Preparation of Silver-Plated Para-Aramid Fiber by Employing Low-Temperature Oxygen Plasma Treatment and Dopamine Functionalization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Silver-Coated PPTA Fibers
2.2.1. Cleaning Oil on The Surface of PPTA Fibers
2.2.2. Oxygen Plasma Treatment of PPTA Fibers
2.2.3. Dopamine Functionalization of PPTA Fibers
2.2.4. Electroless Silver Plating on Fiber Surface
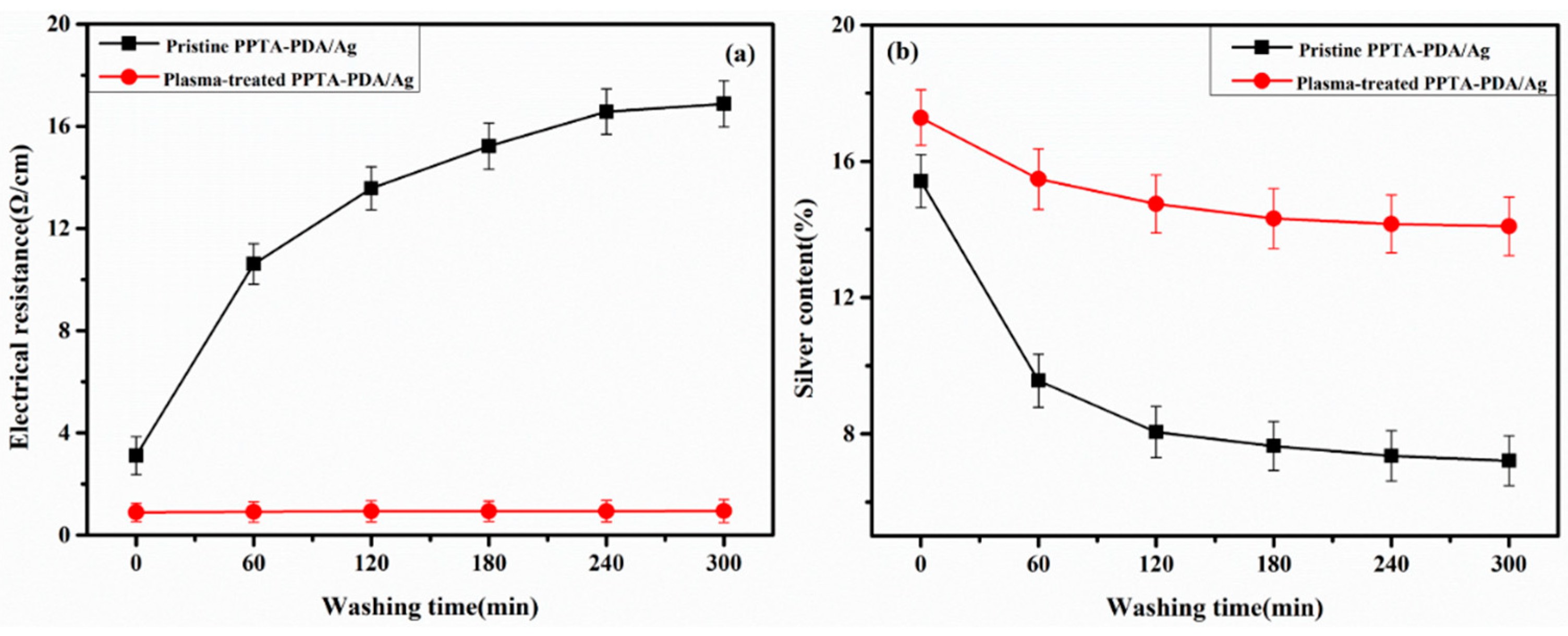
2.3. Washing Fastness Test of Silver-Plated PPTA Fiber Bundles
2.4. Tensile Strain Test of Silver-Plated PPTA Fiber Bundles
2.5. Characterization
3. Results and Discussion
3.1. Oxygen Plasma Treatment and Dopamine Functionalization
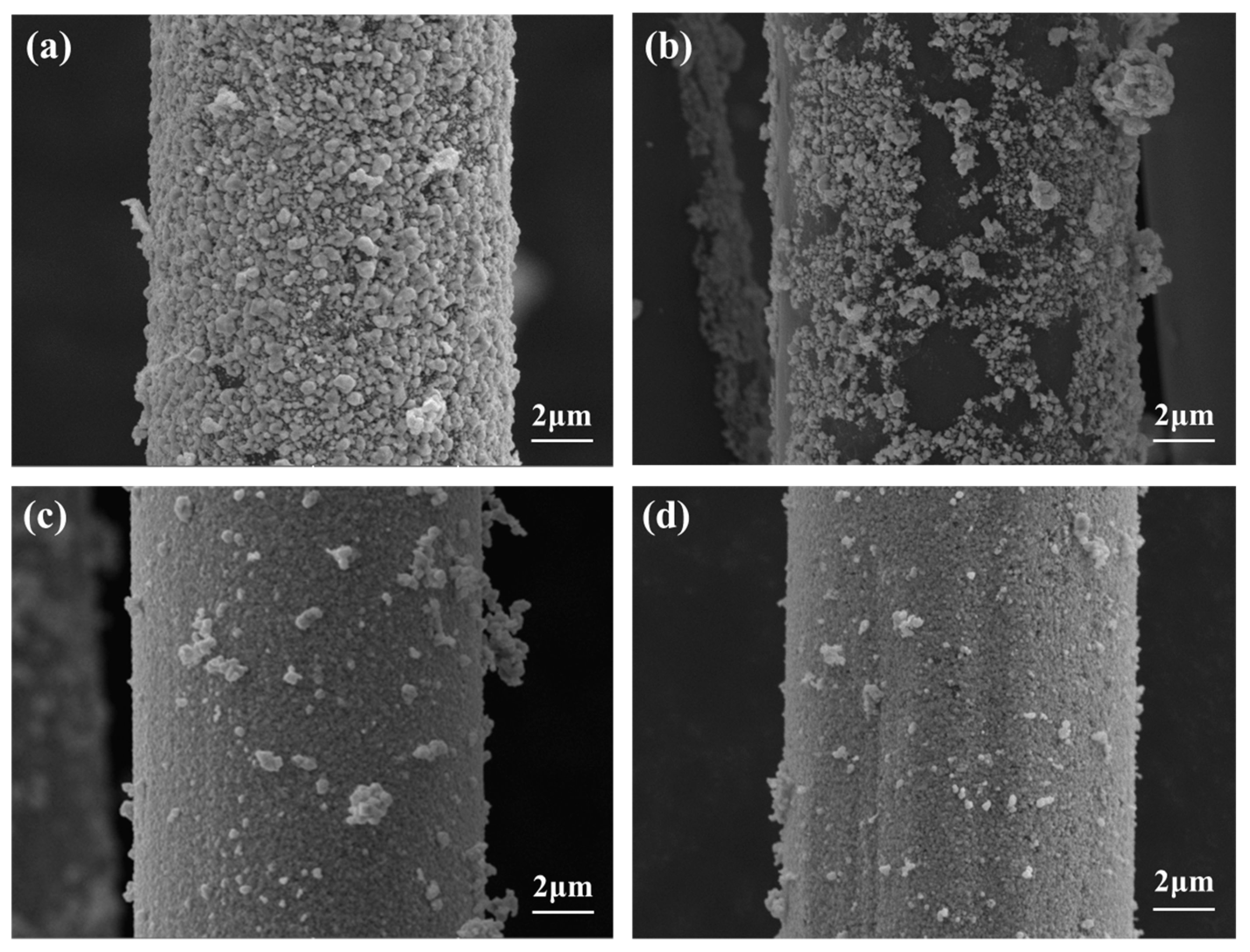
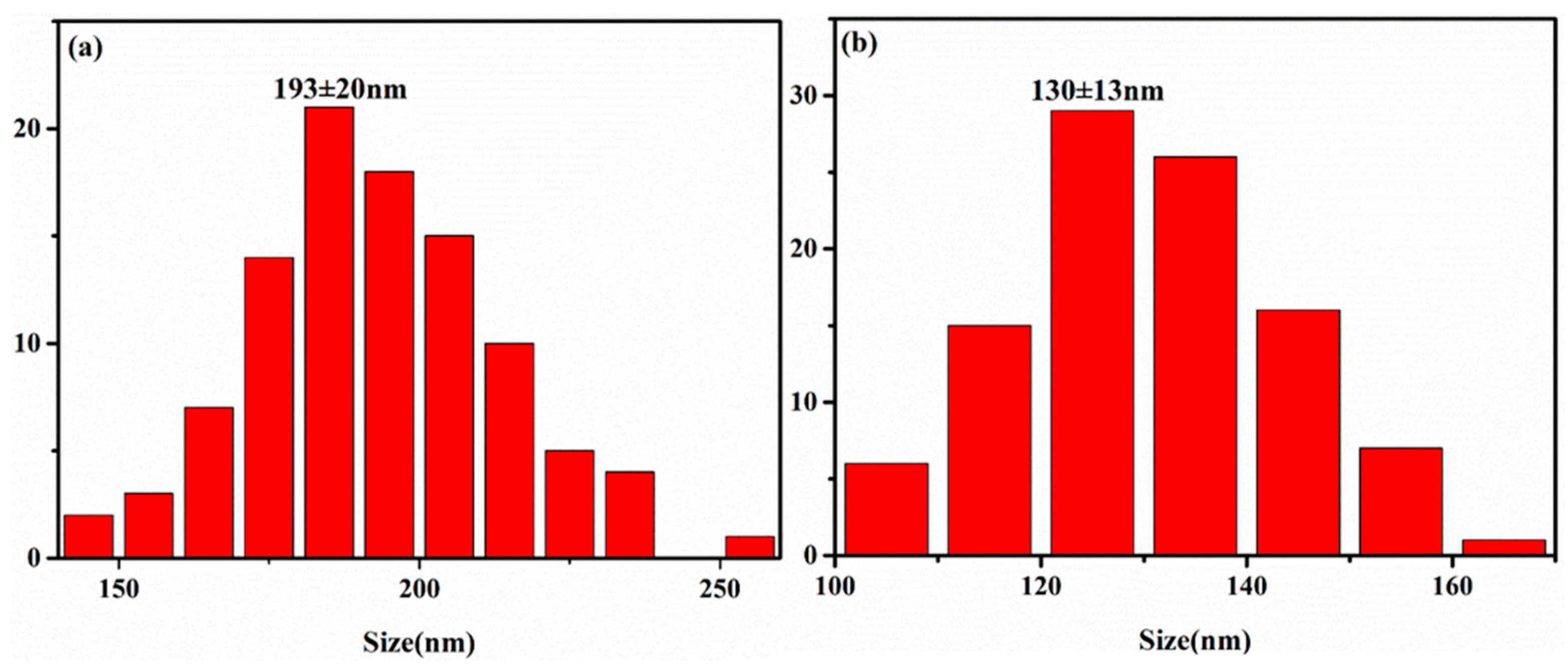
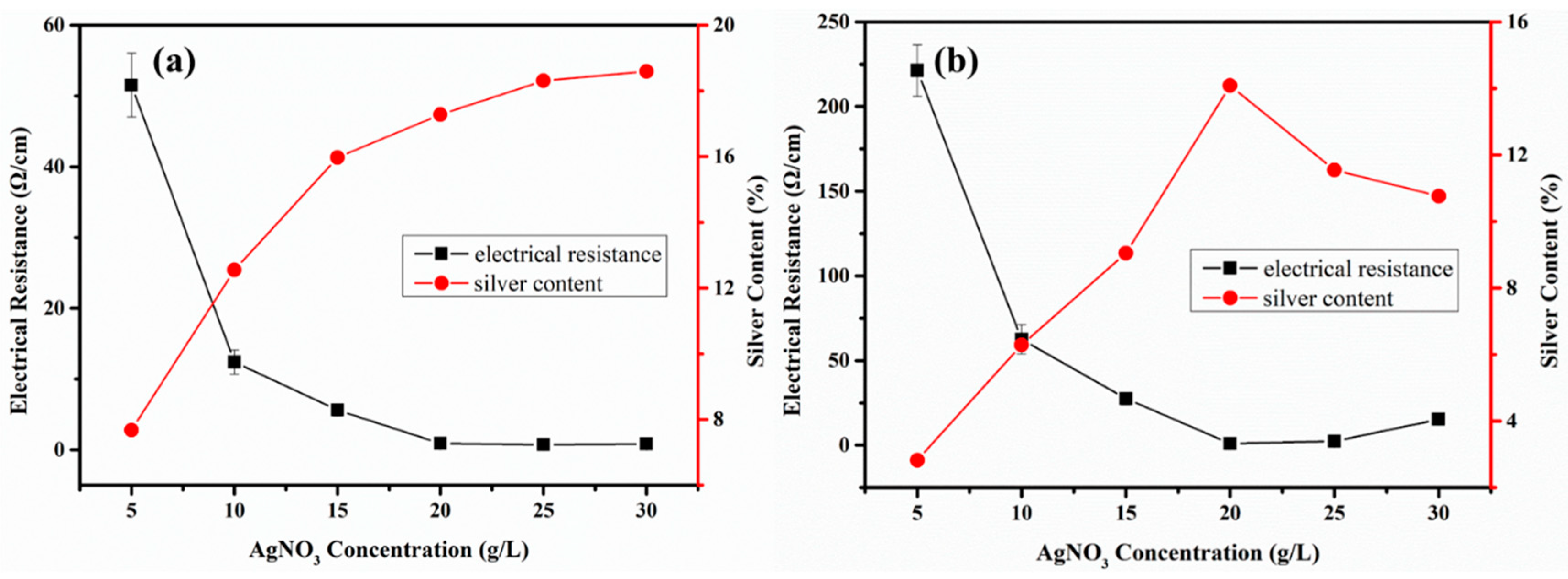
3.2. Electroless Silver Plating on PPTA Fiber Surface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Onggar, T.; Häntzsche, E.; Hund, R.; Cherif, C. Multiple functional coating highly inert fiber surfaces of para-aramid filament yarn. Mater. Res. Express 2018, 5, 095702. [Google Scholar] [CrossRef]
- Trexler, M.M.; Hoffman, C.; Smith, D.A.; Montalbano, T.J.; Yeager, M.P.; Trigg, D.; Nimer, S.; Calderon-Colon, X.; Peitsch, C.; Xia, Z.Y. Synthesis and mechanical properties of para-aramid nanofibers. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 563–573. [Google Scholar] [CrossRef]
- Kang, C.; Kim, S.S.; Ahn, D.; Kim, S.J.; Lee, J. Effective surface attachment Ag nanoparticles on fibers using glycidyltrimethylammonium chloride and improvement of antimicrobial properties. RSC Adv. 2017, 7, 23407–23414. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, X.; Liang, J.; Ma, X.; Tang, Z.; Sun, J. Development of electroless silver plating on para-aramid fibers and growth morphology of silver deposits. J. Appl. Polym. Sci. 2012, 124, 3363–3371. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Y.; Guo, Y.; Jiang, Z.; Chen, S.; Ma, J. Preparation of silver nanoparticle functionalized polyamide fibers with antimicrobial activity and electrical conductivity. J. Appl. Polym. Sci. 2019, 136, 47584. [Google Scholar] [CrossRef]
- Guo, M.; Yi, X.; Rudd, C.; Liu, X. Preparation of highly electrical conductive carbon-fiber composites with interlaminar fracture toughness by using silver-plated interleaves. Compos. Sci. Technol. 2019, 176, 29–36. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, J.; Li, X.; Yue, P.; Gu, Z.; Ogino, K. Electroless plate of polyaniline-silver composite layer on polyester fibers. J. Polym. Eng. 2019, 39, 161–169. [Google Scholar] [CrossRef]
- Mondin, G.; Wisser, F.M.; Leifert, A.; Mohamed-Noriega, N.; Grothe, J.; Dorfler, S.; Kaskel, S. Metal deposition by eletroless plating on polydopamine functionalized micro-and nanoparticles. J. Colloid Interface Sci. 2013, 411, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Zhang, G.; Dai, F.; Zhang, F. Mild surface modification of para-aramid fiber by dilute sulfuric acid under microwave irradiation. Text. Res. J. 2017, 87, 799–806. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Zhang, L.Q.; Wang, W.C. Highly conductive one-dimensional nanofibers: Silvered electrospun silica nanofibers via poly(dopamine) functionalization. ACS Appl. Mater. Interfaces 2014, 6, 5105–5112. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, W.; Tian, M.; Zou, H.; Li, L.; Zhang, L. Preparation of PET/Ag hybrid fibers via a biomimetic surface functionalization method. Eletrochim. Acta 2012, 79, 37–45. [Google Scholar] [CrossRef]
- Liao, Y.; Cao, B.; Wang, W.; Zhang, L.; Wu, D.; Jin, R. A facile method for preparing highly conductive and reflective surface-silvered polyimide films. Appl. Surf. Sci. 2009, 255, 8207–8212. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, Y.; Feng, X.; Wang, W.; Xu, F.; Zhang, L. Antibacterial surfaces through dopamine functionalization and silver nanoparticle immobilization. Mater. Chem. Phys. 2010, 121, 534–540. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, L.; Xue, J.; Hao, L.; Li, J.; He, Y.; Cheng, B. A novel two-step method for fabricating silver plating cotton fabrics. J. Nanomater. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, W.; Yu, D. Conductive, antibacterial, and electromagnetic shielding silver-plated cotton fabrics activated by dopamine. J. Appl. Polym. Sci. 2018, 135, 46766. [Google Scholar] [CrossRef]
- Wang, R.; Shin, C.H.; Park, S.; Cui, L.Z.; Kim, D.; Park, J.-S.; Ryu, M. Enhanced antibacterial activity of silver-coated kapok fibers through dopamine functionalization. Water Air Soil Pollut. 2015, 226, 2241. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, L.; He, Y.; Cheng, B. A novel method for fabricating elastic conductive polyurethane filaments by in-situ reduction of polydopamine and electroless silver plating. Mater. Des. 2017, 113, 254–263. [Google Scholar] [CrossRef]
- Hu, W.; Zeng, Z.; Wang, Z.; Liu, C.; Wu, X.; Gu, Q. Facile fabrication of conductive ultrahigh molecular weight polyethylene fibers via mussel-inspired deposition. J. Appl. Polym. Sci. 2013, 128, 1030–1035. [Google Scholar] [CrossRef]
- Shen, L.; Yu, L.; Wang, M.; Wang, X.; Zhu, M.; Hsiao, B.S. Green fabrication of Ag coated polyacrylonitrile nanofibrous composite membrane with high catalytic efficiency. J. Nanosci. Nanotechnol. 2015, 15, 5004–5012. [Google Scholar] [CrossRef]
- Mao, Y.; Zhu, M.F.; Wang, W.; Yu, D. Well-defined silver conductive pattern fabricated on polyester fabric by screen printing a dopamine surface modifier followed by electroless plating. Soft Matter 2018, 14, 1260–1269. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, X.; Xu, T.; Song, C.; Ogino, K.; Gu, Z. Preparation of conductive polyester fibers using continuous two-step plating silver. Materials 2018, 11, 2033. [Google Scholar] [CrossRef]
- Ma, S.Y.; Liu, L.; Bromberg, V.; Singler, T.J. Fabrication of highly electrically conducting fine patterns via substrate-independent inkjet printing of mussel-inspired organic nano-material. J. Mater. Chem. C 2014, 2, 3885–3889. [Google Scholar] [CrossRef]
- Xu, C.H.; Tian, M.; Liu, L.; Zou, H.; Zhang, L.; Wang, W. Fabrication and properties of silverized glass fiber by dopamine functionalization and electroless plating. J. Electrochem. Soc. 2012, 159, D217–D224. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Tian, M.; Liu, L.; Zou, H.; Zhao, X.; Zhang, L. Surface silverized meta-aramid fibers prepared by bio-inspired poly(dopamine) functionalization. ACS Appl. Mater. Interfaces 2013, 5, 2062–2069. [Google Scholar] [CrossRef]
- Yu, D.; Mu, S.; Liu, L.; Wang, W. Preparation of electroless silver plating on aramid fiber with good conductivity and adhesion strength. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 53–59. [Google Scholar] [CrossRef]
- Lin, F.; Li, W.; Tang, Y.; Shao, H.; Su, C.; Jiang, J.; Chen, N. High-performance polyimide filaments and composites improved by O2 plasma treatment. Polymers 2018, 10, 695. [Google Scholar] [CrossRef]
- Su, M.; Gu, A.J.; Liang, G.Z.; Yuan, L. The effect of oxygen-plasma treatment on Kevlar fibers and the properties of Kevlar fibers/bismaleimide composites. Appl. Surf. Sci. 2011, 257, 3158–3167. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Li, H.; Zhang, C.; Sun, B.; Zhang, X. The analysis of Armos fibers reinforced poly (phthalazinone ether sulfone ketone) composite surfaces after oxygen plasma treatment. Surf. Coat. Technol. 2008, 202, 4986–4991. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Li, H.; Li, W.; Wang, B.; Zhang, C.; Ren, N. Surface characteristic of poly (p-phenylene terephthalamide) fibers with oxygen plasma treatment. Surf. Interface Anal. 2008, 40, 1299–1303. [Google Scholar] [CrossRef]
- Sun, X.; Bu, J.; Liu, W.; Niu, H.; Qi, S.; Tian, G.; Wu, D. Surface modification of polyimide fibers by oxygen plasma treatment and interfacial adhesion behavior of a polyimide fiber/epoxy composite. Sci. Eng. Compos. Mater. 2017, 24, 477–484. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wei, Z.; Zhang, L.; Wang, W.; Tian, M. Surface modification of aramid fibers by bio-inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef]
- Chai, D.; Xie, Z.; Wang, Y.; Liu, L.; Yum, Y.J. Molecular dynamics investigation of the adhesion mechanism acting between dopamine and the surface of dopamine-processed aramid fibers. ACS Appl. Mater. Interfaces 2014, 6, 17974–17984. [Google Scholar] [CrossRef]
- Knorr, D.B.; Tran, N.T.; Gaskell, K.J.; Orlicki, J.A.; Woicik, J.C.; Jaye, C.; Fischer, D.A.; Lenhart, J.L. Synthesis and characterization of aminopropyltriethoxysilane-polydopamine coatings. Langmuir 2016, 32, 4370–4381. [Google Scholar] [CrossRef]
- Nimisha, S.; Nayak, J.; Khushbu, P.; Sahoo, S.K.; Rajender, K. Electrochemical impedance spectroscopy reveals new mechanism based on competitive binding between Tris and protein on conductive biomimetic polydopamine surface. Phys. Chem. Chem. Phys. 2018, 20, 25812–25821. [Google Scholar]
- Ball, V.; Frari, D.D.; Toniazzo, V.; Rush, D. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: Insights in the polydopamine deposition mechanism. J. Colloid Interface Sci. 2012, 386, 366–372. [Google Scholar] [CrossRef]
- Vecchia, N.F.D.; Luchini, A.; Napolitano, A.; D’Errico, G.; Vitiello, G.; Szekely, N.; d’Ischia, M.; Paduano, L. Tris buffer modulates polydopamine growth, aggregation, and paramagnetic properties. Langmuir 2014, 30, 9811–9818. [Google Scholar] [CrossRef]
- GB/T 3921-2008, Textiles-Tests for Colour Fastness-Colour Fastness to Washing with Soap or Soap and Soda; Standards Press of China: Beijing, China, 2008.
- Li, X.; Shan, H.; Cao, M.; Li, B. Mussel-inspired modification of PTFE membranes in a miscible THF-Tris buffer mixture for oil-in-water emulsions separation. J. Membr. Sci. 2018, 555, 237–249. [Google Scholar] [CrossRef]
- Li, S.; Han, K.; Rong, H.; Li, X.; Yu, M. Surface modification of aramid fibers via ammonia-plasma treatment. J. Appl. Polym. Sci. 2014, 131, 40250. [Google Scholar] [CrossRef]
- Xi, M.; Li, Y.; Shang, S.; Li, D.; Yin, Y.; Dai, X. Surface modification of aramid fiber by air DBD plasma at atmospheric pressure with continuous on-line processing. Surf. Coat. Technol. 2008, 202, 6029–6033. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Qiu, Y.; Zhang, C.; Jarrard, B.; Stedeford, R.; Tsai, J.; Park, Y.C.; McCord, M. Effects of atmospheric pressure helium/air plasma treatment on adhesion and mechanical properties of aramid fibers. J. Adhes. Sci. Technol. 2003, 17, 847–860. [Google Scholar] [CrossRef]
- Ma, Z.; Xu, R.; Wang, W.; Yu, D. A wearable, anti-bacterial strain sensor prepared by silver plated cotton/spandex blended fabric for human motion monitoring. Colloids Surf. A Physicochem. Eng. Asp. 2019, 123918. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wang, W.; Yu, D. Pressure responsive PET fabrics via constructing conductive wrinkles at room temperature. Chem. Eng. J. 2017, 330, 146–156. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhou, Y.; Li, W.; Chen, S.; You, S.; Ma, J. Preparation of Silver-Plated Para-Aramid Fiber by Employing Low-Temperature Oxygen Plasma Treatment and Dopamine Functionalization. Coatings 2019, 9, 599. https://doi.org/10.3390/coatings9100599
Sun Z, Zhou Y, Li W, Chen S, You S, Ma J. Preparation of Silver-Plated Para-Aramid Fiber by Employing Low-Temperature Oxygen Plasma Treatment and Dopamine Functionalization. Coatings. 2019; 9(10):599. https://doi.org/10.3390/coatings9100599
Chicago/Turabian StyleSun, Zhenhua, Yanfen Zhou, Wenyue Li, Shaojuan Chen, Shihua You, and Jianwei Ma. 2019. "Preparation of Silver-Plated Para-Aramid Fiber by Employing Low-Temperature Oxygen Plasma Treatment and Dopamine Functionalization" Coatings 9, no. 10: 599. https://doi.org/10.3390/coatings9100599



