Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities
Abstract
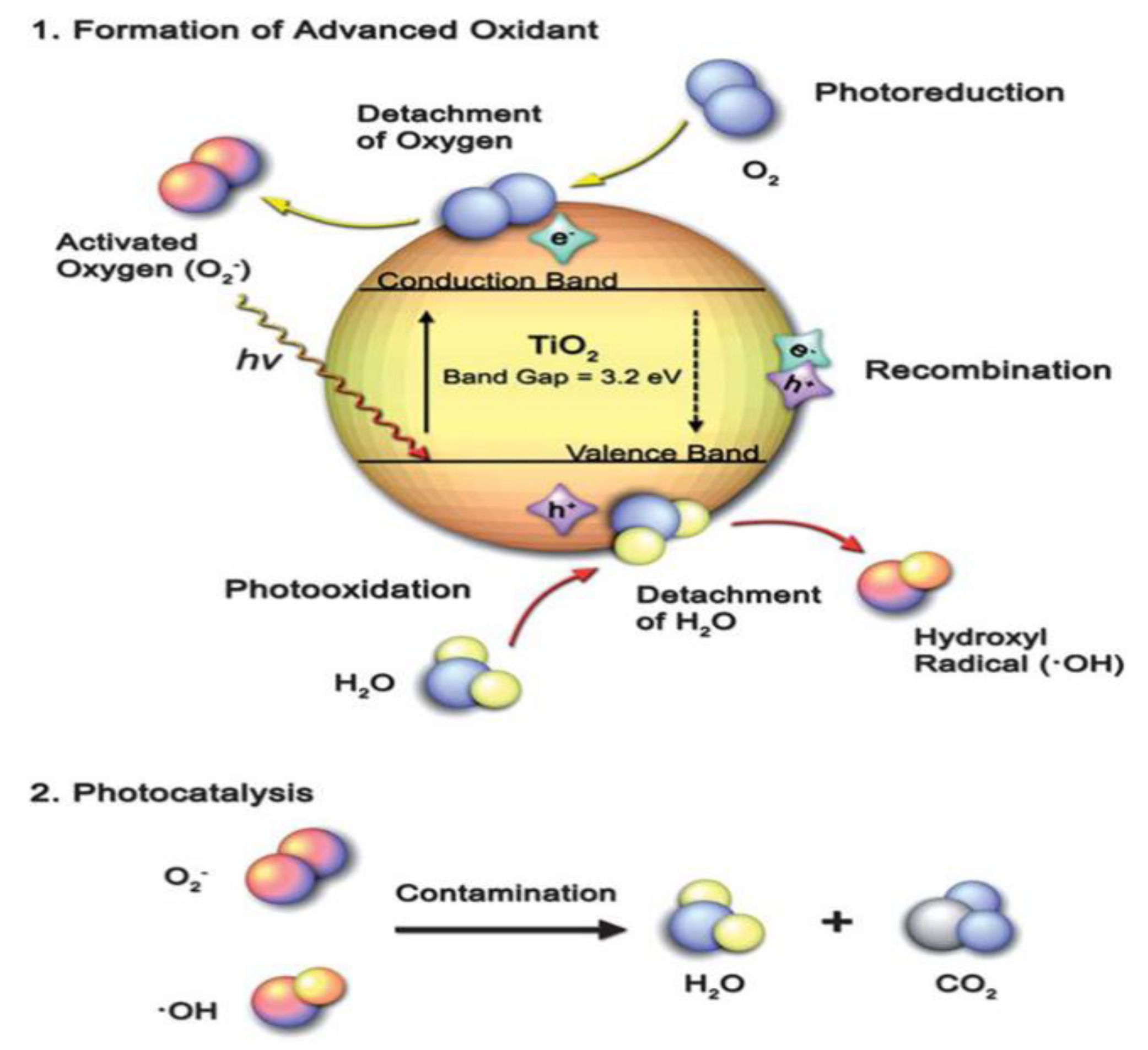
:1. Semiconductor Photocatalysis
2. TiO2 Photocatalyst
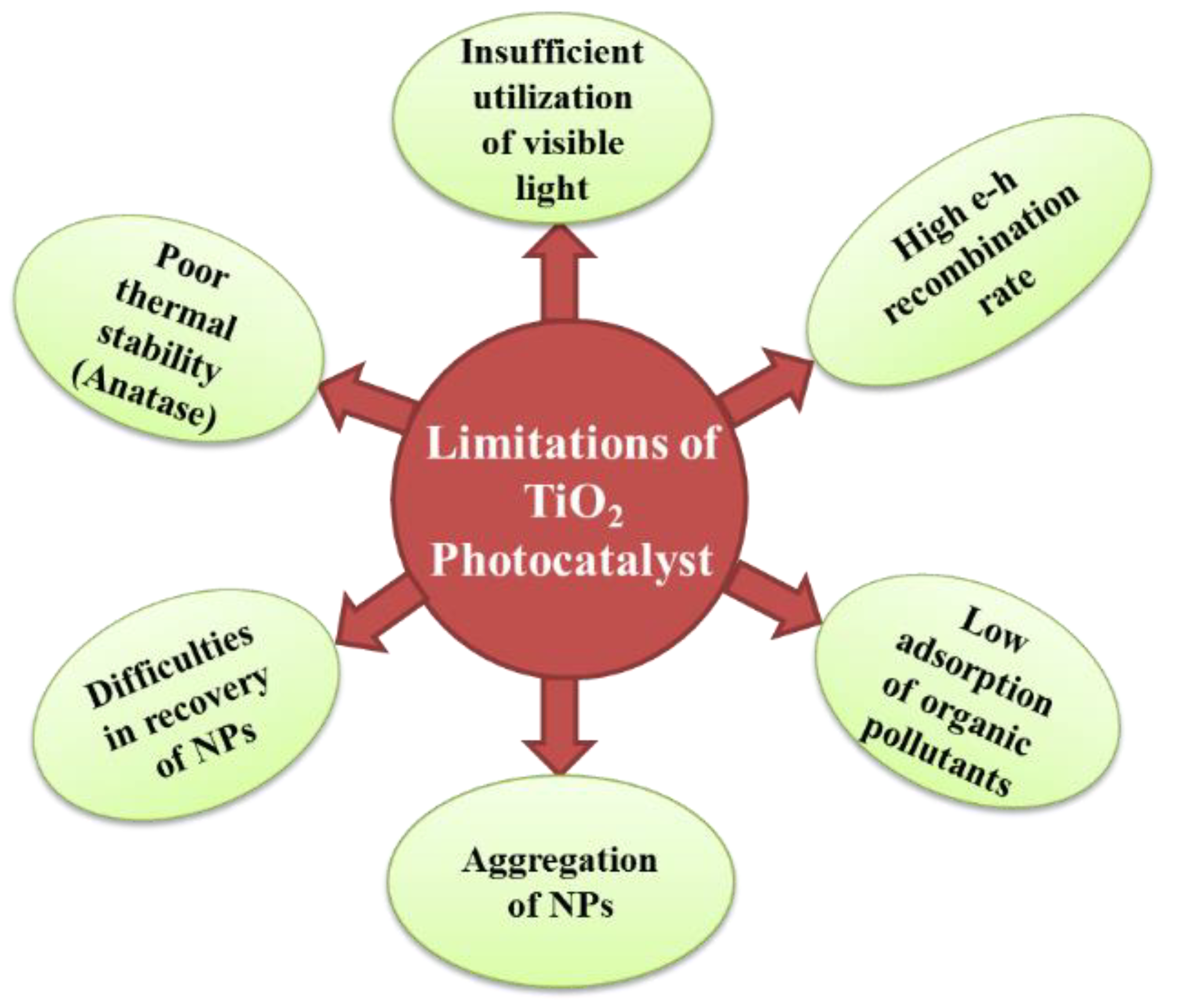
Limitations of TiO2 as a Photocatalyst
3. TiO2 Films
3.1. Preparation of TiO2 Film
3.2. Preparation of TiO2 Film by Sol-gel Method
3.3. TiO2 Film for Organic Dye Removal Application
3.3.1. Undoped TiO2 Films
3.3.2. Modified TiO2 Films
Metal-Doped TiO2 Films
Non-Metal Doped TiO2 Film
Binary Composite
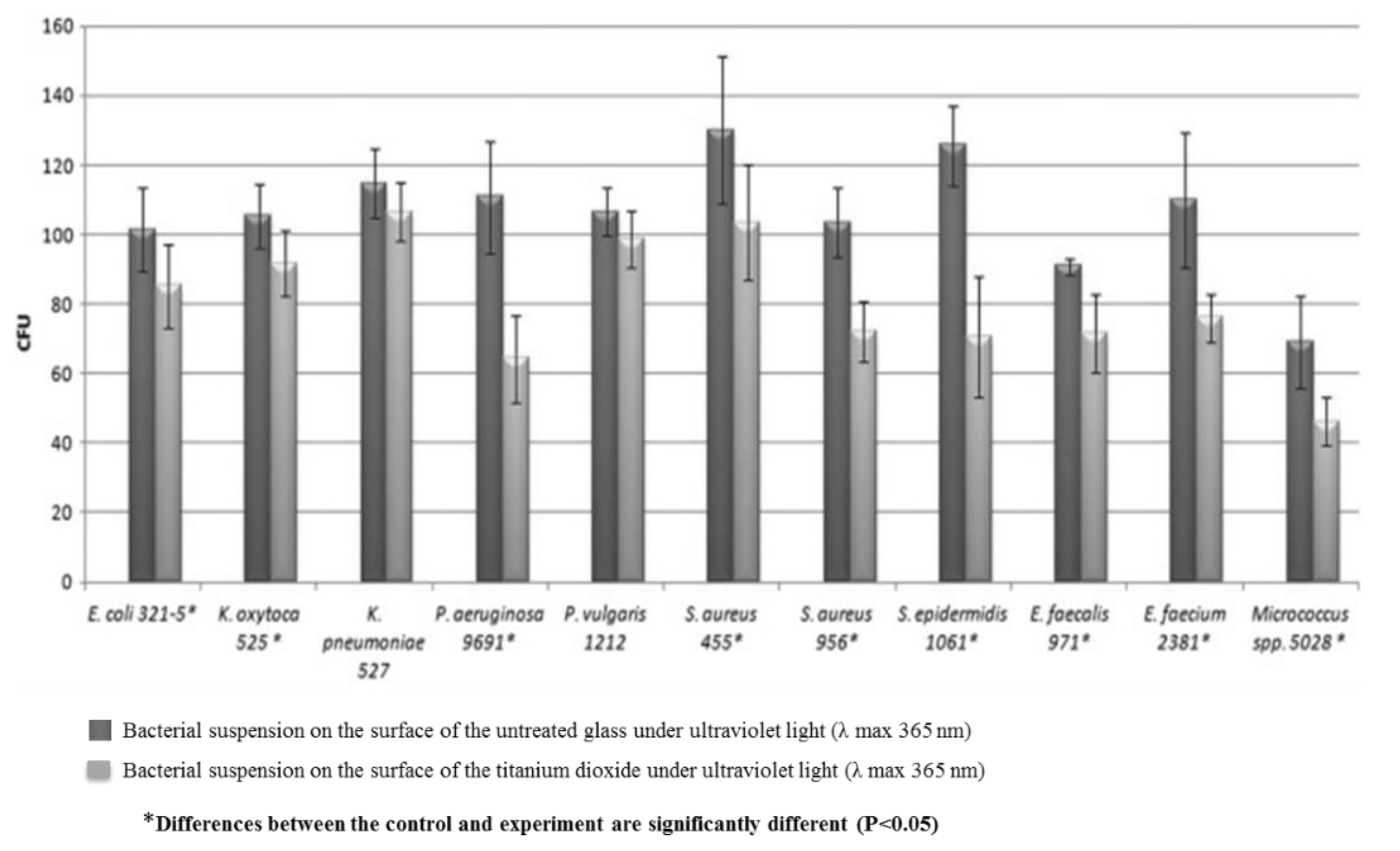
3.4. TiO2 Film for Antibacterial Application
4. Conclusions, Challenges and Future Perspectives
Funding
Conflicts of Interest
References
- Pant, B.; Pant, H.R.; Barakat, N.A.M.; Park, M.; Jeon, K.; Choi, Y.; Kim, H.-Y. Carbon nanofibers decorated with binary semiconductor (TiO2/ZnO) nanocomposites for the effective removal of organic pollutants and the enhancement of antibacterial activities. Ceram. Int. 2013, 39, 7029–7035. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Al-Deyab, S.S.; Kim, H.-C.; Khil, M.-S. Monodispersed 3D MnWO4–TiO2 composite nanoflowers photocatalysts for environmental remediation. Curr. Appl. Phys. 2015, 15, 753–758. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Kim, H.-Y.; Park, S.-J. Ag-ZnO photocatalyst anchored on carbon nanofibers: Synthesis, characterization, and photocatalytic activities. Synth. Met. 2016, 220, 533–537. [Google Scholar] [CrossRef]
- Opoku, F.; Govender, K.K.; van Sittert, C.G.C.E.; Govender, P.P. Recent Progress in the Development of Semiconductor-Based Photocatalyst Materials for Applications in Photocatalytic Water Splitting and Degradation of Pollutants. Adv. Sustain. Syst. 2017, 1, 1700006. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. The advancements in sol–gel method of doped-TiO2 photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J.; Kim, H.-Y. One-pot synthesis of CdS sensitized TiO2 decorated reduced graphene oxide nanosheets for the hydrolysis of ammonia-borane and the effective removal of organic pollutant from water. Ceram. Int. 2016, 42, 15247–15252. [Google Scholar] [CrossRef]
- Tung, W.S.; Daoud, W.A. Self-cleaning fibers via nanotechnology: A virtual reality. J. Mater. Chem. 2011, 21, 7858–7869. [Google Scholar] [CrossRef]
- Hashimoto, K.I.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Barakat, N.A.M.; Pant, H.R.; Park, M.; Saud, P.S.; Kim, J.-W.; Kim, H.-Y. Synthesis and photocatalytic activities of CdS/TiO2 nanoparticles supported on carbon nanofibers for high efficient adsorption and simultaneous decomposition of organic dyes. J. Colloid Interface Sci. 2014, 434, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.-J. Hydrothermal synthesis of Ag2CO3-TiO2 loaded reduced graphene oxide nanocomposites with highly efficient photocatalytic activity. Chem. Eng. Commun. 2019, 1–8. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Fang, T.-H.; Hsiao, Y.-J.; Wu, P.-C. Response and characteristics of TiO2/perovskite heterojunctions for CO gas sensors. J. Alloys Compd. 2019, 794, 576–584. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. TiO2 NPs Assembled into a Carbon Nanofiber Composite Electrode by a One-Step Electrospinning Process for Supercapacitor Applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Saud, P.S.; Park, M.; Park, S.-J.; Kim, H.-Y. General one-pot strategy to prepare Ag–TiO2 decorated reduced graphene oxide nanocomposites for chemical and biological disinfectant. J. Alloys Compd. 2016, 671, 51–59. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Bai, Y.; Mora-Seró, I.; De Angelis, F.; Bisquert, J.; Wang, P. Titanium Dioxide Nanomaterials for Photovoltaic Applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.-J. MoS2/CdS/TiO2 ternary composite incorporated into carbon nanofibers for the removal of organic pollutants from water. Inorg. Chem. Commun. 2019, 102, 113–119. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Park, M.; Liu, Y.; Choi, J.-W.; Barakat, N.A.M.; Kim, H.-Y. Electrospun CdS–TiO2 doped carbon nanofibers for visible-light-induced photocatalytic hydrolysis of ammonia borane. Catal. Commun. 2014, 50, 63–68. [Google Scholar] [CrossRef]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the Relationship between Surface Phases and Photocatalytic Activity of TiO2. Angew. Chem. 2008, 120, 1790–1793. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, X.; Li, W.; Gao, J.; Xue, H.; Li, R.K.Y.; Mai, Y.-W. TiO2 nanoparticle decorated carbon nanofibers for removal of organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 205–211. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Ojha, G.P.; Kim, H.-Y.; Park, M.; Park, S.-J. Fly-ash-incorporated electrospun zinc oxide nanofibers: Potential material for environmental remediation. Environ. Pollut. 2019, 245, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Barakat, N.A.M.; Park, M.; Han, T.-H.; Lim, B.H.; Kim, H.-Y. Incorporation of cadmium sulfide nanoparticles on the cadmium titanate nanofibers for enhanced organic dye degradation and hydrogen release. Ceram. Int. 2014, 40, 1553–1559. [Google Scholar] [CrossRef]
- Javid, A.; Kumar, M.; Ashraf, M.; Lee, J.H.; Han, J.G. Photocatalytic antibacterial study of N-doped TiO2 thin films synthesized by ICP assisted plasma sputtering method. Phys. E: Low-Dimens. Syst. Nanostructures 2019, 106, 187–193. [Google Scholar] [CrossRef]
- Varnagiris, S.; Medvids, A.; Lelis, M.; Milcius, D.; Antuzevics, A. Black carbon-doped TiO2 films: Synthesis, characterization and photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 382, 111941. [Google Scholar] [CrossRef]
- Han, C.; Pelaez, M.; Likodimos, V.; Kontos, A.G.; Falaras, P.; O’Shea, K.; Dionysiou, D.D. Innovative visible light-activated sulfur doped TiO2 films for water treatment. Appl. Catal. B Environ. 2011, 107, 77–87. [Google Scholar] [CrossRef]
- Scuderi, V.; Buccheri, M.A.; Impellizzeri, G.; Di Mauro, A.; Rappazzo, G.; Bergum, K.; Svensson, B.G.; Privitera, V. Photocatalytic and antibacterial properties of titanium dioxide flat film. Mater. Sci. Semicond. Process. 2016, 42, 32–35. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Marques, J.; Coutinho, P.J.G.; Parpot, P.; Tavares, C.J. Photocatalytic thin films coupled with polymeric microcapsules for the controlled-release of volatile agents upon solar activation. J. Phys. Conf. Ser. 2013, 439, 012018. [Google Scholar] [CrossRef]
- Varshney, G.; Kanel, S.R.; Kempisty, D.M.; Varshney, V.; Agrawal, A.; Sahle-Demessie, E.; Varma, R.S.; Nadagouda, M.N. Nanoscale TiO2 films and their application in remediation of organic pollutants. Coord. Chem. Rev. 2016, 306, 43–64. [Google Scholar] [CrossRef]
- Maruyama, T.; Arai, S. Titanium dioxide thin films prepared by chemical vapor deposition. Sol. Energy Mater. Sol. Cells 1992, 26, 323–329. [Google Scholar] [CrossRef]
- Yeung, K.S.; Lam, Y.W. A simple chemical vapour deposition method for depositing thin TiO2 films. Thin Solid Film. 1983, 109, 169–178. [Google Scholar] [CrossRef]
- Miao, H.; Hu, X.; Fan, J.; Li, C.; Sun, Q.; Hao, Y.; Zhang, G.; Bai, J.; Hou, X. Hydrothermal synthesis of TiO2 nanostructure films and their photoelectrochemical properties. Appl. Surf. Sci. 2015, 358, 418–424. [Google Scholar] [CrossRef]
- Kitano, M.; Mitsui, R.; Eddy, D.R.; El-Bahy, Z.M.A.; Matsuoka, M.; Ueshima, M.; Anpo, M. Synthesis of Nanowire TiO2 Thin Films by Hydrothermal Treatment and their Photoelectrochemical Properties. Catal. Lett. 2007, 119, 217–221. [Google Scholar] [CrossRef]
- Kim, E.H.; Lim, M.H.; Lah, M.S.; Koo, S.M. Synthesis and characterization of heteroleptic titanium MOCVD precursors for TiO2 thin films. Dalton Trans. 2018, 47, 2415–2421. [Google Scholar] [CrossRef]
- Saripudin, A.; Arifin, P. Growth mechanism of Co:TiO2 thin film deposited by metal organic chemical vapor deposition technique. Iop Conf. Ser. Mater. Sci. Eng. 2016, 128, 012046. [Google Scholar] [CrossRef]
- Heo, C.H.; Lee, S.-B.; Boo, J.-H. Deposition of TiO2 thin films using RF magnetron sputtering method and study of their surface characteristics. Thin Solid Film. 2005, 475, 183–188. [Google Scholar] [CrossRef]
- Eufinger, K.; Poelman, D.; Poelman, H.; De Gryse, R.; Marin, G.B. Photocatalytic activity of dc magnetron sputter deposited amorphous TiO2 thin films. Appl. Surf. Sci. 2007, 254, 148–152. [Google Scholar] [CrossRef]
- Mallak, M.; Bockmeyer, M.; Löbmann, P. Liquid phase deposition of TiO2 on glass: Systematic comparison to films prepared by sol–gel processing. Thin Solid Film. 2007, 515, 8072–8077. [Google Scholar] [CrossRef]
- Begum, N.S.; Farveez Ahmed, H.M. Synthesis of nanocrystalline TiO2 thin films by liquid phase deposition technique and its application for photocatalytic degradation studies. Bull. Mater. Sci. 2008, 31, 43–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Ke, Y. A novel approach of preparing TiO2 films at low temperature and its application in photocatalytic degradation of methyl orange. J. Hazard. Mater. 2010, 177, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Asenova, I.; Manova, D.; Mändl, S. Incorporation of nitrogen into TiO2 thin films during PVD processes. J. Phys. Conf. Ser. 2014, 559, 012008. [Google Scholar] [CrossRef]
- Garapon, C.; Champeaux, C.; Mugnier, J.; Panczer, G.; Marchet, P.; Catherinot, A.; Jacquier, B. Preparation of TiO2 thin films by pulsed laser deposition for waveguiding applications. Appl. Surf. Sci. 1996, 96, 836–841. [Google Scholar] [CrossRef]
- Murugesan, S.; Kuppusami, P.; Parvathavarthini, N.; Mohandas, E. Pulsed laser deposition of anatase and rutile TiO2 thin films. Surf. Coat. Technol. 2007, 201, 7713–7719. [Google Scholar] [CrossRef]
- Sobczyk-Guzenda, A.; Pietrzyk, B.; Szymanowski, H.; Gazicki-Lipman, M.; Jakubowski, W. Photocatalytic activity of thin TiO2 films deposited using sol–gel and plasma enhanced chemical vapor deposition methods. Ceram. Int. 2013, 39, 2787–2794. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Xian, Y.; Ying, X.; Liu, M.; Jin, L. Preparation and application of TiO2 photocatalytic sensor for chemical oxygen demand determination in water research. Water Res. 2005, 39, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Karuppuchamy, S.; Suzuki, N.; Ito, S.; Endo, T. A novel one-step electrochemical method to obtain crystalline titanium dioxide films at low temperature. Curr. Appl. Phys. 2009, 9, 243–248. [Google Scholar] [CrossRef]
- Pore, V.; Kivelä, T.; Ritala, M.; Leskelä, M. Atomic layer deposition of photocatalytic TiO2 thin films from TiF4 and H2O. Dalton Trans. 2008, 6467–6474. [Google Scholar] [CrossRef] [PubMed]
- Yemmireddy, V.K.; Hung, Y.-C. Using Photocatalyst Metal Oxides as Antimicrobial Surface Coatings to Ensure Food Safety—Opportunities and Challenges. Compr. Rev. Food Sci. Food Saf. 2017, 16, 617–631. [Google Scholar] [CrossRef]
- Jameel, D.A. Thin film deposition process. Int. J. Mod. Phys. Appl. 2015, 1, 193–199. [Google Scholar]
- Patil, M.K.; Shaikh, S.; Ganesh, I. Recent Advances on TiO2 Thin Film Based Photocatalytic Applications (A Review). Curr. Nanosci. 2015, 11, 271–285. [Google Scholar] [CrossRef]
- Mardare, D.; Iacomi, F.; Cornei, N.; Girtan, M.; Luca, D. Undoped and Cr-doped TiO2 thin films obtained by spray pyrolysis. Thin Solid Film. 2010, 518, 4586–4589. [Google Scholar] [CrossRef]
- Deák, Á.; Janovák, L.; Csapó, E.; Ungor, D.; Pálinkó, I.; Puskás, S.; Ördög, T.; Ricza, T.; Dékány, I. Layered double oxide (LDO) particle containing photoreactive hybrid layers with tunable superhydrophobic and photocatalytic properties. Appl. Surf. Sci. 2016, 389, 294–302. [Google Scholar] [CrossRef]
- Merai, L.; Deak, A.; Sebok, D.; Csapo, E.; Kolumban, T.S.; Hopp, B.; Dekany, I.; Janovak, L. Photoreactive composite coating with composition dependent wetting properties. Express Polym. Lett. 2018, 12, 1061–1071. [Google Scholar] [CrossRef]
- Rajendran, K.; Senthil Kumar, V.; Anitha Rani, K. Synthesis and characterization of immobilized activated carbon doped TiO2 thin films. Optik 2014, 125, 1993–1996. [Google Scholar] [CrossRef]
- Dulian, P.; Nachit, W.; Jaglarz, J.; Zięba, P.; Kanak, J.; Żukowski, W. Photocatalytic methylene blue degradation on multilayer transparent TiO2 coatings. Opt. Mater. 2019, 90, 264–272. [Google Scholar] [CrossRef]
- Wang, F.; Ge, W.; Shen, T.; Ye, B.; Fu, Z.; Lu, Y. The effect of bulk/surface defects ratio change on the photocatalysis of TiO2 nanosheet film. Appl. Surf. Sci. 2017, 410, 513–518. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Guillard, C.; Beaugiraud, B.; Dutriez, C.; Herrmann, J.-M.; Jaffrezic, H.; Jaffrezic-Renault, N.; Lacroix, M. Physicochemical properties and photocatalytic activities of TiO2-films prepared by sol–gel methods. Appl. Catal. B: Environ. 2002, 39, 331–342. [Google Scholar] [CrossRef]
- Chen, Y.; Stathatos, E.; Dionysiou, D.D. Microstructure characterization and photocatalytic activity of mesoporous TiO2 films with ultrafine anatase nanocrystallites. Surf. Coat. Technol. 2008, 202, 1944–1950. [Google Scholar] [CrossRef]
- Komaraiah, D.; Madhukar, P.; Vijayakumar, Y.; Ramana Reddy, M.V.; Sayanna, R. Photocatalytic degradation study of methylene blue by brookite TiO2 thin film under visible light irradiation. Mater. Today Proc. 2016, 3, 3770–3778. [Google Scholar] [CrossRef]
- Bozzi, A.; Yuranova, T.; Guasaquillo, I.; Laub, D.; Kiwi, J. Self-cleaning of modified cotton textiles by TiO2 at low temperatures under daylight irradiation. J. Photochem. Photobiol. A Chem. 2005, 174, 156–164. [Google Scholar] [CrossRef]
- Ortelli, S.; Costa, A.L.; Dondi, M. TiO2 Nanosols Applied Directly on Textiles Using Different Purification Treatments. Materials 2015, 8, 7988–7996. [Google Scholar] [CrossRef]
- Huang, F.; Yan, A.; Zhao, H. Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; Cao, W., Ed.; InTech: Rijeka, Croatia, 2016; pp. 31–80. [Google Scholar] [CrossRef]
- Radhiyah, A.A.; Iis, S. Recent Progress on Development of TiO2 Thin Film Photocatalysts for Pollutant Removal. Recent Pat. Mater. Sci. 2009, 2, 88–111. [Google Scholar] [CrossRef]
- Sonawane, R.S.; Kale, B.B.; Dongare, M.K. Preparation and photo-catalytic activity of Fe TiO2 thin films prepared by sol-gel dip coating. Mater. Chem. Phys. 2004, 85, 52–57. [Google Scholar] [CrossRef]
- Gültekin, A.; Karanfil, G.; Özel, F.; Kuş, M.; Say, R.; Sönmezoğlu, S. Synthesis and characterisations of Au-nanoparticle-doped TiO2 and CdO thin films. J. Phys. Chem. Solids 2014, 75, 775–781. [Google Scholar] [CrossRef]
- Yu, J.; Yu, J.C.; Cheng, B.; Zhao, X. Photocatalytic Activity and Characterization of the Sol-Gel Derived Pb-Doped TiO2 Thin Films. J. Sol-Gel Sci. Technol. 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Rapsomanikis, A.; Apostolopoulou, A.; Stathatos, E.; Lianos, P. Cerium-modified TiO2 nanocrystalline films for visible light photocatalytic activity. J. Photochem. Photobiol. A Chem. 2014, 280, 46–53. [Google Scholar] [CrossRef]
- Guillén-Santiago, A.; Mayén, S.A.; Torres-Delgado, G.; Castanedo-Pérez, R.; Maldonado, A.; Olvera, M.d.l.L. Photocatalytic degradation of methylene blue using undoped and Ag-doped TiO2 thin films deposited by a sol–gel process: Effect of the ageing time of the starting solution and the film thickness. Mater. Sci. Eng. B 2010, 174, 84–87. [Google Scholar] [CrossRef]
- Solís-Casados, D.A.; Escobar-Alarcón, L.; Alvarado-Pérez, V.; Haro-Poniatowski, E. Photocatalytic Activity under Simulated Sunlight of Bi-Modified TiO2 Thin Films Obtained by Sol Gel. Int. J. Photoenergy 2018, 2018, 8715987. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Rong, F.; Ji, X.; Fu, D. Carbon-doped mesoporous TiO2 film and its photocatalytic activity. Microporous Mesoporous Mater. 2011, 142, 276–281. [Google Scholar] [CrossRef]
- Hassan, M.E.; Cong, L.; Liu, G.; Zhu, D.; Cai, J. Synthesis and characterization of C-doped TiO2 thin films for visible-light-induced photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2014, 294, 89–94. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Mekprasart, W.; Khumtong, T.; Rattanarak, J.; Techitdheera, W.; Pecharapa, W. Effect of Nitrogen Doping on Optical and Photocatalytic Properties of TiO2 Thin Film Prepared by Spin Coating Process. Energy Procedia 2013, 34, 746–750. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Morales-Luna, M.; Arvizu, M.A.; Tellez-Cruz, M.M. Optical, structural, and morphological properties of photocatalytic TiO2–ZnO thin films synthesized by the sol–gel process. Thin Solid Film. 2015, 594, 304–309. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Santoyo-Salazar, J.; Gallardo-Hernández, S.; Tellez-Cruz, M.M.; Solorza-Feria, O. Sol-gel synthesis of Ag-loaded TiO2-ZnO thin films with enhanced photocatalytic activity. J. Alloy. Compd. 2019, 779, 908–917. [Google Scholar] [CrossRef]
- Sangchay, W. The Self-cleaning and Photocatalytic Properties of TiO2 Doped with SnO2 Thin Films Preparation by Sol-gel Method. Energy Procedia 2016, 89, 170–176. [Google Scholar] [CrossRef]
- Hernández-Torres, M.E.; Ojeda-Carrera, M.T.; Sánchez-Cantú, M.; Silva-González, N.R.; Gracia-Jiménez, J.M. CdS/TiO2 composite films for methylene blue photodecomposition under visible light irradiation and non-photocorrosion of cadmium sulfide. Chem. Pap. 2014, 68, 1257–1264. [Google Scholar] [CrossRef]
- Stoyanov, S.; Mladenova, D.; Dushkin, C. Photocatalytic properties of mixed TiO2/V2O5 films in water purification at varying ph. React. Kinet. Catal. Lett. 2006, 88, 277–283. [Google Scholar] [CrossRef]
- Yuranova, T.; Mosteo, R.; Bandara, J.; Laub, D.; Kiwi, J. Self-cleaning cotton textiles surfaces modified by photoactive SiO2/TiO2 coating. J. Mol. Catal. A Chem. 2006, 244, 160–167. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, W.A. Self-cleaning cotton functionalized with TiO2/SiO2: Focus on the role of silica. J. Colloid Interface Sci. 2013, 401, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, C.; Ruan, L.; Peng, Z.; Zhang, J.; Zhao, J.; Li, Y. Ce-doped SiO2@TiO2 nanocomposite as an effective visible light photocatalyst. J. Alloy. Compd. 2014, 585, 800–804. [Google Scholar] [CrossRef]
- Arabatzis, I.M.; Antonaraki, S.; Stergiopoulos, T.; Hiskia, A.; Papaconstantinou, E.; Bernard, M.C.; Falaras, P. Preparation, characterization and photocatalytic activity of nanocrystalline thin film TiO2 catalysts towards 3,5-dichlorophenol degradation. J. Photochem. Photobiol. A Chem. 2002, 149, 237–245. [Google Scholar] [CrossRef]
- Gelover, S.; Mondragón, P.; Jiménez, A. Titanium dioxide sol-gel deposited over glass and its application as a photocatalyst for water decontamination. J. Photochem. Photobiol. A Chem. 2004, 165, 241–246. [Google Scholar] [CrossRef]
- Yogi, C.; Kojima, K.; Wada, N.; Tokumoto, H.; Takai, T.; Mizoguchi, T.; Tamiaki, H. Photocatalytic degradation of methylene blue by TiO2 film and Au particles-TiO2 composite film. Thin Solid Film. 2008, 516, 5881–5884. [Google Scholar] [CrossRef]
- Peng, Y.-H.; Huang, G.-F.; Huang, W.-Q. Visible-light absorption and photocatalytic activity of Cr-doped TiO2 nanocrystal films. Adv. Powder Technol. 2012, 23, 8–12. [Google Scholar] [CrossRef]
- Kaleji, B.K.; Sarraf-Mamoory, R.; Fujishima, A. Influence of Nb dopant on the structural and optical properties of nanocrystalline TiO2 thin films. Mater. Chem. Phys. 2012, 132, 210–215. [Google Scholar] [CrossRef]
- Gutiérrez, D.J.R.; Mathews, N.R.; Martínez, S.S. Photocatalytic activity enhancement of TiO2 thin films with silver doping under visible light. J. Photochem. Photobiol. A Chem. 2013, 262, 57–63. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aazam, E. Synthesis and characterization of P-doped TiO2 thin-films for photocatalytic degradation of butyl benzyl phthalate under visible-light irradiation. Chin. J. Catal. 2013, 34, 1267–1273. [Google Scholar] [CrossRef]
- Qin, L.Z.; Liang, H.; Liao, B.; Liu, A.D.; Wu, X.Y.; Sun, J. Photocatalytic performance of Fe-, Ni-, or Cu-ion implanted TiO2 films under UV light, visible light and sunlight irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 307, 385–390. [Google Scholar] [CrossRef]
- Hou, X.-G.; Huang, M.-D.; Wu, X.-L.; Liu, A.-D. Preparation and studies of photocatalytic silver-loaded TiO2 films by hybrid sol–gel method. Chem. Eng. J. 2009, 146, 42–48. [Google Scholar] [CrossRef]
- Gnanaprakasam, A.; Sivakumar, V.M.; Thirumarimurugan, M. Influencing Parameters in the Photocatalytic Degradation of Organic Effluent via Nanometal Oxide Catalyst: A Review. Indian J. Mater. Sci. 2015, 2015, 16. [Google Scholar] [CrossRef]
- Raghu, S.; Basha, C.A. Electrochemical treatment of Procion Black 5B using cylindrical flow reactor—A pilot plant study. J. Hazard. Mater. 2007, 139, 381–390. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.; Imam, E.; Ramadan, A. TiO2 Solar Photocatalytic Reactor Systems: Selection of Reactor Design for Scale-up and Commercialization—Analytical Review. Catalysts 2016, 6, 138. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Golubeva, I.S.; Verevkin, Y.K.; Pershin, E.A.; Burenina, V.N.; Korolichin, V.V. Photoinduced bactericidal activity of TiO2 films. Appl. Biochem. Microbiol. 2011, 47, 23–26. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Dasari, T.P.; Pathakoti, K.; Hwang, H.-M. Determination of the mechanism of photoinduced toxicity of selected metal oxide nanoparticles (ZnO, CuO, Co3O4 and TiO2) to E. coli bacteria. J. Environ. Sci. 2013, 25, 882–888. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Van Acker, H.; Coenye, T. The Role of Reactive Oxygen Species in Antibiotic-Mediated Killing of Bacteria. Trends Microbiol. 2017, 25, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Pant, H.R.; Pandeya, D.R.; Panthi, G.; Nam, K.T.; Hong, S.T.; Kim, C.S.; Kim, H.Y. Characterization and antibacterial properties of Ag NPs loaded nylon-6 nanocomposite prepared by one-step electrospinning process. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 94–99. [Google Scholar] [CrossRef]
- Pant, B.; Pokharel, P.; Tiwari, A.P.; Saud, P.S.; Park, M.; Ghouri, Z.K.; Choi, S.; Park, S.-J.; Kim, H.-Y. Characterization and antibacterial properties of aminophenol grafted and Ag NPs decorated graphene nanocomposites. Ceram. Int. 2015, 41, 5656–5662. [Google Scholar] [CrossRef]
- Arellano, U.; Asomoza, M.; Ramírez, F. Antimicrobial activity of Fe–TiO2 thin film photocatalysts. J. Photochem. Photobiol. A Chem. 2011, 222, 159–165. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S.; Sanjines, R.; Pulgarin, C. TiO2 and TiO2-Doped Films Able to Kill Bacteria by Contact: New Evidence for the Dynamics of Bacterial Inactivation in the Dark and under Light Irradiation. Int. J. Photoenergy 2014, 2014, 17. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Golubeva, I.S.; Verevkin, Y.K. Bactericidal activity of titanium dioxide ultraviolet-induced films. Mater. Sci. Eng. C 2016, 59, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Yoriya, S.; Chumphu, A.; Pookmanee, P.; Laithong, W.; Thepa, S.; Songprakorp, R. Multi-Layered TiO2 Films towards Enhancement of Escherichia coli Inactivation. Materials 2016, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Erdural, B.; Bolukbasi, U.; Karakas, G. Photocatalytic antibacterial activity of TiO2–SiO2 thin films: The effect of composition on cell adhesion and antibacterial activity. J. Photochem. Photobiol. A Chem. 2014, 283, 29–37. [Google Scholar] [CrossRef]
- Sangchay, W.; Sikong, L.; Kooptarnond, K. The Photocatalytic and Antibacterial Activity of Cu-Doped TiO2 Thin Films. Walailak J. Sci. Technol. 2013, 10, 19–27. [Google Scholar] [CrossRef]
- Hou, X.; Ma, H.; Liu, F.; Deng, J.; Ai, Y.; Zhao, X.; Mao, D.; Li, D.; Liao, B. Synthesis of Ag ion-implanted TiO2 thin films for antibacterial application and photocatalytic performance. J. Hazard. Mater. 2015, 299, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ubonchonlakate, K.; Sikong, L.; Saito, F. Photocatalytic disinfection of P.aeruginosa bacterial Ag-doped TiO2 film. Procedia Eng. 2012, 32, 656–666. [Google Scholar] [CrossRef]
- Kim, D.S.; Kwak, S.-Y. Photocatalytic Inactivation of E. coli with a Mesoporous TiO2 Coated Film Using the Film Adhesion Method. Environ. Sci. Technol. 2009, 43, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]



| Method | Description | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Sputter deposition | An ionizing plasma sputters the target in a vacuum chamber and the ionized atoms are deposited on the substrate. | —High quality and uniform deposition —Good adhesion | —Risk of substrate damage due to ionic bombardment —Grain size of the sputtered films is typically smaller | [34,53,54] |
| Chemical vapor deposition | A thin film of metal oxide is formed on a heated substrate from a gaseous phase in a closed chamber at a relatively higher temperature. | —Produce uniform, films at low or high rates —Flexible with regards to the shape of the substrate —Compatibility with good adhesion —Simultaneously coat multiple components —Control structure of crystal and generate uniform films with pure materials and high density | —High cost —High reaction temperature —Low deposition rates —Cannot control the stoichiometry of films using more than one material | [53,54,55] |
| Physical Vapor Deposition | It involves the transfer of material on an atomic level onto a solid substrate. This is a physical process such as high temperature vacuum evaporation followed by condensation rather than a chemical reaction among precursors. | —Suitable for any type of inorganic materials —Safer than other methods | —High cost | [54,55] |
| Sol-gel synthesis | This is a wet chemical method that involves hydrolysis and condensation of metallo-organic alkoxide precursors for gel formation followed by dip/spin/spray coating or screen printing. | —Simple, homogeneity, low cost, reliability, reproducibility, controllability —Films are easily anchored on the substrate bearing complicated shapes and a large surface area. —Suitable for deposition on various substrates —Easy method | —Long period of deposition —High temperature —Not possible to attach a thick layer of nanoparticles on the substrate | [34,53,55] |
| Spray pyrolysis | A solution containing a precursor is sprayed by a nanoporous nebulizer onto the hot substrate in the furnace. | —Cost-effective and can be easily performed —Substrates with complex geometries can be coated. —Uniform and high-quality coatings —Low processing temperature —Multilayer fabrication capability | —Coatings are not uniform in thickness. | [34,56] |
| Electrophoretic deposition | Formation of coating on the charged surface takes place by the movement of charge particles in suspension under an appropriate electric field | —Simple and cheap —Uniform coating —Size and shape of nanoparticles can be controlled —High-quality coatings | —Volatile, toxic —Flammability —Costly —High electric field strengths are required. | [53] |
| Hydrothermal | Includes either a single or heterogeneous phase reactions in aqueous solution at elevated temperatures and pressures to crystallize materials directly from solution | —Simple to operate —Ability to grow large, high-quality crystals, maintaining a good control of their chemical composition | —Expensive autoclaves are required —Impossibility of observing the crystal as it grows | [34,55] |
| Doctor-blade | A slurry is placed on a substrate, and the unidirectional shear force is applied by a blade over the substrate. | —Simple and economic —Easy to control film thickness and homogeneity —Suitable for mass production of electro-ceramic thick films | —Slow evaporation —Tendency to aggregate or crystallize at high solution/paste concentration | [34] |
| Plasma-enhanced chemical vapor deposition (CVD) | This method utilizes a plasma to deeply fragment organic precursor molecules, which subsequently deposit onto solid substrates within the reaction chamber, such as nanoparticles. | —Requires much lower temperatures —Good for deposition on multilayer films —Good adhesion and uniformity —High deposition rate —Good mechanical properties —Controllable coating thickness | —Chemical and particle contamination —High cost —Toxic byproducts | [54] |
| Spray coating | The solvent is evaporated during the spraying process. | —Simple —Low-cost —Scalable film forming technique | —The thickness is not uniform. | [57,58] |
| Catalyst | TiO2 Precursor | Substrate | Light Source | Pollutant | Initial Concentration of the Pollutant | Degradation Performance | Ref. |
|---|---|---|---|---|---|---|---|
| TiO2 nanocrystalline thin film | titanium (IV) butoxide and Degussa P25 TiO2 | glass | UV | 3,5-dichlorophenol (3,5-DCP) | 5 ppm | 1600 min | [89] |
| TiO2 film | titanium tetraisopropoxide and Degussa P25 | soda lime glass, pyrex glass | UV | 2-hydroxybutanedioic acid | 50 ppm | 200 min | [63] |
| Fe-doped TiO2 film | titanium tetraisopropoxide | soda lime glass, silica rings, glass helix | sunlight | methyl orange | 100 ppm | 95% in 3 h | [70] |
| TiO2 film | titanium isopropoxide | glass | solar light | 4- chlorophenol and carbaryl | 20 mg/L | 4-chlorophenol: 75% degradation in 3 h, and carbaryl: 65% for degradation in 3 h | [90] |
| Mesoporous TiO2 film | titanium isopropoxide | Tween 20 as template | UV | creatinine | 19.5 mg/L | - | [64] |
| Au-doped TiO2 film | titanium isopropoxide | quartz glass | UV | methylene blue | 1.63 × 10− 5 M | 180 min | [91] |
| S-doped TiO2 film | titanium isopropoxide | borosilicate glass | visible light | hepatotoxin microcystin-LR (MC-LR) | 500 μg L−1 | ∼50% degradation was observed in 5 h | [31] |
| Cr-doped TiO2 film | butyl titanate | glass or silicon | visible light | methyl orange | – | 90% within 5 h | [92] |
| Nb-doped TiO2 film | titanium (IV) butoxide | glazed porcelain | UV | methylene blue | 5 ppm | 76.2% within 120 min | [93] |
| Ag-doped TiO2 film, | titanium butoxide | ITO plates | visible light | methanol and basic orange II (BOII) | 60 × 10−3 mol L−1 | 80% of total organic carbon in 5 h | [94] |
| P-doped TiO2 film | titanium tetrabutyl titanate | glass plates | visible light | butyl benzyl phthalate (BBP) | 20 mg/L | 98% in 240 min | [95] |
| Fe, Ni, and Cu –ion implanted TiO2 film, | tetrabutylorthotitanate | glass | UV, visible, sunlight | methyl orange | – | [96] | |
| Ag/TiO2 films | tetrabutylorthotitanate | glass | UV, visible light | methyl orange | 5 × 10−5 mol/L | UV365 (73%) and visible light (3.8 times) enhanced. | [97] |
| Bi-modified TiO2 film | titanium isopropoxide | borosilicate glass | simulated sunlight | malachite green | 10 μmol/L | 67% 180 min | [75] |
| TiO2 thin films | titanium tetraisopropoxide | glass | visible light | methylene blue | 1 × 10−6 M | 92% 4 h | [65] |
| Pb-doped TiO2 film | titanium (IV) butoxide | soda-lime glass | sunlight | dimethyl-2,2-dichlorovinyl phosphate | 10−4 M | ~30% 6 h | [72] |
| Ce-modified TiO2 film | titanium tetraisopropoxide | glass | UV and visible light | basic blue 41 | 2.5 × 10−5 M | ~85% in 180 min | [73] |
| Catalyst | Substrate | Bacteria | Concentration of Bacteria | Incubation Time | Light Source | Inhibition % | Ref. |
|---|---|---|---|---|---|---|---|
| Fe–TiO2 thin film | sodium glass | E. coli | - | 1 h | Visible light | 100 | [110] |
| Multi-Layered TiO2 film | glass plates | E. coli | 2.59 × 107 CFU/ml | 8 h | Sunlight | 91.9 | [113] |
| nano-TiO2 (anatase)-based thin films | Silicon | E. coli | 108 CFU/mL | 20 min | UV | 100 | [101] |
| SiO2–TiO2 film | glass slides | E. coli | 106–108 per ml | 1 h | Artificial solar radiation | 50 | [114] |
| Cu-doped TiO2 film | glass | E. coli | 103 CFU/ml | 4 h | UV | 100 | [115] |
| TiO2 film | glass plate | S. aureus, S. epidermidis, E. coli | 200 CFU | 15 min | UV | 50 | [102] |
| TiO2 thin film | silica- coated soda- lime glass | E. coli | 2 × 105 CFU/ml | 90 min | UV | 100 | [106] |
| Ag ion-implanted TiO2 thin films | E. coli | 4.46 × 108 CFU/mL | 24 h | fluorescent lamp and in the dark | 100 | [116] | |
| Ag-doped TiO2 film | glass fiber | P. aeruginosa | 1 × 103 CFU/ml | 10 min | UV | 100 | [117] |
| Mesoporous TiO2 film | glass | E. coli | 106 cells mL−1 | 60 min | UV | 99.99 | [118] |
| GO nanosheets on TO2 film | glass | E. coli | 106 CFU/mL | 24 h | Solar light | – | [119] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pant, B.; Park, M.; Park, S.-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings 2019, 9, 613. https://doi.org/10.3390/coatings9100613
Pant B, Park M, Park S-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings. 2019; 9(10):613. https://doi.org/10.3390/coatings9100613
Chicago/Turabian StylePant, Bishweshwar, Mira Park, and Soo-Jin Park. 2019. "Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities" Coatings 9, no. 10: 613. https://doi.org/10.3390/coatings9100613
APA StylePant, B., Park, M., & Park, S.-J. (2019). Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings, 9(10), 613. https://doi.org/10.3390/coatings9100613







