Effect of TiO2 Sol and PTFE Emulsion on Properties of Cu–Sn Antiwear and Friction Reduction Coatings
Abstract
:1. Introduction
2. Experimental Section
2.1. Electroplating Processes and Methods
2.2. Characterization of Plating Solution and Cu–Sn–PTFE–TiO2 Composite Coatings
3. Results and Discussion
3.1. Analysis of Plating Solution
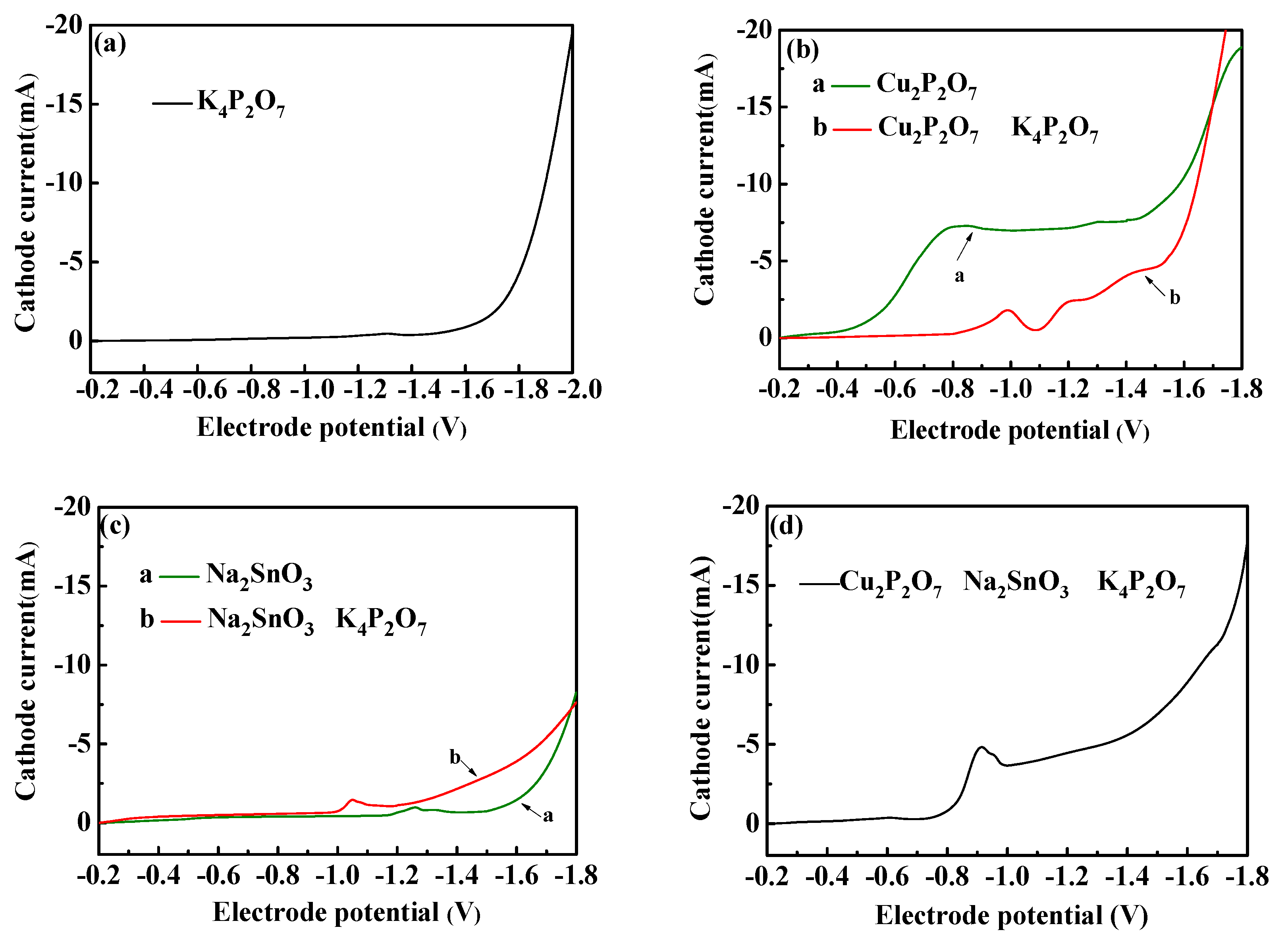
3.1.1. Electrodepositional Behavior of Basic Solution
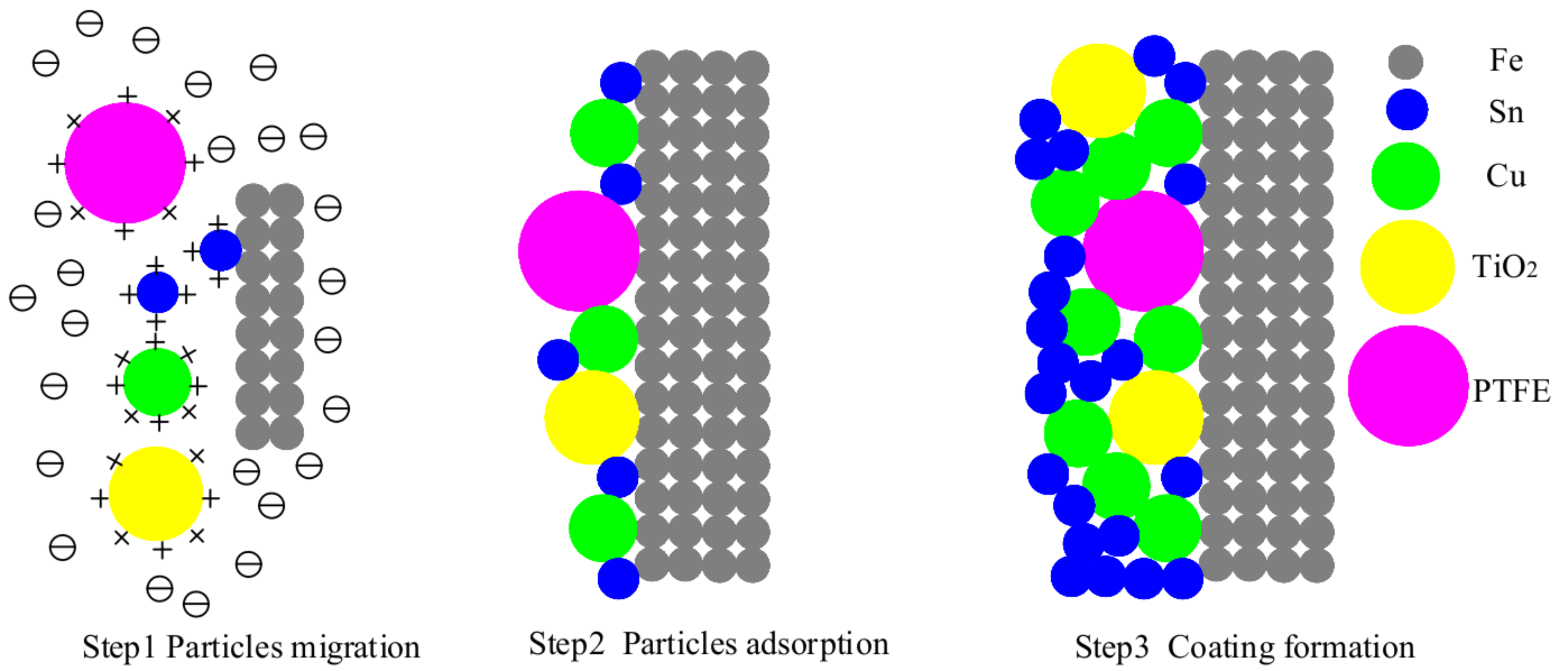
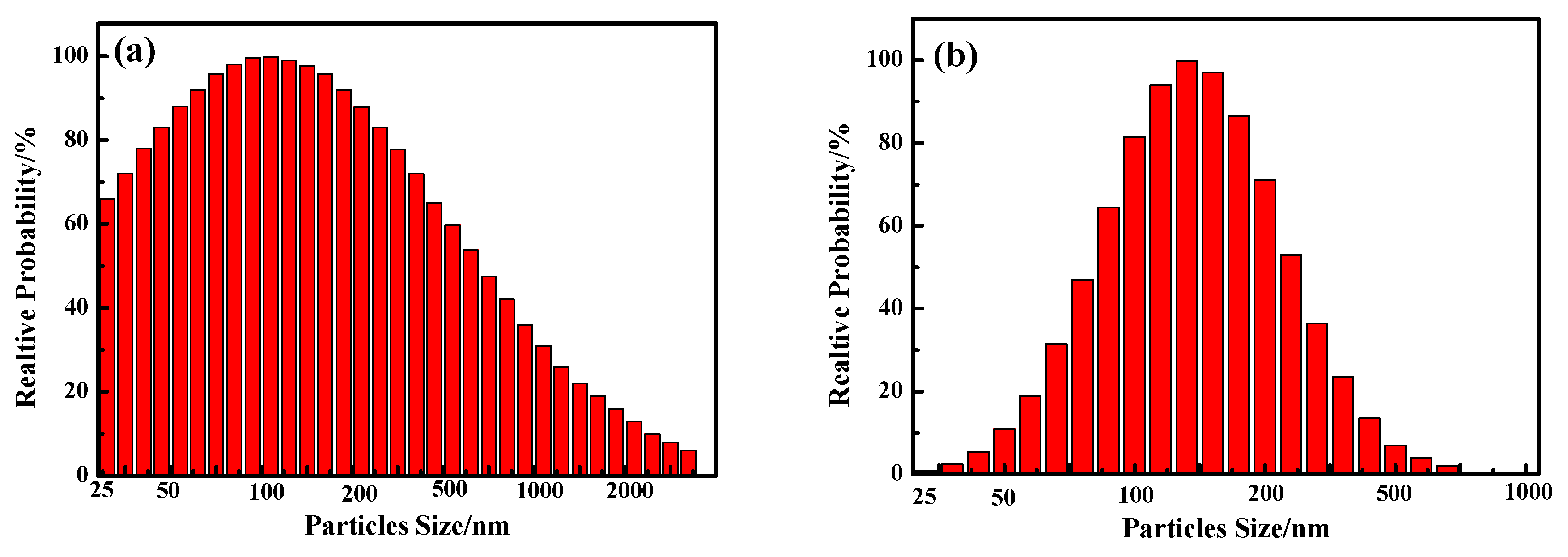
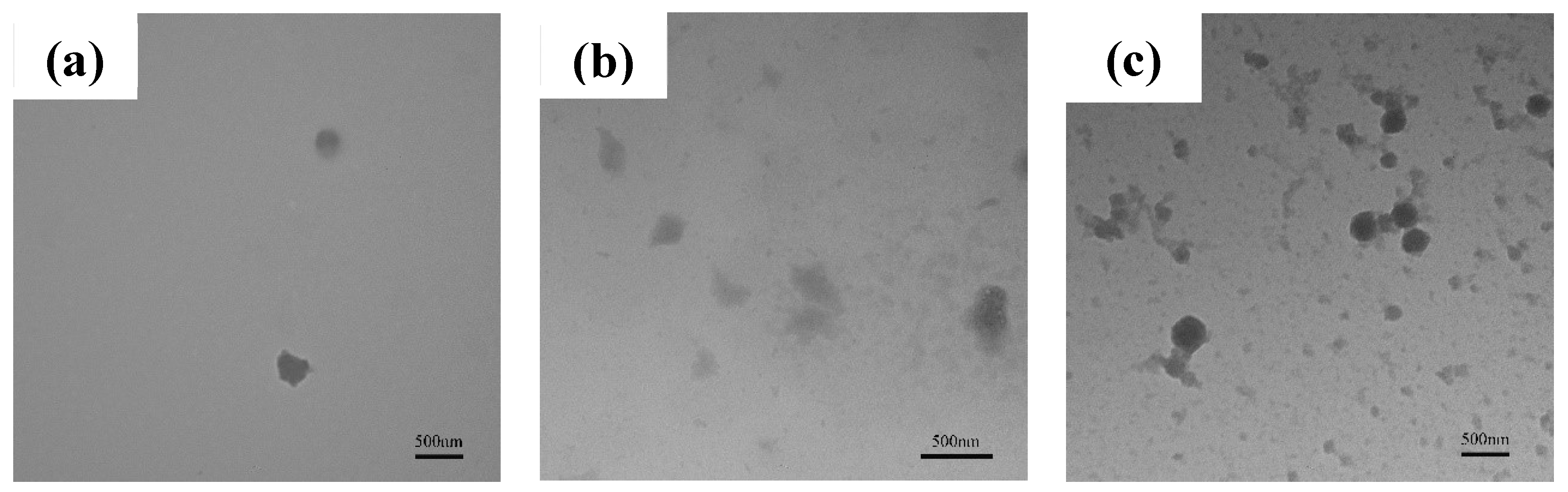
3.1.2. Analysis of TiO2 and PTFE particles
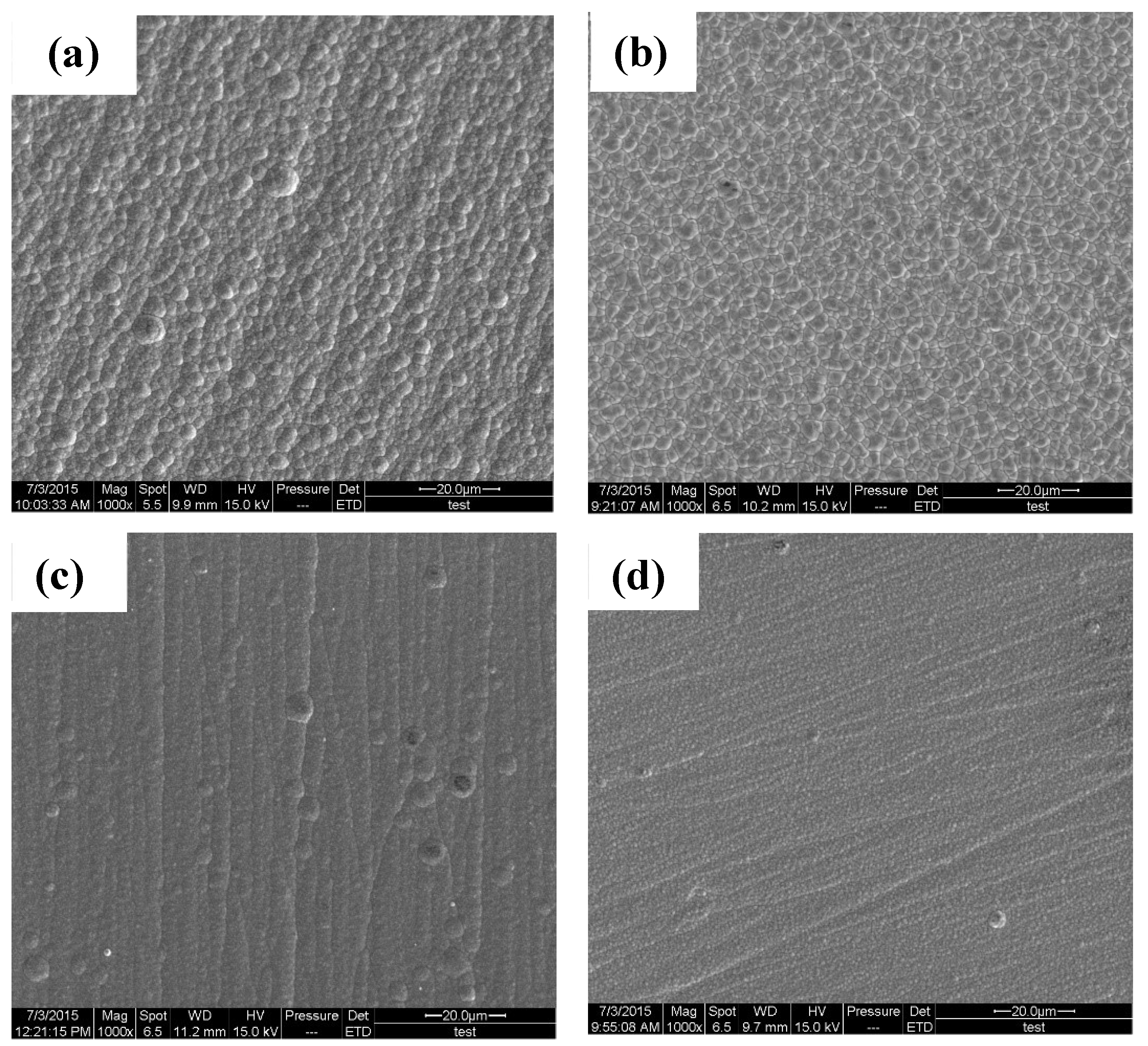
3.2. Microstructure and Composition of the Coatings
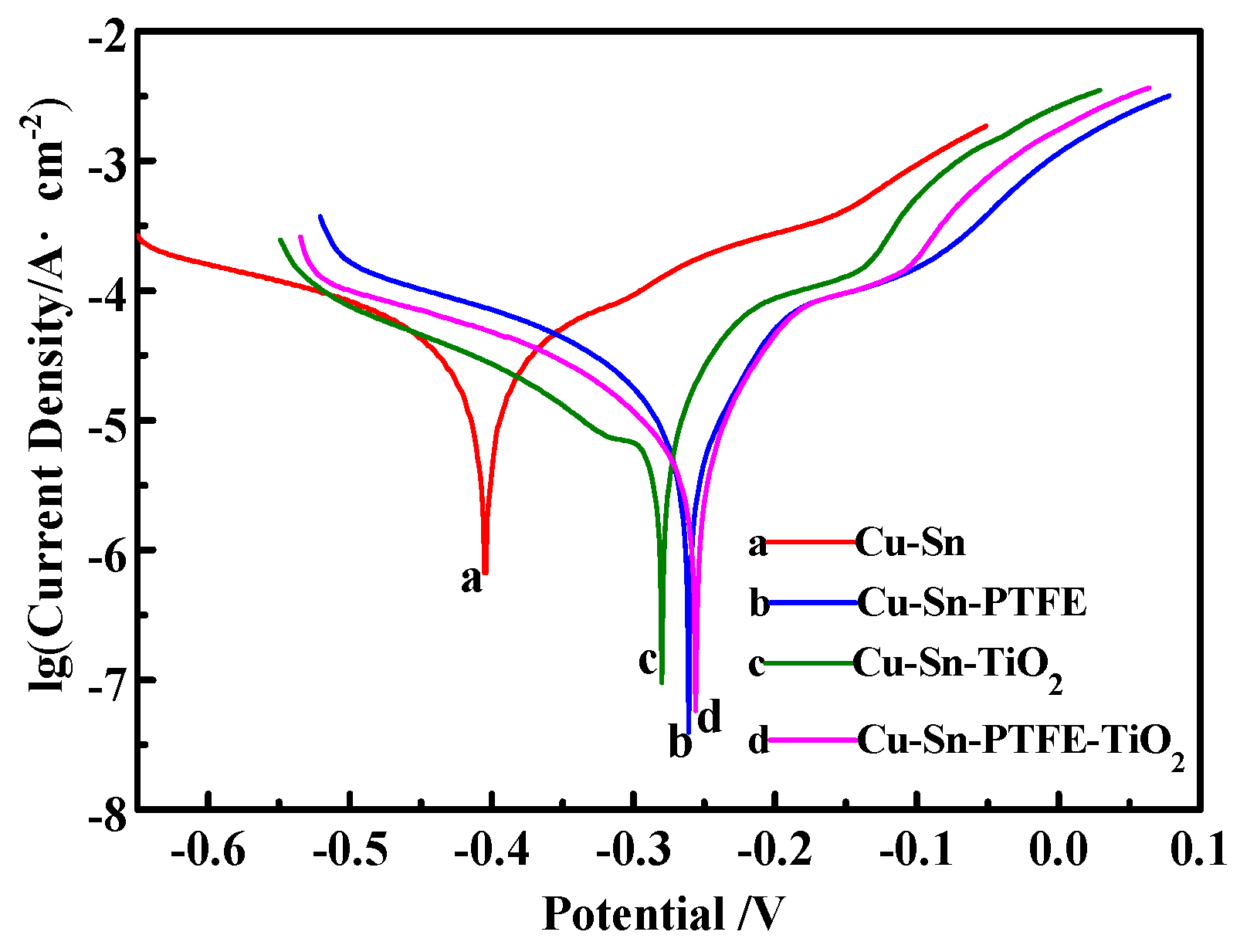
3.3. Corrosion Properties
3.4. Mechanical Properties
3.4.1. Hardness of Coatings
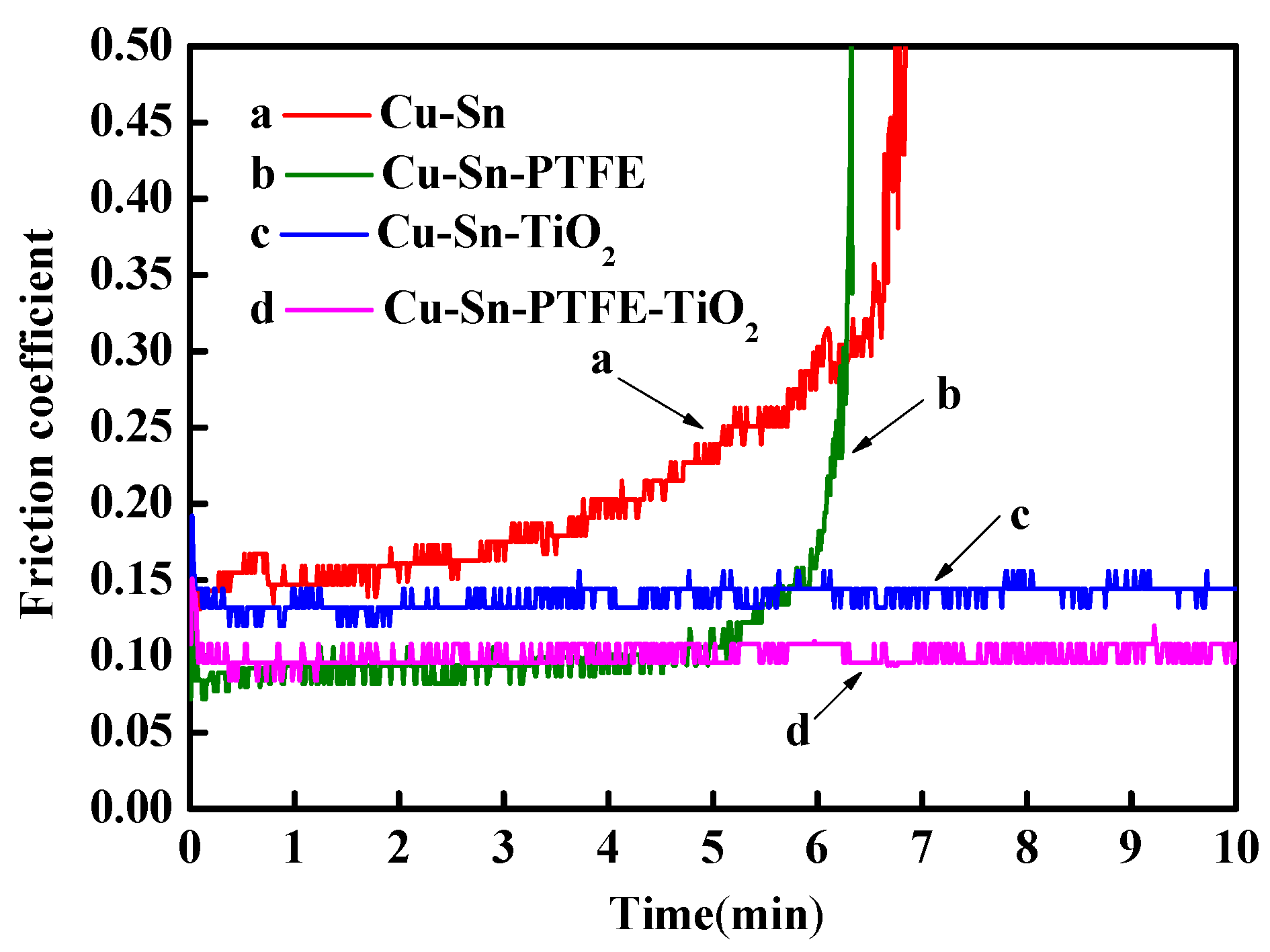
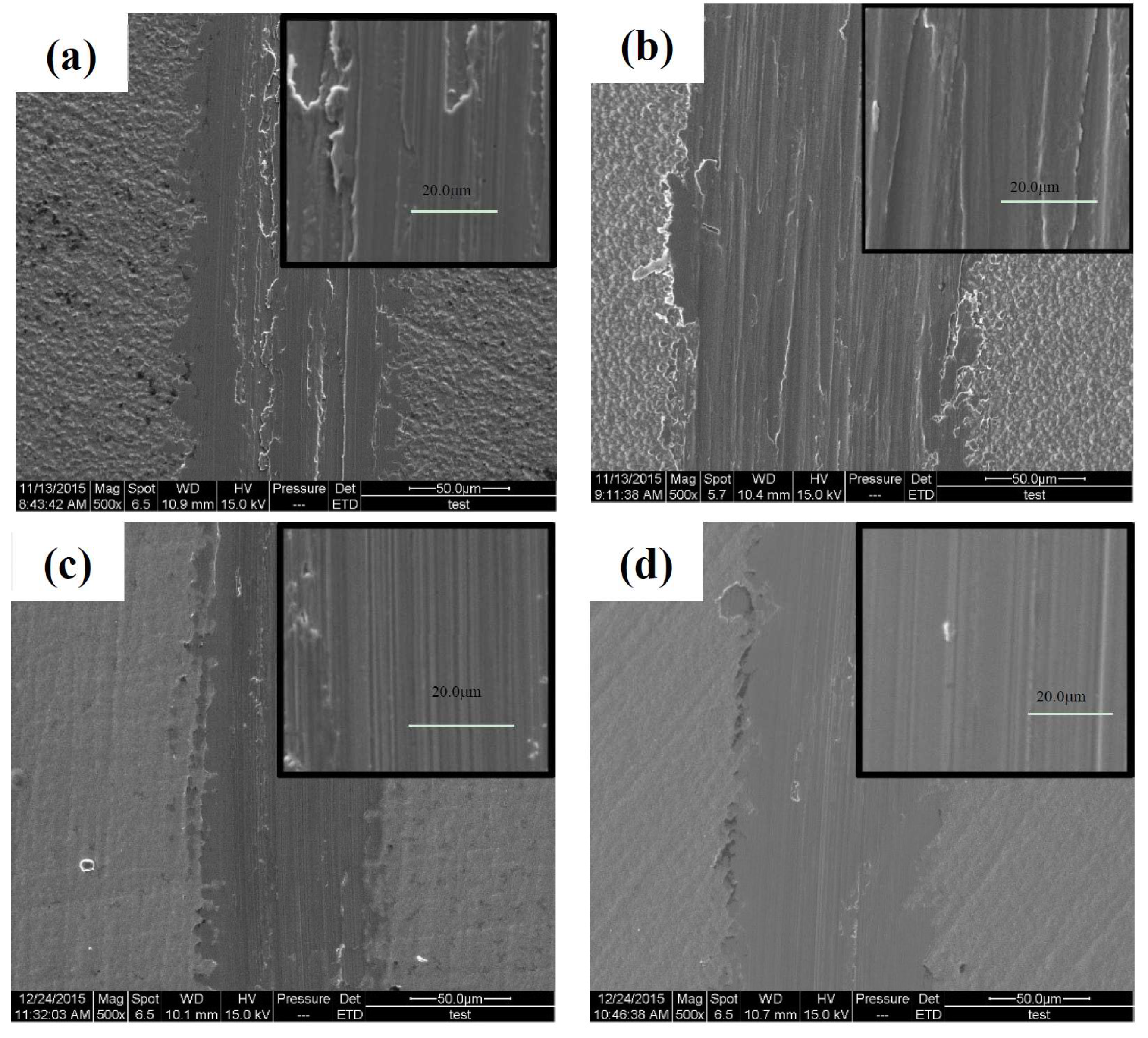
3.4.2. Tribological Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pewnim, N.; Roy, S. Electrodeposition of tin-rich Cu–Sn alloys from a methanesulfonic acid electrolyte. Electrochim. Acta 2013, 90, 498–506. [Google Scholar] [CrossRef]
- Zanella, C.; Xing, S.; Deflorian, F. Effect of electrodeposition parameters on chemical and morphological characteristics of Cu–Sn coatings from a methanesulfonic acid electrolyte. Surf. Coat. Technol. 2013, 236, 394–399. [Google Scholar] [CrossRef]
- Subramanian, B.; Mohan, S.; Jayakrishnan, S. Structural, microstural and corrosion properties of brush plated Copper–Tin alloy coatings. Surf. Coat. Technol. 2006, 201, 1145–1151. [Google Scholar] [CrossRef]
- Bengoa, L.N.; Pary, P.; Conconi, M.S.; Egli, W.A. Electrodeposition of Cu–Sn alloys from a methanesulfonic acid electrolyte containing benzyl alcohol. Electrochim. Acta 2017, 256, 211–219. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Q.; Zhu, S.; Yang, J.; Liu, W. Tribological properties of bronze–graphite composites under sea water condition. Tribol. Int. 2012, 53, 76–86. [Google Scholar] [CrossRef]
- Mallikarjuna, H.M.; Kashyap, K.T.; Koppad, P.G.; Ramesh, C.S.; Keshavamurthy, R. Microstructure and dry sliding wear behavior of Cu–Sn alloy reinforced with multiwalled carbon nanotubes. Trans. Nonferr. Met. Soc. China 2016, 26, 1755–1764. [Google Scholar] [CrossRef]
- Balaji, R.; Pushpavanam, M.; Kumar, K.Y.; Subramanian, K. Electrodeposition of bronze–PTFE composite coatings and study on their tribological characteristics. Surf. Coat. Technol. 2006, 201, 3205–3211. [Google Scholar] [CrossRef]
- Du, C.J.; Li, W.W.; Guo, D.P.; Liu, X.G.; Wang, Y.L.; Zhang, L.; Nan, Y. Electrodeposition of Cu–Sn-Zn–PTFE composite coating and its self-lubricating property. Electroplat. Pollut. Control. 2017, 5, 12–14. (In Chinese) [Google Scholar]
- Banerjee, A.; Kumar, R.; Dutta, M.; Bysakh, S.; Bhowmick, A.K.; Laha, T. Microstructural evolution in Cu–Sn coatings deposited on steel substrate and its effect on interfacial adhesion. Surf. Coat. Technol. 2015, 262, 200–209. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, L.; Shen, B.; Hu, W. Investigation in electroless Ni–P–C (graphite)–SiC composite coating. Surf. Coat. Technol. 2006, 201, 441–445. [Google Scholar] [CrossRef]
- Su, F.; Zhang, S. Tribological properties of polyimide coatings filled with PTFE and surface-modified nano-Si3N4. J. Appl. Polym. Sci. 2014, 131, 383–390. [Google Scholar] [CrossRef]
- Sieh, R.; Le, H. Non-cyanide electrodeposited Ag–PTFE composite coating using direct or pulsed current deposition. Coatings 2016, 6, 31–44. [Google Scholar] [CrossRef]
- Xu, X.; Yao, W.; Xu, J.; Zhang, W.; Yang, L.; Deng, S. The influences of the plating’s hardness, abrasion and antifriction property caused by the adding of nano-Al2O3 and PTFE. Adv. Mater. Res. 2012, 517, 1683–1686. [Google Scholar] [CrossRef]
- Suiyuan, C.; Ying, S.; Hong, F.; Jing, L.; Liu, C.S.; Sun, K. Synthesis of Ni–P–PTFE-nano-Al2O3 composite plating coating on 45 steel by electroless plating. J. Compos. Mater. 2012, 46, 1405–1416. [Google Scholar] [CrossRef]
- Chen, W.; He, Y.; Gao, W. Synthesis of nanostructured Ni–TiO2 composite coatings by sol-enhanced electroplating. Electrochem. Soc. 2010, 157, E122–E128. [Google Scholar] [CrossRef]
- Chen, W.; Gao, W. Thermal stability and tensile properties of sol-enhanced nanostructured Ni–TiO2 composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1627–1634. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Shu, X.; Gao, W.; Lu, W.; Yan, B. Preparation and property of sol-enhanced Ni–B–TiO2 nano-composite coatings. J. Alloy. Compd. 2014, 617, 472–478. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, Y.; Wei, S.; Gao, W.; Lu, W.; Yan, B. Au–Ni–TiO2 nano-composite coatings prepared by sol-enhanced method. J. Electrochem. Soc. 2014, 161, 775–781. [Google Scholar] [CrossRef]
- Ying, L.; Li, Z.; Wu, K.; Lv, X.; Wang, G. Effect of TiO2 sol on the microstructure and tribological properties of Cu–Sn coating. Rare Met. Mater. Eng. 2017, 46, 2801–2806. [Google Scholar] [CrossRef]


| Compositions/Parameters | Quantity |
|---|---|
| K4P2O7·3H2O | 260–270 g/L |
| Cu2P2O7·4H2O | 20 g/L |
| KNaC4H4O6·4H2O | 30–35 g/L |
| Na2SnO3·3H2O | 40 g/L |
| KNO3 | 40 g/L |
| Na3C6H5O7·2H2O | 20 g/L |
| PTFE | 15 g/L |
| TiO2 sol | 1 g/L |
| time | 1 h |
| pH | 9–10 |
| speed | 100 r/min |
| temperature | 35–40 °C |
| Content | Cu–Sn | Cu–Sn–PTFE | Cu–Sn–TiO2 | Cu–Sn–PTFE–TiO2 |
|---|---|---|---|---|
| PTFE (g/L) | 0 | 15 | 0 | 15 |
| TiO2 (g/L) | 0 | 0 | 1 | 1 |
| Conversion Coatings | Cu (wt %) | Sn (wt %) | PTFF (wt %) | TiO2 (wt %) |
|---|---|---|---|---|
| Cu–Sn | 92.42 | 7.58 | — | — |
| Cu–Sn–PTFE | 87.21 | 6.68 | 6.11 | — |
| Cu–Sn–TiO2 | 90.71 | 6.76 | — | 1.54 |
| Cu–Sn–PTFE–TiO2 | 86.84 | 6.46 | 5.50 | 1.20 |
| Coatings | Corrosion Potential/V | Corrosion Current Density/A·cm−2 |
|---|---|---|
| Cu–Sn | −0.405 | 4.067 |
| Cu–Sn–PTFE | −0.261 | 1.856 |
| Cu–Sn–TiO2 | −0.280 | 2.434 |
| Cu–Sn–PTFE–TiO2 | −0.256 | 1.443 |
| Coating | Hardness/HV |
|---|---|
| Cu–Sn | 413 ± 4 |
| Cu–Sn–PTFE | 375 ± 6 |
| Cu–Sn–TiO2 | 485 ± 6 |
| Cu–Sn–PTFE–TiO2 | 465 ± 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, L.; Fu, Z.; Wu, K.; Wu, C.; Zhu, T.; Xie, Y.; Wang, G. Effect of TiO2 Sol and PTFE Emulsion on Properties of Cu–Sn Antiwear and Friction Reduction Coatings. Coatings 2019, 9, 59. https://doi.org/10.3390/coatings9010059
Ying L, Fu Z, Wu K, Wu C, Zhu T, Xie Y, Wang G. Effect of TiO2 Sol and PTFE Emulsion on Properties of Cu–Sn Antiwear and Friction Reduction Coatings. Coatings. 2019; 9(1):59. https://doi.org/10.3390/coatings9010059
Chicago/Turabian StyleYing, Lixia, Zhen Fu, Ke Wu, Chunxi Wu, Tengfei Zhu, Yue Xie, and Guixiang Wang. 2019. "Effect of TiO2 Sol and PTFE Emulsion on Properties of Cu–Sn Antiwear and Friction Reduction Coatings" Coatings 9, no. 1: 59. https://doi.org/10.3390/coatings9010059
APA StyleYing, L., Fu, Z., Wu, K., Wu, C., Zhu, T., Xie, Y., & Wang, G. (2019). Effect of TiO2 Sol and PTFE Emulsion on Properties of Cu–Sn Antiwear and Friction Reduction Coatings. Coatings, 9(1), 59. https://doi.org/10.3390/coatings9010059




