1. Introduction
Over the last few years, holistic approaches are trying to tackle the global graffiti phenomenon [
1]. Any proposed solution is shared with all the involved stakeholders, including those who manage graffiti or utilize street art for city regeneration. The current studies are addressing not only to the graffiti removal, but also to the knowledge and protection of the street art murals [
2,
3,
4].
However, graffiti on building façades, especially those with a cultural and historical value, still remain a vandalism [
5]. Several strategies are used to either remove these graffiti or protect the surfaces against their harmful effects [
6].
Mechanical, chemical or laser techniques are typically employed for the cleaning procedures [
7,
8,
9,
10,
11]; biocleaning methods have been also proposed [
12,
13]. Graffiti removal is expensive and, in some cases, may cause stone damage due to chemical contamination, by-products formation and physical changes. Therefore, preventive actions are preferred, especially on artifacts of historical and artistic relevance.
To this aim, coatings acting as anti-graffiti barrier have been developed in the last years and many products are nowadays commercially available. The anti-graffiti products can be grouped into three main classes: sacrificial, semi-permanent and permanent [
14]. The sacrificial coatings are removed during the cleaning process and they need to be renewed; the semi-permanent systems endure a few cleaning cycles (normally, not more than two or three); the permanent products are not taken away during the cleaning process and they are able to withstand several cleaning cycles. The formulations suitable for application on stone materials [
15,
16,
17,
18,
19,
20,
21,
22,
23] are mainly based on waxes, fluorinated polymers, silicon resins or polyurethanes; more recently, coatings incorporating nano-particles [
17,
24,
25,
26], organic–inorganic hybrid products [
27,
28,
29] and surface functionalization [
30] have been investigated as potential anti-graffiti systems. The fundamental characteristics that these treatments must display are: transparency, permeability to water vapor, durability under outdoor conditions. In addition, non-wettable coatings are preferred to enable the treated surfaces to repel paints and other staining agents [
31].
Wetting is the ability of a liquid to maintain contact with a solid surface; the balance between the intermolecular interactions of adhesive type (liquid to surface) and cohesive type (liquid to liquid) controls this feature. Wettability of a solid surface can be described by the contact angles and sliding angle; models (Young, Wenzel, Cassie–Baxter) have been developed to illustrate the wetting phenomena on surfaces [
32,
33]. The decrease of the wettability corresponds to an increase in liquid-phobicity. Regarding water, a surface is called hydrophobic when water drops on it exhibit contact angles greater than 90°. When the contact angles are 150° or higher, the surface is classified as superhydrophobic. Despite their high level properties, superhydrophobic surfaces have low mechanical wear resistance and poor long-term durability, therefore, their utilization in real applications is still limited [
33,
34,
35]. Water-repellency is achieved in super-hydrophobic surfaces with water contact angle hysteresis <10°, that is a low difference between the advancing and receding contact angles. In these conditions, a water droplet can move with little applied force and easily rolls off from the surface [
36]. Similar considerations are applied to oleo-phobic/-repellent surfaces [
37].
Several methods have been used to design and produce non-wettable stone surfaces, often bioinspired to either plants or insects surfaces. The most common procedures include the application of polymer coatings able to reduce the surface tension of the substrate, sol-gel processes and controlled nano-particle embedding into polymer matrices [
29].
Hydrophobicity and oleophobicity are assumed as basic properties to provide protection against graffiti [
38,
39,
40,
41,
42,
43,
44,
45,
46]; however, the related actual anti-graffiti action is taken for granted and few are the studies where this property has been proved in oleo/hydrophobic coatings applied on stone substrates [
43,
45]. Starting from these issues, an experimental work, aimed at investigating the performance of oleo/hydrophobic coatings and their behavior as anti-graffiti systems applied on building stone materials, has been undertaken. The present study is a part of a wide research on products suitable for superficial protection of stone materials.
Three products have been applied on both compact and porous calcareous stones representative of building materials used in the Mediterranean basin. Two of the used products are already commercially available and are suggested to provide water and anti-graffiti protection to stone surfaces. An experimental nano-filled system, based on fluorine resin containing SiO2 nano-particles, has been also investigated.
Blu-colored spray paint and felt-tip marker were used as staining agents. Traditional and easy-to-apply methods usually used by restores and professionals were chosen for the stain removal. Cleaning procedures by warm water and, subsequently, by acetone, were applied to the stained stone surfaces. The properties of the stone surfaces were, then, analyzed by visual observations, contact angle measurements and color evaluations, on stained and neat surfaces, as well as after the paint removal. The percentage of residual stain and the efficacy of the cleaning procedures, in comparison with the unprotected stone surfaces, were used to measure the paint removal.
2. Materials and Methods
2.1. Protective Products, Stone Materials, and Staining Agents
An experimental formulation and, for comparison purposes, two commercial products were investigated.
The experimental product, hereinafter nanoF, is a water-based fluorine resin (12.7 wt.%) containing SiO
2 nano-particles (10 wt.%), 40–50 nm in dimensions (supplied by Kimia S.p.A., Perugia, Italy); nanoF has density of 1.04 g/cm
3 and pH between 7 and 8; the viscosity, similar to those of the commercial systems, was appropriate for the application by brush [
47]. The first commercial product, F (trade mark Fluoline PE, supplied by C.T.S. S.r.l., Altavilla Vicentina, Italy), is an aqueous dispersion of fluoropolyethers (10 wt.%); this system was selected to compare the experimental nano-filled formulation to a unfilled chemically similar product already on the market (nanoF and F are, indeed, both fluorine-based). The second commercial system, hereinafter SW (trade mark Kimistone DEFENDER, supplied by Kimia S.p.A., Italy), consisting of a mixture of organic silicon compounds and microcrystalline waxes in water solution; SW was included in the study because it belongs to a family of products, i.e., the silicon-based, widely and successfully used in the field of stone conservation. According to the technical sheets, both the commercial systems are able to provide a reversible and hydrophobic coating on the treated surfaces, with dirt-repellent and anti-graffiti properties. Additional information about the protective systems has been reported in a previous study [
47].
The three protective products were tested on two natural calcareous stone materials, representative of construction materials used for historic and civil buildings in many countries in the Mediterranean basin. A highly porous calcarenite (PS), named ‘‘Lecce stone’’, and a compact limestone (CS), known as “Trani stone”, were used.
The principal constituent of ‘‘Lecce stone’’ is calcite (93%–97% [
48]); in this material, very small quantities of clay, phosphates and other non-carbonate minerals are also present [
49,
50]. Petrographically, “Lecce stone” is a packstone [
51], made of microfossils and fossil remains within a groundmass of fine calcareous detritus (
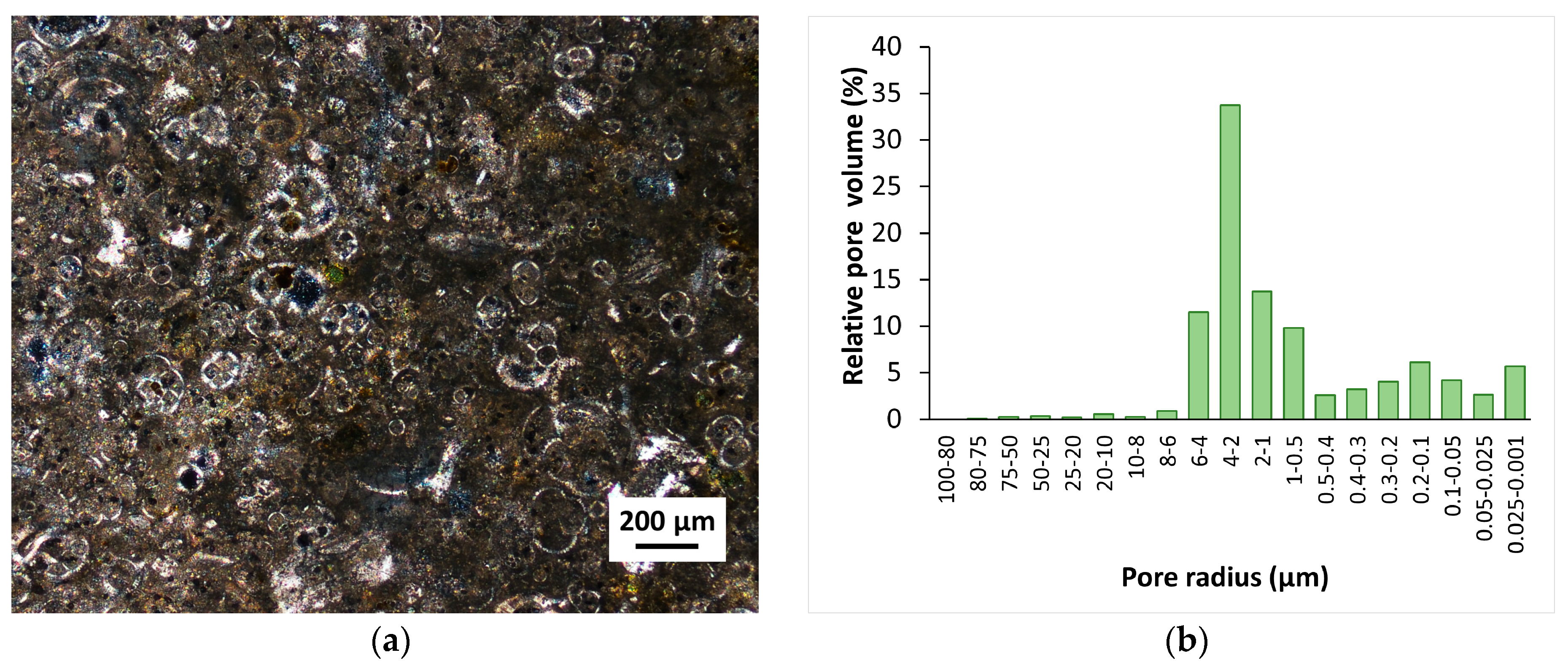
Figure 1a). The used samples exhibited a porosity of 39%, with pore sizes mainly between 0.5 and 6 µm (
Figure 1b), as analyzed by mercury-intrusion porosimetry (MIP) [
52].
“Trani stone” is mainly composed of calcite (>95% [
36]), and low amounts of clay minerals and iron oxides [
33]. “Trani stone” is a packstone [
51] made of calcareous detritic grains, very well cemented by a crystalline cement filling the interparticle porosity (
Figure 2a). The open porosity, measured by MIP, was very low (2%); the pore size was mainly between 0.025 and 0.001 µm (
Figure 2b).
Two staining agents were applied to test the protective action of each coating applied on the stones, i.e., (1) a commercial acrylic spray paint (Cilvani RAL by Cilvani S.r.l., Caivano, Italy), blu-colored (RAL code 5015), provided in a pressurized can; (2) a water-based acrylic paint marker (POSCA by UNI Mitsubishi pencil, Tokyo, Japan), blu-colored (RAL code 5005), having a bullet tip 1.8–2.5 mm wide (PC-5M).
2.2. Stone Specimens
Prismatic specimens of PS and CS stones, with dimensions of 5 cm × 5 cm × 1 cm, were cut by a saw from quarry blocks. According to the UNI10921 standard protocol [
53], the samples were smoothed with abrasive paper (180-grit silicon carbide), cleaned with a soft brush and washed with deionized water in order to remove dust deposits. The stone specimens were completely dried in oven at 60 °C, until the dry weight was achieved, and stored in a desiccator with silica gel (relative humidity (R.H.) = 15%) at 23 ± 2 °C. Before the application of each protective product, the stone specimens were conditioned in equilibrium with the surrounding environment (24 h in the laboratory, at 23 ± 2 °C and 45% ± 5% R.H.).
The treatments were applied by brush on 3 sample surfaces (5 cm × 5 cm) for each product.
For F and SW, the amounts of product suggested in the technical sheets were applied. Preliminary tests were used to verify the optimal amount of nanoF to effectively treat the two stone materials [
47]. Following the minimum intervention criteria, the quantities suitable to obtain highly hydrophobic surfaces along with minimal color changes were identified. For all the products, greater amounts were necessary to guarantee good performances in the highly porous stone (PS).
The actual amount of the applied product was evaluated by weighting the specimens before and after the treatment. After the application of the products, all the specimens were kept in the laboratory at 23 ± 2 °C and 45% ± 5% R.H. for 30 days; then, they were dried in oven at 40 °C until the weight stabilization was achieved, the stabilization being controlled by periodical weight measurements. The treatments’ harmlessness, assessed in terms of surface color variations and reduction in water vapor permeability, was proved in a previous study [
47]; the main results are summarized in
Table 1.
The staining of the surfaces was performed 2 months after the application of the protective coatings, as detailed in
Section 2.3.
During the preparation of the specimens, their subsequent treatments and relative tests, the environmental conditions were monitored by means of a thermo-hygrometer (Mod. EMR812HGN, Oregon Scientific, Hong Kong, China). This instrument is able to collect temperature data from −50 to 70 °C (with resolution of 0.1 °C) and relative humidity data in the range 2%–98% (with resolution of ±1%). All weight measurements were registered using an analytical balance (Model BP 2215, Sartorius, Goettingen, Germany) with an accuracy of ±0.1 mg.
All the procedures carried out on the stone samples are illustrated in
Figure 3.
2.3. Staining Methods and Removal Procedures
2.3.1. Spray Paint
The staining with the spray paint was carried out on untreated and protected stone samples, conditioned in equilibrium with the surrounding environment (24 h in the laboratory, at 23 ± 2 °C and 50% ± 5% R.H.). Two coats of paint were sprayed on specimens placed on a 45° tilted surface (
Figure 4a). The distance between the sample surface and the nebulizer was about 15 cm. In order to limit the deposition of paint to an area of 1.5 cm × 5 cm, the staining was performed with the aid of a stencil and the lateral sides of the specimens were protected with a polyester (PET) film.
After the application of the paint, the samples were stored for 2 days in the laboratory at 23 ± 2 °C and 50% ± 5% R.H. The removal procedures were performed 20 days after the staining. Starting from the data reported in the technical sheet of the SW product, a first cleaning with warm water (at 60 °C and next at 80 °C) and paper towels, was firstly attempted on testing areas. Since this method resulted totally ineffective, it was no more applied to the stained specimens and its effects were not further investigated. Then, following the recommendation reported in the international code [
54], cleaning with an organic solvent was tried. Acetone was selected because it is (alone or in mixture with other organic solvents) a traditional solvent for the cleaning of stone materials affected by graffiti [
6]; it has been successfully used to remove acrylic paints, as reported in previous studies [
9,
55]. Acetone analytical grade, supplied by Carlo Erba Reagents (Val de Reuil, France) was used. For each sample, a wet paper towel was rubbed across the stained area (
Figure 4b) for 25 complete back and forth cycles [
54]; the towel was dunked in acetone every 5 cycles.
2.3.2. Felt-Tip Paint Marker
The staining with the felt-tip marker was performed on untreated and protected stone samples conditioned in equilibrium with the surrounding environment (24 h in the laboratory, at 23 ± 2 °C and 50% ± 5% R.H.). The paint was applied to an area of approximately 1.5 cm × 5 cm in the same specimens stained with the spray paint, but in a different zone (
Figure 5).
The samples were, then, kept in the laboratory conditions (23 ± 2 °C and 50% ± 5% R.H) for 2 days. The same removal procedures used to clean the samples stained with the spray paint (described in
Section 2.3.1) were carried out 18 days after the staining with the marker.
2.4. Analytical Investigations
In order to evaluate hydrophobicity and oleophobicity of the stone surfaces, static contact angle measurements were performed before and after the coatings’ application. A Costech apparatus was used to deposit micro-drops of the wetting liquid on the stone surfaces. The shape of the drop was recorded with a camera and the related contact angle was calculated by means of the “anglometer 2.0” software (Costech). To assure the reproducibility of the test, the image of each drop was acquired 15s after its deposition.
The water-stone contact angles were measured on 30 different positions of the surface of each specimen using deionized water as wetting liquid, according to the European standard [
56]. A commercial olive oil (purchased from a local market) was used to determine the oil-stone static contact angle, following a procedure already proposed in other studies [
57,
58,
59]; 5 measurements were performed on each sample and the results were averaged.
For the unprotected Lecce stone (PS), the absorption of wetting liquids is rapid because of the high stone porosity. Consequently, during the test, the drops of both water and oil were suddenly absorbed inside the stone and the contact angle was not determinable.
The water-stone contact angle measurements were repeated after the staining and after the paint removal.
Color measurements [
60] were performed with a spectrophotometer (mod. CM-700d, Konica Minolta Sensing, Singapore), using CIE Standard illuminant D65 and the target mask 8 mm in diameter. Ten measurements were performed on each sample area and the instrument was recalibrated to a white calibration cap at the start of each measurement session. The color coordinates were measured on the unprotected surfaces, after the coatings’ application, after the staining and after the paint removal.
The colour changes (Δ
E*
ab) were calculated through the
L*
a*
b* (CIE 1976) system, using Equation (1):
where
L* is the lightness/darkness coordinate,
a* the red/green coordinate (+
a* indicating red and −
a* green), and
b* the yellow/blue coordinate (+
b* indicating yellow and −
b* blue).
All the colour variations were determined by the comparison with the untreated surfaces, using the averaged values of L*, a*, and b* for each sample.
The residual stain (RS) after cleaning was evaluated as a percentage by Equation (2):
where (Δ
E*
ab)c is the colour variation of the cleaned surfaces and (Δ
E*
ab)s is the colour variation of the stained surfaces.
In addition, the efficacy of the cleaning procedure, as compared with the unprotected surfaces, was evaluated by Equation (3):
where: RS
u and RS
t are the residual stain values, as defined in Equation (2), for the unprotected and protected surfaces, respectively.
The stained surfaces were examined under a binocular stereomicroscope (Stemi SV11, Zeiss, Oberkochen, Germany) at magnifications of up to 100×.
4. Conclusions
The anti-graffiti behavior of three protective treatments has been tested on compact and porous calcareous stone materials. An experimental formulation, based on fluorine resins and SiO2 nano-particles, and two commercial products were applied. The results of cleaning procedures after the staining by spray paint and felt-tip marker were discussed.
The applied products provided surfaces able to repel both water and oil, thus meeting a fundamental requirement for anti-graffiti systems.
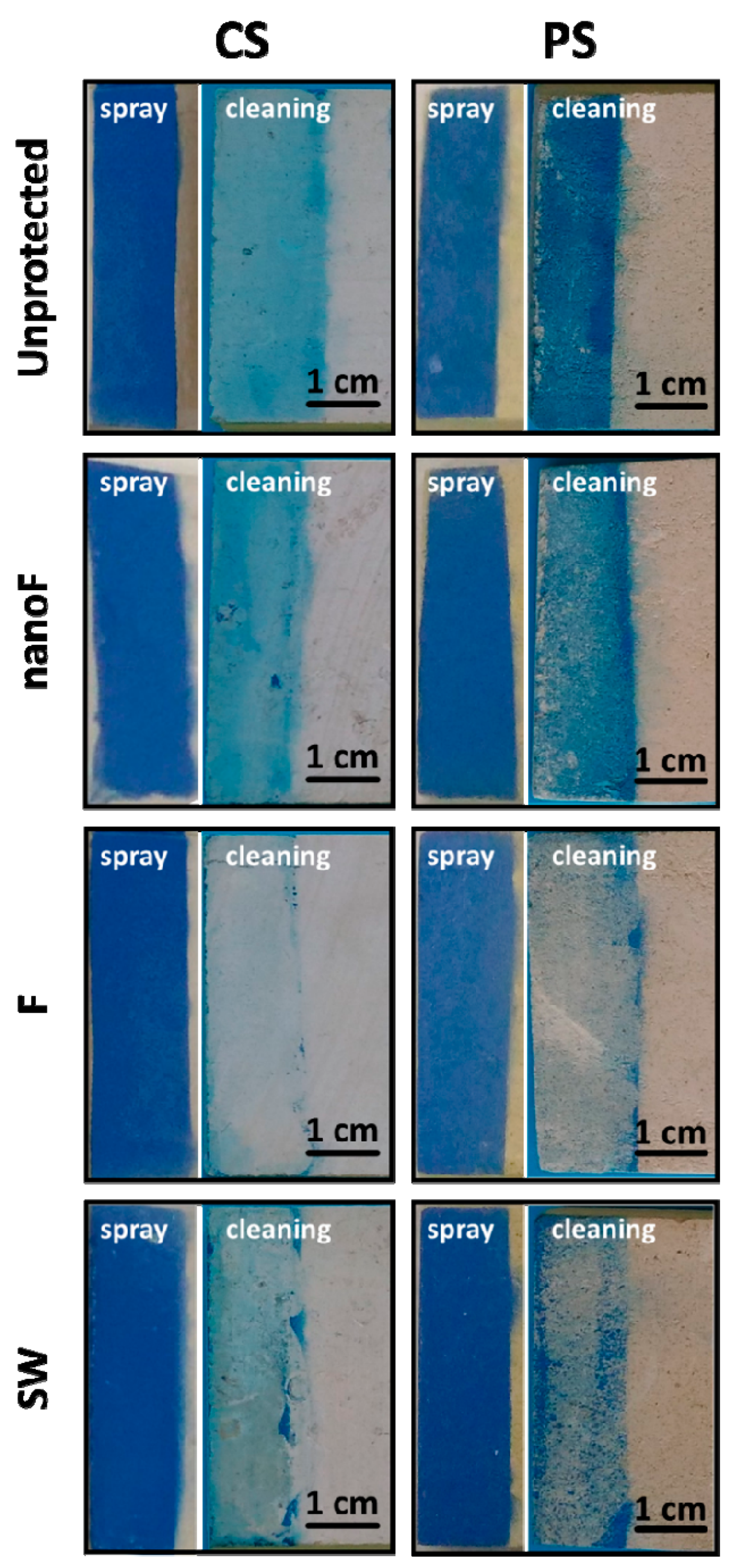
The simulation of staining actions gave rise to a distribution of the paints on the surfaces dependent on the presence and nature of the protective coating. Uniform films covering the surface, totally hiding the stone beneath, were observed in the case of untreated specimens. In most of the protected surfaces, the paint film was affected by cracks due to its shrinkage upon drying. The protective coatings restrained this cracking, which, in fact, was not noticed for the unprotected samples. Evidence of repellence against the stain was optically observed only after the application of the spray paint. In accordance with the higher surface oleophobicity, separate droplets of paint, together with portions of uncovered stone, were seen on the nanoF and F treated-samples. The morphological and porosimetric features of the stone seemed influence the paint spreading on the surface to a limited extent.
Although the removal with acetone affected the paint layer to different degrees, this procedure was not able to supply good results. The residual stain percentage was always above the threshold limit of 20%, except for the compact stone surfaces coated with F product and stained by spray paint. The paint removal was more difficult on the highly porous stone, where greater values of residual stain were found. The presence of protective coatings enhanced the spray paint removal On the contrary, the stain from the felt-tip marker was not removed even where protective coatings were applied, thus confirming a stronger action of this graffiti agent. The relative efficacy of the cleaning was influenced by the type of staining agent rather than the porosity of the substrate. In both cases, the proved hydrophobicity and oleophobicity, as well as the observed paint repellence, did not provide positive outcome of the cleaning.
The protective coatings were not eliminated from the surfaces during the cleaning; rather, the polymer protectives migrated into the porous structure of the stone under the effect of solvent evaporation. In fact, as a consequence of greater amounts of hydrophobic product moved at the surface, increased contact angle values were measured after the cleaning with acetone. This result further supports the inefficacy of the cleaning procedure with neat acetone. The unconfined solvent can spread the dissolved paint into the pores of the substrate. In this case, the stain may affect also the internal part of the stone materials making almost impossible an effective cleaning.
In conclusion, although hydrophobicity and oleophobicity are considered basic requirements of anti-graffiti coatings, these surface properties do not assure good removal of vandalic writings. The success of the anti-graffiti action depends on the applied staining agent, on the used cleaning procedure and, to a more limited extent, on the affected substrate. Therefore, the actual performance of an anti-graffiti system cannot be deduced from the coating’s properties but the effectiveness needs to be assessed in the specific applicative conditions.
Further investigations are in progress to verify the anti-graffiti efficacy under additional cleaning methods, specifically, chemical cleaners in gel matrices; the coatings’ performance after the application of higher amounts of product is another ongoing evaluation.