Superhydrophobic Cerium-Based Coatings on Al-Mg Alloys and Aluminized Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Preparation
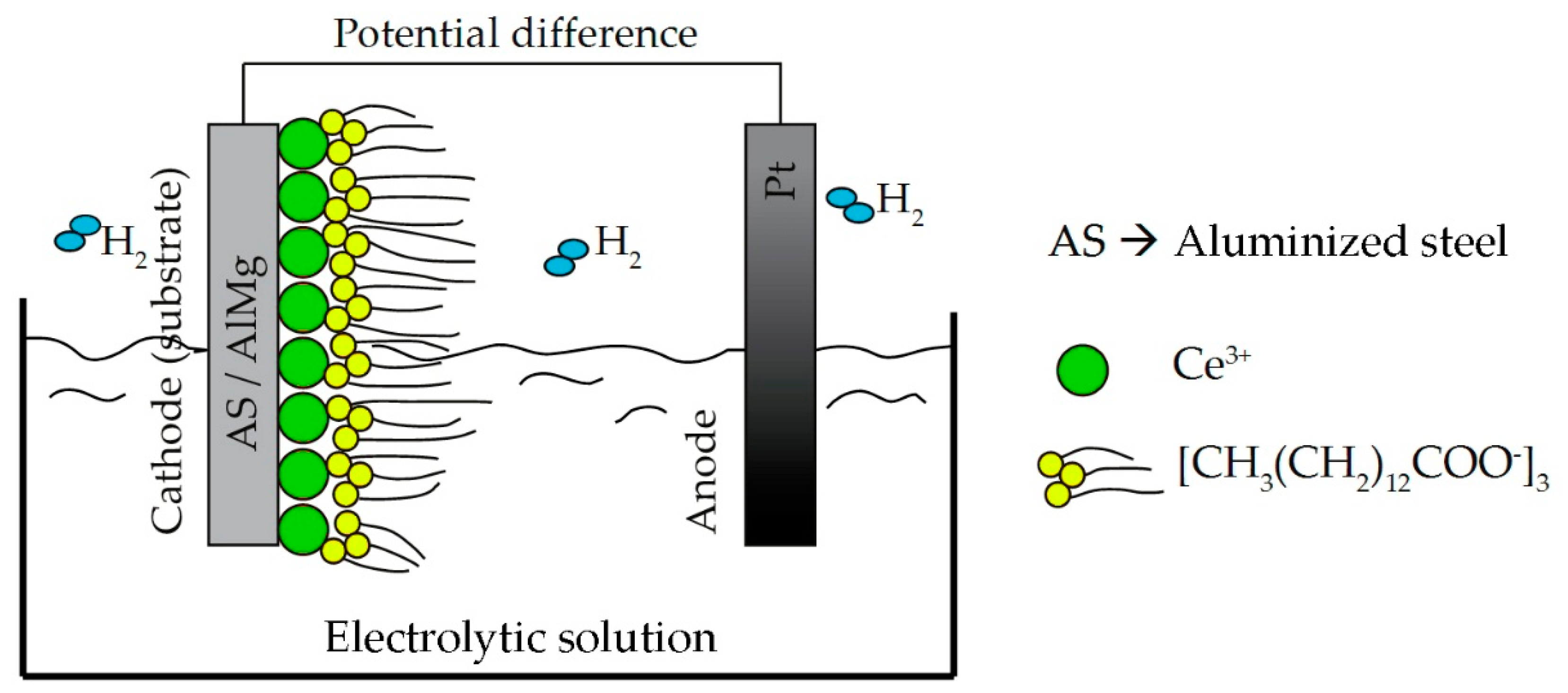
2.1.1. Cathodic Electrodeposition of Cerium-Based Coatings
2.1.2. Deposition of Cerium-Based Coatings by Immersion
2.2. Wettability Analysis
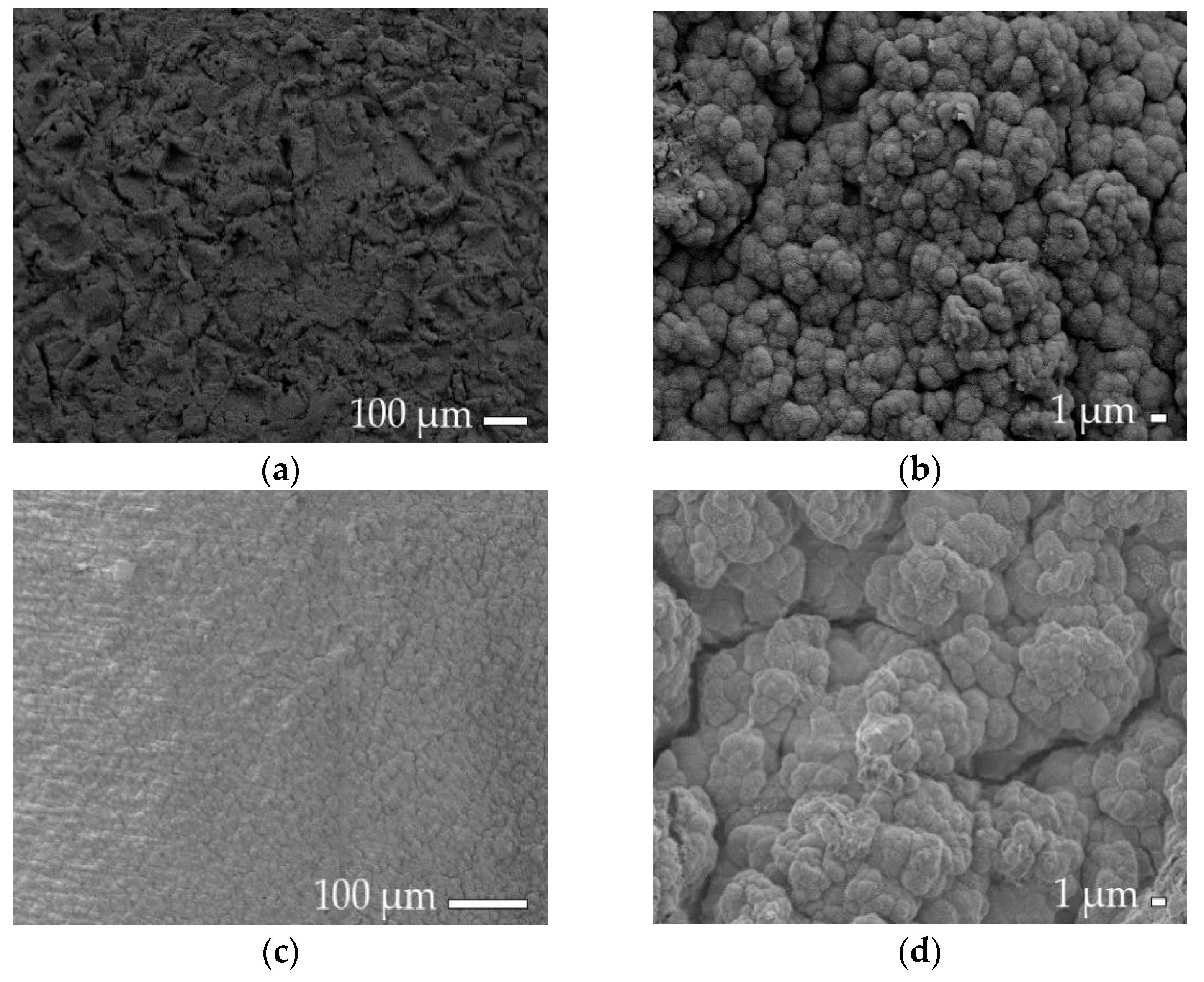
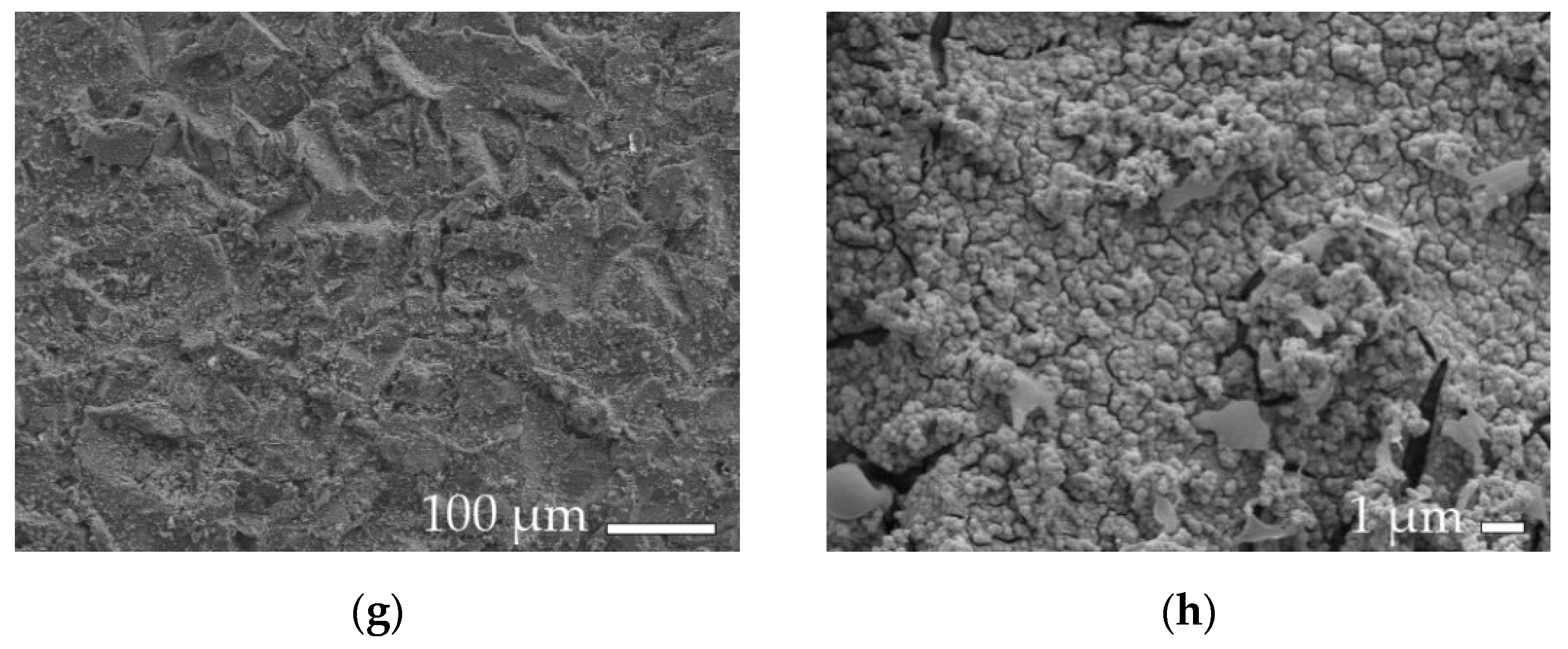
2.3. Roughness and Surface Morphology Analysis
3. Results and Discussion
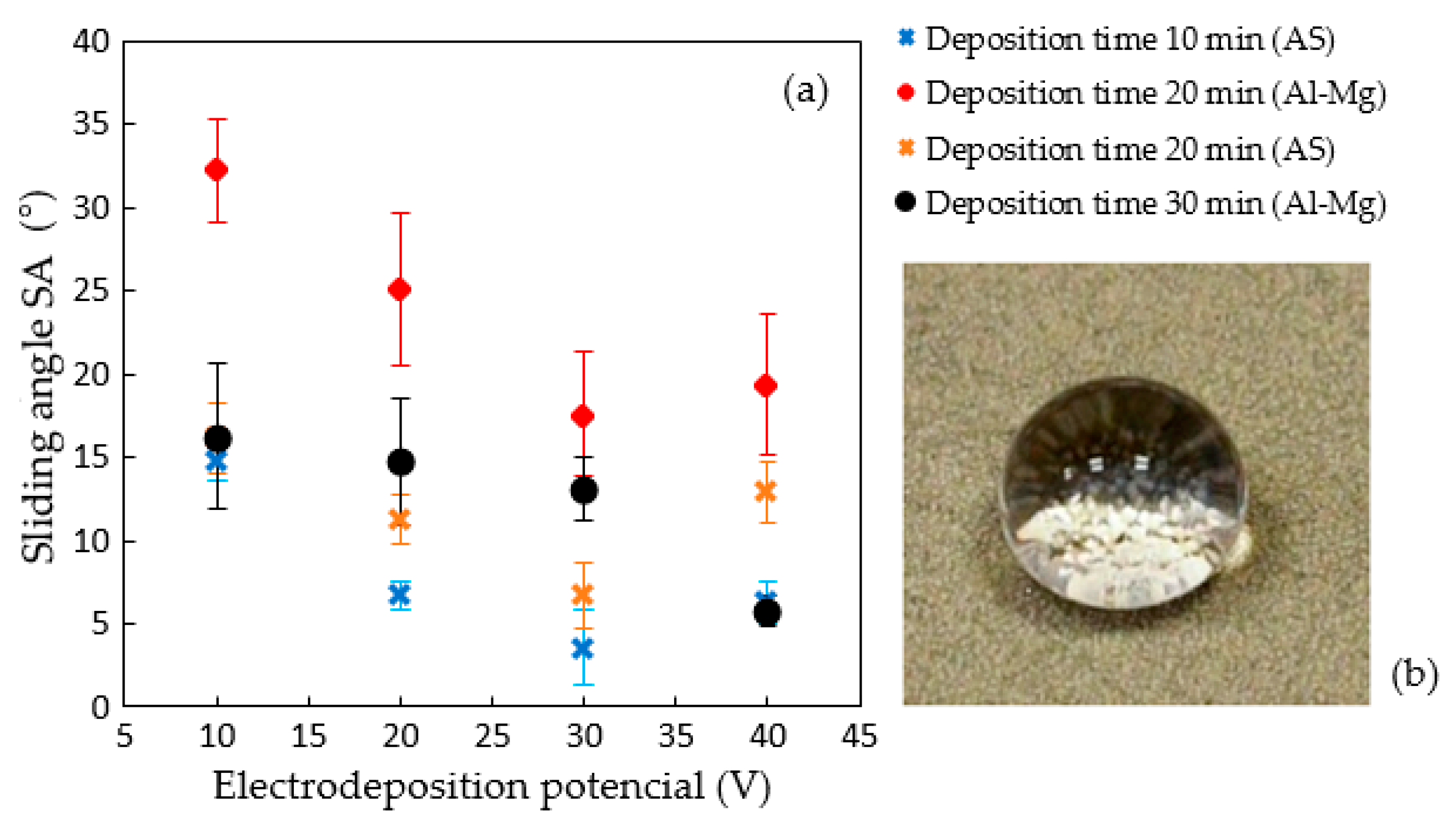
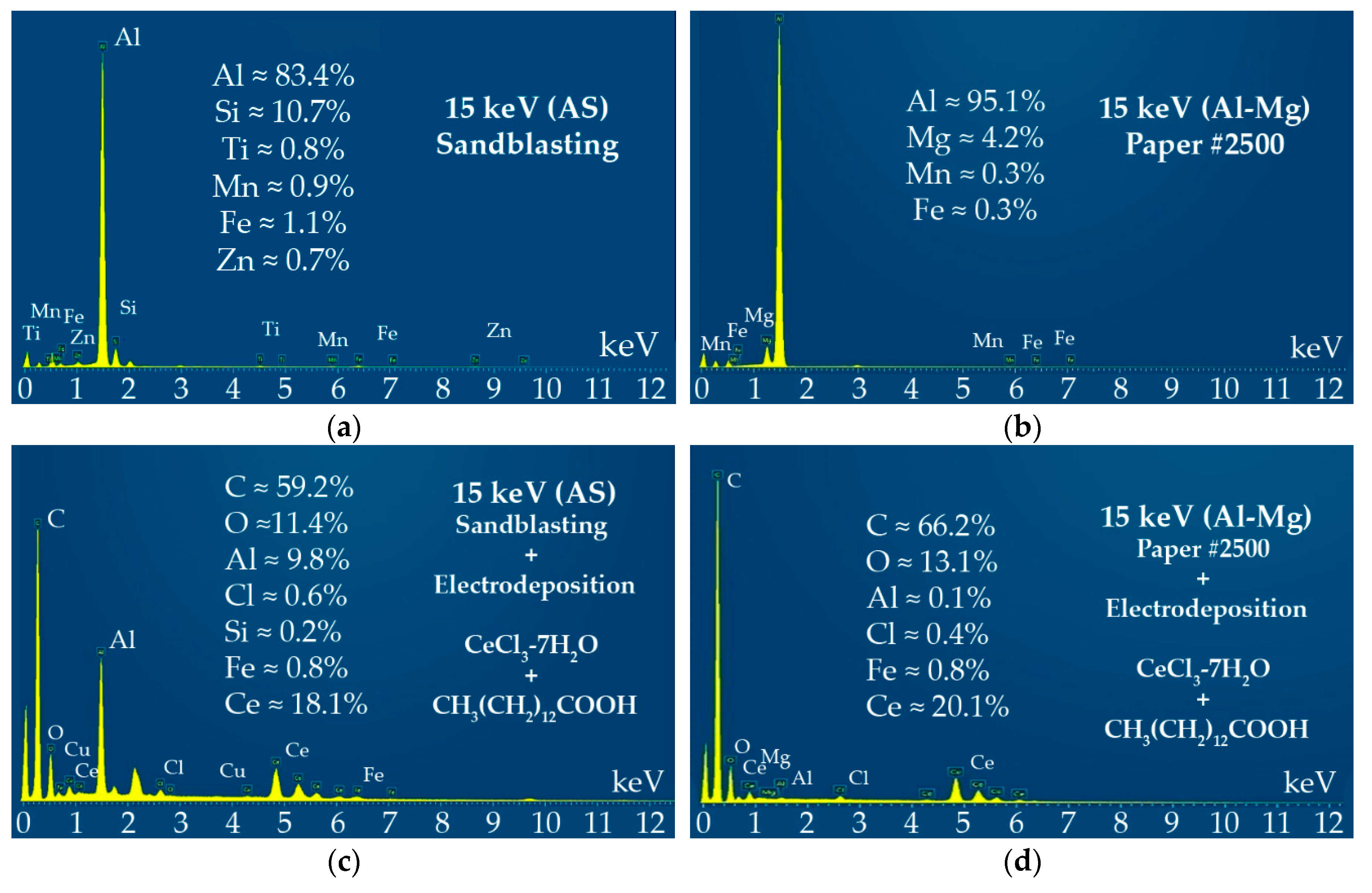
3.1. Cathodic-Electrodeposited Coatings
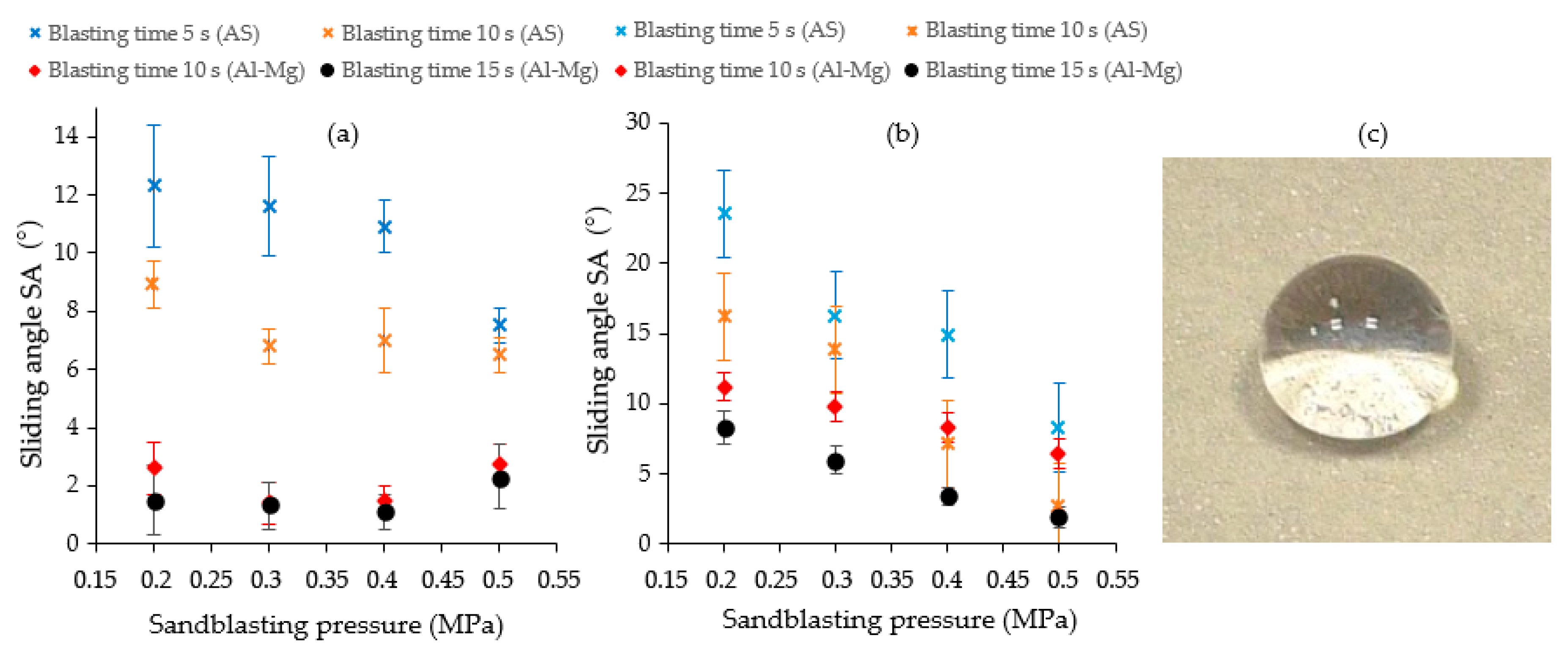
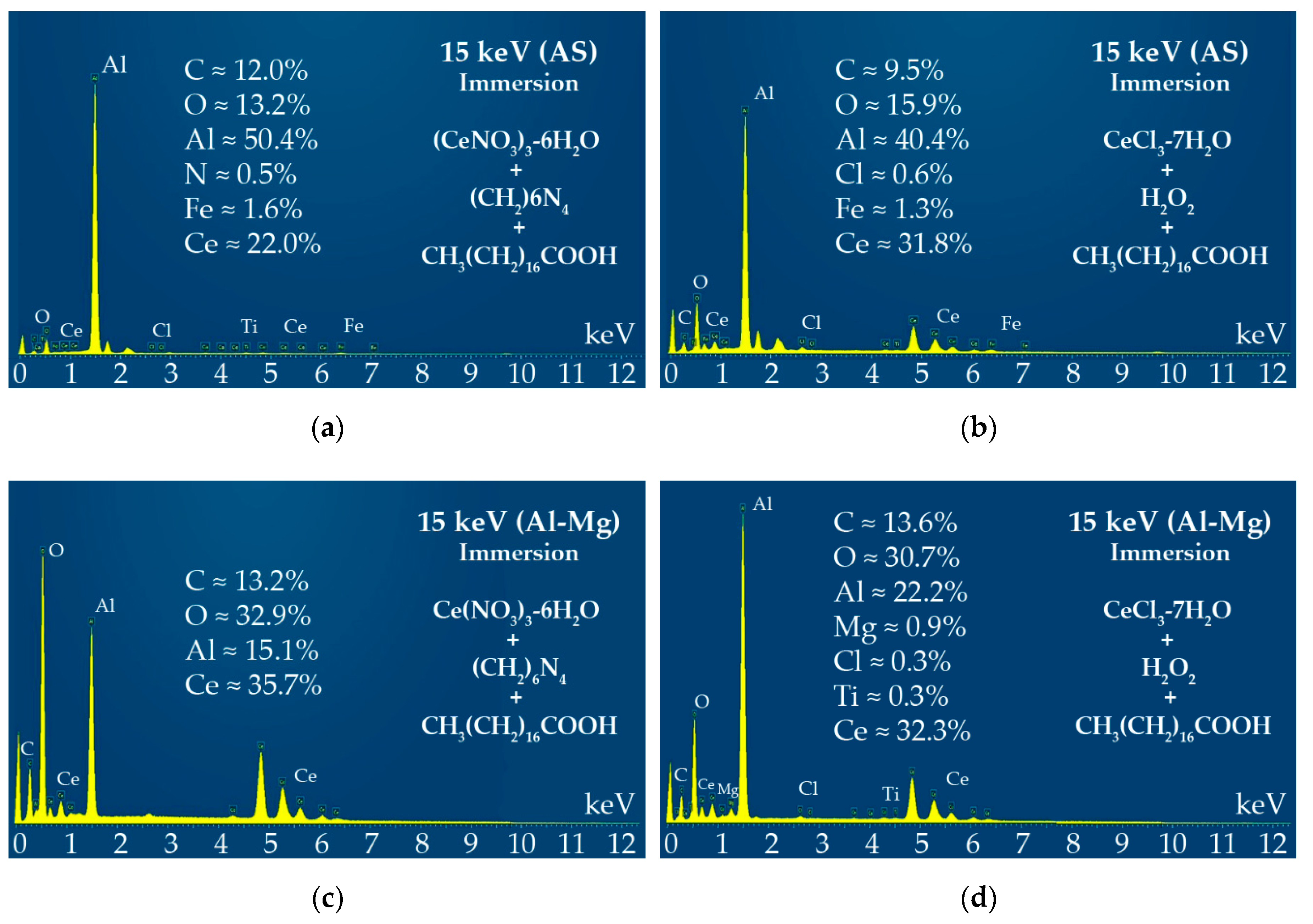
3.2. Immersion in Hexa-Hydrated Cerium Nitrate and Hepta-Hydrated Cerium Chloride
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Alabanda, Ó.; Romero, P.; Guerrero-Vaca, G. Evaluation of substrates of al-mg and aluminized steel coated with non-stick fluoropolymers after the removal of the coating. Materials 2018, 11, 2309. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Gai, G.; Benedetto, R. De Functional and perceptive aspects of non-stick coatings for cookware. Mater. Des. 2014, 53, 782–790. [Google Scholar] [CrossRef]
- Sánchez-Urbano, F.; Paz-Gómez, G.; Rodríguez-Alabanda, O.; Romero, P.E.; Cabrerizo-Vílchez, M.; Rodríguez-Valverde, M.A.; Guerrero-Vaca, G. Non-Stick coatings in aluminium molds for the production of polyurethane foam. Coatings 2018, 8, 301. [Google Scholar] [CrossRef]
- Thomas, P. The use of fluoropolymers for non-stick cooking utensils. Surf. Coat. Int. 1998, 81, 604–609. [Google Scholar] [CrossRef]
- Sethi, S.K.; Manik, G. Recent progress in super hydrophobic/hydrophilic self-cleaning surfaces for various industrial applications: A review. Polym. Plast. Technol. Eng. 2018, 57, 1932–1952. [Google Scholar] [CrossRef]
- Piscitelli, F.; Tescione, F.; Mazzola, L.; Bruno, G.; Lavorgna, M. On a simplified method to produce hydrophobic coatings for aeronautical applications. Appl. Surf. Sci. 2018. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A review on self-cleaning coatings. J. Mate. Chem. 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- Ba, Z.; Dong, Q.; Zhang, X.; Qiang, X.; Cai, Z.; Luo, X. Cerium-based modification treatment of Mg-Al hydrotalcite film on AZ91D Mg alloy assisted with alternating electric field. J. Alloy. Comp. 2017, 695, 106–113. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A. Superhydrophobic surfaces for applications in seawater. Adv. Colloid Interf. Sci. 2015, 222, 291–304. [Google Scholar] [CrossRef]
- Yang, Y.F.; Li, Y.; Li, Q.L.; Wan, L.S.; Xu, Z.K. Surface hydrophilization of microporous polypropylene membrane by grafting zwitterionic polymer for anti-biofouling. J. Membr. Sci. 2010, 362, 255–264. [Google Scholar] [CrossRef]
- Hung, L.H.; Lin, R.; Lee, A.P. Rapid microfabrication of solvent-resistant biocompatible microfluidic devices. Lab Chip 2008, 8, 983. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.; Cannavale, A.; De Marco, L.; Aricò, A.S.; Cingolani, R.; Gigli, G. Durable superhydrophobic and antireflective surfaces by trimethylsilanized silica nanoparticles-based sol−gel processing. Langmuir 2009, 25, 6357–6362. [Google Scholar] [CrossRef] [PubMed]
- Tomšič, B.; Simončič, B.; Orel, B.; Černe, L.; Tavčer, P.F.; Zorko, M.; Jerman, I.; Vilčnik, A.; Kovač, J. Sol–gel coating of cellulose fibres with antimicrobial and repellent properties. J. Sol Gel Sci. Technol. 2008, 47, 44–57. [Google Scholar] [CrossRef]
- Zimmermann, J.; Reifler, F.A.; Fortunato, G.; Gerhardt, L.C.; Seeger, S. A Simple, One-Step approach to durable and robust superhydrophobic textiles. Adv. Funct. Mater. 2008, 18, 3662–3669. [Google Scholar] [CrossRef]
- Lin, D.; Zeng, X.; Li, H.; Lai, X.; Wu, T. One-Pot fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via sol-gel reaction. J. Colloid Interf. Sci. 2019, 533, 198–206. [Google Scholar] [CrossRef]
- Xu, B.; Cai, Z. Fabrication of a superhydrophobic ZnO nanorod array film on cotton fabrics via a wet chemical route and hydrophobic modification. Appl. Surf. Sci. 2008, 254, 5899–5904. [Google Scholar] [CrossRef]
- Guillermo, P.-Z.; Juan Carlos del, C.O.; Oscar, R.-A.; Pablo, E.-R.; Miguel, C.-V.; Guillermo, G.-V.; Miguel Angel, R.-V. Water-Repellent Fluoropolymer-Based Coatings. Coatings 2019, 9, 293. [Google Scholar] [CrossRef]
- Karunakaran, R.G.; Lu, C.H.; Zhang, Z.; Yang, S. Highly transparent superhydrophobic surfaces from the coassembly of nanoparticles (≤100 nm). Langmuir 2011, 27, 4594–4602. [Google Scholar] [CrossRef]
- Cai, Y.; Chang, W.; Luo, X.; Qin, Y. Hydrophobicity of pyramid structures fabricated by micro milling. In Proceedings of the 2017 World Congress on Micro and Nano Manufacturing, Taiwan, China, 27 March 2017. [Google Scholar]
- Ming, W.; Wu, D.; van Benthem, R.; de With, G. Superhydrophobic Films from Raspberry-like Particles. Nano Lett. 2005, 5, 2298–2301. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, D.; Kang, Z. One-Step electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interf. 2015, 7, 1859–1867. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, F.J.M.; Rodríguez-Criado, J.C.; Cabrerizo-Vílchez, M.; Rodríguez-Valverde, M.A.; Guerrero-Vacas, G. Towards super-nonstick aluminized steel surfaces. Prog. Org. Coat. 2017, 109, 135–143. [Google Scholar] [CrossRef]
- Nouri, N.M.; Saadat-Bakhsh, M. Fabrication method of large-scale and mechanically durable superhydrophobic silicon rubber/aerogel coating on fibrous substrates. J. Coat. Technol. Res. 2017, 14, 477–488. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J.; Huang, P. Facile fabrication of superhydrophobic cerium coating with micro-nano flower-like structure and excellent corrosion resistance. Surf. Coat. Technol. 2014, 258, 580–586. [Google Scholar] [CrossRef]
- Liang, J.; Hu, Y.; Fan, Y.; Chen, H. Formation of superhydrophobic cerium oxide surfaces on aluminum substrate and its corrosion resistance properties. Surf. Interf. Anal. 2013, 45, 1211–1216. [Google Scholar] [CrossRef]
- Kaphle, A.; Navya, P.N.; Umapathi, A.; Daima, H.K. Nanomaterials for agriculture, food and environment: Applications, toxicity and regulation. Environ. Chem. Lett. 2018, 16, 43–58. [Google Scholar] [CrossRef]
- Chen, Z.; Li, F.; Hao, L.; Chen, A.; Kong, Y. One-step electrodeposition process to fabricate cathodic superhydrophobic surface. Appl. Surf. Sci. 2011, 258, 1395–1398. [Google Scholar] [CrossRef]
- Pedraza, F.; Mahadik, S.A.; Bouchaud, B. Synthesis of ceria based superhydrophobic coating on Ni20Cr substrate via cathodic electrodeposition. Phys. Chem. Chem. Phys. 2015, 17, 31750–31757. [Google Scholar] [CrossRef]
- Wan, B.; Ou, J.; Lv, D.; Xue, M.; Wang, F.; Wu, H. Superhydrophobic ceria on aluminum and its corrosion resistance. Surf. Interf. Anal. 2016, 48, 173–178. [Google Scholar] [CrossRef]
- Liu, Q.; Kang, Z. One-step electrodeposition process to fabricate superhydrophobic surface with improved anticorrosion property on magnesium alloy. Mater. Lett. 2014, 137, 210–213. [Google Scholar] [CrossRef]
- Rivera, B.F.; Johnson, B.Y.; O’Keefe, M.J.; Fahrenholtz, W.G. Deposition and characterization of cerium oxide conversion coatings on aluminum alloy 7075-T6. Surf. Coat. Technol. 2004, 176, 349–356. [Google Scholar] [CrossRef]



| Material | Mg | Si | Fe | Mn | Cu | Zn | Cr | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| Al-Mg alloy | 2.5 | 0.46 | 0.56 | 0.27 | - | - | - | - | Rest |
| AS | 0.01 | 8.3 | 0.45 | 0.1 | 0.05 | - | - | - | Rest |
| Substrate | Micro-Texturing | Time (min)/Voltage (V) | Ra (µm) | ACA/RCA/SA (°) | Bounces |
|---|---|---|---|---|---|
| AS | - | - | 0.41 ± 0.03 | 122/91/29 | - |
| AS | Sandpaper and blasting at 0.3 MPa/10 s | 10/30 | 4.57 ± 0.41 | 155.5/142.3/3.6 | 9 |
| Al-Mg | - | - | 0.23 ± 0.03 | 119/87/34 | - |
| Al-Mg | Sandpaper | 30/40 | 2.41 ± 0.32 | 157.5/131.8/6.2 | 6 |
| Substrate | Blasting (MPa)/Time (s) | Salt | Ra (µm) | ACA/RCA/SA (°) | Bounces |
|---|---|---|---|---|---|
| AS | 0.5/10 | Cerium nitrate | 3.37 ± 0.33 | 142.7/129.3/6.5 | 8 |
| AS | 0.5/10 | Cerium chloride | 4.03 ± 0.74 | 150.9/137.3/2.7 | 14 |
| Al-Mg | 0.4/10 | Cerium nitrate | 4.45 ± 0.27 | 155.5/138.3/1.5 | 16 |
| Al-Mg | 0.5/15 | Cerium chloride | 3.69 ± 0.61 | 155.1/136.3/2.3 | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Vaca, G.; Rodríguez-Valverde, M.A.; Castilla-Montilla, P.; Alguacil-Salamanca, F.; Rodríguez-Alabanda, Ó.; Romero, P.E.; Molero-Romero, E.; Montes Ruiz-Cabello, F.J. Superhydrophobic Cerium-Based Coatings on Al-Mg Alloys and Aluminized Steel. Coatings 2019, 9, 774. https://doi.org/10.3390/coatings9120774
Guerrero-Vaca G, Rodríguez-Valverde MA, Castilla-Montilla P, Alguacil-Salamanca F, Rodríguez-Alabanda Ó, Romero PE, Molero-Romero E, Montes Ruiz-Cabello FJ. Superhydrophobic Cerium-Based Coatings on Al-Mg Alloys and Aluminized Steel. Coatings. 2019; 9(12):774. https://doi.org/10.3390/coatings9120774
Chicago/Turabian StyleGuerrero-Vaca, Guillermo, Miguel A. Rodríguez-Valverde, Pedro Castilla-Montilla, Francisco Alguacil-Salamanca, Óscar Rodríguez-Alabanda, Pablo E. Romero, Esther Molero-Romero, and F. Javier Montes Ruiz-Cabello. 2019. "Superhydrophobic Cerium-Based Coatings on Al-Mg Alloys and Aluminized Steel" Coatings 9, no. 12: 774. https://doi.org/10.3390/coatings9120774
APA StyleGuerrero-Vaca, G., Rodríguez-Valverde, M. A., Castilla-Montilla, P., Alguacil-Salamanca, F., Rodríguez-Alabanda, Ó., Romero, P. E., Molero-Romero, E., & Montes Ruiz-Cabello, F. J. (2019). Superhydrophobic Cerium-Based Coatings on Al-Mg Alloys and Aluminized Steel. Coatings, 9(12), 774. https://doi.org/10.3390/coatings9120774







