Tribological Performance of a Ni-Based Composite Coating in Artificial Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tribocorrosion Tests
3. Results and Discussion
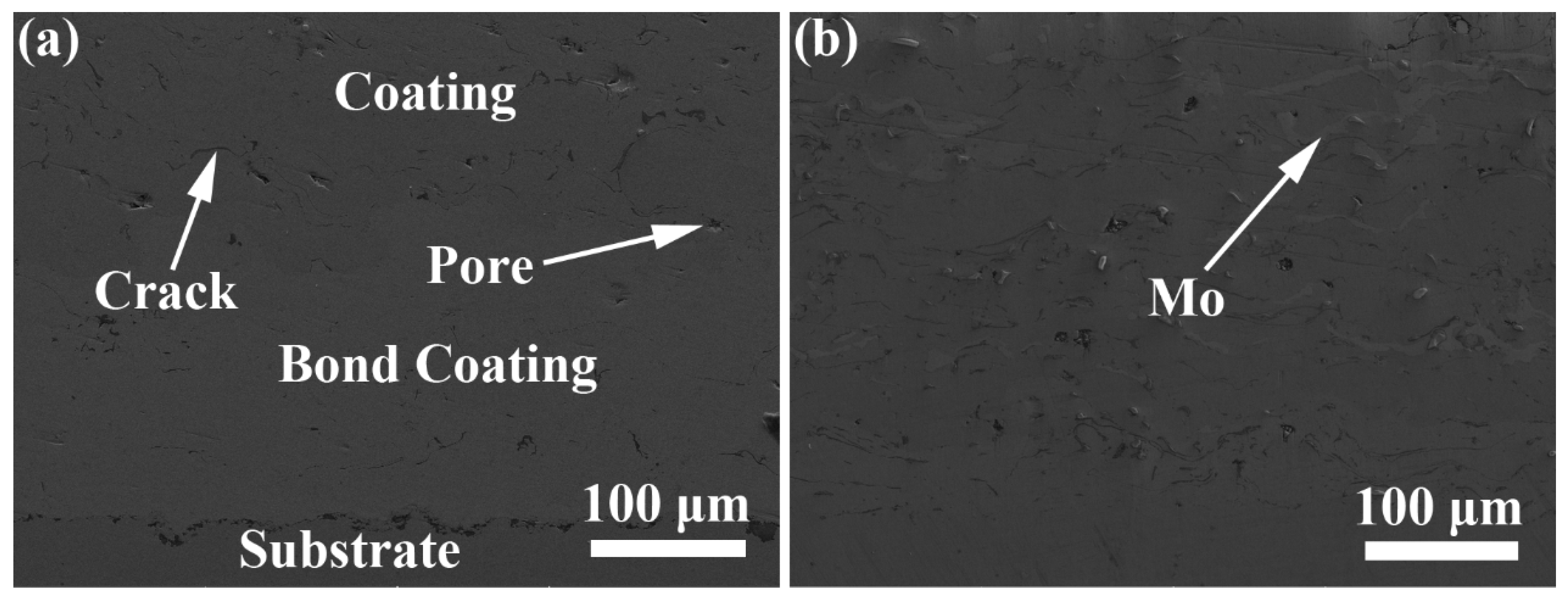
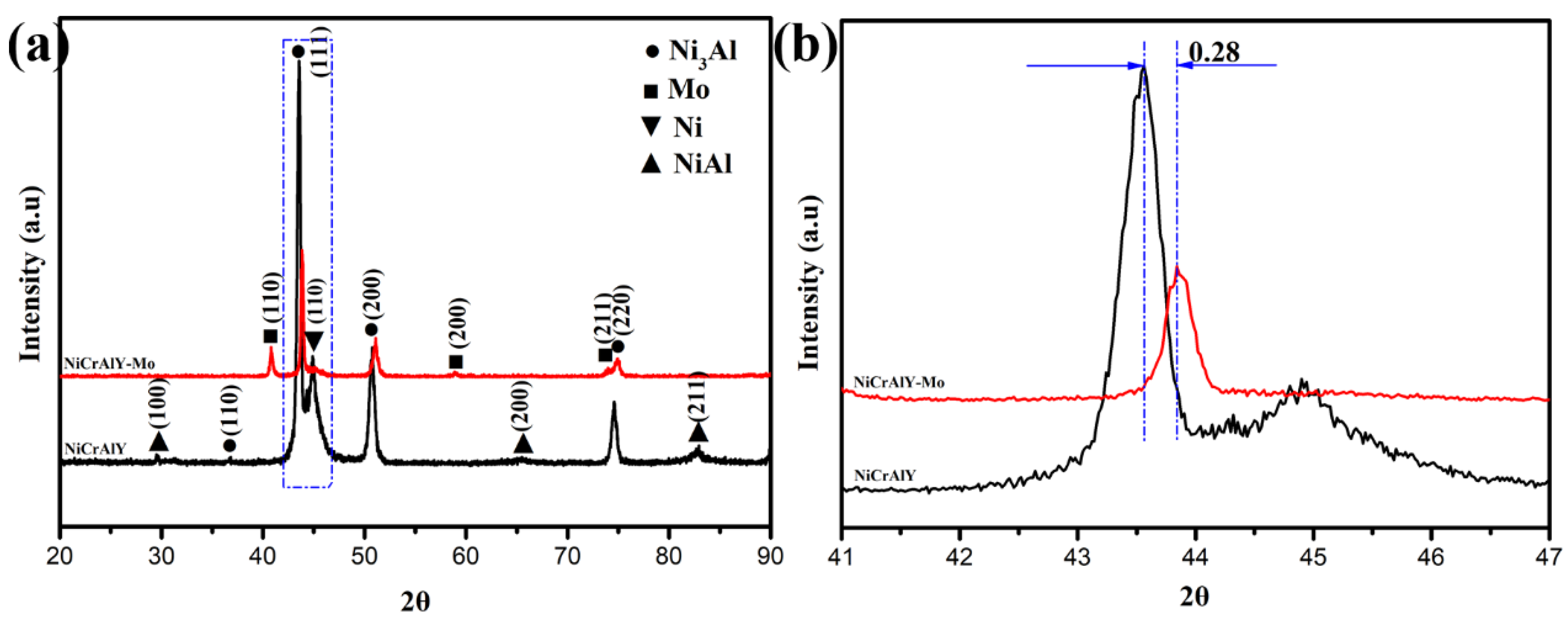
3.1. Microstructure and Microhardness of Coatings
3.2. Electrochemical Behavior of Coatings
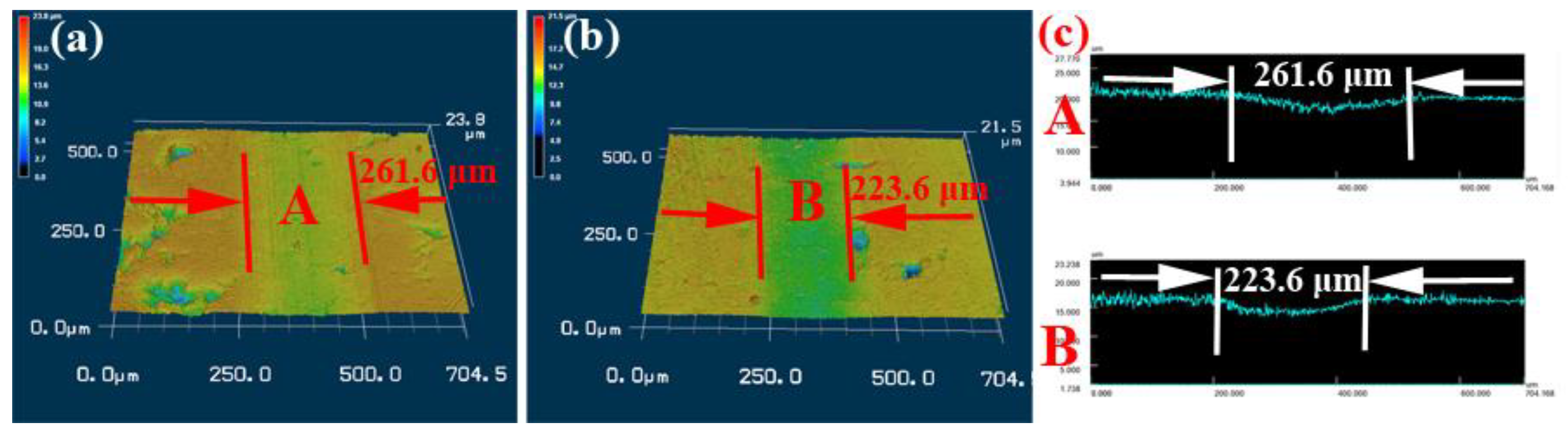
3.3. Friction and Wear Behavior of Coatings
4. Conclusions
- (1)
- Adding the Mo element, the diffraction peaks of Ni3Al shift to the right and the microhardness of the coating is improved from 329.8 HV to 342.5 HV.
- (2)
- The NiCrAlY−Mo has a lower friction coefficient and wear rate of 0.26 and 3.69 × 10−6 mm3/Nm than the NiCrAlY coating of 0.37 and 4.67 × 10−6 mm3/Nm.
- (3)
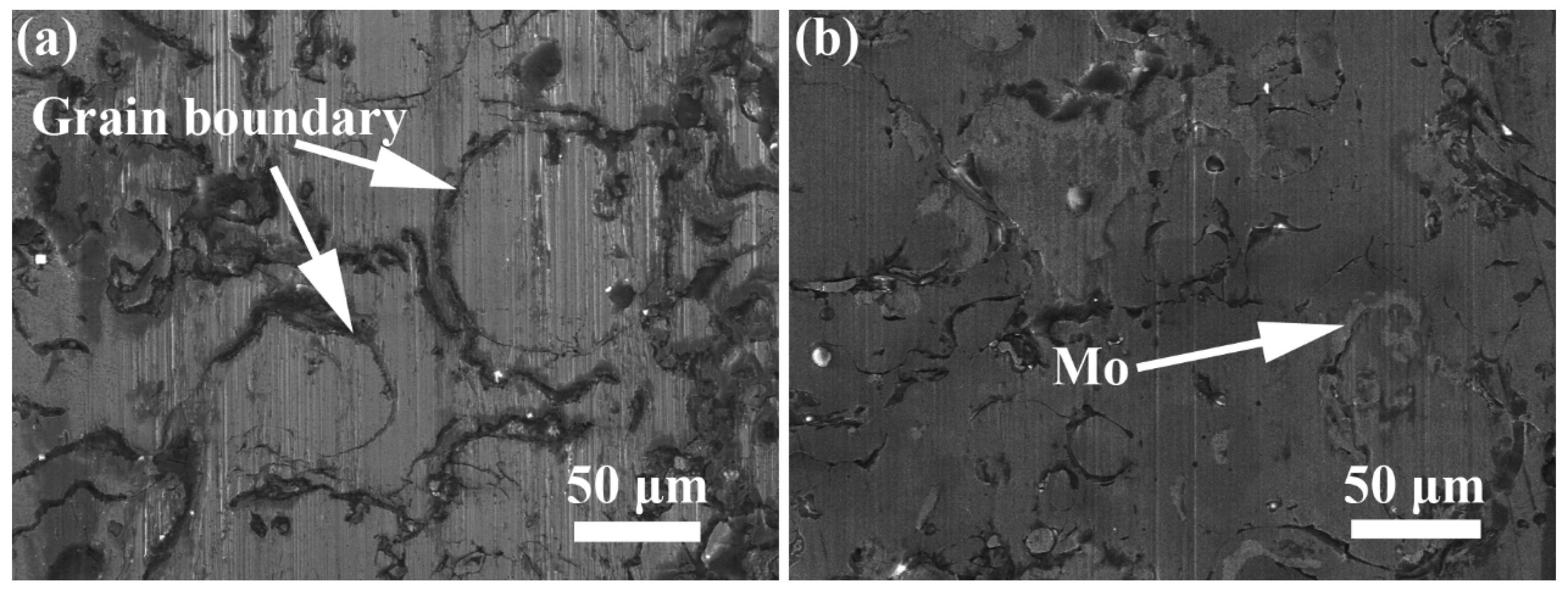
- The NiCrAlY coating has severe corrosion and the corrosion mainly occurs in the grain boundary. Adding the Mo element, the Mo element is distributed in the grain boundary and the coating has a lower corrosion rate and visibly slighter corrosion.
- (4)
- The NiCrAlY-Mo coating had excellent corrosion-wear properties in artificial seawater.
Author Contributions
Funding
Conflicts of Interest
References
- Shan, L.; Wang, Y.; Zhang, Y.; Zhang, Q.; Xue, Q. Tribocorrosion behaviors of PVD CrN coated stainless steel in seawater. Wear 2016, 362, 97–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, X.Y.; Yan, F.Y. Tribocorrosion behaviour of type S31254 steel in seawater: Identification of corrosion-wear components and effect of potential. Mater. Chem. Phys. 2016, 179, 273–281. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; An, Y.; Hou, G.; Li, S.; Deng, W.; Zhou, H.; Chen, J. Effects of loads on corrosion-wear synergism of NiCoCrAlYTa coating in artificial seawater. Tribol. Int. 2018, 118, 421–431. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Structural, mechanical and tribocorrosion behaviour in artificial seawater of CrN/AlN nano-multilayer coatings on F690 steel substrates. Appl. Surf. Sci. 2018, 428, 404–414. [Google Scholar] [CrossRef]
- Silva, R.C.C.; Nogueira, R.P.; Bastos, I.N. Tribocorrosion of UNS S32750 in chloride medium: Effect of the load level. Electrochim. Acta 2011, 56, 8839–8845. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, F.; Zhao, X.; Yang, Q.; Guo, L.; Jiang, L. Effect of phosphorus content on mechanical properties of polymeric nickel composite materials with a diamond-structure microlattice. RSC Adv. 2018, 8, 33025–33029. [Google Scholar] [CrossRef]
- Bonache, V.; Salvador, M.D.; García, J.C.; Sánchez, E.; Bannier, E. Influence of plasma intensity on wear and erosion resistance of conventional and nanometric WC-Co coatings deposited by APS. J. Therm. Spray Technol. 2010, 20, 549–559. [Google Scholar] [CrossRef]
- Boztepe, E.; Alves, A.C.; Ariza, E.; Rocha, L.A.; Cansever, N.; Toptan, F. A comparative investigation of the corrosion and tribocorrosion behaviour of nitrocarburized, gas nitrided, fluidized-bed nitrided, and plasma nitrided plastic mould steel. Surf. Coat. Technol. 2018, 334, 116–123. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Bagheri, A.; Souza, J.C.M.; Silva, F.S.; Henriques, B. Tribocorrosion behavior of hot pressed CoCrMo alloys in artificial saliva. Tribol. Int. 2016, 97, 423–430. [Google Scholar] [CrossRef]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Daroonparvar, M.; Kasiri-Asgarani, M.; Jabbarzare, S.; Medraj, M. Microstructural, mechanical properties and corrosion behavior of plasma sprayed NiCrAlY/nano-YSZ duplex coating on Mg-1.2Ca-3Zn alloy. Ceram. Int. 2015, 41, 15272–15277. [Google Scholar] [CrossRef]
- Anwar, S.; Zhang, Y.; Khan, F. Electrochemical behaviour and analysis of Zn and Zn-Ni alloy anti-corrosive coatings deposited from citrate baths. RSC Adv. 2018, 8, 28861–28873. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Daroonparvar, M.; Yajid, M.A.M.; Medraj, M. Preparation and characterization of NiCrAlY/nano-YSZ/PCL composite coatings obtained by combination of atmospheric plasma spraying and dip coating on Mg–Ca alloy. J. Alloys Compd. 2016, 658, 440–452. [Google Scholar] [CrossRef]
- Mishra, S.B.; Chandra, K.; Prakash, S. Erosion-corrosion performance of NiCrAlY coating produced by plasma spray process in a coal-fired thermal power plant. Surf. Coat. Technol. 2013, 216, 23–34. [Google Scholar] [CrossRef]
- Peng, X.; Jiang, S.; Gong, J.; Sun, X.; Sun, C. Preparation and Hot Corrosion Behavior of a NiCrAlY + AlNiY Composite Coating. J. Mater. Sci. Technol. 2016, 32, 587–592. [Google Scholar] [CrossRef]
- Liu, X.; An, Y.; Li, S.; Zhao, X.; Hou, G.; Zhou, H.; Chen, J. An assessment of tribological performance on NiCoCrAlYTa coating under corrosive environments. Tribol. Int. 2017, 115, 35–44. [Google Scholar] [CrossRef]
- Castillo-Rodríguez, M.; Nó, M.L.; Jiménez, J.A.; Ruano, O.A.; San Juan, J. High temperature internal friction in a Ti–46Al–1Mo–0.2Si intermetallic, comparison with creep behavior. Acta Mater. 2016, 103, 46–56. [Google Scholar] [CrossRef]
- Li, B.; Gao, Y.; Jia, J.; Han, M.; Guo, H.; Wang, W. Influence of heat treatments on the microstructure as well as mechanical and tribological properties of NiCrAlY–Mo–Ag coatings. J. Alloys Compd. 2016, 686, 503–510. [Google Scholar] [CrossRef]
- Guo, X.; Niu, Y.; Huang, L.; Ji, H.; Zheng, X. Microstructure and Tribological Property of TiC–Mo Composite Coating Prepared by Vacuum Plasma Spraying. J. Therm. Spray Technol. 2012, 21, 1083–1090. [Google Scholar] [CrossRef]
- Ivannikov, A.Y.; Kalita, V.I.; Komlev, D.I.; Radyuk, A.A.; Bagmutov, V.P.; Zakharov, I.N.; Parshev, S.N. The effect of electromechanical treatment on structure and properties of plasma sprayed Fe–6W–5Mo–4Cr–2V–C coating. Surf. Coat. Technol. 2018, 335, 327–333. [Google Scholar] [CrossRef]
- Klimashin, F.F.; Euchner, H.; Mayrhofer, P.H. Computational and experimental studies on structure and mechanical properties of Mo–Al–N. Acta Mater. 2016, 107, 273–278. [Google Scholar] [CrossRef]
- Li, B.; Jia, J.; Han, M.; Gao, Y.; Wang, W.; Li, C. Microstructure, mechanical and tribological properties of plasma-sprayed NiCrAlY-Mo-Ag coatings from conventional and nanostructured powders. Surf. Coat. Technol. 2017, 324, 552–559. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, H.; Liu, T.; Niu, S.; Ma, J. Improved mechanical and tribological properties of bismaleimide composites by surface-functionalized reduced graphene oxide and MoS2 coated with cyclotriphosphazene polymer. RSC Adv. 2015, 5, 97883–97890. [Google Scholar] [CrossRef]
- Li, F.; Zhu, S.; Cheng, J.; Qiao, Z.; Yang, J. Tribological properties of Mo and CaF2 added SiC matrix composites at elevated temperatures. Tribol. Int. 2017, 111, 46–51. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Xing, X.; Yuan, Z.; Yang, S.; Han, Z.; Yuan, G. Effects of Ni addition on tribocorrosion property of TiCu alloy. Tribol. Int. 2017, 107, 39–47. [Google Scholar] [CrossRef]
- Castillejo, F.E.; Marulanda, D.M.; Olaya, J.J.; Alfonso, J.E. Wear and corrosion resistance of niobium-chromium carbide coatings on AISI D2 produced through TRD. Surf. Coat. Technol. 2014, 254, 104–111. [Google Scholar] [CrossRef]
- Dhandapani, V.S.; Subbiah, R.; Thangavel, E.; Arumugam, M.; Park, K.; Gasem, Z.M.; Veeraragavan, V.; Kim, D.E. Tribological properties, corrosion resistance and biocompatibility of magnetron sputtered titanium-amorphous carbon coatings. Appl. Surf. Sci. 2016, 371, 262–274. [Google Scholar] [CrossRef]
- Kannan, A.S.; Muralidharan, S.; Sarangapani, K.B.; Balaramachandran, V.; Kapali, V. Corrosion and anodic behaviour of zinc and its ternary alloys in alkaline battery electrolytes. J. Power Sources 1995, 57, 93–98. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Plasma gas flow Ar, L/min | 40 |
| Secondary gas flow H2, L/min | 5 |
| Spraying angle | 90° |
| Powder feed rate, g/min | 42 |
| Current, A | 500 |
| Voltage, V | 60 |
| Spray distance, mm | 110 |
| Constituent | Concentration (g/L) |
|---|---|
| NaCl | 24.530 |
| Na2SO4 | 4.090 |
| CaCl2 | 1.160 |
| MgCl2·6H2O | 11.110 |
| KCl | 0.695 |
| NaHCO3 | 0.201 |
| KBr | 0.100 |
| H3BO3 | 0.027 |
| SrCl2·6H2O | 0.042 |
| NaF | 0.003 |
| Coatings | NiCrAlY | NiCrAlY–Mo |
|---|---|---|
| Vickers hardness (HV) | 330 ± 16.9 | 343 ± 17.3 |
| Coatings | Ecorr (V, vs. SCE) | icorr (A/cm2) | βa (V/dec) | −βc (V/dec) | Rp (Ω) |
|---|---|---|---|---|---|
| NiCrAlY | −0.538 ± 0.013 | 2.243 × 10−5 ± 1.168 × 10−6 | 0.041 | 0.036 | 3.711 × 102 |
| NiCrAlY-Mo | −0.490 ± 0.011 | 8.030 × 10−6 ± 0.325 × 10−6 | 0.030 | 0.042 | 9.463 × 102 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Li, C.; Gao, Y.; Guo, H.; Kang, Y.; Zhao, S. Tribological Performance of a Ni-Based Composite Coating in Artificial Seawater. Coatings 2019, 9, 747. https://doi.org/10.3390/coatings9110747
Li B, Li C, Gao Y, Guo H, Kang Y, Zhao S. Tribological Performance of a Ni-Based Composite Coating in Artificial Seawater. Coatings. 2019; 9(11):747. https://doi.org/10.3390/coatings9110747
Chicago/Turabian StyleLi, Bo, Cong Li, Yimin Gao, Hongjian Guo, Yunchuan Kang, and Siyong Zhao. 2019. "Tribological Performance of a Ni-Based Composite Coating in Artificial Seawater" Coatings 9, no. 11: 747. https://doi.org/10.3390/coatings9110747
APA StyleLi, B., Li, C., Gao, Y., Guo, H., Kang, Y., & Zhao, S. (2019). Tribological Performance of a Ni-Based Composite Coating in Artificial Seawater. Coatings, 9(11), 747. https://doi.org/10.3390/coatings9110747





