A Micro-Tomographic Insight into the Coating Systems of Historical Bowed String Instruments
Abstract
:1. Introduction
2. Experimental
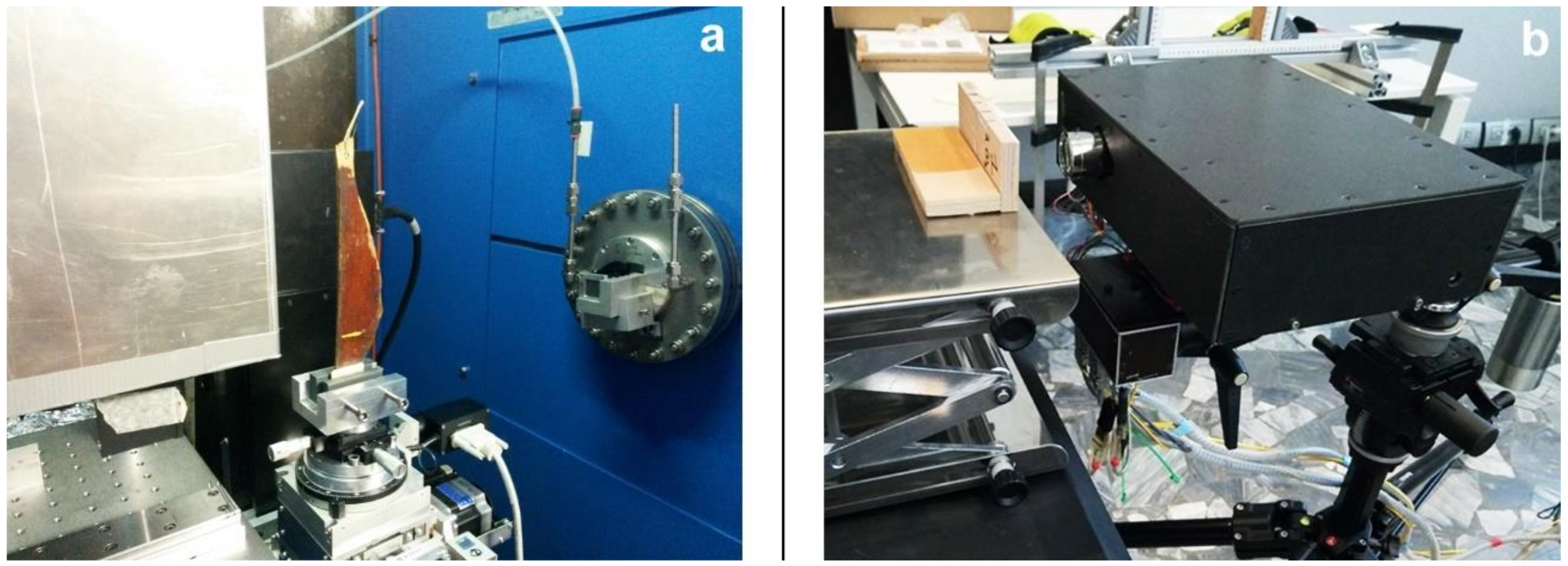
2.1. Synchrotron Radiation Micro-Computed Tomography
2.2. Optical Coherence Tomography
3. Results and Discussion
3.1. F01-Jacobus Stainer
3.2. F13-Gasparo da Salò
3.3. F16-Gasparo da Salò
3.4. F20-Giovanni Paolo Maggini
3.5. F21-Lorenzo Guadagnini
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Setragno, F.; Zanoni, M.; Antonacci, F.; Sarti, A.; Malagodi, M.; Rovetta, T.; Invernizzi, C. Feature-based analysis of the impact of ground coat and varnish on violin tone qualities. Acta Acust. United Acust. 2017, 103, 80–93. [Google Scholar] [CrossRef]
- Jalovec, K. Beautiful Italian Violins; P. Hamlyn: London, UK, 1963. [Google Scholar]
- Fichera, G.V.; Albano, M.; Fiocco, G.; Invernizzi, C.; Licchelli, M.; Malagodi, M.; Rovetta, T. Innovative monitoring plan for the preventive conservation of historical musical instruments. Stud. Conserv. 2017, 63, 351–354. [Google Scholar] [CrossRef]
- Invernizzi, C.; Fichera, G.V.; Licchelli, M.; Malagodi, M. A non-invasive stratigraphic study by reflection FT-IR spectroscopy and UV-induced fluorescence technique: The case of historical violins. Microchem. J. 2018, 138, 273–281. [Google Scholar] [CrossRef]
- Invernizzi, C.; Rovetta, T.; Licchelli, M.; Malagodi, M. Mid and Near-Infrared Reflection Spectral Database of Natural Organic Materials in the Cultural Heritage Field. Int. J. Anal. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rovetta, T.; Invernizzi, C.; Licchelli, M.; Cacciatori, F.; Malagodi, M. The elemental composition of Stradivari’s musical instruments: New results through non-invasive EDXRF analysis. X-ray Spectrom. 2018, 47, 159–170. [Google Scholar] [CrossRef]
- Dondi, P.; Lombardi, L.; Invernizzi, C.; Rovetta, T.; Malagodi, M.; Licchelli, M. Automatic analysis of UV induced fluorescence imagery of historical violins. JOCCH 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Invernizzi, C.; Daveri, A.; Vagnini, M.; Malagodi, M. Non-invasive identification of organic materials in historical stringed musical instruments by reflection infrared spectroscopy: A methodological approach. Anal. Bioanal. Chem. 2017, 409, 3281–3288. [Google Scholar] [CrossRef]
- Daher, C.; Drieu, L.; Bellot-Gurlet, L.; Percot, A.; Paris, C.; Le Hô, A.-S. Combined approach of FT-Raman, SERS and IR micro-ATR spectroscopies to enlighten ancient technologies of painted and varnished works of art. J. Raman Spectrosc. 2014, 45, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Rovetta, T.; Invernizzi, C.; Fiocco, G.; Albano, M.; Licchelli, M.; Gulmini, M.; Alf, G.; Fabbri, D.; Rombolà, A.G.; Malagodi, M. The case of Antonio Stradivari 1718 ex-San Lorenzo violin: History, restorations and conservation perspectives. J. Archaeolog. Sci. Rep. 2019, 23, 443–450. [Google Scholar] [CrossRef]
- Fichera, G.V.; Rovetta, T.; Fiocco, G.; Alberti, G.; Invernizzi, C.; Licchelli, M.; Malagodi, M. Elemental analysis as statistical preliminary study of historical musical instruments. Microchem. J. 2018, 137, 309–317. [Google Scholar] [CrossRef]
- Fiocco, G.; Rovetta, T.; Gulmini, M.; Piccirillo, A.; Licchelli, M.; Malagodi, M. Spectroscopic analysis to characterize finishing treatments of ancient bowed string instruments. Appl. Spectrosc. 2017, 71, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Echard, J.P.; Lavedrine, B. Review on the Characterisation of ancient stringed musical instruments varnishes and implementation of an analytical strategy. J. Cult. Heritage 2008, 9, 420–429. [Google Scholar] [CrossRef]
- Barlow, C.Y.; Woodhouse, J. Of old wood and varnish: Peering into the can of worms. Catgut. Acoust. Soc. J. Ser. II 1989, 1, 2–9. [Google Scholar]
- Nagyvary, J.; Guillemette, R.N.; Spiegelman, C.H. Mineral preservatives in the wood of Stradivari and Guarneri. PLoS ONE 2009, 4, e4245. [Google Scholar] [CrossRef] [PubMed]
- Echard, J.P.; Bertrand, L.; von Bohlen, A.; Le Ho, A.S.; Paris, C.; Bellot-Gurlet, L.; Soulier, B.; Lattuati-Derieux, A.; Thao, S.; Robinet, L.; et al. The nature of the extraordinary finish of Stradivari’s instruments. Angew. Chem. 2010, 49, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Tai, B.H. Stradivari’s Varnish: A review of scientific findings—Part I. J. Violin Soc. Am. 2007, 21, 119–144. [Google Scholar]
- Fiocco, G.; Rovetta, T.; Gulmini, M.; Piccirillo, A.; Canevari, C.; Licchelli, M.; Malagodi, M. Approaches for detecting madder lake in multi-layered coating systems of historical bowed string instruments. Coatings 2018, 8, 171. [Google Scholar] [CrossRef]
- Caruso, F.; Chillura Martino, D.F.; Saverwyns, S.; Van Bos, M.; Burgio, L.; Di Stefano, C.; Peschke, G.; Caponetti, E. Micro-analytical identification of the components of varnishes from South Italian historical musical instruments by PLM, ESEM-EDX, microFTIR, GC–MS, and Py–GC–MS. Microchem. J. 2014, 116, 31–40. [Google Scholar] [CrossRef]
- Tai, B.H. Stradivari’s Varnish: A review of scientific findings—Part II. J. Violin Soc. Am. 2009, 22, 1–31. [Google Scholar]
- Echard, J.P. In situ multi-element analyses by energy-dispersive X-ray fluorescence on varnishes of historical violins. Spectrochim. Acta B 2004, 59, 1663–1667. [Google Scholar] [CrossRef]
- Michelman, J. Violin Varnish: A Plausible Re-Creation of the Varnish Used by the Italian Violin Makers Between the Years 1550 and 1750, A.D., 1st ed.; Joseph Michelman: Cincinnati, OH, USA, 1946; pp. 58–154. [Google Scholar]
- Morigi, M.P.; Casali, F.; Bettuzzi, M.; Brancaccio, R.; D’Errico, V. Application of X-ray Computed Tomography to Cultural Heritage diagnostics. Appl. Phys. A 2010, 100, 653. [Google Scholar] [CrossRef]
- Borman, T.; Stoel, B. Review of the Uses of Computed Tomography for Analyzing Instruments of the Violin Family with a Focus on the Future. J. Violin Soc. Am. VSA Pap. 2009, 22, 1–12. [Google Scholar]
- Latour, G.; Echard, J.P.; Soulier, B.; Emond, I.; Vaiedelich, S.; Elias, M. Structural and optical properties of wood and wood finishes studied using optical coherence tomography: Application to an 18th century Italian violin. Appl. Opt. 2009, 48, 6485–6491. [Google Scholar] [CrossRef] [PubMed]
- Sedighi-Gilani, M.; Pflaum, J.; Hartmann, S.; Kaufmann, R.; Baumgartner, M.; Schwarze, F.W.M.R. Relationship of vibro-mechanical properties and microstructure of wood and varnish interface in string instruments. Appl. Phys. A 2016, 122, 260. [Google Scholar] [CrossRef]
- Lämmlein, S.L.; Mannes, D.; Schwarze, F.W.M.R.; Burgert, I.; Sedighi-Gilani, M. Combined Experimental and Numerical Investigation of Vibro-Mechanical Properties of Varnished Wood for Stringed Instruments. In Model Validation and Uncertainty Quantification, Volume 3; Barthorpe, R., Platz, R., Lopez, I., Moaveni, B., Papadimitriou, C., Eds.; Springer: Berlin, Germany, 2017; pp. 81–83. [Google Scholar]
- Re, A.; Lo Giudice, A.; Nervo, M.; Buscaglia, P.; Luciani, P.; Borla, M.; Greco, C. The importance of tomography studying wooden artefacts: A comparison with radiography in the case of a coffin lid from Ancient Egypt. Int. J. Conserv. Sci. 2016, 7, 935–944. [Google Scholar]
- Re, A.; Corsi, J.; Demmelbauer, M.; Martini, M.; Mila, G.; Ricci, C. X-ray tomography of a soil block: A useful tool for the restoration of archaeological finds. Heritage Sci. 2015, 3, 4. [Google Scholar] [CrossRef]
- Re, A.; Albertin, F.; Avataneo, C.; Brancaccio, R.; Corsi, J.; Cotto, G.; De Blasi, S.; Dughera, G.; Durisi, E.; Ferrarese, W.; et al. X-ray tomography of large wooden artworks: The case study of “Doppio corpo” by Pietro Piffetti. Heritage Sci. 2014, 2, 19. [Google Scholar] [CrossRef]
- Sodini, N.; Dreossi, D.; Chen, R.; Fioravanti, M.; Giordano, A.; Herrestal, P.; Rigon, L.; Zanini, F. Non-invasive microstructural analysis of bowed stringed instruments with synchrotron radiation X-ray microtomography. J. Cult. Heritage 2012, 13, S44–S49. [Google Scholar] [CrossRef]
- Sodini, N.; Dreossi, D.; Giordano, A.; Kaiser, J.; Zanini, F.; Zikmund, T. Comparison of different experimental approaches in the tomographic analysis of ancient violins. J. Cult. Heritage 2017, 27, S88–S92. [Google Scholar] [CrossRef]
- Fiocco, G.; Rovetta, T.; Malagodi, M.; Licchelli, M.; Gulmini, M.; Lanzafame, G.; Zanini, F.; Lo Giudice, A.; Re, A. Synchrotron radiation micro-computed tomography for the investigation of finishing treatments in historical bowed string instruments: Issues and perspectives. Eur. Phys. J. Plus 2018, 133, 525. [Google Scholar] [CrossRef]
- Liang, H.; Cid, M.G.; Cucu, R.G.; Dobre, G.M.; Podoleanu, A.G.; Pedro, J.; Saunders, D. En-face optical coherence tomography—A novel application of non-invasive imaging to art conservation. Opt. Express 2005, 13, 6133–6144. [Google Scholar] [CrossRef] [PubMed]
- Targowski, P.; Rouba, B.J.; Wojtkowski, M.; Kowalczyk, A. Application of optical coherence tomography to non-destructive examination of museum objects. Stud. Conserv. 2004, 49, 107–114. [Google Scholar] [CrossRef]
- Targowski, P.; Iwanicka, M.; Rouba, B.J.; Frosinini, C. OCT for Examination of Artwork. In Optical Coherence Tomography: Technology and Applications; Drexler, W., Fujimoto, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 2473–2495. [Google Scholar]
- Targowski, P.; Iwanicka, M. Optical Coherence Tomography: Its role in the non-invasive structural examination and conservation of cultural heritage objects—A review. Appl. Phys. A 2012, 106, 265–277. [Google Scholar] [CrossRef]
- Spotti, G. Gaetano e Pietro Sgarabotto. Liutai-Violin Makers, (1878–1990); Turris: Cremona, Italy, 1991. [Google Scholar]
- Cloetens, P.; Pateyron-Salomé, M.; Buffiere, J.Y.; Peix, G.; Baruchel, J.; Peyrin, F.; Schlenker, M. Observation of microstructure and damage in materials by phase sensitive radiography and tomography. J. Appl. Phys. 1997, 81, 5878–5886. [Google Scholar] [CrossRef]
- Brun, F.; Massimi, L.; Fratini, M.; Dreossi, D.; Billè, F.; Accardo, A.; Pugliese, R.; Cedola, A. SYRMEP Tomo Project: A graphical user interface for customizing CT reconstruction workflows. Adv. Struct. Chem. Imaging 2017, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Brun, F.; Accardo, A.; Kourousias, G.; Dreossi, D.; Pugliese, R. Effective implementation of ring artifacts removal filters for synchrotron radiation microtomographic images. In Proceedings of the International Symposium on Image and Signal Processing and Analysis (ISPA 2013), Trieste, Italy, 4–6 September 2013; Ramponi, G., Loncaric, S., Carini, A., Egiazarian, K., Eds.; IEEE: Trieste, Italy, 2013; Volume 17, pp. 672–676. [Google Scholar]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, G.T. Image Reconstruction from Projections: The Fundamentals of Computerized Tomography, 1st ed.; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Iwanicka, M.; Lanterna, G.; Lalli, C.G.; Innocenti, F.; Sylwestrzak, M.; Targowski, P. On the application of optical coherence tomography as a complimentary tool in an analysis of the 13th century Byzantine Bessarion reliquary. Microchem. J. 2016, 125, 75–84. [Google Scholar] [CrossRef]
- Oct4art—Optical Choerence Tomography for Examination of Work of Art. Available online: http://www.oct4art.eu/ (accessed on 20 December 2018).






| Fragment | Layer | Microscopic Examination & Chemical Analyses [11,12] | SR-Micro-CT | OCT |
|---|---|---|---|---|
| J. Stainer F01-Cello | D | Not detected | 10-µm thick layer with particles | 26–33 µm thick layer with particles |
| C | 30-µm thick oil-based varnish containing Fe-based pigments | 20-µm thick layer with particles | ||
| B | 15-µm thick proteinaceous binder containing carbonate-based particles | 10–15 µm thick layer with particles | 12-µm thick layer | |
| A | Maple wood | Maple wood | Wood substrate (not recognized) | |
| Gasparo da Salò F13-Cello | D | Layer with organic black particles | 10–15 µm thick layer with particles | 15-µm thick layer composed by two thin layers with particles |
| 10-µm thick layer with inorganic particles (5 µm) | ||||
| C | 5-µm thick layer with inorganic particles | 10–15 µm thick layer with particles | ||
| 10-µm thick layer with Fe-based pigments and gypsum grains (5–10 µm) | ||||
| B | Proteinaceous binder with black particles and Fe-based particles | Not detected | Thin layer detected | |
| A | Maple wood | Maple wood | Wood substrate (not recognized) | |
| Gasparo da Salò F16-Cello | C | 5-µm thick layer embedding organic black particles | Layer with two types of particles identified through grayscale variations | 10–19 µm thick layer with particles |
| B | 20-µm thick layer with Fe-based (2–3 µm) and Pb-based (15–20 µm) particles | 23–26 µm thick layer with particles | ||
| A | Maple wood | Maple wood | Wood substrate (not recognized) | |
| G.P. Maggini F20-Double Bass | C | 30-µm thick varnish layer with Fe-based particles (2–3 µm) | 30-µm thick layer with particles (2–3 µm) | 19–36 µm thick layer |
| B | Not detected | Not detected | 9–12 µm thick layer | |
| A | Maple wood | Maple wood | Wood substrate (not recognized) | |
| L. Guadagnini F21-Double Bass | C | 50-µm thick layer | Layer with cracks, air bubbles and particles | 30–50 µm thick layer with cracks and particles |
| B | 10 µm thick layer with Fe-based and gypsum particles | Layer with particles | 12–15 µm thick layer | |
| A | Spruce wood | Spruce wood | Wood substrate (not recognized) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiocco, G.; Rovetta, T.; Invernizzi, C.; Albano, M.; Malagodi, M.; Licchelli, M.; Re, A.; Lo Giudice, A.; Lanzafame, G.N.; Zanini, F.; et al. A Micro-Tomographic Insight into the Coating Systems of Historical Bowed String Instruments. Coatings 2019, 9, 81. https://doi.org/10.3390/coatings9020081
Fiocco G, Rovetta T, Invernizzi C, Albano M, Malagodi M, Licchelli M, Re A, Lo Giudice A, Lanzafame GN, Zanini F, et al. A Micro-Tomographic Insight into the Coating Systems of Historical Bowed String Instruments. Coatings. 2019; 9(2):81. https://doi.org/10.3390/coatings9020081
Chicago/Turabian StyleFiocco, Giacomo, Tommaso Rovetta, Claudia Invernizzi, Michela Albano, Marco Malagodi, Maurizio Licchelli, Alessandro Re, Alessandro Lo Giudice, Gabriele N. Lanzafame, Franco Zanini, and et al. 2019. "A Micro-Tomographic Insight into the Coating Systems of Historical Bowed String Instruments" Coatings 9, no. 2: 81. https://doi.org/10.3390/coatings9020081
APA StyleFiocco, G., Rovetta, T., Invernizzi, C., Albano, M., Malagodi, M., Licchelli, M., Re, A., Lo Giudice, A., Lanzafame, G. N., Zanini, F., Iwanicka, M., Targowski, P., & Gulmini, M. (2019). A Micro-Tomographic Insight into the Coating Systems of Historical Bowed String Instruments. Coatings, 9(2), 81. https://doi.org/10.3390/coatings9020081













