F.; Billard, A. Ag composition gradient CuCr0.93Mg0.07O2/Ag/CuCr0.93Mg0.07O2 coatings with improved p-type optoelectronic performances. J. Mater. Sci. 2017, 52, 11537–11546. [Google Scholar] [CrossRef]
- Cao, L.; Li, X.Y.; Wang, D.X.; Zhu, L.P. Li-doped NiMgO thin films as a promising p-type transparent conductive material with wide band-gap. Mater. Lett. 2013, 110, 73–75. [Google Scholar] [CrossRef]
- Sarmadian, N.; Saniz, R.; Partoens, B.; Lamoen, D. Easily doped p-type, low hole effective mass, transparent oxides. Sci. Rep. 2016, 6, 20446. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Yum, J.H.; Moon, S.J.; Chen, P. Inorganic p-type semiconductors: Their applications and progress in dye-sensitized solar cells and perovskite solar cells. Energies 2016, 9, 331. [Google Scholar] [CrossRef]
- Han, M.J.; Wang, J.; Deng, Q.L.; Wang, J.Y.; Li, W.W.; Zhang, P.; Li, C.Q.; Hu, Z.G. Effect of annealing temperature on structural optoelectronic properties and interband transitions of CuCrO2 nanocrystalline films prepared by the sol-gel method. J. Alloys Compd. 2015, 647, 1028–1034. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Chen, C.L. Improved electrical properties of p-type CuGaO2 semiconductor thin films through Mg and Zn doping. Ceram. Int. 2017, 43, 2563–2567. [Google Scholar] [CrossRef]
- Zhang, Y.D. Thermal oxidation fabrication of NiO film for optoelectronic devices. Appl. Surf. Sci. 2015, 344, 33–37. [Google Scholar] [CrossRef]
- Wong, T.K.S.; Zhuk, S.; Masudy-Panah, S.; Dalapati, G.K. Current status and future prospects of copper oxide heterojunction solar cells. Materials 2016, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Loukil, A.; Boukhachem, A.; Ben Amor, M.; Ghamnia, M.; Raouadi, K. Effects of potassium incorporation on the structural, optical, vibrational and electrical properties of NiO sprayed thin films for p-type optical windows. Cream. Int. 2016, 42, 8274–8289. [Google Scholar] [CrossRef]
- Amiruddin, R.; Santhosh Kumar, M.C. Role of p-NiO electron blocking layers in fabrication of (P-N):ZnO/Al:ZnO UV photodiodes. Curr. Appl. Phys. 2016, 16, 1052–1061. [Google Scholar] [CrossRef]
- Sun, H.; Chen, S.C.; Chen, P.J.; Ou, S.L.; Liu, C.Y.; Xin, Y.Q. p-type conductive NiOx: Cu thin films with high carrier mobility deposited by ion beam assisted deposition. Cream. Int. 2018, 44, 3291–3296. [Google Scholar] [CrossRef]
- Iyengar, P.; Das, C.; Balasubramaniam, K.R. Photoelectrochemical performance of NiO-coated ZnO–CdS core-shell photoanode. J. Phys. D Appl. Phys. 2017, 50, 10LT01. [Google Scholar] [CrossRef]
- Dong, D.M.; Wang, W.W.; Dong, G.B.; Zhang, F.; He, Y.C.; Yu, H.; Liu, F.M.; Wang, M.; Diao, X.G. Electrochromic properties and performance of NiOx films and their corresponding all-thin-film flexible devices preparedby reactive DC magnetron sputtering. Appl. Surf. Sci. 2016, 383, 49–56. [Google Scholar] [CrossRef]
- Turgut, E.; Coban, O.; Saritas, S.; Tuzemen, S.; Yildirim, M.; Gur, E. Oxygen partial pressure effects on the RF sputtered p-type NiO hydrogen gas sensors. Appl. Surf. Sci. 2018, 435, 880–885. [Google Scholar] [CrossRef]
- Mei, B.; Permyakova, A.A.; Frydendal, R.; Bae, D.; Pedersen, T.; Malacrida, P.; Hansen, O.; Stephens, I.E.L.; Vesborg Peter, C.K.; Seger, B.; et al. Iron-treated NiO as a highly transparent p-type protection layer for efficient Si-based photoanodes. J. Phys. Chem. Lett. 2014, 5, 3456–3461. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; McDowell, M.T.; Nielander, A.C.; Hu, S.; Shaner, M.R.; Yang, F.; Brunschwig, B.S.; Lewis, N.S. Stable solar-driven water oxidation to O2(g) by Ni-oxide-coated silicon photoanodes. J. Phys. Chem. Lett. 2015, 6, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.G.; Rosso, K.M.; Bruemmer, S.M. Charge and ion transport in NiO and aspects of Ni oxidation from first principles. J. Phys. Chem. C 2012, 116, 1948–1954. [Google Scholar] [CrossRef]
- Dawson, J.A.; Guo, Y.; Robertson, J. Energetics of intrinsic defects in NiO and the consequences for its resistive random access memory performance. Appl. Phys. Lett. 2015, 107, 122110. [Google Scholar] [CrossRef]
- Grilli, M.L.; Menchini, F.; Dikonimos, T.; Nunziante, P.; Pilloni, L.; Yilmaz, M.; Piegari, A.; Mittiga, A. Effect of growth parameters on the properties of RF-sputtered highly conductive and transparent p-type NiOx films. Semicond. Sci. Technol. 2016, 31, 055016. [Google Scholar] [CrossRef]
- Predanocy, M.; Hotov, I.; Caplovicova, M. Structural, optical and electrical properties of sputtered NiO thin films for gas detection. Appl. Surf. Sci. 2017, 395, 208–213. [Google Scholar] [CrossRef]
- Li, L.; Wang, W.; He, L.; Zhang, J.; Wu, Z.; Zhang, B.; Liu, Y. Synthesis and characterization of p-type NiO films suitable for normally-off AlGaN/GaN HFETs application. Mater. Sci. Semicond. Process. 2017, 67, 141–146. [Google Scholar] [CrossRef]
- Chen, S.C.; Wen, C.K.; Kuo, T.Y.; Peng, W.C.; Lin, H.C. Characterization and properties of NiO films produced by rf magnetron sputtering with oxygen ion source assistance. Thin Solid Films 2014, 572, 51–55. [Google Scholar] [CrossRef]
- Ai, L.; Fang, G.J.; Yuan, L.Y.; Liu, N.S.; Wang, M.J.; Li, C.; Zhang, Q.L.; Li, J.; Zhao, X.Z. Influence of substrate temperature on electrical and optical properties of p-type semitransparent conductive nickel oxide thin films deposited by radio frequency sputtering. Appl. Surf. Sci. 2008, 254, 2401–2405. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, T.Y.; Sun, T.H. Microstructures, electrical and optical properties of non-stoichiometric p-type nickel oxide films by radio frequency reactive sputtering. Surf. Coat. Technol. 2010, 205, S236–S240. [Google Scholar] [CrossRef]
- Reddy, A.M.; Reddy, A.S.; Lee, K.S.; Reddy, P.S. Effect of oxygen partial pressure on the structural, optical and electrical properties of sputtered NiO films. Ceram. Int. 2011, 37, 2837–2843. [Google Scholar] [CrossRef]
- Fujii, E.; Tomozawa, A.; Torii, H.; Talayama, R. Preferred orientations of NiO films prepared by plasma-enhanced metalorganic chemical vapor deposition. Jpn. J. Appl. Phys. 1996, 35, L328–L330. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1987, 11, 102–113. [Google Scholar] [CrossRef]
- Nandy, S.; Saha, B.; Mitra, M.K.; Chattopadhyay, K.K. Effect of oxygen partial pressure on the electrical and optical properties of highly (200) oriented p-type Ni1−xO films by DC sputtering. J. Mater. Sci. 2007, 42, 5766–5772. [Google Scholar] [CrossRef]
- Reddy, Y.A.K.; Reddy, A.S.; Reddy, P.S. Influence of oxygen partial pressure on the structural, optical and electrical properties of Cu-doped NiO thin films. Phys. Scr. 2013, 87, 015801. [Google Scholar] [CrossRef]
- Demichelis, F.; Kaniadakis, G.; Tagliaferro, A.; Tresso, E. New approach to optical analysis of absorbing thin solid films. Appl. Opt. 1987, 26, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Xin, Y.Q.; Zhao, J.F.; Song, S.M.; Chen, S.C.; Lu, Y.B.; Sun, H. High transmittance in IR region of conductive ITO/AZO multilayers deposited by RF magnetron sputtering. Ceram. Int. 2018, 44, 6769–6774. [Google Scholar] [CrossRef]
- Ghosh, A.; Nelson, C.M.; Abdallah, L.S.; Zollner, S. Optical constants and band structure of trigonal NiO. J. Vac. Sci. Technol. A 2015, 33, 061203. [Google Scholar] [CrossRef]
- Gillen, R.; Robertson, J. Accurate screened exchange band structures for the transition metal monoxides MnO, FeO, CoO and NiO. J. Phys. Condens. Matter 2013, 25, 165502. [Google Scholar] [CrossRef] [PubMed]
- Sawatzky, G.A.; Allen, J.W. Magnitude and origin of the band gap of NiO. Phys. Rev. Lett. 1984, 53, 2339–2342. [Google Scholar] [CrossRef]
- Kurmaev, E.Z.; Wilks, R.G.; Moewes, A.; Finkelstein, L.D.; Shamin, S.N.; Kunes, J. Oxygen x-ray emission and absorption spectra as a probe of the electronic structure of strongly correlated oxides. Phys. Rev. B 2008, 77, 165127. [Google Scholar] [CrossRef]








| Film | Thickness (nm) | Transmittance (%) | Conductivity (S·cm−1) | Carrier Concentration (cm−3) | Carrier Mobility (V·cm−1·s−1) | Ref. |
|---|---|---|---|---|---|---|
| NiO | 100 | ~20–50 (visible region) | 0.5 | 7.26 × 1016 | 45.1 | [21] |
| NiO | 50 | ~83–88 (visible region) | – | ~4.4 × 1019 | – | [25] |
| NiO | 35 | 39 (at 550 nm) | 21.74 | 1.1 × 1021 | 0.1 | [29] |
| NiO | 100 | 73.4 (at 550 nm) | 2.86 × 10−5 | 1.13 × 1013 | 6.41 | [30] |
| NiO | – | ~40–50 (visible region) | – | 3.8 × 1017 | – | [31] |
| Parameters | Value | Parameters | Value | |||
|---|---|---|---|---|---|---|
| Background pressure (Pa) | <6.7 × 10−5 | Substrate | Corning 1737F glass | |||
| Working pressure (Pa) | 0.67 | Ion source | O2 | |||
| Working gas | Ar, O2 | Ion source power (W) | 150 | |||
| Target | NiO | Target power (W) | 400 | |||
| Hot substrate | No | Oxygen flow ratio | ||||
| Films thickness (nm) | 100 | O2/(O2+Ar) (%) | 0 | 10 | 50 | 100 |
| Ion Source Power (W) | Oxygen Content (at.%) |
|---|---|
| 80 | 59.5 |
| 100 | 67.5 |
| 120 | 68.6 |
| 150 | 69.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Chen, S.-C.; Peng, W.-C.; Wen, C.-K.; Wang, X.; Chuang, T.-H. The Influence of Oxygen Flow Ratio on the Optoelectronic Properties of p-Type Ni1?xO Films Deposited by Ion Beam Assisted Sputtering. Coatings 2018, 8, 168. https://doi.org/10.3390/coatings8050168
Sun H, Chen S-C, Peng W-C, Wen C-K, Wang X, Chuang T-H. The Influence of Oxygen Flow Ratio on the Optoelectronic Properties of p-Type Ni1?xO Films Deposited by Ion Beam Assisted Sputtering. Coatings. 2018; 8(5):168. https://doi.org/10.3390/coatings8050168
Chicago/Turabian StyleSun, Hui, Sheng-Chi Chen, Wen-Chi Peng, Chao-Kuang Wen, Xin Wang, and Tung-Han Chuang. 2018. "The Influence of Oxygen Flow Ratio on the Optoelectronic Properties of p-Type Ni1?xO Films Deposited by Ion Beam Assisted Sputtering" Coatings 8, no. 5: 168. https://doi.org/10.3390/coatings8050168
APA StyleSun, H., Chen, S.-C., Peng, W.-C., Wen, C.-K., Wang, X., & Chuang, T.-H. (2018). The Influence of Oxygen Flow Ratio on the Optoelectronic Properties of p-Type Ni1?xO Films Deposited by Ion Beam Assisted Sputtering. Coatings, 8(5), 168. https://doi.org/10.3390/coatings8050168