Influence of ALD Coating Layers on the Optical Properties of Nanoporous Alumina-Based Structures
Abstract
:1. Introduction
2. Materials and Methods
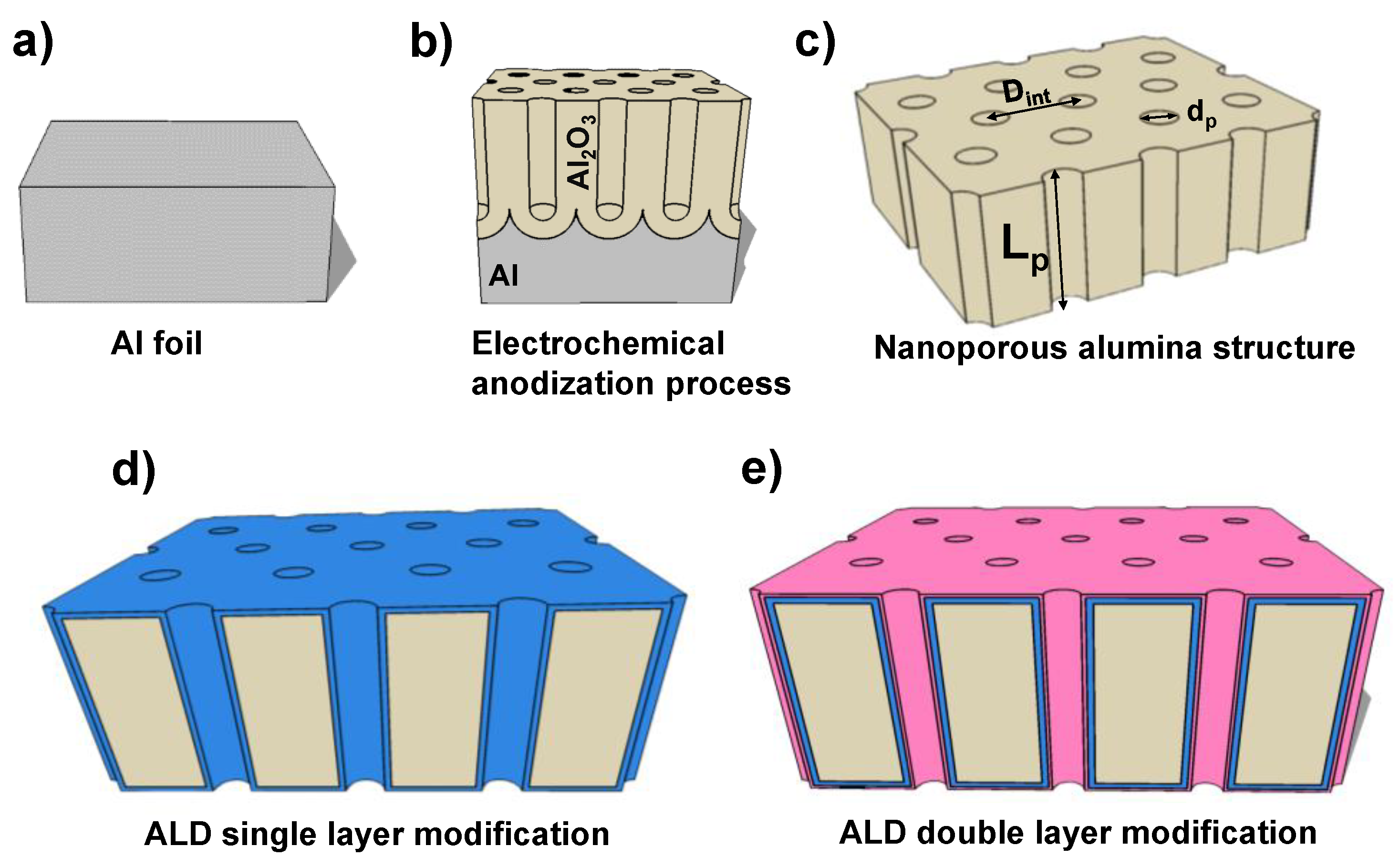
2.1. NPAS Fabrication and Surfaces Coating by Atomic Layer Deposition Method
2.2. Chemical Surface Analysis
2.3. Spectroscopic Ellipsometry Measurements
2.4. Transmittance Measurements
3. Results and Discussion
3.1. Chemical Characterization of the Nanoporous Alumina-Based Structures
3.2. Optical Characterization of the Nanoporous Alumina-Based Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- McInnes, S.J.; Irani, Y.; Williams, K.A.; Voelcker, N.H. Controlled drug delivery from composites of nanostructured porous silicon and poly (l-lactide). Nanomedicine 2012, 7, 995–1016. [Google Scholar] [CrossRef] [PubMed]
- Law, C.S.; Lim, S.Y.; Abell, A.D.; Voelcker, N.H.; Santos, A. Nanoporous anodic alumina photonic crystals for optical chemo- and biosensing: Fundamentals, advances, and perspectives. Nanomaterials 2018, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Ingham, C.J.; ter Maat, J.; de Vos, W.M. Where bio meets nano: The many uses for nanoporous aluminum oxide in biotechnology. Biotechnol. Adv. 2012, 30, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Valeev, R.G.; Petukhov, D.I.; Kriventsov, V.V. Structure and optical properties of thin porous anodic alumina films synthesized on a glass surface. Phys. Procedia 2016, 84, 415–420. [Google Scholar] [CrossRef]
- Santos, A.; Formentín, P.; Pallarès, J.; Ferré-Borrull, J.; Marsal, L.F. Structural engineering of nanoporous anodic alumina funnels with high aspect ratio. J. Electroanal. Chem. 2011, 655, 73–78. [Google Scholar] [CrossRef]
- Loh, J.Y.Y.; Kherani, N. Design of nano-porous multilayer antireflective coatings. Coatings 2017, 7, 134. [Google Scholar] [CrossRef]
- Kumeria, T.; Santos, A.; Losic, D. Nanoporous anodic alumina platforms: Engineered surface chemistry and structure for optical sensing applications. Sensors 2014, 14, 11878–11918. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Hernández-Vélez, M.; Manova, E.; Ruiz-Hitzky, E. Structured thin organic active layers and their use in electrochemical biosensors. Thin Solid Films 2006, 495, 321–326. [Google Scholar] [CrossRef]
- Schwirn, K.; Lee, W.; Hillebrand, R.; Steinhart, M.; Nielsch, K.; Gösele, U. Self-ordered anodic aluminum oxide formed by H2SO4 hard anodization. ACS Nano 2008, 2, 302–310. [Google Scholar] [CrossRef]
- Romero, V.; Vega, V.; García, J.; Prida, V.M.; Hernando, B.; Benavente, J. Ionic transport across tailored nanoporous anodic alumina membranes. J. Colloids Interface Sci. 2012, 376, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Velleman, L.; Kant, K.; Kumeria, T.; Gulati, K.; Shapter, J.G.; Beattie, D.A.; Simovic, S. Self-ordering electrochemistry: A simple approach for engineering nanopore and nanotube arrays for emerging applications. Aust. J. Chem. 2011, 64, 294–301. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Simovic, S. Self-ordered nanopore and nanotube platforms for drug delivery applications. Exp. Opin. Drug Deliv. 2009, 6, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Porta-i-Batalla, M.; Xifré-Pérez, E.; Eckstein, C.; Ferré-Borrull, J.; Marsal, L.F. 3D nanoporous anodic alumina structures for sustained drug release. Nanomaterials 2017, 7, 227. [Google Scholar] [CrossRef] [PubMed]
- Velleman, L.; Triani, G.; Evans, P.J.; Shapter, J.G.; Losic, D. Structural and chemical modification of porous alumina membranes. Microporous Mesoporous Mater. 2009, 126, 87–94. [Google Scholar] [CrossRef]
- Romero, V.; Vega, V.; García, J.; Zierold, R.; Nielsch, K.; Prida, V.M.; Hernando, B.; Benavente, J. Changes in morphology and ionic transport induced by ALD SiO2 coating of nanoporous alumina membranes. ACS Appl. Mater. Interfaces 2013, 5, 3556–3564. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Homola, T.; Bryukvin, A.; Cameron, D.C. Catalytic performance of Ag2O and Ag doped CeO2 prepared by atomic layer deposition for diesel soot oxidation. Coatings 2018, 8, 237. [Google Scholar] [CrossRef]
- Elam, J.W.; Routkevitch, D.; Mardilovich, P.P.; George, S.M. Conformal coating on ultrahigh-aspect-ratio nanopores of anodic alumina by atomic layer deposition. Chem. Mater. 2003, 15, 3507–3517. [Google Scholar] [CrossRef]
- Vega, V.; Gelde, L.; González, A.S.; Prida, V.M.; Hernando, B.; Benavente, J. Diffusive transport through surface functionalized nanoporous alumina membranes by atomic layer deposition of metal oxides. J. Ind. Eng. Chem. 2017, 52, 66–72. [Google Scholar] [CrossRef]
- Weber, M.; Julbe, A.; Ayral, A.; Miele, P.; Bechelany, M. Atomic layer deposition for membranes: Basics, challenges, and opportunities. Chem. Mater. 2018, 30, 7368–7390. [Google Scholar] [CrossRef]
- Moghadam, H.; Samimi, A.; Behzadmehr, A. Effect of nanoporous anodic aluminum oxide (AAO) characteristics on solar absorptivity. Transp. Phenom. Nano Micro Scales 2013, 1, 110–116. [Google Scholar] [CrossRef]
- Thompson, D.W.; Snyder, P.G.; Castro, L.; Yan, L.; Kaipa, P.; Woollam, J.A. Optical characterization of porous alumina from vacuum ultraviolet to midinfrared. J. Appl. Phys. 2005, 97, 113511. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Finch, D.S.; Oreskovic, T.; Ramadurai, K.; Herrmann, C.F.; George, S.M.; Mahajan, R.L. Biocompatibility of atomic layer-deposited alumina thin films. J. Biomed. Mater. Res. A 2008, 87A, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernández, E.; Baeza, A.; Vallet-Regí, M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Mundle, R.M.; Santiago, K.; Skuza, J.R.; Xiao, B.; Song, K.D.; Bahoura, M.; Cheaito, R.; Hopins, P.E. Extreme tunability in aluminum doped Zinc Oxide plasmonic materials for near-infrared applications. Sci. Rep. 2014, 4, 6415. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, J.; Zierold, R.; Chong, Y.T.; Hauert, R.; Sturm, C.; Schmidt-Grund, R.; Rheinländer, B.; Grundmann, M.; Gösele, U.; Nielsch, K. A practical, self-catalytic, atomic layer deposition of silicon dioxide. Angew. Chem. Int. Ed. 2008, 47, 6177–6179. [Google Scholar] [CrossRef]
- Gelde, L.; Cuevas, A.L.; Martínez de Yuso, M.D.V.; Benavente, J.; Vega, V.; González, A.S.; Prida, V.M.; Hernando, B. Influence of TiO2-coating layer on nanoporous alumina membranes by ALD technique. Coatings 2018, 8, 60. [Google Scholar] [CrossRef]
- Meng, X.; Banis, M.N.; Geng, D.; Li, X.; Zhang, Y.; Li, R.; Abou-Rachidb, H.; Sun, X. Controllable atomic layer deposition of one-dimensional nanotubular TiO2. Appl. Surf. Sci. 2013, 266, 132–140. [Google Scholar] [CrossRef]
- Iglesias, L.; Vega, V.; García, J.; Hernando, B.; Prida, V.M. Development of electrostatic supercapacitors by atomic layer deposition on nanoporous anodic aluminum oxides for energy harvesting applications. Front. Phys. 2005, 3, 12. [Google Scholar] [CrossRef]
- Banerjee, P.; Lee, W.-J.; Bae, K.-R.; Lee, S.B.; Rubloff, G.W. Structural, electrical, and optical properties of atomic layer deposition Al-doped ZnO films. J. Appl. Phys. 2010, 108, 043504. [Google Scholar] [CrossRef]
- Fernández-Menéndez, L.J.; González, A.S.; Vega, V.; Prida, V.M. Electrostatic supercapacitors by atomic layer deposition on nanoporous anodic alumina templates for environmentally sustainable energy storage. Coatings 2018, 8, 403. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Cumpson, P.J. Angle-resolved XPS and AES: Depth-resolution limits and a general comparison of properties of depth-profile reconstruction methods. J. Electron. Spectrosc. Relat. Phenom. 1995, 73, 25–52. [Google Scholar] [CrossRef]
- Ariza, M.J.; Benavente, J.; Rodríguez-Castellón, E. The capability of X-ray photoelectron spectroscopy in the characterization of membranes: Correlation between surface chemical and transport properties in polymeric membranes. In X-ray Photoelectron Spectroscopy; Wagner, J.M., Ed.; Nova Publishers: Hauppauge, NY, USA, 2009; pp. 257–290. [Google Scholar]
- La Flamme, K.E.; Popat, K.C.; Leoni, L.; Markiewicz, E.; LaTempa, T.J.; Roman, B.B.; Grimes, C.A.; Desai, T.A. Biocompatibility of nanoporous alumina membranes for immunoisolation. Biomaterials 2007, 28, 2638–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheow, P.-S.; Liu, L.; Toh, C.-S. Grafting of nanoporous alumina membranes and films with organic acids. Surf. Interface Anal. 2007, 39, 601–610. [Google Scholar] [CrossRef]
- Hou, Q.; Meng, F.; Sun, J. Electrical and optical properties of Al-doped ZnO and ZnAl2O4 films prepared by atomic layer deposition. Nanoscale Res. Lett. 2013, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, W.H.; Cook, E.L. Compilation of energy band gaps in elemental and binary compound semiconductors and insulators. J. Phys. Chem. Ref. Data 1973, 2, 163–199. [Google Scholar] [CrossRef]
- Losurdo, M.; Begmair, M.; Bruno, G.; Cattelan, D.; Cobet, C.; De Martina, A.; Fleischer, K.; Dohcevic-Mitrovic, Z.; Esser, N.; Galliet, M.; et al. Spectroscopic ellipsometry and polarimetry for materials and systems analysis at the nanoscale: State-of-art, potential, and perspectives. J. Nanopart. Res. 2009, 11, 1521–1554. [Google Scholar] [CrossRef] [PubMed]
- Gâlcä, A.C.; Kooij, E.S.; Wormeester, H.; Salm, C.; Leca, V.; Rector, J.H.; Poelsema, B. Structural and optical characterizations of porous anodic aluminium oxide. J. Appl. Phys. 2003, 94, 4296–4305. [Google Scholar] [CrossRef]
- RefractiveIndex.INFO. Available online: https://refractiveindex.info/ (accessed on 14 January 2019).
- Tompkins, G.; Hilfiker, J.N. Spectroscopic Ellipsometry: Practical Application to Thin Film Characterization, 1st ed.; Momentum Press: New York, NY, USA, 2016; pp. 57–70. [Google Scholar]
- Taherniya, A.; Roufi, D. Thickness dependence on structural, optical and morphological properties of sol-gel derived TiO2 thin film. Mater. Res. Express 2019, 6, 016417. [Google Scholar] [CrossRef]






| Material (Substrate Temperature) | Precursors (Precursor Temperature) | t1 (s) | t2 (s) | t3 (s) |
|---|---|---|---|---|
| ZnO (200 °C) | H2O (60 °C) | 0.1 | 90 | 180 |
| (C2H5)2Zn (25 °C) | 0.05 | 90 | 180 | |
| Al2O3 (200 °C) | H2O (60 °C) | 0.1 | 90 | 180 |
| Al2(CH3)6 (25 °C) | 0.05 | 90 | 180 | |
| TiO2 (200 °C) | H2O (60 °C) | 1 | 60 | 120 |
| Ti[OCH(CH3)2]4 (75 °C) | 1 | 60 | 60 | |
| SiO2 (150 °C) | H2O (60 °C) | 1 | 60 | 120 |
| O3 (25 °C) | 0.1 | 60 | 120 | |
| H2N(CH2)3Si(OC2H5)3 (100 °C) | 2 | 60 | 120 |
| Sample | O (%) | Al (%) | Si (%) | Ti (%) | Zn (%) |
|---|---|---|---|---|---|
| NPAS + SiO2 | 28.8 | 2.1 | 4.0 | – | – |
| NPAS + TiO2 | 52.2 | 1.5 | – | 21.4 | – |
| NPAS + SiO2 + Al2O3 | 29.1 | 6.5 | 0.3 | – | – |
| NPAS + SiO2 + AZO | 30.5 | 9.0 | 2.3 | – | 0.7 |
| Sample | T (%) at 550 nm | T (%) at 950 nm | Band Gap (nm/eV) |
|---|---|---|---|
| NPAS + SiO2 | 92.0 | 94.6 | 281.8/4.40 |
| NPAS + TiO2 | 83.3 | 86.1 | 346.8/3.58 |
| NPAS + SiO2 + Al2O3 | 86.4 | 89.1 | 308.8/4.02 |
| NPAS + SiO2 + AZO | 78.0 | 90.1 | 312.5/3.87 |
| Sample | n (at 550 nm) | k (at 550 nm) | n (at 950 nm) | k (at 950 nm) |
|---|---|---|---|---|
| NPAS + SiO2 | 1.312 | 0.675 | 1.364 | 1.109 |
| NPAS + TiO2 | 1.693 | 0.663 | 1.704 | 0.837 |
| NPAS + SiO2 + Al2O3 | 1.398 | 0.357 | 1.527 | 0.592 |
| NPAS + SiO2 + AZO | 0.892 | 0.800 | 1.560 | 1.095 |
| Sample | εr (at 550 nm) | εr (at 950 nm) | εi (at 550 nm) | εi (at 950 nm) |
|---|---|---|---|---|
| NPAS + SiO2 | 1.282 | 0.630 | 1.779 | 3.025 |
| NPAS + TiO2 | 2.428 | 2.205 | 2.243 | 2.853 |
| NPAS + SiO2 + Al2O3 | 1.826 | 1.982 | 0.997 | 1.807 |
| NPAS + SiO2 + AZO | 0.156 | 1.235 | 1.427 | 3.418 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, A.L.; Martínez de Yuso, M.d.V.; Vega, V.; González, A.S.; Prida, V.M.; Benavente, J. Influence of ALD Coating Layers on the Optical Properties of Nanoporous Alumina-Based Structures. Coatings 2019, 9, 43. https://doi.org/10.3390/coatings9010043
Cuevas AL, Martínez de Yuso MdV, Vega V, González AS, Prida VM, Benavente J. Influence of ALD Coating Layers on the Optical Properties of Nanoporous Alumina-Based Structures. Coatings. 2019; 9(1):43. https://doi.org/10.3390/coatings9010043
Chicago/Turabian StyleCuevas, Ana L., María del Valle Martínez de Yuso, Víctor Vega, Ana Silvia González, Víctor M. Prida, and Juana Benavente. 2019. "Influence of ALD Coating Layers on the Optical Properties of Nanoporous Alumina-Based Structures" Coatings 9, no. 1: 43. https://doi.org/10.3390/coatings9010043
APA StyleCuevas, A. L., Martínez de Yuso, M. d. V., Vega, V., González, A. S., Prida, V. M., & Benavente, J. (2019). Influence of ALD Coating Layers on the Optical Properties of Nanoporous Alumina-Based Structures. Coatings, 9(1), 43. https://doi.org/10.3390/coatings9010043







