Surface Characterization and Copper Release of a-C:H:Cu Coatings for Medical Applications
Abstract
:1. Introduction
2. Materials and Methods
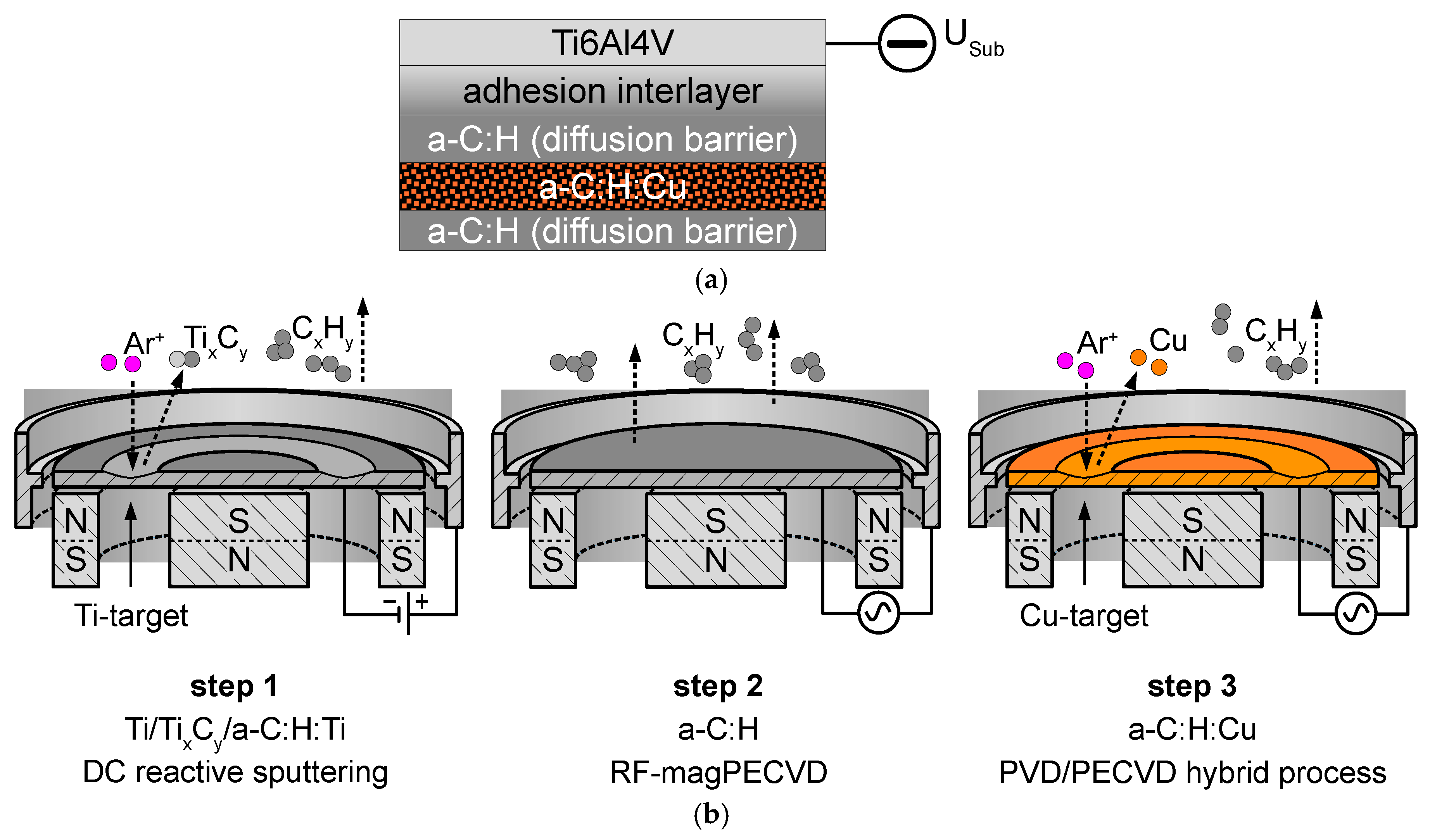
2.1. Deposition Methods
2.2. Chemical and Structural Characterisation
2.3. Surface Characterization
2.4. Release of Cu2+ Ions
3. Results and Discussion
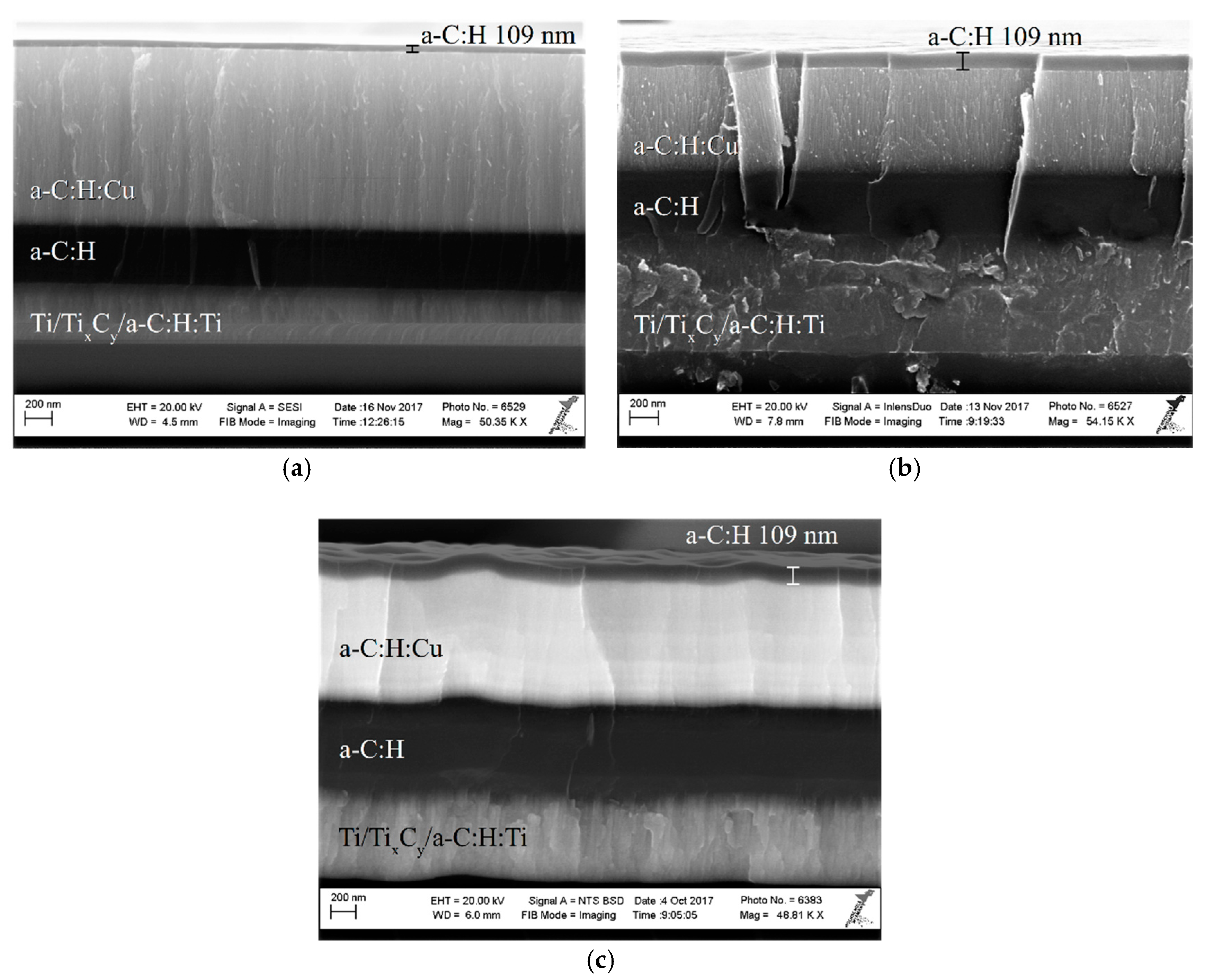
3.1. Surface Topography
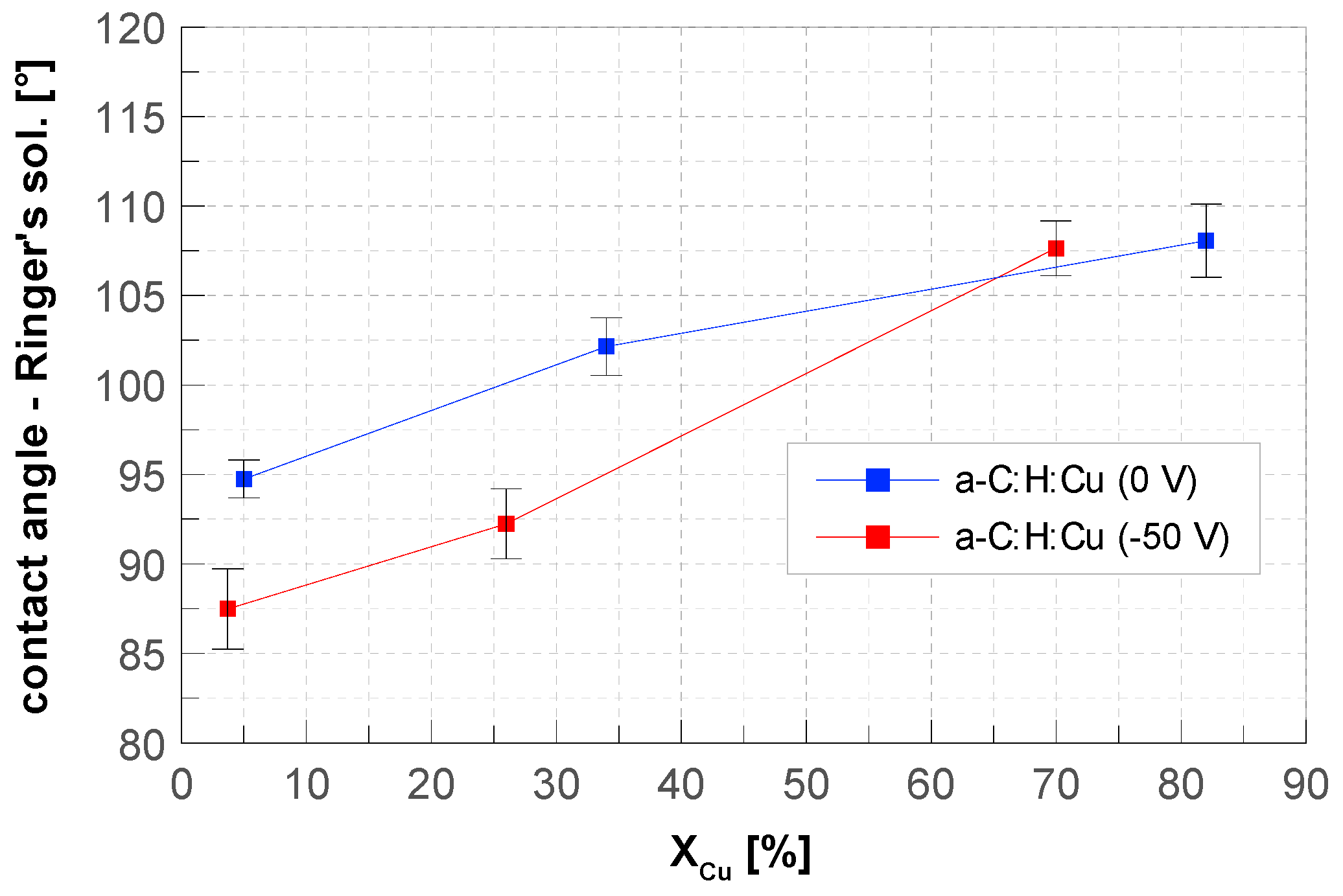
3.2. Contact Angle
3.3. Release Kinetics of Cu2+ Ions
(aq) aqua = dissolved (s) solid = film
- top-layer (XCu = 90%, nCu = 1.225 μmol);
- bottom-layer (XCu = 55%, nCu = 2.31 μmol);
- dual-layer (nCu = 3.71 μmol) consisting of top and bottom layer.
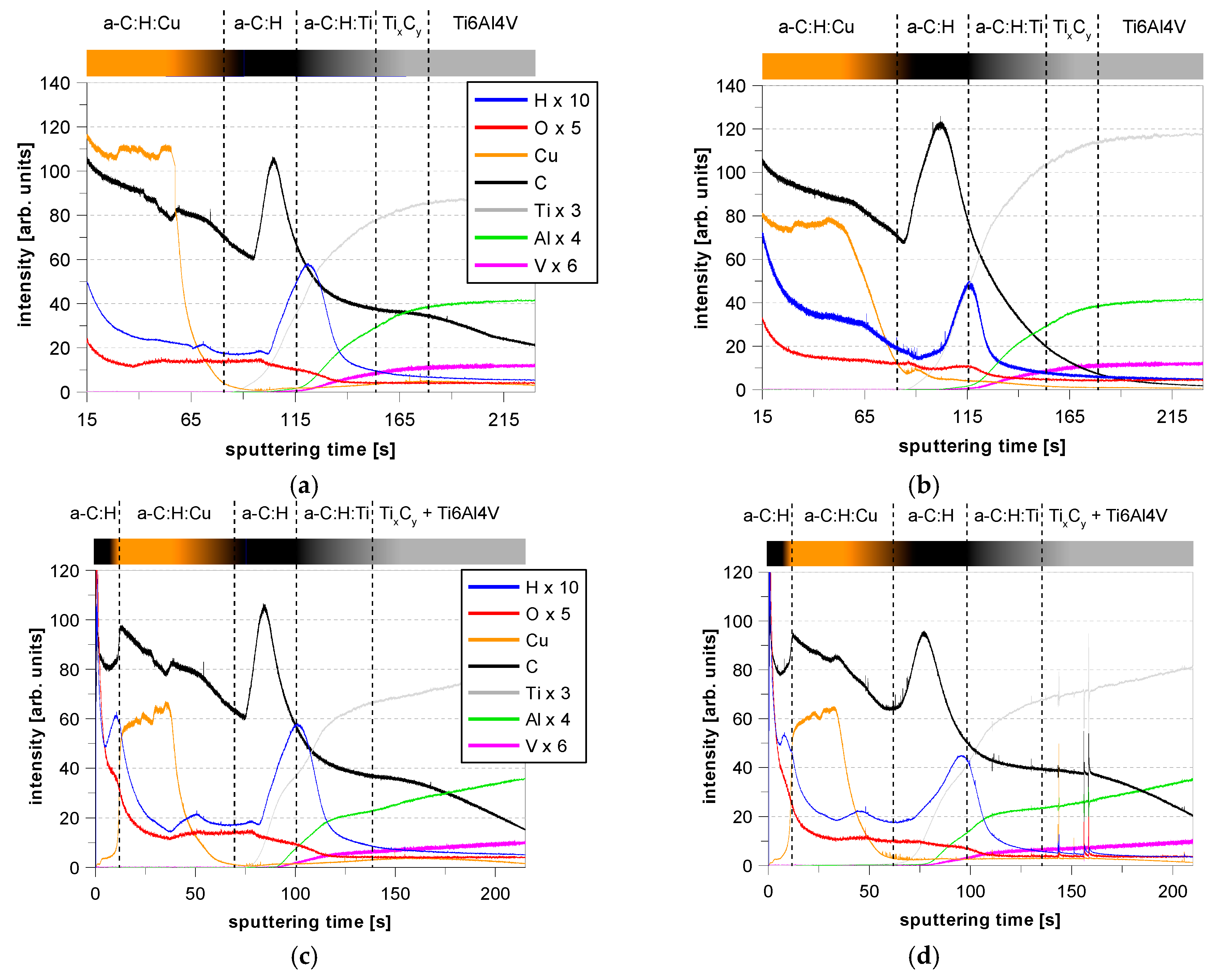
3.4. GDOES Depth Profiles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aslam, S.; Darouiche, R.O. Prosthetic joint infection. Curr. Infect. Dis. Rep. 2012, 14, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Neogi, D.S.; Yadav, C.S.; Madhuri V, P. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampin-based regimen. Acta Orthop. 2008, 79, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.E.; Crane, T.P.; Noy, M.; Elliott, T.S.J.; Grimer, R.J. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: A 15-year prospective survey. J. Bone Jt. Surg. 2006, 88, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Jämsen, E.; Huhtala, H.; Puolakka, T.; Moilanen, T. Risk factors for infection after knee arthroplasty: A register-based analysis of 43,149 cases. J. Bone Jt. Surg. 2009, 91, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Peersman, G.; Laskin, R.; Davis, J.; Peterson, M. Infection in total knee replacement. Clin. Orthop. Relat. Res. 2001, 392, 15–23. [Google Scholar] [CrossRef]
- Crismani, A.G.; Bertl, M.H.; Čelar, A.G.; Bantleon, H.P.; Burstone, C.J. Miniscrews in orthodontic treatment-Review and analysis of published clinical trials. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 108–113. [Google Scholar] [CrossRef]
- Dalessandri, D.; Salgarello, S.; Dalessandri, M.; Lazzaroni, E.; Piancino, M. Determinants for success rates of temporary anchorage devices in orthodontics: A meta-analysis (n > 50). Eur. J. Orthod. 2014, 36, 303–313. [Google Scholar] [CrossRef]
- Cunha, A.C.; da Veiga, A.M.A.; Masterson, D.; Mattos, C.T.; Nojima, L.I. How do geometry-related parameters influence the clinical performance of orthodontic mini-implants? A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 1539–1551. [Google Scholar] [CrossRef]
- Mohammadi, A.; Zarghami, A.; Sadrhaghighi, A.H. The effect of mini-implant design on peri-implantitis and pain level after insertion in orthodontic treatment: A prospective clinical study. J. Periodontol. Implant Dent. 2016, 8, 55–59. [Google Scholar]
- Park, H.S.; Jeong, S.H.; Kwon, O.W. Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, S.; Koyama, I.; Inoue, M.; Mishima, K.; Sugahara, T. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 373–378. [Google Scholar] [CrossRef]
- Coculescu, B.I. Antimicrobial resistance induced by genetic change. J. Med. Life 2009, 2, 114–123. [Google Scholar] [PubMed]
- Balsalobre, L.C.; Dropa, M.; Matté, M.H. An overview of antimicrobial resistance and its public health significance. Braz. J. Microbiol. 2014, 45, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Shi, Z.L.; Chua, P.H.; Kang, E.T.; Neoh, K.G. Functionalization of titanium surfaces via controlled living radical polymerization: From antibacterial surface to surface for osteoblast adhesion. Ind. Eng. Chem. Res. 2007, 46, 9077–9086. [Google Scholar] [CrossRef]
- Antoci, V.; Adams, C.S.; Parvizi, J.; Ducheyne, P.; Shapiro, I.M. Covalently attached vancomycin provides a nanoscale antibacterial surface. Clin. Orthop. Relat. Res. 2007, 461, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol-gel films on titanium alloy fracture plate material. Biomaterials 2007, 28, 1721–1729. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Agrawal, A.; Knabe, C.; Ducheyne, P. Sol-gel silica controlled release thin films for the inhibition of methicillin-resistant Staphylococcus aureus. Biomaterials 2014, 35, 509–517. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Mihailescu, I.N.; Bociaga, D.; Socol, G.; Stan, G.E.; Chifiriuc, M.C. Fabrication of antimicrobial silver-doped carbon structures by combinatorial pulsed laser deposition. Int. J. Pharm. 2016, 515, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Adesanya, K.; di Silvio, L.; Catauro, M.; Ambrosio, L. The biocompatibility of silver-containing Na2O·CaO·2SiO2 glass prepared by sol-gel method: In vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Stranak, V.; Wulff, H.; Ksirova, P.; Zietz, C.; Drache, S. Ionized vapor deposition of antimicrobial Ti–Cu films with controlled copper release. Thin Solid Films 2014, 550, 389–394. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrini, P.; Arciola, C.R.; Pezzali, I.; Bozzini, S.; Montanaro, L. Antibacterial activity of zinc modified titanium oxide surface. Int. J. Artif. Organs 2018, 29, 434–442. [Google Scholar] [CrossRef]
- Hempel, F.; Finke, B.; Zietz, C.; Bader, R.; Weltmann, K.D. Antimicrobial surface modification of titanium substrates by means of plasma immersion ion implantation and deposition of copper. Surf. Coat. Technol. 2014, 256, 52–58. [Google Scholar] [CrossRef]
- Hauert, R.; Gampp, R.; Müller, U.; Schroeder, A.; Blum, J. Surface analysis and bioreactions on silver-containing amorphous hydrogenated carbon films. Polym. Repr. 1997, 38, 994–995. [Google Scholar]
- Agarwal, A.; Weis, T.L.; Schurr, M.J.; Faith, N.G.; Czuprynski, C.J. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials 2010, 31, 680–690. [Google Scholar] [CrossRef] [Green Version]
- Mass, A.; Bruno, A.; Bosetti, M.; Biasibetti, A.; Cannas, M. Prevention of pin track infection in external fixation with silver coated pins-Clinical and microbiological results. J. Biomed. Mater. Res. 2000, 53, 600–604. [Google Scholar] [CrossRef]
- Shirai, T.; Tsuchiya, H.; Shimizu, T.; Ohtani, K.; Zen, Y. Prevention of pin tract infection with titanium-copper alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Cloutier, M.; Harnagea, C.; Hale, P.; Seddiki, O.; Rosei, F. Long-term stability of hydrogenated DLC coatings: Effects of aging on the structural, chemical and mechanical properties. Diam. Relat. Mater. 2014, 48, 65–72. [Google Scholar] [CrossRef]
- Cloutier, M.; Turgeon, S.; Busby, Y.; Tatoulian, M.; Pireaux, J.J. Controlled distribution and clustering of silver in Ag-DLC nanocomposite coatings using a hybrid plasma approach. ACS Appl. Mater. Interfaces 2016, 8, 21020–21027. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Manninen, N.K.; Fialho, L.; Henriques, M.; Carvalho, S. Morphology and oxygen incorporation effect on antimicrobial activity of silver thin films. Appl. Surf. Sci. 2016, 371, 1–8. [Google Scholar] [CrossRef]
- Serrano, C.; García-Fernández, L.; Fernández-Blázquez, J.P.; Barbeck, M.; Ghanaati, S. Nanostructured medical sutures with antibacterial properties. Biomaterials 2015, 52, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Elashnikov, R.; Lyutakov, O.; Ulbrich, P.; Svorcik, V. Light-activated polymethylmethacrylate nanofibers with antibacterial activity. Mater. Sci. Eng. C 2016, 64, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Lyutakov, O.; Goncharova, I.; Rimpelova, S.; Kolarova, K.; Svanda, J. Silver release and antimicrobial properties of PMMA films doped with silver ions, nano-particles and complexes. Mater. Sci. Eng. C 2015, 49, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H. Preparation and properties of electrospun poly(vinyl alcohol)/silver fiber web as wound dressings. Polym. Eng. Sci. 2007, 47, 43–49. [Google Scholar] [CrossRef]
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol–gel coatings. J. Mater. Chem. 2005, 15, 4385–4398. [Google Scholar] [CrossRef]
- Rujitanaroj, P.O.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Körner, E.; Aguirre, M.H.; Fortunato, G.; Ritter, A.; Rühe, J. Formation and distribution of silver nanoparticles in a functional plasma polymer matrix and related Ag+ release properties. Plasma Process. Polym. 2010, 7, 619–625. [Google Scholar] [CrossRef]
- Vasilev, K.; Sah, V.; Anselme, K.; Ndi, C.; Mateescu, M. Tunable antibacterial coatings that support mammalian cell growth. Nano Lett. 2010, 10, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Jelínek, M.; Zemek, J.; Remsa, J.; Mikšovský, J.; Kocourek, T. Hybrid laser technology and doped biomaterials. Appl. Surf. Sci. 2017, 417, 73–83. [Google Scholar] [CrossRef]
- Ming, M.Y.; Jiang, X.; Piliptsou, D.G.; Zhuang, Y.; Rogachev, A.V. Chromium-modified a-C films with advanced structural, mechanical and corrosive-resistant characteristics. Appl. Surf. Sci. 2016, 379, 424–432. [Google Scholar] [CrossRef]
- Lee, N.R.; Jun, Y.S.; Moon, K.I.; Lee, C.S. Ti-doped hydrogenated diamond like carbon coating deposited by hybrid physical vapor deposition and plasma enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 2017, 56, 35506. [Google Scholar] [CrossRef]
- Xuemin, W.; Weidong, W.; Shengyin, L.; Li, B.; Linhong, C. Properties of W incorporated diamond-like carbon films prepared by pulsed-laser deposition. J. Alloy. Compd. 2009, 479, 741–745. [Google Scholar] [CrossRef]
- Müller, I.C.; Sharp, J.; Rainforth, W.M.; Hovsepian, P.; Ehiasarian, A. Tribological response and characterization of Mo–W doped DLC coating. Wear 2017, 376–377, 1622–1629. [Google Scholar]
- Marciano, F.R.; Bonetti, L.F.; Santos, L.V.; Da-Silva, N.S.; Corat, E.J. Antibacterial activity of DLC and Ag–DLC films produced by PECVD technique. Diam. Relat. Mater. 2009, 18, 1010–1014. [Google Scholar] [CrossRef]
- Wang, C.; Yu, X.; Hua, M. Microstructure and mechanical properties of Ag-containing diamond-like carbon films in mid-frequency dual-magnetron sputtering. Appl. Surf. Sci. 2009, 256, 1431–1435. [Google Scholar] [CrossRef]
- Sun, L.; Guo, P.; Ke, P.; Li, X.; Wang, A. Synergistic effect of Cu/Cr co-doping on the wettability and mechanical properties of diamond-like carbon films. Diam. Relat. Mater. 2016, 68, 1–9. [Google Scholar] [CrossRef]
- Pang, X.; Shi, L.; Wang, P.; Xia, Y.; Liu, W. Effects of Al incorporation on the mechanical and tribological properties of Ti-doped a-C:H films deposited by magnetron sputtering. Curr. Appl. Phys. 2011, 11, 771–775. [Google Scholar] [CrossRef]
- Lan, W.C.; Ou, S.F.; Lin, M.H.; Ou, K.L.; Tsai, M.Y. Development of silver-containing diamond-like carbon for biomedical applications. Part I: Microstructure characteristics, mechanical properties and antibacterial mechanisms. Ceram. Int. 2013, 39, 4099–4104. [Google Scholar] [CrossRef]
- Bonilla-Gameros, L.; Cloutier, M.; Montaño-Machado, V.; Chevallier, P.; Mantovani, D. Controlling silver ion release from Ag-based nanocoatings by plasma surface engineering. MSF 2018, 941, 1625–1631. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Kmeth, R.; Obermeier, A.; Bauer, A.T.; Halter, N. Silver nanoparticle-enriched diamond-like carbon implant modification as a mammalian cell compatible surface with antimicrobial properties. Sci. Rep. 2016, 6, 22849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, K.; Hatada, R.; Flege, S.; Ensinger, W.; Shibata, Y. Preparation and antibacterial properties of Ag-containing diamond-like carbon films prepared by a combination of magnetron sputtering and plasma source ion implantation. Vacuum 2013, 89, 179–184. [Google Scholar] [CrossRef]
- Chan, Y.H.; Huang, C.F.; Ou, K.L.; Peng, P.W. Mechanical properties and antibacterial activity of copper doped diamond-like carbon films. Surf. Coat. Technol. 2011, 206, 1037–1040. [Google Scholar] [CrossRef]
- Endrino, J.L.; Anders, A.; Albella, J.M.; Horton, J.A.; Horton, T.H. Antibacterial efficacy of advanced silver-amorphous carbon coatings deposited using the pulsed dual cathodic arc technique. J. Phys. Conf. Ser. 2010, 252, 12012. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.Y.; Wang, D.Y.; Wu, W. Catalysis effect of metal doping on wear properties of diamond-like carbon films deposited by a cathodic-arc activated deposition process. Thin Solid Films 2002, 420–421, 241–247. [Google Scholar] [CrossRef]
- Zou, C.W.; Wang, H.J.; Feng, L.; Xue, S.W. Effects of Cr concentrations on the microstructure, hardness, and temperature-dependent tribological properties of Cr-DLC coatings. Appl. Surf. Sci. 2013, 286, 137–141. [Google Scholar] [CrossRef]
- Polak, M.; Ohl, A.; Quaas, M.; Lukowski, G.; Lüthen, F. Oxygen and water plasma-immersion ion implantation of copper into titanium for antibacterial surfaces of medical implants. Adv. Eng. Mater. 2010, 12, B511–B518. [Google Scholar] [CrossRef]
- Chu, P.K. Applications of plasma-based technology to microelectronics and biomedical engineering. Surf. Coat. Technol. 2009, 203, 2793–2798. [Google Scholar] [CrossRef]
- Schröder, K.; Finke, B.; Polak, M.; Lüthen, F.; Nebe, B.; Rychly, J.; Bader, R.; Lukowski, G.; Walschus, U.; Schlosser, M.; et al. Gas-discharge plasma-assisted functionalization of titanium implant surfaces. MSF 2010, 638–642, 700–705. [Google Scholar]
- Jelinek, M.; Zemek, J.; Vandrovcova, M.; Bacakova, L.; Kocourek, T. Bonding and bio-properties of hybrid laser/magnetron Cr-enriched DLC layers. Mater. Sci. Eng. C 2016, 58, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Foong, Y.M.; Koh, A.T.T.; Lim, S.R.; Chua, D.H.C.; Ng, H.Y. Properties of laser fabricated nanostructured Cu/diamond-like carbon composite. J. Mater. Res. 2011, 26, 2761–2771. [Google Scholar] [CrossRef]
- Zhang, H.S.; Endrino, J.L.; Anders, A. Comparative surface and nano-tribological characteristics of nanocomposite diamond-like carbon thin films doped by silver. Appl. Surf. Sci. 2008, 255, 2551–2556. [Google Scholar] [CrossRef] [Green Version]
- Bociąga, D.; Jakubowski, W.; Komorowski, P.; Sobczyk-Guzenda, A.; Jędrzejczak, A. Surface characterization and biological evaluation of silver-incorporated DLC coatings fabricated by hybrid RF PACVD/MS method. Mater. Sci. Eng. C 2016, 63, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Harrasser, N.; Jüssen, S.; Obermeir, A.; Kmeth, R.; Stritzker, B. Antibacterial potency of different deposition methods of silver and copper containing diamond-like carbon coated polyethylene. Biomater. Res. 2016, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, I.; Lüthen, F.; Prinz, C.; Kreikemeyer, B.; Zietz, C. A dual function of copper in designing regenerative implants. Biomaterials 2015, 44, 36–44. [Google Scholar] [CrossRef]
- Zietz, C.; Fritsche, A.; Finke, B.; Stranak, V.; Haenle, M. Analysis of the release characteristics of Cu-treated antimicrobial implant surfaces using atomic absorption spectrometry. Bioinorg. Chem. Appl. 2012, 2012, 850390. [Google Scholar] [CrossRef]
- Finke, B.; Polak, M.; Hempel, F.; Rebl, H.; Zietz, C. Antimicrobial potential of copper-containing titanium surfaces generated by ion implantation and dual high power impulse magnetron sputtering. Adv. Eng. Mater. 2012, 14, B224–B230. [Google Scholar] [CrossRef]
- Patenge, N.; Arndt, K.; Eggert, T.; Zietz, C.; Kreikemeyer, B. Evaluation of antimicrobial effects of novel implant materials by testing the prevention of biofilm formation using a simple small scale medium-throughput growth inhibition assay. Biofouling 2012, 28, 267–277. [Google Scholar] [CrossRef]
- Stranak, V.; Wulff, H.; Rebl, H.; Zietz, C.; Arndt, K. Deposition of thin titanium–copper films with antimicrobial effect by advanced magnetron sputtering methods. Mater. Sci. Eng. C 2011, 31, 1512–1519. [Google Scholar] [CrossRef]
- Amberg, M.; Vandenbossche, M.; Hegemann, D. Controlled Ag release from electrically conductive coating systems. Surf. Coat. Technol. 2018, 336, 29–33. [Google Scholar] [CrossRef]
- Nißen, S.; Heeg, J.; Wienecke, M.; Behrend, D.; Warkentin, M. Enhancing adhesion strength of a-C:H:Cu composite coatings on Ti6Al4V by graded copper deposition in a rf-PVD/PECVD hybrid process. Surf. Coat. Technol. 2018, 350, 659–671. [Google Scholar] [CrossRef]
- Nelis, T.; Payling, R. Glow discharge optical emission spectroscopy: A practical guide. R. Soc. Chem. 2007. [Google Scholar]
- March, G.; Nguyen, T.D.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Sun, L.; Li, X.; Xu, S.; Ke, P. Structural properties and surface wettability of Cu-containing diamond-like carbon films prepared by a hybrid linear ion beam deposition technique. Thin Solid Films 2015, 584, 289–293. [Google Scholar] [CrossRef]
- Sun, L.; Guo, P.; Li, X.; Wang, A. Comparative study on structure and wetting properties of diamond-like carbon films by W and Cu doping. Diam. Relat. Mater. 2017, 73, 278–284. [Google Scholar] [CrossRef]
- Ma, W.J.; Ruys, A.J.; Mason, R.S.; Martin, P.J.; Bendavid, A. DLC coatings: Effects of physical and chemical properties on biological response. Biomaterials 2007, 28, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Salgueiredo, E.; Vila, M.; Silva, M.A.; Lopes, M.A.; Santos, J.D. Biocompatibility evaluation of DLC-coated Si3N4 substrates for biomedical applications. Diam. Relat. Mater. 2008, 17, 878–881. [Google Scholar] [CrossRef]
- Kwok, S.C.H.; Wang, J.; Chu, P.K. Surface energy, wettability, and blood compatibility phosphorus doped diamond-like carbon films. Diam. Relat. Mater. 2005, 14, 78–85. [Google Scholar] [CrossRef]
- Yang, P.; Huang, N.; Leng, Y.X.; Yao, Z.Q.; Zhou, H.F. Wettability and biocompatibility of nitrogen-doped hydrogenated amorphous carbon films: Effect of nitrogen. Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 242, 22–25. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Wang, C.; Wang, S. Bacterial adhesion on silicon-doped diamond-like carbon films. Diam. Relat. Mater. 2007, 16, 1682–1687. [Google Scholar] [CrossRef]
- Logothetidis, S. Haemocompatibility of carbon based thin films. Diam. Relat. Mater. 2007, 16, 1847–1857. [Google Scholar] [CrossRef]
- Jelínek, M.; Smetana, K.; Kocourek, T.; Dvořánková, B.; Zemek, J.; Remsa, J.; Luxbacher, T. Biocompatibility and sp3/sp2 ratio of laser created DLC films. Mater. Sci. Eng. B 2010, 169, 89–93. [Google Scholar]
- Wei, C.; Pan, W.J.; Hung, M.S. The effects of substrate roughness and associated surface properties on the biocompatibility of diamond-like carbon films. Surf. Coat. Technol. 2013, 224, 8–17. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Huang, M.S.; Chen, L.K.; Shen, Y.D.; Lin, M.H. Surface properties of copper-incorporated diamond-like carbon films deposited by hybrid magnetron sputtering. Ceram. Int. 2013, 39, 8335–8340. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]







| Sample | Ra (nm) | σR (nm) | Rq (nm) |
|---|---|---|---|
| polished Ti6Al4V substrate | 1.36 | 0.017 | 1.94 |
| Ti/TixCy/a-C:H:Ti/a-C:H | 2.23 | 0.012 | 3.61 |
| a-C:H:Cu XCu = 12% | 3.01 | 0.013 | 5.35 |
| a-C:H:Cu XCu = 31% | 3.11 | 0.018 | 6.23 |
| a-C:H:Cu XCu = 55% | 4.04 | 0.014 | 6.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nißen, S.; Heeg, J.; Wienecke, M.; Behrend, D.; Warkentin, M.; Rokosz, K.; Gaiaschi, S.; Chapon, P. Surface Characterization and Copper Release of a-C:H:Cu Coatings for Medical Applications. Coatings 2019, 9, 119. https://doi.org/10.3390/coatings9020119
Nißen S, Heeg J, Wienecke M, Behrend D, Warkentin M, Rokosz K, Gaiaschi S, Chapon P. Surface Characterization and Copper Release of a-C:H:Cu Coatings for Medical Applications. Coatings. 2019; 9(2):119. https://doi.org/10.3390/coatings9020119
Chicago/Turabian StyleNißen, Stefan, Jan Heeg, Marion Wienecke, Detlef Behrend, Mareike Warkentin, Krzysztof Rokosz, Sofia Gaiaschi, and Patrick Chapon. 2019. "Surface Characterization and Copper Release of a-C:H:Cu Coatings for Medical Applications" Coatings 9, no. 2: 119. https://doi.org/10.3390/coatings9020119
APA StyleNißen, S., Heeg, J., Wienecke, M., Behrend, D., Warkentin, M., Rokosz, K., Gaiaschi, S., & Chapon, P. (2019). Surface Characterization and Copper Release of a-C:H:Cu Coatings for Medical Applications. Coatings, 9(2), 119. https://doi.org/10.3390/coatings9020119







